Abstract
Mouse models for the study of autistic-like behaviors are increasingly needed to test hypotheses about the causes of autism, and to evaluate potential treatments. Both the automated 3-chambered social approach test and social transmission of food preference have been proposed as mouse behavioral assays with face validity to diagnostic symptoms of autism, including aberrant reciprocal social interactions and impaired communication. Both assays measure aspects of normal social behavior in the mouse. However, little is known regarding the salient cues present in each assay that elicit normal social approach and communication. To deconstruct the critical components, we focused on delivering discrete social and non-social olfactory and visual cues within the context of each assay. Results indicate that social olfactory cues were sufficient to elicit normal sociability in the 3-chambered social approach test On social transmission of food preference, isolated social olfactory cues were sufficient to induce social investigation, but not sufficient to induce food preference. These findings indicate that olfactory cues are important in mouse social interaction, but that additional sensory cues are necessary in certain situations. The present evidence that both the 3-chambered social approach assay and the social transmission of food preference assay require socially relevant cues to elicit normal behavior supports the use of these two assays to investigate autism-related behavioral phenotypes in mice.
Keywords: mouse, autism, social behavior, 3-chambered social approach task, social transmission of food preference
INTRODUCTION
Mice are naturally social creatures. High tendencies for social interaction in mice have been utilized by biomedical researchers to model aspects of human social behaviors [16, 52, 66]. The automated 3-chambered social approach task [44] and the social transmission of food preference (STFP) task [62, 63] have been applied as assays for modeling components of autistic-like behaviors in mice [15, 16, 39–42, 44]. Little is known, however, about which salient cues in these tasks are necessary to drive the social interactions. The present experiments were designed to identify the essential sensory cues necessary to elicit normal social behavior in the two assays.
Mice rely heavily upon olfaction during typical social interactions. Olfactory cues assist in mate choice, distinguish between individuals and determine health status [3, 4, 10, 11, 20, 22, 61]. Anosmic mice show abnormal aggressive, afilliative and social behavior [2, 28, 31, 33]. In mice, olfactory cues are detected by both the main olfactory system and the accessory olfactory system [9, 51]. The accessory olfactory bulb processes non-volatile pheromones whereas the main olfactory bulb primarily processes volatile odorants and in mice is responsive to social cues present in urine [31, 36, 51, 57]. It is likely that both systems play a role in social behavior [28, 36, 56].
In addition to olfaction, mice employ other senses to gather social cues. Visual and auditory deprivation both depress normal aggressive behavior in mice [59]. Ultrasonic vocalizations in separated pups elicit retrieval behaviors by parents [18, 25, 58]. Adult mice of both sexes will vocalize in social situations such as aggressive encounters or copulation, and vocalizations can be elicited by exposing mice to urine or environments conditioned to be viewed as social [27, 48, 50]. Tactile input, including information gathered via the vibrissae, is likely employed in aggressive, affiliative and sexual interactions [1, 6, 33, 49].
Social interactions in mice have become an important focus for the development of mouse models of autism [7, 12, 14, 26, 37, 39, 55, 66]. We have generated an automated 3-chambered social approach task that provides the subject mouse with a choice between spending time with a novel mouse or spending time with a novel non-social object [16, 17, 39–42, 44, 64, 65]. This task has face validity to the tendency of autistic children to spend more time playing with an inanimate toy than engaged in social interactions with other children [35, 38]. Versions of this assay have been used to measure social behavior in rats [46] and pine voles [19], as well as mouse models of autism [29, 34, 39, 41, 42, 55, 64, 65]. However, the specific sensory cues that attract the subject to spend time with the stranger in this task have not yet been determined.
The STFP task has also been identified as an assay with potential face validity to the symptoms of autism [16, 39]. The observation that rodents can communicate dietary information among individuals was first reported in rats [13, 24]. It was subsequently demonstrated that mice show a similar pattern of behaviors [32, 60, 62, 63]. Rodents are naturally neophobic, and will avoid a completely novel food. However, rats and mice will readily consume food previously smelled and/or tasted on the breath, muzzle, and whiskers of a conspecific. In this assay, the subject “observer” mouse is allowed to freely interact with a “demonstrator” cagemate who recently ate a novel flavored food. The subject observer forms a food preference based on cues transferred between the mice during the interaction session. The food preference is subsequently measured in a choice test for consumption of the novel flavored food that the demonstrator ate versus a second, completely novel flavored food. Originally developed as a memory task [23, 54], STFP may be used without a temporal delay between the interaction session and the choice session, to measure aspects of social communication between conspecifics [39, 53]. Successful development of the food preference requires the subject mouse to interact with, and gather relevant olfactory and taste cues from, a conspecific. However, the specific sensory cues that are most critical for the transmission of the preference for the more familiar food are not completely known for mice. The present experiments employ adult male mice of the commonly used C57BL/6J inbred strain to evaluate olfactory and visual cues that contribute to performance in the social approach and STFP tasks.
MATERIALS AND METHODS
Adult C57BL/6J mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in a vivarium at the University of North Carolina at Chapel Hill. All mice were weaned on postnatal day 21 and subsequently housed with same-sex littermates. Animals were maintained on a 12L:12D circadian schedule with lights on at 7AM. The housing room was maintained at 20–24°C and 40%–50% relative humidity. Dams were fed ad libitum on Purina PicoLab Mouse Diet 20. Adults and weaned pups were fed ad libitum on Purina ProLab IsoPro 3000. Mice were housed in standard polycarbonate cages containing laboratory grade Bed-O-Cob bedding and were given water ad libitum. A small section of PVC pipe was present in each cage for enrichment. All breeding and testing procedures were conducted in strict compliance with the Guide for the Care and Use of Laboratory Animals (1996) and approved by the Institutional Animal Care and Use Committee of the University of North Carolina School of Medicine.
Adult male mice were behaviorally tested at 3–4 months of age, first in the automated three chambered social approach task, then two weeks later in the social transmission of food preference task. Behavioral testing was conducted in rooms within a dedicated mouse behavioral testing facility, between 8 AM and 2 PM.
