Introduction
The well-studied nematode Caenorhabditis elegans has been widely used as a model for research in many areas of biology, including development, genetics, and aging, among other fields (see Johnson Subficld History). As a model organism that has been the subject of detailed investigations for more than 50 years, it is now arguably the world’s best understood multicellular animal (1–5). The utility of C. elegans for studies of aging (6–9) and age-specific patterns of mortality (10 is due in part to a number of well-characterized mutations that dramatically affect longevity in vitro (6, 7, 10) (see Genes/Interventions Database). Virtually nothing, however, is known about its natural habitat (8, 9, 11, 12), the nematode’s life history in the field, its seasonal abundance, global distribution, or association with other organisms as commensal, parasite, host, or prey.
Publications about C. elegans often begin with a sentence very similar to one of the following: (i) “Caenorhabditis elegans is a small, free-living soil nematode found commonly in many parts of the world” (4); (ii) “Caenorhabditis elegans is a free-living nematode worm that lives in the soil, across most of the temperate regions of the world, feeding on microorganisms” (13); (iii) “C. elegans is a ubiquitous, small (1 to 1.5 mm), free-living nematode that lives in the soil” (9); or, generally, (iv) “C. elegans is a cosmopolitan, soil-dwelling, bacterial-feeding nematode.” Such statements about the natural habitat of this nematode are usually not referenced, suggesting that the “soil” life history is considered common knowledge by the extensive C. elegans research community. This supposition is somewhat surprising given that “There exists a large contrast between extensive knowledge of C. elegans as an experimental system, and great ignorance about its natural environment and ecology” (14). What is known about the natural history of C. elegans has been inferred from those substrates where it has been collected, often from decaying vegetable matter and compost (3, 14–16); however, this organism is not necessarily easy to collect in nature (15, and nematologists working with multispecies communities of soil nematodes do not frequently observe C. elegans in soil samples. Observations made on C. elegans in the laboratory are sometimes presumed to indicate how C. elegans might behave in its putative natural environment--the soil (8, 9, 16). Given that the actual natural environment is uncertain (9, 16), inferences arising from experiments in the laboratory or involving the introduction of C. elegans into soil may be confounded by lack of proper ecological context (see “Death in the Dirt”).
What Is the Natural Ecology of C. elegans?
The canonical wild-type C. elegans strain is N2, a strain originally isolated in 1956 from mushroom compost in England by Warwick Nicholas. This strain arose from the progeny of a single hermaphrodite picked from a nutrient agar plate (17). This strain was moved from the United Kingdom to the Berkeley, California, lab of Ellsworth Dougherty, where research on Caenorhabditis was under way (1, 18–20). An axenic culture of the strain (that is, a culture free of other organisms) was sent from the Dougherty laboratory to Sydney Brenner, who began growing the strain on Escherichia coli. From such culture, “a hermaphrodite was isolated and its progeny used to establish two lines” with the code name N2 (2). Thus, N2 has passed through at least two bottlenecks, and N2 and most other C. elegans isolates (11, 14) were apparently in “domestication” for generations before they were frozen as stock cultures (5, 14). Although methods for maintaining cultures vary, sometimes single gravid hennaphrodites are used to initiate new cultures, causing a founder effect and potentially resulting in inadvertent selection and genetic drift. The in vitro culture of wild-type strains prior to deposition in the Caenorhabditis Genetics Center (CGC) possibly subjected worms to many generations of inadvertent selection for characteristics favored by laboratory conditions (see Reznick Perspective, Spencer Perspective, and “Get Wild”). Long-term culturing and subculturing might select lab strains that are different from wild worms, leading to questions such as how much allelic variation among gerontogenes (see Johnson Subfield History for a derivation of this term) exists in wild populations of C. elegans and how long the worm’s life span is in the wild (see “How Can We Craft a Better Theory to Explain the Evolution of Aging?”).
