Abstract
It is possible that antifungal drugs with novel modes of action will provide favorable options to treat fungal infections. In the course of our screening for antifungal compounds acting on the cell wall, a pyridobenzimidazole derivative with unique activities, named D75-4590, was discovered. During treatment of Saccharomyces cerevisiae with D75-4590, (i) incorporation of [14C]glucose into the β-1,6-glucan component was selectively reduced, (ii) proteins released from the cell had lost the β-1,6-glucan moiety, and (iii) cells tended to clump, resulting in impaired cell growth. Genetic analysis of a D75-4590-resistant mutant of S. cerevisiae indicated that its primary target was Kre6p, which is considered to be one of the β-1,6-glucan synthases. These results strongly suggest that D75-4590 is a specific inhibitor of β-1,6-glucan synthesis. D75-4590 showed potent activities against various Candida species. It inhibited hyphal elongation of C. albicans as well. KRE6 is conserved in various fungi, but no homologue has been found in mammalian cells. These lines of evidence indicate that D75-4590 is a promising lead compound for novel antifungal drugs. To our knowledge, this is the first report of a β-1,6-glucan inhibitor.
Fungal infections have increased in frequency over the past several decades due to a growing number of immunocompromised patients (8, 9, 35). Present therapeutic options are limited, however, to three classes of compounds: polyenes, azoles, and recently introduced candins (3, 9, 48). The utility of polyenes is limited by their nephrotoxicity (35, 48). Although azoles are safer and most commonly used, the broad usage of these drugs has probably allowed the increase of less-susceptible species of Candida, such as C. glabrata (2, 17). In addition, drug-drug interactions and teratogenicity limit their clinical usage. Candins have solved some of these problems; however, they have not completely satisfied unmet medical needs mainly due to their poor oral absorption and limited spectrum (48). In addition, incidents of resistance to these drugs have also emerged (14, 28). Therefore, development of an orally active antifungal drug with a novel mode of action is desirable.
The fungal cell wall is an attractive target for antifungal agents because it is an essential, fungal-specific organelle that is absent from human cells. The cell wall of Saccharomyces cerevisiae is basically composed of β-1,3-glucan, β-1,6-glucan, chitin, and highly mannosylated glycoproteins, which are interconnected (5, 20). Many fungal-specific enzymes, such as Fks1p, Kre6p, and Chs1p, are involved in the synthesis of β-1,3-glucan, β-1,6-glucan, and chitin, respectively (5). In addition to the synthases of cell wall components, several enzymes have been shown to be involved in the interconnection of these components (5). A number of β-1,3-glucan synthase inhibitors, such as echinocandins, papulacandins, and enfumafungin, have already been reported (21, 34, 44). Several chitin synthase inhibitors have been reported as well (13, 42). In contrast, an inhibitor of β-1,6-glucan synthase or the enzymes involved in the interconnection of cell wall components has not been reported.
Genetic analyses of S. cerevisiae and C. albicans have provided us with valuable information regarding β-1,6-glucan synthesis (16, 27, 30, 39). Many proteins encoded by KRE genes, such as KRE6, KRE9, and KRE1, are involved in the biosynthesis in a sequential manner (5). However, no precise functions, either catalytic or regulatory, have been definitively assigned to any KRE gene products. Lack of enzymatic information hampers the discovery of their inhibitors. Structural and biochemical analyses of the yeast cell wall, however, have provided a way to obtain inhibitors. Most cell wall proteins are glycosylphosphatidylinositol (GPI) dependent and are attached to β-1,3-glucan and/or chitin via β-1,6-glucan (19, 22, 23). Recent progress in genetic technology allowed us to attach heterologous protein to the cell wall by constructing a gene of interest fused to a secretion signal and GPI attachment signal (46, 47). Using this technology, we have developed a cell-based assay system for screening various inhibitors of cell wall components, including β-1,6-glucan (A. Kitamura, K. Someya, and R. Nakajima, U.S. patent application 20040091949 [PCT/JP01/03630]). In the course of screening for antifungal compounds using this system, we discovered the compound D75-4590, which has unique activities. To gain a better insight into this compound, we studied the nature of its antifungal activities and its mechanism of action.
MATERIALS AND METHODS
Strains and media.
S. cerevisiae YPH500 (Matα ade2 his3 leu2 lys2 typ1 ura3) (40), AY-10 (isogenic strain derived from YPH500; Matα ade2 his3 lys2 ura3), AY-10c (AY-10 skn1::URA3) (Kitamura et al., U.S. patent application 20040091949 [PCT/JP01/03630]), and the 15 pathogenic fungi listed in Table 1 were used in this study. These pathogenic fungi were purchased from the American Type Culture Collection (Rockville, MD), the Institute for Fermentation Osaka (Osaka, Japan), or the Teikyo Institute of Medical Mycology (Tokyo, Japan).
