Abstract
WCK 771 demonstrated MIC50 and MIC90s of 0.03 and 1 μg/ml, respectively, against 297 recent U.S. community-acquired and hospital strains of Staphylococcus aureus, irrespective of quinolone or glycopeptide resistance. Against quinolone-resistant strains, MIC90s of WCK 771 and moxifloxacin were 1 and 16 μg/ml, respectively.
Methicillin-resistant Staphylococcus aureus (MRSA) accounts for approximately 48 to 60% of the staphylococcal strains isolated from inpatients and outpatients in the United States, with >50% being multidrug resistant (17). Recently, the epidemiology of MRSA has changed, with emergence of quinolone-resistant community-acquired and multidrug-resistant hospital-acquired MRSA strains. Additionally, recognition of heteroresistant vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA) has caused concern; hVISA and VISA strains have been associated with vancomycin clinical failures (1, 7, 16). Compromised activity against VISA (10) and lack of broad-spectrum activity and bacteriostatic action are limitations of the currently employed anti-MRSA agents and point toward the need for new alternatives from other antibacterial classes.
The increase in quinolone resistance among methicillin-susceptible S. aureus (MSSA) and lack of sufficient activity against MRSA have compromised the therapeutic utility of all clinically available quinolones for the treatment of staphylococcal infections (5, 13). WCK 771 is a broad-spectrum intravenous quinolone active against MRSA and quinolone-resistant S. aureus and is currently being studied in phase 2 clinical trials (2, 8, 14). Its oral form is under development in phase 1 clinical studies. The present study was undertaken to establish the in vitro activity of WCK 771 against recent (2005 to 2007) U.S. clinical isolates of S. aureus with various resistance phenotypes, including vancomycin nonsusceptibility, originating from community and hospital settings and to determine its activity relative to currently utilized anti-MRSA agents, such as vancomycin. Moxifloxacin was included as a comparator quinolone, since among approved quinolones it shows significantly improved activity against S. aureus and possesses a superior pharmacokinetic-pharmacodynamic profile compared to other available quinolones.
(Portions of this work were previously presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2007 [12].)
A total of 101 methicillin-susceptible and 196 methicillin-resistant strains were tested: 110 isolates were hospital acquired. Among 86 community-acquired MRSA strains, 73 produced Panton-Valentin leukocidin as identified by PCR using methods described previously (11); 2 hVISA, 25 VISA, and 7 VRSA strains were also included in the study. Vancomycin-susceptible strains were isolated in Pennsylvania, Texas, and Ohio, and a few strains were from western Europe. Both hVISA isolates were obtained from sputum of patients at Hershey Medical Center (HMC) who had received prior vancomycin therapy. Five of the VISA strains were isolated from blood of patients at HMC, and the rest were obtained from the Network on Antimicrobial Resistance in S. aureus (NARSA). Reference VISA strains MU3 and MU50 were obtained from ATCC. One of the seven VRSA strains was an HMC isolate, and the balance were from NARSA. MICs were determined as per CLSI recommendations on Mueller-Hinton agar containing serial twofold dilutions of the drugs (3). Vancomycin MICs were read after a full 24-h incubation. Genomic DNA isolation, PCR amplification (of the quinolone resistance-determining region [QRDR]) of vancomycin-susceptible MRSA, VISA, and VRSA, and sequencing were performed as described previously (2, 4, 6).