Automated Three-Chambered Social Approach Test
Behavioral testing was conducted as previously described [44]. All the animals were run with the automated 3-chamber apparatus in the same location in the same testing room. The location and position of the apparatus in the test room were held constant, after determining the configuration that prevented side bias between the left and right chambers. The number of transitions between chambers and the time spent in each chamber was automatically recorded. The experimenter sat approximately two meters from the chamber and manually scored time spent sniffing each novel object using a computer keyboard and software developed by Josephine M. Johns at the University of North Carolina, Chapel Hill and Larry W. Means at East Carolina University, Greenville [30]. The maze was wiped down with water between each trial and cleaned with alcohol at the end of each testing day.
On the day of testing, the home cage was brought into the testing room at least thirty minutes prior to testing. Each mouse was given ten minutes to acclimate to the empty apparatus, with the doors open to all three chambers. Following the ten minute habituation session, the targets were placed in each of the side chambers. The non-social novel object was an inverted wire pencil cup (Galaxy Pencil/Utility Cup, Item #315, Chrome, Kitchen Plus, http://www.kitchen-plus.com/proddetail.asp?prod=315). The novel “stranger” mouse, social cue objects, and control stimuli are described below. The test mouse was then allowed to freely explore all three chambers for ten minutes. The amount of time spent in each chamber and the number of entries into each chamber were automatically recorded. In every experiment, the stranger mouse was matched to the test mouse by age and was the same C57BL/6J strain. Stranger mice had previously been habituated to the wire container or Plexiglas cylinder for 10 minutes at least once. Habituated mice that did not sit quietly in the wire container were not used as strangers. Six experiments were conducted:
1) Comparison of approach to a male versus approach to a female stranger mouse by a male subject, to evaluate sex-specific cues. Adult male subject mice were randomly assigned for testing with either a male stranger or a female stranger, but not both. The stranger was placed randomly into one of the two wire cups, in either the left or the right side chamber, and the second cup was left empty. Previous experiments from our laboratory have tested male subjects with novel females, and female subjects with novel males [41]. Results were qualitatively and quantitatively similar, when compared to experiments in which same-sex social testing was conducted. This earlier study showed that strong social approach by the subject male toward the novel female was uniformly obtained without controlling for estrous cycle and we therefore did not control for the estrous cycle of the female mouse here.
2) Comparison of approach to a stranger male versus approach to a nestlet from another cage of mice, to evaluate social olfactory cues. A nestlet (Ancare Corp., Bellmore, NY; 5cm × 5cm × 0.5 cm initial dimensions) was soiled by leaving it in a cage containing at least three male mice for 24 hours prior to testing and placed randomly into one of the two wire cups. The stranger was placed into the other cup.
3) Comparison of approach to a social odor nestlet versus approach to an almond-scented nestlet, to evaluate interest in social versus non-social olfactory cues. The social odor nestlet was generated as described above. The almond-scented nestlet was created by taking a clean nestlet and manually shredding the nestlet square using forceps until its shape and appearance were similar to the soiled nestlet. 0.5 ml of dilute almond extract (McCormick & Co. Inc., Sparks, MD; 1:100 in tap water), previously found to elicit sniffing in mice [63] was then gently dropped into the dry, shredded nestlet. This amount of liquid is readily absorbed by the nestlet and produces an easily identifiable odor.
4) Comparison of approach to a social odor nestlet versus approach to a clean nestlet, to evaluate interest in the non-social visual aspects of the non-odoriferous nest material. The soiled nestlet was generated as described above. The clean nestlet was created by manually shredding a new nestlet square as described above. The clean nestlet was always handed with clean gloves and stored in a clean plastic beaker.
5) Comparison of approach to a social odor nestlet versus a stranger male mouse restrained in a clear Plexiglas cylinder, to evaluate social visual cues. The soiled nestlet was generated as described above. The Plexiglas cylinder was 15.5 cm high, 8.5 cm inner diameter with a wall thickness of 7 mm. The cylinder was placed on end with the stranger mouse contained inside. Plexiglas disks (11cm diameter, 0.5 cm thickness) were placed over both ends to block olfactory cues.
6) Comparison of approach to a stranger male mouse restrained in a clear Plexiglas cylinder versus approach to a clean empty wire cage, to further evaluate visual cues. The Plexiglas cylinder was identical to the cylinder used in experiment 5.
Social Transmission of Food Preference
Behavioral testing was conducted as previously described [62, 63]. Prior to testing, all mice were housed three or four per cage. Test cages were placed in the same position in the same testing room for all subjects. The experimenter scored social interactions with a silent stopwatch and observed from a distance of approximately one meter. Twenty-four hours prior to the onset of testing, mouse chow food was removed from the home cage and a specially designed food jar (Dyets Inc., Bethlehem, PA), filled with bedding and topped with powdered chow, was placed into each cage, to acclimate each mouse to the novel feeding apparatus. Eighteen hours prior to testing, the feeding jars were removed and the mice remained food deprived until testing. Thirty minutes prior to testing, the subject mice were brought to the testing room and individually housed in cages with clean bedding.
To begin testing, a “demonstrator” mouse was chosen and given a food jar filled with bedding and topped with flavored, powdered chow. The flavored food was prepared by mixing either 1% ground cinnamon (McCormick & Co. Inc., Sparks, MD) or 2% powdered cocoa (The Hershey Co., Hershey, PA) by weight into powdered Purina 5001 rodent chow on the morning of testing. After one hour, the food jar was removed and weighed to ensure that at least 0.2 grams of food had been consumed. The demonstrator mouse was immediately placed into the cage of the “observer” mouse. Interactions between the demonstrator and the observer were scored for thirty minutes, and the number of mutual nose sniffs was recorded. Immediately following the thirty minute interaction period, the demonstrator was removed and two jars of powdered food one of each flavor) were placed into the cage with the observer mouse. The mouse was scored for one hour and the number of jar climbs and eating bouts were recorded. At the end of the one hour choice session, the food jars were removed and weighed. All feeding jars were thoroughly cleaned with soap and hot water in between testing.