TABLE 1.
Antifungal activities of D75-4590 and fluconazolea
| Strain | D75-4590
|
FLC (MIC-2) | ||
|---|---|---|---|---|
| MIC-2 | MIC-0 | MFC | ||
| S. cerevisiae YPH500 | 1 | 2 | >32 | 4 |
| C. albicans ATCC 24433 | 8 | 32 | >32 | 0.25 |
| C. albicans ATCC MYA-573 | 16 | 32 | NT | >64 |
| C. glabrata IFO0622 | 1 | 2 | 32 | 2 |
| C. glabrata ATCC200918 | 4 | 4 | NT | 16 |
| C. krusei TIMM0269 | 8 | 16 | >32 | 16 |
| C. tropicalis TIMM0313 | 1 | 8 | >32 | 0.25 |
| C. tropicalis ATCC 200956 | 1 | 1 | NT | >64 |
| C. parapsilosis IFO1396 | 4 | >32 | NT | 1 |
| C. lusitaniae ATCC 200951 | 8 | 16 | NT | 0.063 |
| C. kefyr ATCC 46764 | 1 | 2 | NT | 0.25 |
| C. neoformans ATCC 90112 | >32 | >32 | NT | 2 |
| T. asahii ATCC 90039 | >32 | >32 | NT | 4 |
| A. fumigatus ATCC 36607 | >32 | >32 | NT | >64 |
| A. flavus ATCC 64025 | >32 | >32 | NT | >64 |
| A. terreus ATCC 28301 | >32 | >32 | NT | >64 |
MIC-0s, MIC-2s, and MFCs (in micrograms per milliliter) were determined by using the microdilution method described in Materials and Methods. NT, not tested; FLC, fluconazole.
Sabouraud dextrose agar (SDA; Difco, Detroit, MI), RPMI 1640 (Sigma, St. Louis, MO), RPMIB (RPMI 1640 supplemented with 1 M sorbitol, 100 mM potassium phosphate buffer [pH 6.5], 2% glucose, 40 μg/ml adenine, 20 μg/ml uracil), YNB (0.67% yeast nitrogen base with amino acids [Difco], 2% glucose), hyphal forming medium 7 (HFM-7; 5 g/liter glucose, 0.26 g/liter Na2HPO4·12H2O, 0.66 g/liter KH2PO4, 0.08 g/liter MgSO4·7H2O, 0.33 g/liter NH4Cl, 16 mg/liter biotin, 4% fetal bovine serum) (18), and Lee's medium were used for various tests. If appropriate, 40 μg/ml adenine and 20 μg/ml uracil were added to each medium for S. cerevisiae to grow. Escherichia coli DH5α was used for propagation of plasmids and was grown in Luria broth or agar (Difco) with 100 μg/ml ampicillin (Sigma) when appropriate.
Chemicals.
D75-4590 {2-ethyl-(2-N′,N′-DEAE)amino-3-methylpyrido[1,2-a]benzimidazole-4-carbonitrile} is a compound in our chemical libraries which was synthesized in our laboratories. Its structure is shown in Fig. 1. Amphotericin B, 5-flucytosine, fluconazole, and calcofluor white were purchased from Sigma. Tunicamycin and aculeacin A were purchased from Funakoshi (Tokyo, Japan) and Wako Pure Chemical (Osaka, Japan), respectively. Drugs were dissolved in dimethyl sulfoxide and were used for biological testing at final dimethyl sulfoxide concentrations of less than 0.5% for experiments with human cells and less than 1% for other experiments.
FIG. 1.

Chemical structure of D75-4590.
In vitro antifungal and antibacterial activity and cytotoxicity.
The MIC for each fungal strain was measured by the microdilution method described by the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS), except that the incubation temperature was 30°C (31). The MIC-0 (the lowest drug concentration producing an optically clear well) was used as an endpoint for the experiments with S. cerevisiae. The MIC-2 (the lowest drug concentration producing a prominent reduction in turbidity) was used for fluconazole. For D75-4590, both MIC-0 and MIC-2 were used as endpoints. Since D75-4590-treated cells often grew well but were severely clumped and could not be uniformly mixed, it was difficult to visually define the endpoint. For that reason, an oxidation-reduction indicator, alamar blue (Biosource, Camarillo, CA), was used to define their MIC-2 (6, 43). The MIC-2 is defined as a 50% reduction compared with the drug-free control in absorbance at 570 nm. The optical density at 570 nm was measured with a Wallac 1420 ARVOsx (Wallac, Tokyo, Japan) multilabel counter. The initial cell densities were from 1 × 103 to 3 × 103 cells/ml.