Table 1 provides MIC ranges, MIC50s, and MIC90s of WCK 771 against all groups of S. aureus strains. Against all S. aureus strains, WCK 771 demonstrated MIC50 and MIC90 values of 0.03 μg/ml and 1 μg/ml, respectively. Except for one isolate with an MIC of 8 μg/ml, the MIC range of WCK 771 was 0.015 to 2 μg/ml. The range, MIC50, and MIC90 of moxifloxacin were 0.06 to >32 μg/ml, 0.25 μg/ml, and 16 μg/ml, respectively. Thus, WCK 771 was 8 to 16 times superior in potency compared to moxifloxacin. The MIC range of vancomycin was 0.125 to 8 μg/ml. Higher MIC50 and MIC90 values of moxifloxacin for hospital-acquired MRSA strains (4 and 16 μg/ml, respectively) indicated elevated quinolone resistance in the hospital environment. For community-acquired and hospital-acquired MRSA strains, the WCK 771 MIC50 and MIC90 were 0.03 and 0.5 μg/ml and 0.5 and 1 μg/ml, respectively. Against hVISA/VISA strains, WCK 771 was 32 times more active than moxifloxacin. Vancomycin showed decreased susceptibility for hVISA/VISA strains, with MIC50 and MIC90 values of 4 and 4 μg/ml and 2 and 4 μg/ml, respectively. Against quinolone-resistant S. aureus the MIC range, MIC50, and MIC90 of WCK 771 were 0.03 to 8 μg/ml, 0.5 μg/ml, and 1.0 μg/ml, respectively. Higher values in the WCK 771 MIC range indicate some degree of cross-resistance; however, WCK 771 was consistently more potent than moxifloxacin (MIC range, 2 to >32 μg/ml; MIC50, 4 μg/ml; MIC90, 16 μg/ml).
TABLE 1.
Activities of WCK 771 and other antibacterial agents against recent U.S. isolates of S. aureus
| Organism, resistance type or source (no. of isolates) | MIC values (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| WCK 771
|
Moxifloxacin
|
Vancomycin
|
|||||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |
| All S. aureus (297) | 0.015-8 | 0.03 | 1 | 0.03 to >32 | 0.25 | 16 | 0.5-4 | 1 | 1 |
| MSSA (101) | 0.015-2 | 0.03 | 0.25 | 0.03-16 | 0.125 | 2 | 0.5-4 | 1 | 1 |
| MRSA, community acquired (86)a | 0.015-1 | 0.03 | 0.5 | 0.06-8 | 0.125 | 2 | 0.5-1 | 1 | 1 |
| MRSA, hospital acquired (110) | 0.015-8 | 0.5 | 1 | 0.06 to >32 | 4 | 16 | 0.5-4 | 1 | 4 |
| hVISA + VISA (27)b | 0.03-1 | 0.25 | 0.5 | 0.125-16 | 8 | 16 | 1-4 | 4 | 4 |
| Quinolone-resistant S. aureus (117)c | 0.03-8 | 0.5 | 1 | 2 to >32 | 4 | 16 | 0.5-4 | 1 | 4 |
Seventy-three isolates produced Panton-Valentin leukocidin.
Includes 2 hVISA and 25 VISA.
Moxifloxacin MICs were ≥2 μg/ml.
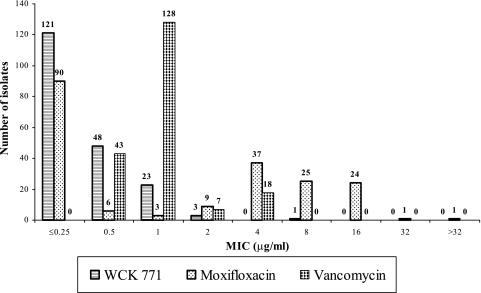
Figure 1 shows the MIC distribution data for the MRSA strains. The number of MRSA strains that were inhibited by WCK 771, moxifloxacin, and vancomycin at ≤2 μg/ml were 195, 108, and 178, respectively. Similarly, the numbers of strains inhibited at >2 μg/ml were 1 for WCK 771, 88 for moxifloxacin, and 18 for vancomycin.
FIG. 1.
MIC distribution of 196 MRSA strains (86 community-acquired and 110 hospital-acquired strains). Values indicated at the tops of bars show the number of strains exhibiting an MIC at that concentration for each of the drugs.
Since the activities of WCK 771 and moxifloxacin are expected to be modulated by the number and variety of QRDR mutations, we chose two vancomycin-susceptible MRSA strains, two VISA strains, and seven VRSA strains (all quinolone resistant; ciprofloxacin MICs of ≥16 μg/ml) to analyze QRDRs. Table 2 shows the mutations in QRDR and corresponding MICs of WCK 771, moxifloxacin, and other agents. MRSA strains, VISA (Mu3 and Mu50) strains, and one VRSA strain (VRS2) showed double mutations, one each in gyrA and grlA. VRS3, VRS4, and VRS6 showed triple mutations, one in gyrA and two in grlA. WCK 771 demonstrated an MIC of 0.5 μg/ml against all these strains possessing two or three mutations. VRS5 (a recent VRSA isolate from Michigan) (1) revealed four mutations, two each in gyrA (Ser84Leu and Glu88Lys) and grlA (Ser80Tyr and Glu84Gly). WCK 771 and moxifloxacin MICs for VRS5 were 8 and >32 μg/ml, respectively.