Two experiments were conducted to evaluate odor cues during social transmission of food preference. Each subject and observer mouse was used only once.
1) Comparison of STFP after standard social interaction versus exposure to food odor + social odor, to evaluate the importance of a live conspecific versus social olfactory cue pairing with the novel food flavor on later food preference. During the 30 minute interaction period, the test mice were given access to a novel flavored food placed within a small section of PVC pipe (4.5 cm diameter × 1.5 cm height) closed off by wire mesh (2 mm square openings). This allowed the mice to smell the food, but not taste it. In this experiment, one gram of the novel flavored food was mixed with one gram of soiled bedding (the social odor cue). The soiled bedding was taken from the cage of unfamiliar male mice. Immediately after the 30 minute exposure, the observer mice were given food cups containing the now-familiar flavored food and a second new flavored food for one hour, as described above.
2) Comparison of STFP after standard social interaction versus exposure to food odor + nonsocial odor, to evaluate the importance of a live conspecific versus food containing no social odor. During the 30 minute interaction period, the test mice were given access to one gram of a novel flavored food placed within a small section of PVC and wire mesh. No social odors were associated with the novel flavored food. Immediately after this exposure, food preference between the now-familiar flavored food and a new flavored food was then tested as described above.
Statistical Analyses
PC SAS (Cary, NC) was used for all statistical analyses. Two-way Analysis of Variance (ANOVA) was used to determine main treatment effects. Post-hoc analyses were conducted with Dunnett’s test for individual comparisons following a significant ANOVA. For the 3-chambered social approach analyses, only time spent in the right and left chambers were compared. The time spent in the center chamber is included in the figures for illustrative purposes only.
RESULTS
Automated 3-Chambered Social Approach Test
Figure 1 shows the comparison of male versus female stranger mice. Similar levels of social approach by male subject mice were seen when the stranger was a male versus a female. In both cases, subject mice (n = 10 per group) spent more time in the chamber containing the stranger mouse than in the chamber containing a non-social novel object, the empty wire container (F1,18 = 3.54; p = 0.0024 for males; F1,18 = 2.3054; p = 0.0333 for females) and spent more time sniffing the stranger mouse than the novel object (F1,18 = 3.18; p = 0.0052 for males; F1,18 = 2.82; p = 0.0115 for females). The number of entries into the two side chambers did not differ (F1,18 = 0.61; p = 0.5496 for males; F1,18 = 0.52; p = 0.6068 for females). The sex of the stranger mouse had no effect on the duration of time spent in the chamber (F1,9 = 4.07; p = 0.074), the time spent sniffing the stranger (F1,9 = 2.40; p = 0.156), or the number of entries in the chamber with the stranger (F1,9 = 2.01; p = 0.190).
Figure 1.
Similar sociability when the stranger mouse is the same sex versus the opposite sex. Male C57BL/6J mice were given a choice between an unfamiliar male mouse (Male Stranger) and a non-social novel object, an empty inverted wire pencil cup. A second group of male C57BL/6J mice was given a choice between an unfamiliar female mouse (Female Stranger) and non-social novel object. (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the stranger mouse or the novel object. (C) Number of entries into each side chamber during the ten minute test. N = 10 in each group. In all Figure 1–Figure 8, data are expressed as mean + the standard error of the mean. * p < 0.05 vs. Novel Object.
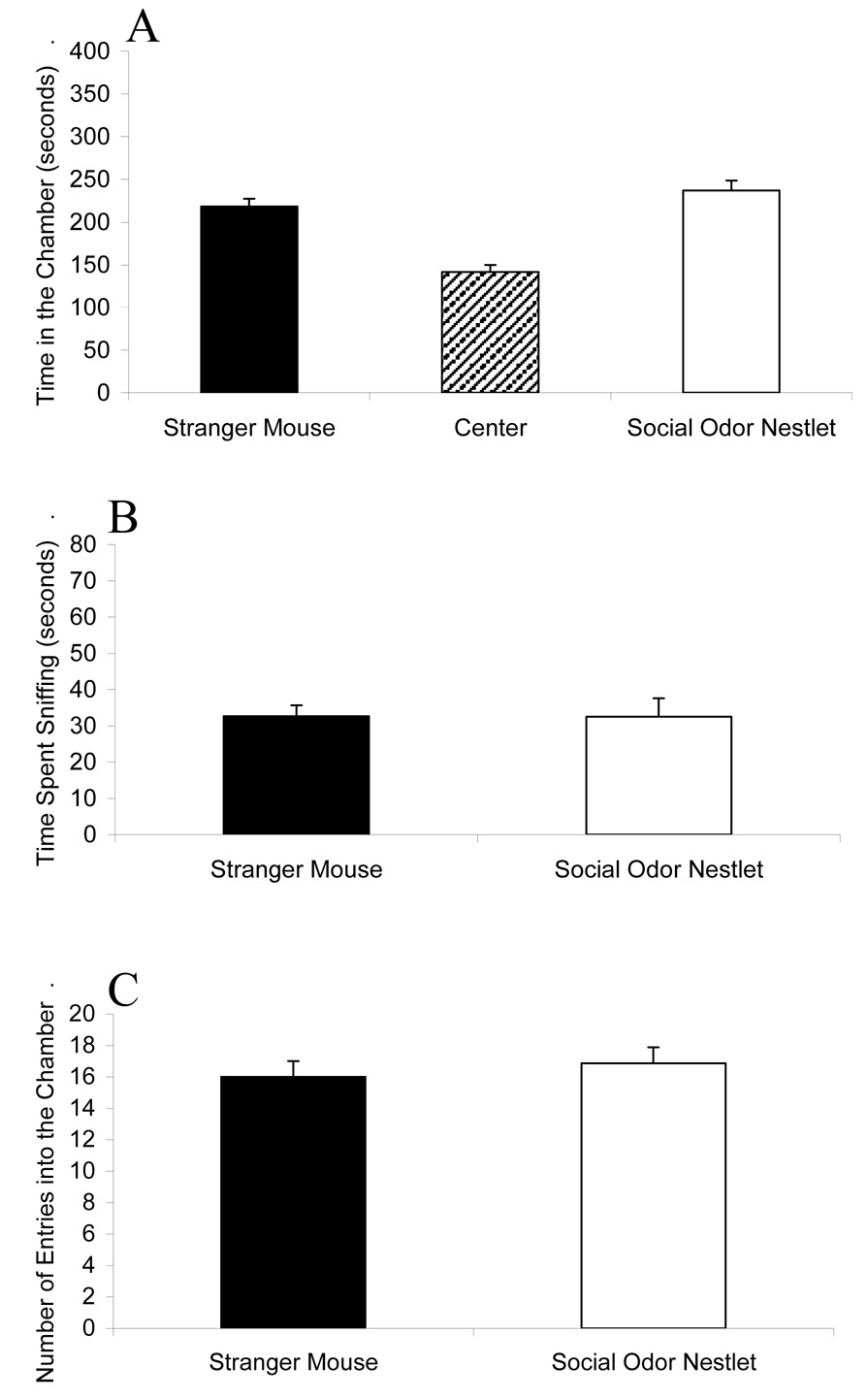
Figure 2 shows the comparison of stranger mouse versus nestlet containing social odors The test mice (n = 22) showed similar amounts of time spent in each chamber (F1,42 = 1.62; p = 0.210), and similar amounts of time spent sniffing the stranger mouse and the nestlet containing social olfactory cues (F1,42 = 0.01; p = 0.909). The number of entries into the two side chambers did not differ (F1,42 = 0.36; p = 0.550).