For evaluation of the minimal fungicidal concentrations (MFCs) against various fungi, 4-μl portions of the mixtures were pipetted from a 96-well microtiter plate after the MIC measurements and added to 200 μl of MOPS (morpholinepropanesulfonic acid)-buffered RPMI. These were observed after 24 h of incubation at 30°C, and MFCs were defined as the lowest drug concentration producing no visible fungal growth.
The MIC for the bacterial strains was measured by the CLSI method (32). Cytotoxicity of D75-4590 against PC-6 (lung cancer) cells was measured by a procedure described elsewhere (26).
Morphological analysis.
Exponentially growing cells of C. albicans ATCC 24433, C. glabrata IFO0622, or S. cerevisiae YPH500 were suspended in RPMI 1640 with or without drug. After 18 h of incubation with gentle shaking at 30°C, the cells were fixed with 3% glutaraldehyde in 0.1 M phosphate buffer and were examined with an inverted light microscope (Olympus model IX7; Olympus, Tokyo, Japan).
To evaluate the inhibitory effect of the drug against hyphal growth of C. albicans ATCC 24433, HFM-7 and Lee's medium were used instead of RPMI 1640. Cell suspensions with or without drug were cultured in type I collagen-coated 24-well plates (Iwaki, Tokyo, Japan). After 6 or 18 h of incubation without shaking at 37°C, the cells were examined with a light microscope (Leica model DMLB100; Leica, Solms, Germany). Images were acquired with a digital charge-coupled-device camera (Olympus model DP70).
Time-kill study.
Exponentially growing cells of C. glabrata IFO0622 were suspended in RPMI 1640 with or without drug at a cell density of 1 × 104 cells/ml. Samples were taken after 2, 4, 8, 24, and 48 h of incubation with gentle shaking at 30°C. The samples were diluted appropriately and spread on SDA plates. The number of viable cells in each sample was measured by counting the colonies on each plate after an overnight incubation at 30°C.
Incorporation studies with growing cells.
The effects of D75-4590 on macromolecular synthesis were evaluated by pulse-labeling the cells with radioactive precursors of specific macromolecules. The assay procedures were conducted based on the methods described by Yamaguchi et al. (49) with some modifications. Exponentially growing cells of C. glabrata IFO0622 or S. cerevisiae AY-10 were suspended in RPMI 1640 medium to give approximately 0.6 absorbance at 595 nm. After the drug solution and radiolabeled precursor were added, the reaction tubes were incubated at 30°C with occasional shaking. After 1 and 3 h of incubation, samples were taken for evaluation. The precursor used and the preparation methods for each fraction were as follows, and the radioactivity of each fraction was counted with a toluene scintillator.
(i) Glucose (precursor of cell wall components).
[14C]glucose (Daiichi Pure Chemicals, Tokyo, Japan) was added to a cell suspension at a concentration of 2 μCi/ml. At each time point, a 10-ml sample was taken and crude fractions of β-1,3-glucan, chitin, mannan, and β-1,6-glucan were prepared based on the method of Umeyama et al. (45). The harvested cells were suspended in 2 ml of 3% NaOH and heated for 1 h at 80°C. Insoluble material was harvested, and another extraction was done by the same procedure. The supernatants were gathered, and the mannan fraction was prepared by using Fehling's reaction (4). Insoluble material was washed with water and was suspended in 1.5 ml of 10 mM Tris-HCl buffer (pH 7.5), and then 0.5 ml of Zymolyase 100T (Seikagaku-kougyou, Tokyo, Japan) suspension in the same buffer was added to the samples at a final concentration of 1 mg/ml. Since Zymolyase 100T contains β-1,3-glucanase but does not have β-1,6-glucanase or chitinase activity, β-1,3-glucan was digested into oligosaccharide to make it soluble; β-1,6-glucan remains undigested but becomes soluble and chitin remains insoluble. After overnight incubation at 30°C, the insoluble material was harvested by centrifugation as the chitin fraction; 0.5 ml of supernatant was taken as glucan fractions (the β-1,3-glucan fraction and the β-1,6-glucan fraction); and 1.5 ml of sample was dialyzed overnight against 10 mM Tris-HCl buffer (pH 7.5) to remove the digested β-1,3-glucan. After dialysis, a 1-ml sample was taken as the β-1,6-glucan fraction. The radioactivity of the β-1,3-glucan fraction was calculated by subtracting that of the β-1,6-glucan fraction from that of the glucan fraction. To confirm that the “chitin fraction” actually contained chitin, it was digested with chitinase and the radioactivity of the remaining insoluble material was measured. No significant radioactivity was detected (data not shown).