TABLE 2.
Activities of WCK 771 and other antibacterial agents against vancomycin-susceptible MRSA, VISA, and VRSA with genetically defined QRDRa
| Strain | Phenotype | QRDRb substitution(s)
|
MIC (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| GyrA | GrlA | WCK 771 | MXF | VAN | ||
| MRSA32 | VANs | S84L | S80Y | 0.5 | 2 | 1 |
| MRSA34 | VANs | S84L | S80F | 0.5 | 2 | 1 |
| Mu3 | VISA | S84L | S80F | 1 | 4 | 4 |
| Mu50 | VISA | S84L | S80F | 1 | 4 | 8 |
| VRS1c | VRSA | S84L | S80F | 0.5 | 8 | 128 |
| VRS2 | VRSA | S84L | S80F | 0.5 | 4 | 32 |
| VRS3d | VRSA | S84L | S80Y E84K | 0.5 | 8 | 64 |
| VRS5 | VRSA | S84L E88K | S80Y E84G | 8 | >32 | 64 |
| VRS6 | VRSA | S84L | S80Y E84G | 0.5 | 8 | 128 |
Strain overlap occurs (for example, quinolone-resistant organisms also include VISA and VRSA). Abbreviations: VAN, vancomycin; MXF, moxifloxacin.
None of the strains showed mutations in QRDR of grlB except VRS1, which showed a D432V mutation. None of the strains showed a mutation in QRDR of gyrB.
VRS7 showed QRDR mutations and drug susceptibilities similar to VRS1.
VRS4 showed QRDR mutations and drug susceptibilities similar to VRS3.
The activity of WCK 771 reported in this study shows that recent community-acquired and hospital-acquired S. aureus strains continue to be susceptible to WCK 771 in the face of evolving quinolone resistance, particularly in hospital settings. The MIC90 of vancomycin against MRSA was usually two times higher than against MSSA, probably indicating the steady accumulation of a nonsusceptible population within MRSA. Previous reports regarding suboptimal clinical responses of vancomycin for infections caused by VISA are probably linked to such a phenomenon (7, 15, 16). The true incidence of vancomycin-nonsusceptible strains from clinical isolates is unknown because of technical problems in their detection, but it might be higher than currently reported.
Against QRDR-characterized, quinolone-resistant MRSA, VISA, and VRSA strains, unlike moxifloxacin, WCK 771 retained high potency for strains possessing two or three mutations in quinolone target genes. Taken together the earlier studies and our present observations with VRS5 suggest that WCK 771 MICs of ≥4 μg/ml (moxifloxacin MIC, >32 μg/ml) would be displayed only by those strains that possess four concurrent mutations, two each in gyrA and grlA, which probably is a rare phenomenon (2). However, this study supports the current understanding that such a high level of quinolone resistance in S. aureus is still not widely prevalent (1 out of 297 strains tested in this study).
The potent activity of WCK 771 against an older collection of quinolone-resistant S. aureus strains not including community-acquired MRSA and vancomycin-nonsusceptible strains was described previously (8). Unlike other quinolones such as levofloxacin and moxifloxacin, WCK 771 demonstrates distinctive antistaphylococcal properties, such as preferential action toward DNA gyrase, unaltered activity against strains exhibiting quinolone efflux, low most probably counts, and induction of growth-defective, noninfectious, small colony variants, resulting in superior pharmacodynamic features against quinolone-resistant strains (2, 8, 14) (Wockhardt Research Center, unpublished data). During phase 1 clinical trials in India, WCK 771 administered as an intravenous infusion at 800 mg, twice a day, attained mean 24-h total areas under the concentration-time curves (AUCs) of 390 μg · h/ml, with 58.5 μg · h/ml being unbound (9). Thus, based on WCK 771's MIC, the unbound AUC/MIC ratio between 58.5 and 117 (9) indicates that most S. aureus strains employed in this study would potentially be treatable with WCK 771 at a suggested breakpoint of 2 μg/ml (9, 14). Results of the current MIC study, taken together with interesting antimicrobial features and clinical pharmacokinetic data, point to a potentially useful place for WCK 771 in the therapy of infections caused by multidrug-resistant S. aureus.
