Abstract
Indian Leishmania donovani isolates (n = 19) from regional zones representing various levels of antimony resistance displayed significantly (P < 0.01) correlated results with respect to in vitro susceptibility to the antileishmanial drugs sodium antimony gluconate, amphotericin B, and Miltefosine, raising the possibility of cross-resistance mechanisms operating in the field isolates. The results of gene expression analysis of LdMT and LdRos3 were suggestive of alternate mechanisms of Miltefosine susceptibility in the isolates.
A high (>60%) proportion of non-antimony-responsive cases of Kala azar in India and the anthroponotic mode of transmission of the parasite causing the disease increase the chances of the generation and spreading of drug-resistant parasites (15, 17). The second-line antileishmanials amphotericin B (AmB) and Miltefosine (MIL) are highly effective for treatment of antimony-resistant patients but are of limited utility because of adverse reactions and high cost. A recent report of unresponsiveness to Ambisome in Sudanese patients of VL is worrisome and indicates the emergence of AmB-resistant parasites (9). Preliminary data from a phase IV trial with MIL suggested a doubling of the relapse rate, indicating lower drug efficacy than in phase II and III trials and providing a warning about the emergence of resistance (3, 18, 19).
Earlier studies using isolates from responsive and nonresponsive patients indicated that resistance to antimonials is an intrinsic property of the parasite (4, 8, 15, 16). Antimony resistance varies among zones representing differing levels of endemicity, emphasizing the acquired nature of resistance in the region (15). Sodium antimony gluconate (SAG)-resistant isolates exhibited cross-resistance to AmB and MIL, with HSP83 and a calpain-related protein being implicated in resistance by modulating drug-induced programmed cell death (21). Since the use of MIL for VL treatment has been introduced only recently, resistance has not yet been reported in the field; however, a wide range of 50% effective doses (ED50) of MIL has been observed for parasite isolates from Nepal and Peru (23). The results of earlier studies revealed a role in MIL uptake and susceptibility for the LdMT-LdRos3-dependent flippase machinery at the plasma membrane (10-12). The present study was aimed at (i) evaluating the in vitro natural susceptibility of field isolates of Leishmania donovani to SAG, AmB, and MIL and (ii) correlating MIL susceptibility with the mRNA expression of LdMT and LdRos3 to explore their role in MIL resistance and potential as markers of MIL resistance in field isolates.
The present study considered 19 L. donovani isolates from VL patients representing regional zones with various degrees of disease endemicity. In vitro susceptibility of parasites from SAG-treated patients (responsive and nonresponsive) and AmB-treated patients (all responded to treatment, and no clinical resistance was observed) was studied. Informed consent based on the guidelines of the Ethical Committee, Safdarjung Hospital, New Delhi, India, was obtained from the patients. The field isolates were investigated for susceptibility to SAG (Albert David Ltd., India), AmB (Sigma), and MIL (Cayman Chemical Company) at the intracellular amastigote and promastigote stages as described previously (15). The clinical profiles of VL patients and in vitro susceptibilities of parasite isolates are summarized in Table 1.
TABLE 1.