(ii) Leucine (precursor of protein).
[3H]leucine (Daiichi Pure Chemicals) was added to cell suspensions at a concentration of 2.0 μCi/ml, and 1-ml samples were taken at each point. Harvested cells were suspended in 2 ml of 5% trichloroacetic acid (TCA) and heated at 90°C for 30 min. After the reaction, the insoluble material was collected on glass-fiber filters (GF/C; Whatman, Kent, United Kingdom), which were washed with water and 95% ethanol and then dried (protein fraction).
(iii) Uridine (precursor of RNA).
[3H]uridine (Daiichi Pure Chemicals) was added to the cell suspensions at a concentration of 4.0 μCi/ml, and 2-ml samples were taken at each point. For each sample, 2 ml of ice cold 10% TCA was added and the mixture was incubated on ice for 1 h. Insoluble material was collected on glass-fiber filters (GF/C; Whatman), which were washed with 5% TCA and 95% ethanol and then dried (RNA fraction).
(iv) Acetic acid (precursor of sterol).
[14C]acetic acid (Daiichi Pure Chemicals) was added to the cell suspensions at a concentration of 2.0 μCi/ml, and 5-ml samples were taken at each point. The harvested cells were suspended in 2 ml of a mixture of ethanol and ether (3:1, vol/vol) and incubated at 55°C for 4 h. The supernatant was collected by centrifugation (sterol fraction).
Western analysis of proteins released into medium by treatment of drugs.
Exponentially growing cells of S. cerevisiae AY-10 were harvested by centrifugation and suspended in fresh RPMIB medium to give an absorbance of approximately 0.7 at 595 nm. This cell suspension (150 μl) was mixed with 50 μl of drug solution in 96-well plates and incubated at 30°C for 6 h with occasional shaking. Supernatant from four wells was collected using polyvinylidene difluoride membrane filter plates (multiscreen MAHVN4550; Corning, Corning, NY) and combined. The collected supernatant was concentrated 50 times using ultrafiltration (UFC3LTK; Millipore, Tokyo, Japan). Samples were applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then blotted to a polyvinylidene difluoride membrane (Millipore). Polyclonal anti-β-1,6-glucan antibodies were generated in rabbits against pustulan (Calbiochem, La Jolla, CA). The immunoglobulin G fraction of antiserum was purified via Affi-gel protein A affinity chromatography (Millipore) and was used as the first antibody. Alkaline phosphatase conjugated anti-rabbit immunoglobulin G (goat) antibody was purchased from Sigma and was used as the secondary antibody. Immunodetection of β-1,6-glucan was performed with an alkaline phosphatase detection kit (Bio-Rad, Richmond, CA) according to the manufacturer's instructions.
Generation and analysis of D75-4590-resistant mutants.
Exponentially growing cells of S. cerevisiae AY-10 (1 × 108 cells) were spread onto RPMIB agar containing D75-4590 (64 μg/ml). Plates were exposed to UV irradiation to give approximately 10% survival. After 3 days of incubation at 30°C, 28 colonies had emerged. Genomic DNA was collected from three strains, and their genomic libraries were made using the XhoI, XbaI, HindIII, NheI, SalI, or SpeI site of single-copy vector pRS416 (Stratagene, Cedar Creek, TX). They were transformed into S. cerevisiae AY-10, and resistant strains were selected on RPMIB agar containing D75-4590 (64 μg/ml). Plasmids were recovered from resistant strains and transformed into E. coli DH5α for amplification. Nucleotide sequencing was done using an Applied Biosystems model 3100 sequencer.
Construction of S. cerevisiae CY-1a and CY-2a.