Most research on C. elegans is performed using laboratory strains (5, 8, 9, 14), and laboratory experiments using the wild-type strains from the CGC are the basis for most published work directed at understanding the natural history of C. elegans, with a few recent notable exceptions (15, 16). Although “wild-type” worms are often studied, they are not necessarily worms collected recently from a wild state in nature.
Why Natural History Is Important in Aging-Related Studies
Aging-related research often focuses on one of two approaches. The first is more mechanistic, with emphasis on the genetics and biochemistry that govern aging. In this approach, aging is considered relative to the function of certain genes during the life of an individual (“ecological time”). The second approach attempts to understand how and why aging occurs as a life-history phenomenon in essentially all organisms--that is, the evolution of life span, aging, and senescence. These questions are addressed relative to the fitness of genotypes and associated phenotypes over longer, geologic-time intervals (“evolutionary time”). The natural history and ecology of C. elegans is important because environment provides the context for the evolution of life-history traits [the ecological theater in which the evolutionary play is performed, to use Hutchinson’s (21) compelling tenninology]. Ecological developmental biology emphasizes the need to forge links between ecology and development and phenotypic plasticity, through the concept that the genome not only acts but reacts in response to environment to yield context-dependent phenotypes (22, 23). The life history of C. elegans includes important context-dependent phenotypes, including androdioecy (a system in which both males and hermaphrodites are present in a breeding population), a dormant dauer stage, and, under some conditions, bagging (facultative vivipary, in which eggs hatch inside the parent, generally resulting in the parent’s death) (24). These traits certainly reflect adaptations to the natural environs and may relate to the evolution of many biological phenomena of interest to C. elegans researchers, including senescence and aging. Understanding C. elegans’ natural history may help researchers complete an improved picture of gene function as it relates to aging, but these points raise the following questions: What are the natural environs of C. elegans, and what are the characteristics of wild C. elegans?
The Search for Wild C. elegans
To determine how to easily and consistently obtain C. elegans from field populations for our research on aging in the wild (see “Get Wild”), we conducted a search of northern California habitats and locations. We collected samples from locations in northern California in an attempt to isolate C. elegans, including one location from which C. elegans was collected previously (Palo Alto, California) (14). Our samples included soil, compost piles, garden soils high in organic matter, mushroom fruiting bodies in various stages of decomposition, and surface layers of forest floors (some of the latter included fungal fruiting bodies or mycelia). Nematodes were extracted from soil by a range of methods, including flotation, sieving, and the use of a Baermann funnel (a nematode collection device). We obtained many bacterial and fungal-feeding nematodes, but not C. elegans.
Based on reports of Caenorhabditis spp. in terrestrial gastropods (25, 26) and conversations with colleagues, we expanded our search to include snails, resampling some of our original locations. Snails were chopped into pieces, and the emerging nematodes were collected and placed on E. coli (strain OP-50) growing on nematode growth medium agar (2). From these, we selected nematodes with morphological characteristics similar to those of C. elegans (26) and then identified them conclusively as such using a combination of characters, including (i) the presence of hermaphrodites with rare males, (ii) the presence of dauers, (iii) bagging (24), and (iv) the fertility of hermaphrodites crossed with N2 males provided by the CGC (and vice versa) (18, 27), with resultant increased frequency of males (or an increase in the “andric index”) as compared with the frequency of males produced by a population of hermaphrodites (18). Additional confirmation was obtained through analysis of 28S ribosomal DNA (rDNA), internal transcribed spacer rDNA, and amplified fragment length polymorphism sequences.