Clinical profiles of VL patients from LR and HR regions and in vitro susceptibility of parasite isolates to SAG, AmB, and MIL, with expression indices of MIL transporters LdMT and LdRos3
| Strain | Sex/age (yr)a | Area in India/region category or source and/or description | Treatment (response)b | Susceptibility (ED50 [μg/ml])c
|
Fold decrease in expressiond
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAG (Amas) | AmB
|
MIL
|
LdMT | LdRoS3 | ||||||
| Amas | Pro | Amas | Pro | |||||||
| LdAG83 | Standard Indian L. donovani strain | 2.06 ± 0.23 | 0.017 ± 0.01 | 0.023 ± 0.01 | 0.85 ± 0.03 | 1.05 ± 0.07 | 1 | 1 | ||
| K59 | F/21 | Vaishali/HR | SAG (NR) | 14.66 ± 3.29 | 0.180 ± 0.02 | 0.50 ± 0.006 | 1.86 ± 0.07 | 1.19 ± 0.07 | 2.84 ± 0.22 | 4.06 ± 0.21 |
| K131 | M/22 | Saharsha/HR | SAG (NR) | 19.38 ± 1.68 | 0.35 ± 0.08 | 0.43 ± 0.035 | 0.51 ± 0.06 | 0.63 ± 0.03 | 2.43 ± 0.25 | 11.1 ± 0.23 |
| K149 | M/20 | Saran/HR | AmB (R) | 15.70 ± 4.01 | 0.28 ± 0.10 | 1.46 ± 0.12 | 1.57 ± 0.16 | 1.56 ± 0.11 | 1.08 ± 0.56 | 7.69 ± 0.16 |
| K192 | M/24 | Saran/HR | AmB (R) | 20.30 ± 0.84 | 0.67 ± 0.10 | 0.78 ± 0.12 | 1.99 ± 0.17 | 1.91 ± 0.08 | 0.23e ± 0.04 | 1.9 ± 0.13 |
| K251 | M/11 | Saran/HR | NDf | 11.82 ± 1.28 | 0.55 ± 0.01 | 1.42 ± 0.09 | 1.59 ± 0.07 | 0.40 ± 0.05 | 7.68 ± 0.10 | 52.63 ± 0.06 |
| K417 | F/8 | Muzaffarpur/HR | AmB (R) | 14.65 ± 0.67 | 0.44 ± 0.01 | 0.69 ± 0.007 | 1.90 ± 0.04 | 1.72 ± 0.11 | 1.88 ± 0.23 | 5.02 ± 0.23 |
| K429 | M/26 | Saharsha/HR | AmB (R) | 13.76 ± 0.82 | 0.37 ± 0.10 | 1.34 ± 0.17 | 1.24 ± 0.18 | 1.26 ± 0.06 | 1.26 ± 0.52 | 8.85 ± 0.25 |
| K439 | M/16 | Muzaffarpur/HR | AmB (R) | 12.88 ± 0.12 | 0.77 ± 0.08 | 1.62 ± 0.134 | 1.07 ± 0.1 | 0.87 ± 0.04 | 4.76 ± 0.20 | 3.12 ± 0.20 |
| K481 | M/32 | Muzaffarpur/HR | AmB (R) | 17.53 ± 0.34 | 0.55 ± 0.07 | 0.69 ± 0.016 | 2.32 ± 0.14 | 1.90 ± 0.12 | ND | ND |
| K498 | F/55 | Madhubani/HR | AmB (R) | 15.82 ± 0.24 | 0.54 ± 0.01 | 1.16 ± 0.06 | 1.65 ± 0.05 | 1.32 ± 0.08 | 1.38 ± 0.24 | 7.63 ± 0.24 |
| K516 | F/60 | Motihari/HR | AmB (R) | 16.48 ± 0.61 | 0.62 ± 0.04 | 1.26 ± 0.029 | 2.02 ± 0.10 | 1.62 ± 0.16 | 8 ± 0.12 | 83.33 ± 0.17 |
| K509 | F/4 | Madhubani/HR | AmB (R) | 16.84 ± 0.26 | 0.65 ± 0.06 | ND | 2.16 ± 0.13 | 1.94 ± 0.11 | ND | ND |
| K80 | F/40 | Bhagalpur/LR | SAG (NR) pentamidine | 10.42 ± 2.17 | 0.18 ± 0.02 | 0.50 ± 0.006 | 1.32 ± 0.04 | 1.58 ± 0.16 | 1.24 ± 0.48 | 1.71 ± 0.48 |
| K111 | F/36 | Siwan/LR | SAG (R) | 5.63 ± 0.57 | 0.2 ± 0.01 | 1.02 ± 0.07 | 0.85 ± 0.19 | 0.47 ± 0.06 | 3.4 ± 0.12 | 5.26 ± 0.19 |
| K132 | F/24 | Munger/LR | ND | 3.95 ± 0.28 | 0.22 ± 0.01 | 1.22 ± 0.11 | 0.48 ± 0.05 | 0.53 ± 0.03 | 5.5 ± 0.16 | 55.55 ± 0.16 |
| K133 | M/20 | West Bengal/LR | SAG (R) | 3.45 ± 0.28 | 0.21 ± 0.01 | 0.65 ± 0.18 | 0.93 ± 0.10 | 0.83 ± 0.04 | 1.5 ± 0.26 | 4.52 ± 0.26 |
| K135 | F/45 | Gopalganj/LR | SAG (R) | 4.22 ± 0.38 | 0.25 ± 0.04 | 0.31 ± 0.014 | 0.72 ± 0.03 | 0.86 ± 0.03 | 2.86 ± 0.29 | 1.67 ± 0.29 |
| K216 | M/14 | West Bengal/LR | SAG (R) | 2.14 ± 0.28 | 0.25 ± 0.03 | 0.52 ± 0.018 | 0.91 ± 0.19 | 0.76 ± 0.02 | 1.4 ± 0.27 | 9.43 ± 0.26 |
| K435 | M/17 | Kushinagar/LR | AmB (R) | 11.82 ± 1.39 | 0.17 ± 0.01 | 0.57 ± 0.012 | 1.08 ± 0.25 | 1.16 ± 0.19 | 2.67 ± 0.19 | 4.76 ± 0.21 |
| K59M20 | Lab generated, MIL resistant | NAg | ND | ND | ND | >15 | >15 | 13.33 ± 0.2 | 100 ± 0.03 | |
| K417M20 | Lab generated, MIL resistant | NA | ND | ND | ND | >15 | >15 | 11.3 ± 0.17 | 100 ± 0.02 | |
M, male; F, female.