Figure 2.
Similar sociability in mice given a choice between an unfamiliar male mouse (Stranger Mouse) and a nestlet soiled with male mouse cage odors (Social Odor Nestlet). (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the wire cage containing either the unfamiliar male or social odor nestlet. (C) Number of entries into each outer chamber during the ten minute test. No significant differences were detected between the two side chambers for any of the three behavioral parameters. N = 22.
Figure 3 shows the comparison of a nestlet containing social odors versus a nestlet containing a non-social odor, almond. The test mice (n = 15), spent more time in the chamber containing the nestlet soiled with social olfactory cues than in the chamber containing the nestlet with a non-social odor cue (F1,28 = 68.71; p < 0.0001) and spent more time sniffing the soiled nestlet than sniffing the almond-scented nestlet (F1,28 = 48.71; p < 0.0001). The number of entries into the two side chambers did not differ (F1,28 = 0.40; p = 0.5325).
Figure 3.
Higher approach to a nestlet soiled with male cage odors (Social Odor Nestlet) than to an almond-scented nestlet (Almond Nestlet). (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the wire cage containing either the soiled nestlet or the almond-scented nestlet. (C) Number of entries into each outer chamber during the ten minute test. * p < 0.05 cage vs. Almond Nestlet. N = 15.
Figure 4 shows the comparison of a nestlet containing social odors versus a clean nestlet: The test mice (n = 17), spent more time in the chamber containing the nestlet with social odors than in the chamber containing the clean nestlet (F1,32 = 43.09, p < 0.0001) and spent more time sniffing the soiled nestlet than sniffing the clean nestlet (F1,32 = 34.24; p < 0.0001). The number of entries into the two side chambers did not differ (F1,32 = 0.41; p = 0.526).
Figure 4.