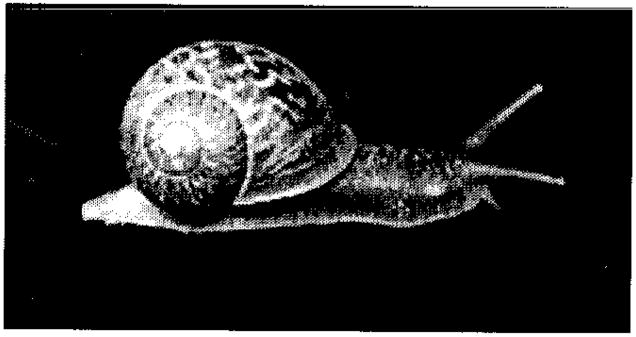
We recovered C. elegans from snails (Helix aspersa) (Fig. 1) in -50% of the California locations where we made these collections, including Davis, Palo Alto, Berkeley, Orange County, and San Diego, and in -25% of the snails assessed. In a recent report on the population genetics of wild C. elegans in France, the worm was collected from compost and invertebrates, including H. aspersa, although the frequency of occurrence was not included (15). Interestingly, H. aspersa is native to Europe and was introduced to San Jose, California, from France in the 1850s (28). It has since become widely established in California and other areas of the United States. Based on our observations, we contend that the association between C. elegans and snails is more than an incidental or casual occurrence. Given that the self-dispersal capabilities of Caenorhabditis spp. are probably quite limited, especially in environments with low humidity and high temperatures, a phoretic relation (in which one organism is transported by another) with snails would presumably enhance C. elegans’ capacity to avoid stressful environments and aid their dispersal to new food sources. Although genetic diversity has been detected among natural isolates of C. elegans (15, 16), and migration among sites suggested, the exact mechanism for spread among sites is uncertain (16) Based on our observations, we infer that C. elegans has a symbiotic association with pulmonate gastropods (members of a subclass of Gastropoda that contain an air-breathing organ). An association with an invasive species of snail, and associated possible movement by man and predators, may help to explain the geographic distribution of C. elegans and its association with human habitation (15).
Fig. 1.
The snail Helix aspersa.
Nematode associations with terrestrial gastropods are not unusual. The phylum Nematoda has eight families with members parasitic to terrestrial gastropods (29). The closely related nematodes C. remanei, C. vulgaris, and C. formosana are considered possible necromenic associates of snails (that is, organisms that become associated with a carrier when it is alive and finish their life cycle when the carrier is dead) (25, 26, 30), and C. remanei also has been recovered from terrestrial isopods, crustaceans that are usually scavengers, including the common pill bug or sow bug (30). On the basis of phylogenetic analyses, it has been suggested that the ability to infect gastropods has evolved independently at least five times in the phylum (29). Comparative assessment of both snail and C. elegans phylogenies and biogeographic histories might be mutually informative.
A symbiotic relation between C. elegans and snails provides a new context for understanding the evolution of the worm and its life-history traits. Certainly, global gene expression patterns are influenced by environmental cues, and investigating environmentally appropriate signals may provide insights into pathways influencing aging. For example, some of the daf genes are well documented as involved in dauer formation and longevity extension, but the natural history of the dauer in C. elegans has generally not been documented, other than that dauers are collected from nature (15). Appropriate ecological context may well be important in defining the life span of wild C. elegans 11 and the influence of longevity genes on fitness (31–33). The comparison of life spans of wild and N2 worms may offer new insights on C. elegans as a nematode paradigm in aging-related research (8).
Ecology of Aging of the Worm
The potential for linking the tremendous understanding of C. elegans genetics with knowledge of its natural history and ecology represents an intriguing opportunity to combine detailed genetic understanding with new insights from ecology. Sydney Brenner noted that in late 1962, as he and Francis Crick discussed their future research, “Both of us felt very strongly that most of the classical problems of molecular biology had been solved and that the future lay in tackling more complex biological problems.” Further, Brenner noted, “I had come to believe that most of molecular biology had become inevitable and that, as I put it in a draft paper, we must move on to other problems of biology which are new, mysterious and exciting” (34). Molecular genetics has opened the door to substantial understanding of genes involved in aging. Brenner’s vision is readily extended to the consideration of other new, mysterious, and exciting aspects of the biology of aging by addressing the complex ecological problems yet to be resolved of how and why organisms age in their natural habitats. Improved understanding of the ecological context of life histories will illuminate questions in development and evolution and present fascinating opportunities for understanding the context for mechanistic theories of aging.