Responses were noted 30 days after treatment with SAG infusions (20 mg/kg of body weight) or with AmB infusions (1 mg/kg of body weight) on alternate days for 1 month. Patients with an absence of fever and with a reduction in spleen size were designated responders (R); patients who did not exhibit those outcomes were considered nonresponders (NR).
Mean ED50s ± standard deviations of the results from three separate assays. Amas, amastigotes; Pro, promastigotes.
Expression levels indicative of decreases relative to those seen with strain LdAG83.
4.28-fold increase relative to the results seen with strain LdAG83.
ND, not determined.
NA, not applicable.
For SAG, ED50 values ranged from 2.14 ± 0.28 (mean ± standard deviation) to 20.30 ± 0.84 μg/ml, with a mean ED50 of 12.18 ± 5.68 μg/ml. The mean ED50 of isolates from the high-resistance (HR) region (15.81 ± 2.50 μg/ml) was significantly (P < 0.001) higher than that of the low-resistance (LR)-region isolates (5.46 ± 3.69 μg/ml). We observed a strong correlation of in vitro SAG susceptibility with the endemicity zones (rrank = 0.998) and with the clinical response (rrank = 0.982), based on the criteria defined earlier (15, 20). The ED50 values for AmB at the amastigote stage ranged from 0.17 ± 0.01 (mean ± standard deviation) to 0.77 ± 0.08 μg/ml, with a mean of 0.39 ± 0.19 μg/ml. The ED90 values ranged from 0.37 ± 0.02 to 2.55 ± 0.42 μg/ml (mean, 1.29 ± 0.63 μg/ml). The mean ED50 (0.49 ± 0.17 μg/ml) of isolates from the HR region was significantly higher (P < 0.001) than the mean ED50 (0.21 ± 0.03 μg/ml) for LR zone isolates. At the promastigote stage, the ED50 for AmB (n = 18) ranged from 0.312 ± 0.014 to 1.62 ± 0.134 μg/ml (mean, 0.89 ± 0.44 μg/ml). Antileishmanial activity of AmB was partially correlated (r = 0.596) at the amastigote and promastigote stages.
The field isolates (n = 19) showed various levels of susceptibility to MIL, with ED50 values at the amastigote stage ranging from 0.48 ± 0.05 to 2.32 ± 0.14 μg/ml (mean = 1.38 ± 0.55 μg/ml) and ED90 values ranging from 3.53 ± 0.12 to 12.83 ± 1.2 μg/ml (mean = 6.59 ± 3.22 μg/ml). The ED50 values for promastigotes (n = 19) ranged from 0.40 ± 0.05 to 1.94 ± 0.11 μg/ml (mean ED50 = 1.18 ± 0.11 μg/ml), whereas ED90 values ranged from 1.64 ± 0.34 to 8.83 ± 2.67 μg/ml (mean ED90 = 3.98 ± 0.87 μg/ml). The antileishmanial activity of MIL revealed a strong correlation between the susceptibility of amastigotes and that of promastigotes (rrank = 0.82). The isolates from HR zones (n = 12) showed a mean ED50 of 1.65 ± 0.14 μg/ml, which was significantly higher than that of the isolates from LR region, for which the corresponding value was 0.90 ± 0.1 μg/ml (P < 0.001). Overall, we observed a significant positive correlation for the SAG susceptibility profile with AmB (r = 0.599, P < 0.01) or MIL (r = 0.66, P < 0.01), indicating the possibility of a development of resistance to MIL and AmB. Susceptibility profiles of the field isolates for MIL and AmB were also positively correlated (r = 0.57, P < 0.01).