Higher approach to a nestlet soiled with male mouse cage odors (Social Odor Nestlet) than to a clean nestlet (Clean Nestlet). (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the wire container with the soiled nestlet versus the wire container with the clean nestlet. (C) Number of entries into each side chamber during the ten minute test. * p < 0.05 vs. Clean Nestlet. N = 17.
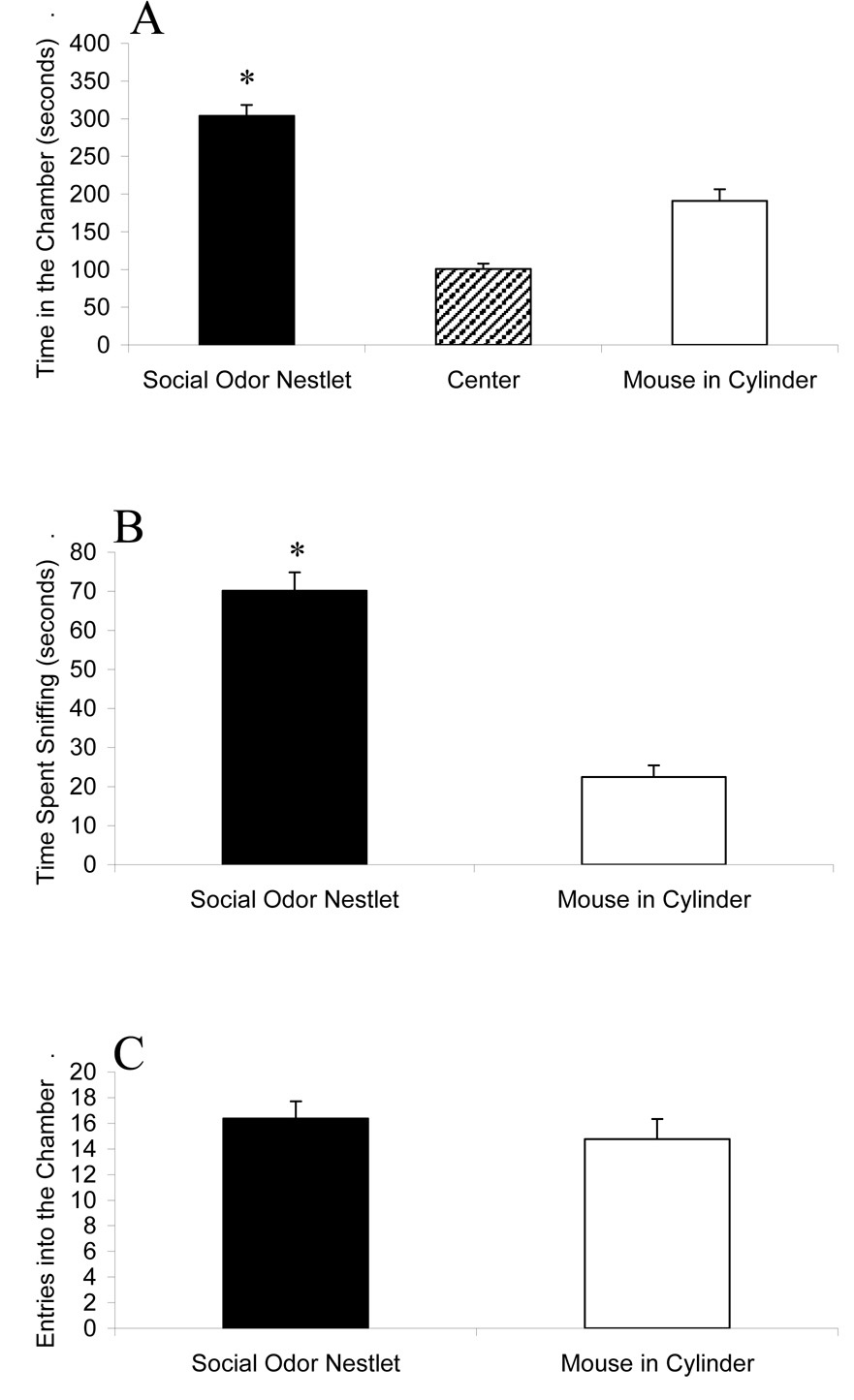
Figure 5 shows the comparison of nestlet containing social odors versus a live mouse contained in a Plexiglas cylinder that blocks olfactory cues. The test mice (n = 13) spent more time in the chamber containing the nestlet with social olfactory cues than in the chamber containing the mouse restrained in the Plexiglas cylinder (F1,24 = 28.90; p < 0.0001) and spent more time sniffing the nestlet than the Plexiglas cylinder (F1,24 = 73.55; p < 0.0001). The number of entries into the two side chambers did not differ (F1,24 = 0.62; p = 0.4397).
Figure 5.
Higher approach to a nestlet soiled with male mouse cage odors (Social Odor Nestlet) than to an unfamiliar male mouse enclosed in a solid Plexiglas cylinder that blocked olfactory cues (Mouse in Cylinder). (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the wire cage containing the soiled nestlet versus the cylinder containing the mouse. (C) Number of entries into each outer chamber during the ten minute test. * p < 0.05 vs. the Mouse in Cylinder. N = 13.
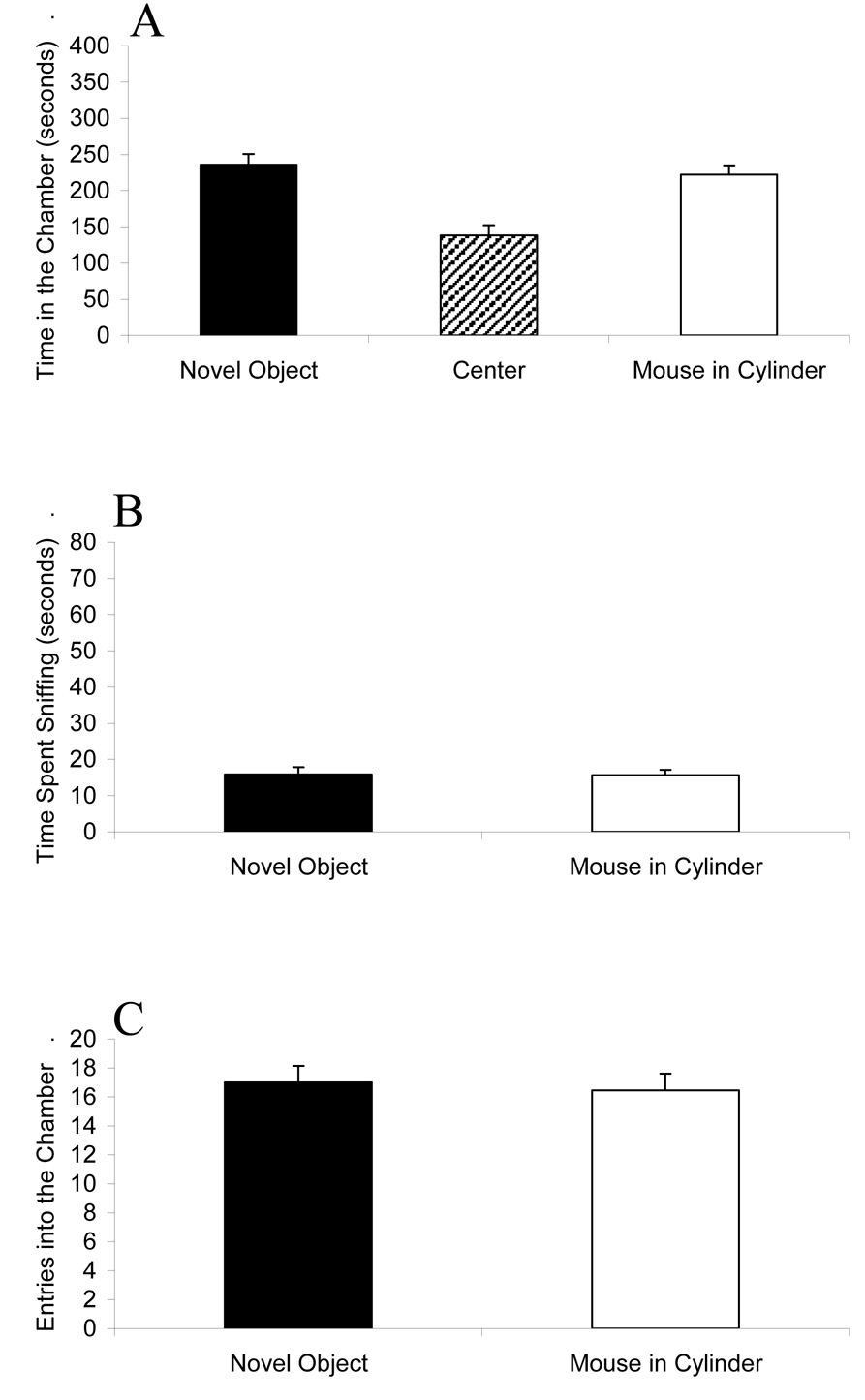
Figure 6 shows the comparison of an empty wire cage versus a live mouse contained in a Plexiglas cylinder that blocks olfactory cues. The test mice (n = 11) showed similar amounts of time spent in each chamber (F1,20 = 0.75; p = 0.3983), and similar amounts of time spent sniffing the Plexiglas cylinder and the empty wire cage (F1,20 = 0.48; p = 0.4943). The number of entries into the two side chambers did not differ (F1,20 = 0.00; p = 1.00).