Footnotes
Citation: E. P. Caswell-Chen, J. Chen, E. E. Lewis, G. W. Douhan, S. A. Nadler, J. R. Carey, Revising the Standard Wisdom of C. elegans Natural History: Ecology of Longevity. Sci. Aging Knowl. Environ. 2005 (40), pe30 (2005).
References
- 1.Dougherty EC, Calhoun HG. Possible significance of free-living nematodes in genetic research. Nature. 1948;161:29. doi: 10.1038/161029a0. [DOI] [PubMed] [Google Scholar]
- 2.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. JAbstractlFree Full Textl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgkin J. What does a worm want with 20,000 genes? Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-11-comment2008. Comment2008 [Medlinej. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood WB. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Plainview, NY: 1988. [Google Scholar]
- 5.Johnson TE. Advantages and disadvantages of Caenorhabditis elegans for aging research. Exp Gerontol. 2003;38:1329–1332. doi: 10.1016/j.exger.2003.10.020. [CrossRefI[Medlinel. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenyon C, Chang J, Gensch E. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [CrossRefl[Medlinel. [DOI] [PubMed] [Google Scholar]
- 8.Reznick AZ, Gershon D. Experimentation with nematodes. In: Yu BP, editor. Methods in Aging Research. CRC Press; Boca Raton, FL: 1999. pp. 167–190. [Google Scholar]
- 9.Gershon H, Gershon D. Caenorhabditis elegans--A paradigm for aging research: Advantages and limitations. Mech Ageing Develop. 2002;123:261–274. doi: 10.1016/s0047-6374(01)00401-8. rCrossRefl [Medlinej. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TE, Wu D, Tedesco P, Dames S, Vaupel JW. Age-specific demographic profiles of longevity mutants in Caenorhabditis elegans show segmental effects. J Gerontol Biol Sci. 2001;56A:B331–B339. doi: 10.1093/gerona/56.8.b331. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 11.Gems D, Riddle DL. Defining wild-type life span in Caenorhabditis elegans. J Gerontol Biol Sci. 2000;55A:13215–13219. doi: 10.1093/gerona/55.5.b215. [Medlinel. [DOI] [PubMed] [Google Scholar]
- 12.Denver DR, Morris K, Thomas WK. Phylogenetics in Caenorhabditis elegans: An analysis of divergence and outcrossing. Mol Biol Evol. 2003;20:393–400. doi: 10.1093/molbev/msg044. [Abstract/Free Full Textl. [DOI] [PubMed] [Google Scholar]
- 13.Hope IA. In: Background on Caenorhabditis elegans, in C. elegans: A Practical Approach, I. Hope A, editor. Oxford Univ. Press; Oxford: 1999. pp. 1–15. [Google Scholar]
- 14.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [Abstract/Frec Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [CrossRefl[Medlinej. [DOI] [PubMed] [Google Scholar]
- 16.Haber M, Schungel M, Putz A, Muller S, Hasert B, Schulenburg H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: Evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Mol Biol Evol. 2005;22:160–173. doi: 10.1093/molbev/msh264. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 17.W. Nicholas, personal communication.