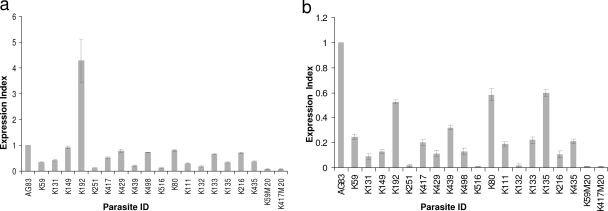
The gene expression levels of LdMT and LdRos3 were determined for VL field isolates (n = 17) in comparison with the expression levels seen with the standard L. donovani LdAG83 strain and two MIL-resistant LdM20 parasite strains (generated by a stepwise increase in the concentration of MIL up to 20 μg/ml) by real-time PCR using SYBR green and analyzed by the 2−ΔΔCt method. In comparison to strain LdAG83, the majority of the isolates, except one (K192), revealed decreased expression of LdMT and LdRos3. In general, the levels of expression of LdMT and LdRos3 were correlated and expression of LdRos3 was higher than that of LdMT, though the ratios of expression of LdMT and LdRos3 differed greatly (from 0.67- to-10 fold) among the isolates. In comparison to the results seen with the two laboratory-generated MIL-resistant LdM20 parasite strains, the levels of expression of LdMT and LdRos3 differed more than threefold for the majority (14/17 [82.3%]) of the isolates (Tables 1 and 2 and Fig. 1).
TABLE 2.
Oligonucleotide sequences of genes amplified in this study
| Gene | Primer description | Primer sequence (5′→3′) | Product size (bp) |
|---|---|---|---|
| GAPDHa | Forward | GAA GTA CAC GGT GGA GGC TG | 206 |
| Reverse | CGC TGA TCA CGA CCT TCT TC | ||
| LdMTb | Forward | CAA GTG CCT TTC CAC CAG AAT C | 228 |
| Reverse | CTC ACC TTT TTG AAC TCC AAC AGG | ||
| LdRos3c | Forward | ACG ACA CGG CTT GAT TTT CG | 238 |
| Reverse | GAG TAG TCC ACG GAG GCA GTA AAG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
LdMT, L. donovani putative miltefosine transporter.
LdRos3, β-subunit of LdMT.
FIG. 1.
Expression of LdMT and LdRos3 in different field isolates. (a) Real-time reverse transcription-PCR expression analysis of an L. donovani MIL transporter (LdMT). The graph shows the expression index, defined as ratios of gene expression relative to that of strain LdAG83. (b) Real-time reverse transcription-PCR expression analysis of an L. donovani MIL transporter (LdRos3). The graph shows the expression index, defined as ratios of gene expression relative to that of strain LdAG83. Data represent the means of the results of three independent experiments.
Considering that drug resistance is a manifestation of multifactorial phenomena, various determinants may be responsible for variations in the drug susceptibility of field isolates. Differences in membrane sterol content (1, 5) and lipid content (2) have been demonstrated to lead to distinct drug susceptibility profiles. The extensive use of SAG in areas of hyperendemicity may have changed the biochemical composition of these parasites' membranes in ways that might affect drug susceptibility. Both AmB and MIL are known to interact with the plasma membrane of the cells (5, 13), and membrane modifications have also been suggested as a mechanism of resistance in SAG-resistant isolates (6). Previous studies indicated that some common mechanism of resistance, such as permanent modification in the membranes or drug transporters, etc., may be operating that may modulate drug-induced cell death and may lend cross-resistance to the drugs (6, 7, 14, 21, 22).
The present study highlights the possibility of the occurrence of cross-resistance to three drugs, i.e., SAG, AmB, and MIL, in field isolates, emphasizing the need for novel strategies for treatment of VL. Development of antimony resistance in the anthroponotic VL cycle suggests that resistance to other antileishmanial drugs could also develop once they are widely used as single agents.