Figure 6.
Similar approach to an unfamiliar male mouse enclosed in a solid Plexiglas cylinder that blocked any olfactory cues (Mouse in Cylinder) and an empty wire cage (Novel Object). (A) Amount of time spent in each of the three chambers during the ten minute test. (B) Amount of time spent sniffing the wire object versus the cylinder containing the mouse. (C) Number of entries into each outer chamber during the ten minute test. No significant differences were detected between the two side chambers for any of the three behavioral parameters. N = 11.
Social Transmission of Food Preference
Figure 7 shows the preference for a novel flavored cued food when this cued food is paired with a stranger mouse or paired with inanimate social odors. The observer subject mice exposed to inanimate social odors (n = 11) ate equal amounts of the cued and non-cued foods (F1,20 = 0.25, p = 0.6195) and had an equal number of feeding bouts with each food source (F1,20 = 0.85, p = 0.3686), whereas the observers that interacted with the demonstrator mouse (n = 11) ate more of the cued than the non-cued foods (F1,20 = 9.96, p = 0.0050) and had more feeding bouts with the cued food (F1,20 = 15.49, p = 0.0008). Observer mice sniffed the PVC tubes with the flavored food and isolated social odors more often than the control mice sniffed the demonstrator mice (F1,20 = 22.22; p = 0.0001).
Figure 7.
Significant social transmission of food preference in mice exposed to a cagemate that had previously eaten novel flavored powdered chow, but no significant social transmission of food preference in mice exposed to the novel flavored powdered chow associated with a social odor. One group of “observer” mice was exposed to a same-sex stranger “demonstrator” mouse that had previously eaten a flavored powdered chow and had the odor of cinnamon or cocoa flavored food on its breath (Demonstrator Mouse). A second group of mice was exposed to 1 gram of flavored powdered food mixed with 1 gram of bedding containing social cage odors (Powdered Food + Social Odor Bedding). After the 30 minute exposure period, the “observer” mice were immediately given 60 minutes of access to two food jars. One food jar contained powdered chow mixed with the novel flavor, e.g. cinnamon, previously consumed by the demonstrator or mixed with the bedding (Cued). The other jar contained powdered chow mixed with a completely novel flavor (Non-Cued), e.g. cocoa. Half the observers received cinnamon as the initial flavor cue and cocoa as the flavor for the uncued choice; the other half of the observers received cocoa as the cued initial flavor and cinnamon as the flavor for the uncued. (A) Amount of cued and non-cued food eaten. (B) Number of feeding bouts at each food jar during the preference test. (C) Number of investigative sniffs during the social interaction. * p < 0.05 vs. Non-Cued. N = 11 in both groups.
Figure 8 shows the preference for a novel flavored cued food when this cued food is paired with a stranger mouse versus not associated with any other odors. Observer mice exposed to the unassociated food odor (n = 20) ate the same amount of cued and uncued food (F1,38 = 0.16, p = 0.6917) and had the same number of feeding bouts at both food sources (F1,38 = 1.00, p = 0.3243) whereas observers that interacted with a demonstrator mouse (n = 15) ate significantly more cued than non-cued food (F1,28 = 11.33, p = 0.0022) and had more feeding bouts at the cued food (F1,18 = 15.12, p = 0.0011. Observer mice sniffed the PVC tubes with the flavored food more often than the control mice sniffed the demonstrator mice (F1,20 = 10.81; p = 0.0037).
Figure 8.
Social transmission of food preference in mice exposed to a demonstrator mouse, but not in mice exposed to only flavored food odor with no associated odor. One group of observer mice was exposed to 1 gram of powdered food mixed with either cocoa or cinnamon for 30 minutes and then immediately given a test to determine their preference for this cued food (Powdered Food + No Associated Odor). The other group of observer mice was exposed to a same-sex stranger mouse who had just consumed cinnamon or cocoa flavored food for 30 minutes (Demonstrator Mouse). Each group was then immediately given a 60 minute choice test to determine their preference for the cued food. (A) Amount of cued and non-cued food eaten. (B) Number of feeding bouts in each food jar during the preference test. (C) Number of investigative sniffs during the social interaction. * p < 0.05 vs. Non-Cued. N = 20 in the Powdered Food + No Associated Odor group and N = 15 in the Demonstrator Mouse group.
DISCUSSION
Generating new mouse behavioral assays requires careful attention to the methods and parameters that will optimize the tasks. In particular, identifying the critical components of social approach, social interaction, and social communication tasks in mice may advance our ability to evaluate mouse behavioral traits relevant to the diagnostic symptoms of autism. This in turn will create greater power to test genetic and environmental hypotheses about the causes of autism, and to develop effective treatments. The present study was designed to identify some of the critical sensory cues required to elicit normal social behavior in both the automated 3-chambered social approach assay and the social transmission of food preference assay.