- 18.Nigon V, Dougherty EC. Attempts at hybridizing two closely related hermaphroditic species of the nematode genus. Rhabditis Anatom Rec. 1949;105:532–533. [Google Scholar]
- 19.Nicholas WL, Dougherty EC, Hansen EL. Axenic cultivation of Caenorhabditis briggsae (Nematoda: Rhabditidae) with chemically undefined supplements: Comparative studies with related nematodes. Ann N Y Acad Sci. 1959;77:218–235. [Google Scholar]
- 20.Hansen EL, Yarwood EA, Nicholas WL, Sayre FW. Differential nutritional requirements for reproduction of two strains of Caenorhabditis elegans in axenic culture. Nematologica. 1960;5:27–31. [Google Scholar]
- 21.Hutchinson GE. The Ecological Theater and the Evolutionarv Play. Yale Univ. Press; New Haven, CT: 1965. [Google Scholar]
- 22.Gilbert SF. Ecological developmental biology: Developmental biology meets the real world. Develop Biol. 2001;233:1–12. doi: 10.1006/dbio.2001.0210. [CrossRe][Medlinej. [DOI] [PubMed] [Google Scholar]
- 23.Dusheck J. It’s the ecology, stupid! Nature. 2002;418:578–579. doi: 10.1038/418578a. [CrossRefI[Medline] [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Caswell-Chen EP. Facultative vivipary is a life-history trait in C. elegans. J Nematol. 2004;36:107–113. [PMC free article] [PubMed] [Google Scholar]
- 25.Andrassy I. A Taxonomic Review of the Suborder Rhabditina. Nematoda; Secernentia: ORSTOM; Paris: 1983. [Google Scholar]
- 26.Baird SE, Fitch DHA, Emmons SW. Caenorhabditis vulgaris n. sp. (Nematoda: Rhabditidae): A necromenic associate of pill bugs and snails. Nematologica. 1994;4:1–11. [Google Scholar]
- 27.Baird SE, Sutherlin ME, Emmons SW. Reproductive isolation in Rhabditidae (Nematoda: Secernentea): Mechanisms that isolate six species of three genera. Evolution. 1992;46:585–594. doi: 10.1111/j.1558-5646.1992.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 28.Steams REC. Exotic mollusca in California. Science. 1900;11:655–659. doi: 10.1126/science.11.278.655. [DOI] [PubMed] [Google Scholar]
- 29.Morand S, Wilson MJ, Glen DM. Nematodes (Nematoda) parasitic in terrestrial gastropods. In: Barker GM, editor. Natural Enemies of Terrestrial Mollusks. Commonwealth Agricultural Bureau; Wallingford, UK: 2004. pp. 525–557. [Google Scholar]
- 30.Baird SE. Natural and experimental associations of Caenorhabditis remanei with Trachelipus rathkii and other terrestrial isopods. Nematology. 1999;1:471–475. [CrossRefl. [Google Scholar]
- 31.Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ. Evolution of lifespan in. C elegans Nature. 2000;405:296–297. doi: 10.1038/35012693. [CrossRefI[Medlinel. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins NL, McColl G, Lithgow GJ. Fitness cost of extended lifespan in Caenorhabditis elegans. Proc R Soc Lond B. 2004;271:2523–2526. doi: 10.1098/rspb.2004.2897. [CrossRefI[Medlinel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Voorhies WA, Fuchs J, Thomas S. The longevity of Caenorhabditis elegans in soil. Biol Lett. 2005;1:247–249. doi: 10.1098/rsbl.2004.0278. [CrossRef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner S. Foreword. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Lab. Press; Plainview, NY: 1988. p. ix. [Google Scholar]
- 35.We thank J. Chitambar and W. Moore for assistance in identification of worms; C. Johnson, R. Clover, P. Grewal, L. Carta, V. Williamson, and M.-A. Felix for comments on sampling, including considering snails; T. Doniach for information on the Palo Alto worm; W. Nicholas for information on the origins of the N2 strain; R. M. Davis, D. Rizzo, M. Smith, V. Williamson, and D. Levitis for assistance collecting; B. Jaffee; T. Johnson, K. Thomas, and H. Caswell for comments and suggestions on the manuscript; and D. Wall for helpful comments and insights. Funding by NIH/NIA grant #PO1 AG022500-01 is gratefully acknowledged.