Acknowledgments
Financial support by Indian Council of Medical Research, India, is gratefully acknowledged. D.K. and A.K. are grateful to CSIR for financial support. R.S. was supported by a UNESCO L'Oreal for Women in Science fellowship.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Beach, D. H., L. J. Goad, and G. G. Holz, Jr. 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31:149-162. [DOI] [PubMed] [Google Scholar]
- 2.Beach, D. H., G. G. Holz, Jr., and G. E. Anekwe. 1979. Lipids of Leishmania promastigotes. J. Parasitol. 65:201-216. [PubMed] [Google Scholar]
- 3.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dube, A., N. Singh, S. Sundar, and N. Singh. 2005. Refractoriness to the treatment of sodium stibogluconate in Indian kala-azar field isolates persist in in vitro and in vivo experimental models. Parasitol. Res. 96:216-223. [DOI] [PubMed] [Google Scholar]
- 5.Goad, L. J., G. G. Holz, Jr., and D. H. Beach. 1984. Sterols of Leishmania species. Implications for biosynthesis. Mol. Biochem. Parasitol. 10:161-170. [DOI] [PubMed] [Google Scholar]
- 6.Kothari, H., P. Kumar, S. Sundar, and N. Singh. 2007. Possibility of membrane modification as a mechanism of antimony resistance in Leishmania donovani. Parasitol. Int. 56:77-80. [DOI] [PubMed] [Google Scholar]
- 7.Lee, N., S. Bertholet, A. Debrabant, J. Muller, R. Duncan, and H. L. Nakhasi. 2002. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 9:53-64. [DOI] [PubMed] [Google Scholar]
- 8.Lira, R., S. Sundar, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sacks. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 9.Mueller, M., K. Ritmeijer, M. Balasegaram, Y. Koummuki, M. R. Santana, and R. Davidson. 2007. Unresponsiveness to AmBisome in some Sudanese patients with kala-azar. Trans. R. Soc. Trop. Med. Hyg. 101:19-24. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Victoria, F. J., M. P. Sanchez-Canete, S. Castanys, and F. Gamarro. 2006. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J. Biol. Chem. 281:23766-23775. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Victoria, F. J., M. P. Sanchez-Canete, K. Seifert, S. L. Croft, S. Sundar, S. Castanys, and F. Gamarro. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Updat. 9:26-39. [DOI] [PubMed] [Google Scholar]
- 13.Rakotomanga, M., P. M. Loiseau, and M. Saint-Pierre-Chazalet. 2004. Hexadecylphosphocholine interaction with lipid monolayers. Biochim. Biophys. Acta 1661:212-218. [DOI] [PubMed] [Google Scholar]
- 14.Sereno, D., P. Holzmuller, I. Mangot, G. Cuny, A. Ouaissi, and J. L. Lemesre. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 45:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh, R., D. Kumar, V. Ramesh, N. S. Negi, S. Singh, and P. Salotra. 2006. Visceral leishmaniasis, or kala azar (KA): high incidence of refractoriness to antimony is contributed by anthroponotic transmission via post-KA dermal leishmaniasis. J. Infect. Dis. 194:302-306. [DOI] [PubMed] [Google Scholar]
- 16.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 17.Sundar, S. 2001. Treatment of visceral leishmaniasis. Med. Microbiol. Immunol. 190:89-92. [DOI] [PubMed] [Google Scholar]
- 18.Sundar, S., and H. W. Murray. 2005. Availability of miltefosine for the treatment of kala-azar in India. Bull. W.H.O. 83:394-395. [PMC free article] [PubMed] [Google Scholar]
- 19.Sundar, S., and P. L. Olliaro. 2007. Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther. Clin. Risk Manag. 3:733-740. [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur, C. P., S. Narayan, and A. Ranjan. 2004. Epidemiological, clinical & pharmacological study of antimony-resistant visceral leishmaniasis in Bihar, India. Indian J. Med. Res. 120:166-172. [PubMed] [Google Scholar]
- 21.Vergnes, B., B. Gourbal, I. Girard, S. Sundar, J. Drummelsmith, and M. Ouellette. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell. Proteomics 6:88-101. [DOI] [PubMed] [Google Scholar]
- 22.Verma, N. K., and C. S. Dey. 2004. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob. Agents Chemother. 48:3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yardley, V., S. L. Croft, S. De Doncker, J. C. Dujardin, S. Koirala, S. Rijal, C. Miranda, A. Llanos-Cuentas, and F. Chappuis. 2005. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am. J. Trop. Med. Hyg. 73:272-275. [PubMed] [Google Scholar]