The results of experiment 1 show that the sex of the stranger mouse had no impact on the amount of social approach and interaction by the C57BL/6J male subject mouse in the automated social approach apparatus, supporting previous indications [41] and showing that the mice in this project display similar behavior as in the past. As a result of this finding, we chose to use only male strangers for the rest of the experiments described herein. It appears likely that future studies investigating C57BL/6J male mouse social behavior in the 3-chambered apparatus can use either male or female mice as strangers with no impact on sociability scores. However, it is possible that female subject mice, or mice from other inbred strains, might exhibit differences in levels of sociability with male or female strangers in the three-chambered apparatus.
Social odor alone was found to be sufficient to elicit significant social approach in male C57BL/6J mice. Subjects spent equal amounts of time with a social odor imbued nestlet and with an unfamiliar mouse. This indicates that male mice are socially motivated primarily by odor when choosing where to spend time in a three chambered apparatus. One interpretation of this finding is that visual, tactile, and auditory signals associated with a stranger mouse do not contribute greatly to social investigation, at least when compared to a discrete concentration of social odors. This interpretation is reinforced by our findings of more time spent with a social odor than with a non-social odor (the almond scented nestlet), or with a clean, unscented nestlet. Thus, the visual appearance of nesting material was not sufficient to elicit social investigation in the absence of accompanying social odors.
The importance of visual cues from an awake, behaving conspecific mouse appeared to be minimal in eliciting social approach, at least as compared to social odors. More time was spent in the chamber containing a social odor nestlet than in the chamber containing a stranger mouse that was enclosed in a solid Plexiglas cylinder that prevented detection of social odors. Further, the visual cues of a stranger mouse in the Plexiglas cylinder were not sufficient to elicit any more investigation than the visual cues of an empty wire container. This was an unpredicted finding, as the stranger mouse was still clearly visible to the test mouse, and audible squeaks were heard on occasion from the mouse within the tube. In male mice, these visual and auditory cues were apparently insufficient to elicit attention in the absence of relevant social olfactory cues.
Several methodological factors merit consideration. First, the number of entries into the two side chambers did not differ in any of the experiments, confirming general exploratory activity and lack of aversive properties of any stimulus. Second, a potentially confounding factor was that the nestlets were soiled by multiple males, contributing various individual odors, whereas stranger mice were presented singly during the test session. However, stranger mice were group housed, and therefore should have brought along the odors of multiple cagemate mice as well. Third, the soiled nestlets used in this experiment were placed in home cages for 24 hours prior to being used. These nestlets were likely soiled with urine and feces and potentially with food, saliva and hair. Urine is a highly relevant social stimulus for mice [3–5, 8–10, 28, 36], and likely plays a key role in the social approach seen here. However, we did not directly address which social odors were important in eliciting approach. Future studies will be needed to identify the critical individual social cues found in a soiled nestlet.
The STFP assay is more complex, in that mice obtain relevant social cues during a 30 minute session, and then apply this information to a novel food preference test during a subsequent 60 minute session. Absence of a time delay between the first and second sessions reduces the memory component of this task, so that the primary variable is the amount of information transmitted during the social interaction between demonstrator and observer. Social odor alone was not sufficient to transmit food preference in male observer mice. Significant preference for the cued food information was detected only when the observers were paired with demonstrator mice. It is interesting to note that social odor was sufficient to elicit investigation by the test mouse, as measured by sniffing bouts, but did not transmit food preference. In addition, a 30 minute exposure to the novel food odor alone was not sufficient to produce a subsequent food preference, even though the novel flavored food elicited high levels of sniffing investigation. These findings are in agreement with a previous report in adult rats [22]. In adult rats, transmission of food preference has been linked to the presence of carbon disulfide, a chemical normally found in exhaled air [21]. Since the specific salient cues which drive food preference in adult mice were not identified in the present study, a follow-up investigation of carbon disulfide and STFP in mice will be interesting to pursue.
The social behaviors of female subject mice were not tested in the present experiments The focus on males was consistent with the more common use of male mice in both social interaction and STFP assays, and in modeling autism, which has a 4:1 male:female prevalence [36, 38]. Follow-up studies focused on female mouse social behavior, as well as in other strains of mice, will be needed to confirm the present findings with male subject mice.
In conclusion, social approach in male C57BL/6J mice was found to be driven primarily by social olfactory cues, with visual cues appearing to play a remarkably minor role. In contrast, the communication of food preference was found to require social interactions rather than social odor cues. The more complex demands of the STFP task, as compared to the simpler social approach task, may explain the different sensory and communication requirements for these two assays. The automated 3-chambered apparatus appears to provide a robust assay, in which stranger mice can be of the same or opposite sex, and social cage odor cues may be sufficient stimuli. It may prove possible to modify existing protocols to eliminate the stranger mouse entirely, and use only social olfactory cues, delivered on nestlet squares or other media, to elicit social approach. Modifications that remove live strangers from the assay may be especially useful for mouse strains with high anxiety-like traits or aggression towards strangers. Our results, showing that normal behavior in the 3-chambered social approach and STFP tests rely on the presence of social stimuli, support the use of these assays in phenotyping autistic-like behaviors in mice, as tools to test hypotheses about the causes of autism, and to evaluate potential treatments. The use of multiple social tasks such as these will yield a more complete understanding of social and communication traits in mouse models of autism.