Acknowledgments
This study was funded by a grant from Wockhardt Research Center, Aurangabad, India.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Appelbaum, P. C. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398-408. [DOI] [PubMed] [Google Scholar]
- 2.Bhagwat, S. S., L. A. Mundkur, S. V. Gupte, M. V. Patel, and H. F. Khorakiwala. 2006. The anti-methicillin-resistant Staphylococcus aureus quinolone WCK 771 has potent activity against sequentially selected mutants, a narrow mutant selection window against quinolone-resistant Staphylococcus aureus, and preferentially targets DNA gyrase. Antimicrob. Agents Chemother. 50:3568-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 7th ed., vol. 26. CLSI, Wayne, PA.
- 4.Ferrero, L., B. Cameron, B. Manse., D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 4:651-653. [DOI] [PubMed] [Google Scholar]
- 5.Flamm, R. K., C. Vojtko, D. T. W. Chu, Q. L. J. Beyer, D. Hensey, N. Ramer, J. J. Clement, and S. K. Tanaka. 1995. In vitro evaluation of ABT-719, a novel DNA gyrase inhibitor. Antimicrob. Agents Chemother. 39:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gootz, T., R. P. Zaniewski, S. L. Haskell, F. S. Kaczmarek, and A. E. Maurice. 1999. Activities of trovafloxacin compared with those of other fluoroquinolones against purified topoisomerases and gyrA and grlA mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1845-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howden, B. P., P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cross, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayball, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521-528. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs, M. R., S. Bajaksouzian, A. Windau, P. C. Appelabaum, M. V. Patel, S. V. Gupte, S. S. Bhagwat, N. J. DeSouza, and H. F. Khorakiwala. 2004. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob. Agents Chemother. 48:3338-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha, R., M. Kumar, S. Deshmukh, Y. Chugh, R. Yeole, M. Patel, H. Khorakiwala, T. R. K Rao, P. Usha, and M. U. R. Naidu. 2006. WCK 771: a phase I safety, tolerability and pharmacokinetics of 800 mg multiple dose intravenous infusion, abstr. A-1093. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lina, G., Y. Piemont., F. Godail-Gamot., M. Bes., M. O. Peter, and V. Gaudchon. 1999. Involvement of Panton-Valentin leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 12.McGhee, P., and P. C. Appelbaum. 2006. Comparative activity of WCK 771 against S. aureus with raised vancomycin and daptomycin MICs and other resistotypes, abstr. F1-2129. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Otani, T., M. Tanaka, E. Ito, Y. Kurosaka, Y. Murakami, K. Onodera, T. Akasaka, and K. Sato. 2003. In vitro and in vivo antibacterial activities of DK-507k, a novel fluoroquinolone. Antimicrob. Agents. Chemother. 47:3250-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel, M. V., N. J. De Souza, S. V. Gupte, M. Jafri, S. S. Bhagwat, Y. Chugh, H. F. Khorakiwala, M. R. Jacobs, and P. C. Appelbaum. 2004. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob. Agents Chemother. 48:4754-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plipat, N., G. Livni, H. Bertram, and R. B. Thomson, Jr. 2005. Unstable vancomycin heteroresistance is common among clinical isolates of methicilin-resistant Staphylococcus aureus. 2005. J. Clin. Microbiol. 43:2494-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybak, M. J., S. N. Leonard, K. L. Rossi, C. M. Cheung, H. S. Sader, and R. N. Jones. 16 July 2008, posting date. Characterization of vancomycin heteroresistant Staphylococcus aureus (hVISA) from the Detroit metropolitan area over a 22-year period (1986-2007). J. Clin. Microbiol. doi: 10.1128/JCM.00582-08. [DOI] [PMC free article] [PubMed]
- 17.Styers, D., D. J. Sheehan, P. Hogan, and D. F. Sahm. 2006. Laboratory based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microbiol. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]