ACKNOWLEDGEMENTS
Support for this project was provided by the Neurodevelopmental Disorders Research Center at University of North Carolina at Chapel Hill NICHD training grant #T32-HD40127 (BCR), NICHD grant P30-HD03110 (SSM), and the National Institute of Mental Health Intramural Research Program (JNC). The authors would also like to thank Randy Nonneman for assistance with animal care and Joe Piven for advice on autism phenotypes and assay design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ahl AS. The role of vibrissae in behavior: a status review. Vet Res Commun. 1986;10:245–268. doi: 10.1007/BF02213989. [DOI] [PubMed] [Google Scholar]
- 2.Alberts JR. Producing and interpreting experimental olfactory deficits. Physiol Behav. 1974;12:657–670. doi: 10.1016/0031-9384(74)90216-9. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp GK, Yamazaki K. Chemical signaling in mice. Biochem Soc Trans. 2003;31:147–151. doi: 10.1042/bst0310147. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp GK, Yamazaki K. Individual differences and the chemical senses. Chem Senses. 2005;30 Suppl. 1:i6–i9. doi: 10.1093/chemse/bjh086. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard RJ, Dulloog L, Markham C, Nishimura O, Compton JN, Jun A, Han C, Blanchard DC. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav. 2001;72:245–254. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- 7.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behaviors in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan PA. The nose knows who’s who: chemosensory individuality and mate recognition in mice. Horm Behav. 2004;46:231–240. doi: 10.1016/j.yhbeh.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- 11.Broad KD, Keverne EB. More to pheromones than meets the nose. Nature Neurosci. 2008;11:128–129. doi: 10.1038/nn0208-128. [DOI] [PubMed] [Google Scholar]
- 12.Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and non-spatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- 14.Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Ehret G. Infant rodent ultrasounds – a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 19.Engell MD, Godwin J, Young LJ, Vandenbergh JG. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol. 2006;28:103–110. doi: 10.1016/j.ntt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 21.Galef BG, Jr, Mason JR, Preti G, Bean NJ. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 1988;42:119–124. doi: 10.1016/0031-9384(88)90285-5. [DOI] [PubMed] [Google Scholar]
- 22.Galef BG, Jr, Kennett DJ. Different mechanisms for social transmission of food preference in rat pups of different ages. Dev Psychobiol. 1987;20:209–215. doi: 10.1002/dev.420200209. [DOI] [PubMed] [Google Scholar]
- 23.Galef BG, Jr, Whiskin EE. Socially transmitted food preference can be used to study long-term memory in rats. Learn Behav. 2003;31:160–164. doi: 10.3758/bf03195978. [DOI] [PubMed] [Google Scholar]
- 24.Galef BG, Jr, Wigmore SW, Kennett DJ. A failure to find socially mediated taste aversion learning in Norway rats (R. norvegicus) J Comp Psychol. 1983;97:358–363. [PubMed] [Google Scholar]
- 25.Hahn ME, Lavooy MJ. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35:31–52. doi: 10.1007/s10519-004-0854-7. [DOI] [PubMed] [Google Scholar]
- 26.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:2177–2186. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol Behav. 2008;93:467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varagueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johns JM, Noonan LR, Zimmerman LI, McMillan BA, Means LW, Walker CH, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and their prenatally exposed offspring. Ann NY Acad Sci. 1998;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- 31.Keverne EB. Importance of olfactory and vomernasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- 33.Kolunie JM, Stern JM. Maternal aggression: disruption by perioral anesthesia in lactating Long-Evans rats (Rattus norvegicus) J Comp Psychol. 1990;104:352–360. doi: 10.1037/0735-7036.104.4.352. [DOI] [PubMed] [Google Scholar]
- 34.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lainhart JE, Piven J. Diagnosis, treatment, and neurobiology of autism in children. Curr Opin Pediatr. 1995;7:392–400. doi: 10.1097/00008480-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Lin DY, Zhang S-Z, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- 38.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 39.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 40.Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Med Genet. 2006;142:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- 41.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 42.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mucignat-Caretta C, Bondi M, Caretta A. Time course of alterations after olfactory bulbectomy in mice. Physiol Behav. 2006;89:637–643. doi: 10.1016/j.physbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantification of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 45.Neckers LM, Zarrow MX, Myers MM, Denenberg VH. Influence of olfactory bulbectomy and the serotonergic system upon intermale aggression and maternal behavior in the mouse. Pharmacol Biochem Behav. 1975;3:545–550. doi: 10.1016/0091-3057(75)90170-7. [DOI] [PubMed] [Google Scholar]
- 46.Nicolas LB, Prinssen EP. Social approach-avoidance behavior of a high-anxiety strain of rats: effects of benzodiazepine receptor ligands. Psychopharmacology. 2006;184:65–74. doi: 10.1007/s00213-005-0233-y. [DOI] [PubMed] [Google Scholar]
- 47.Otmakova NA, Gurevich EV, Katkov YA, Nesterova IV, Bobkova NV. Dissociation of multiple behavioral effects between olfactory bulbectomized C57Bl/6J and DBA/2J mice. Physiol Behav. 1992;52:441–448. doi: 10.1016/0031-9384(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 48.Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellis SM, McKenna MM, Field EF, Pellis VC, Prusky GT, Whishaw IQ. Use of vision by rats in play fighing and other close-quarter social interactions. Physiol Behav. 1996;59:905–913. doi: 10.1016/0031-9384(95)02162-0. [DOI] [PubMed] [Google Scholar]
- 50.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 51.Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views of the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Rustay NR, Wrenn CC, Kinney JW, Holmes A, Bailey KR, Sullivan TL, Harris AP, Long KC, Saavedra MC, Starosta G, Innerfield CE, Yang RJ, Dreiling JL, Crawley JN. Galanin impairs performance on learning and memory tasks: findings from galanin transgenic and GAL-R1 knockout mice. Neuropeptides. 2005;39:239–243. doi: 10.1016/j.npep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Andrande G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. J Reprod Dev. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- 55.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strasser S, Dixon AK. Effects of visual and acoustic deprivation on agonistic behaviour of the albino mouse (M. musculus L.) Physiol Behav. 1986;36:773–778. doi: 10.1016/0031-9384(86)90367-7. [DOI] [PubMed] [Google Scholar]
- 60.Valsecchi P, Galef BG., Jr Social influences on the food preference of house mice (Mus musculus) Int J Comp Psychol. 1989;2:245–256. [Google Scholar]
- 61.Wersinger SR, Temple JL, Caldwell HK, Young WS., 3rd Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–121. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wrenn CC. Social transmission of food preference in mice. Curr Protocols Neurosci. 2004 Suppl. 28:8.5G.1–8.5G.7. doi: 10.1002/0471142301.ns0805gs28. [DOI] [PubMed] [Google Scholar]
- 63.Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- 64.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR + tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1–9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M, Zhodizishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiatry. 2002;7 Suppl. 2:S38–S39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]