SKN1 (homologue of KRE6) of S. cerevisiae AY-10 was disrupted using a method similar to that described by Roemer et al. (37), resulting in S. cerevisiae AY-10c (Kitamura et al., U.S. patent application 20040091949 [PCT/JP01/03630]). S. cerevisiae CY-1a and CY-2a were constructed from S. cerevisiae AY-10c as follows. A HIS3 cassette obtained by digesting pRS403 (Stratagene) with SspI and PstI was inserted into the HincII and PstI sites of pUC19 to generate pUXS4. Wild-type KRE6 was amplified by PCR (template, S. cerevisiae YPH500 genomic DNA; primers, SCKRE6-Sen3 [5′-CGCGGCCGTAACAAAACGAACAACATGAGACAAAACCCG-3′] and SCKRE6-Anti3 [5′-CGAGGCCTTTAGTTCCCTTTATGACCCGATTTGAAC-3′]) and subcloned into pGEM-T (Promega, Madison, WI) to generate pUAO1. A fragment obtained by digestion of pUAO1 and pRS416-31 with SphI and NheI (KRE6 without a promoter region) was inserted into the XbaI site (within the region of the KRE6 open reading frame [ORF]) of pUXS-1 to generate pUAE1 and pUAE2, respectively. pUAE1 and pUAE2 were digested with XbaI and were introduced into the chromosomal DNA of S. cerevisiae AY-10c to generate S. cerevisiae CY-1a and CY-2a, respectively. Since the KRE6s of pUAE1 and pUAE2 do not have promoter regions, S. cerevisiae CY-1a expresses wild-type KRE6 and CY-2a expresses KRE6 with a resistant mutation.
RESULTS
Discovery of D75-4590.
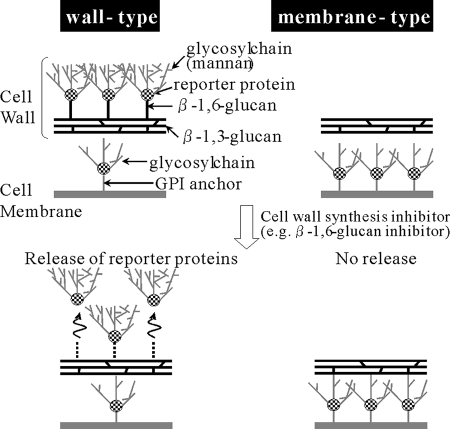
D75-4590 (Fig. 1) was discovered from our chemical library in a high-throughput manner using our novel cell-based assay system described elsewhere (Kitamura et al., U.S. patent application 20040091949 [PCT/JP01/03630]). Briefly, we constructed two types of yeasts, namely “wall-type arming yeast” and “membrane-type arming yeast,” each having an easily detectable protein (reporter protein) fixed on the cell wall via β-1,6-glucan or on the cell membrane via a GPI anchor by means of genetic engineering (11, 47). Compounds inhibiting the process of protein fixation are expected to accelerate the release of a reporter protein from a cell. Therefore, such compounds can be screened by the detection of a reporter protein in the medium. Although the process of protein fixation to the cell membrane is generally conserved in eukaryotic cells, fixation to the cell wall is considered to be unique to fungi. Therefore, screening for the compounds which accelerate the release of the reporter protein from “wall-type arming yeast” but have no effect on “membrane-type arming yeast” would be an efficient way to find the compounds that inhibit fungus-specific processes (Fig. 2). The results of validation studies of our screening system show that several compounds which act on the cell wall, such as aculeacin A (β-1,3-glucan synthesis inhibitor) and tunicamycin (mannan synthesis inhibitor), significantly accelerate the release of the reporter protein from only “wall-type arming yeast” and that other compounds, including nikkomycin Z, fluconazole, 5-flucytosine, and cycloheximide, slightly accelerated or did not accelerate the release of the reporter protein from both arming yeasts (Kitamura et al., U.S. patent application 20040091949 [PCT/JP01/03630]). It was also found that disruption by KRE6 of “wall-type arming yeast” accelerated the release of the reporter protein (Kitamura et al., U.S. patent application 20040091949 [PCT/JP01/03630]). Therefore, several types of compounds acting on the cell wall should be screened by using our system.
FIG. 2.
The principle of the screening system. Reporter proteins are fixed onto the cell wall (wall-type arming yeast) or onto the cell membrane (membrane-type arming yeast) by means of genetic engineering. Both yeasts are treated with the test compound. If the compound acts on the process of protein fixation to the cell wall (e.g., β-1,6-glucan synthesis), reporter proteins will be released from the wall-type arming yeast only. If the compounds act on the process of protein fixation to cell membranes, reporter proteins will be released from both yeasts. If the compounds do not act in protein fixation, no reporter proteins will be released from either yeast.
Antifungal activities of D75-4590.
Table 1 shows the antifungal activities of D75-4590 and fluconazole against various fungi. D75-4590 has activities against a variety of Candida species, including fluconazole-resistant strains. Most strains of C. albicans, C. tropicalis, and C. parapsilosis displayed trailing growth phenomena similar to those observed in the presence of azoles (36). Interestingly, visible morphological changes, which resemble aggregation of S. cerevisiae, were observed in these strains at a much lower concentration of D75-4590 than its MIC-2 values (data not shown). No significant effect was found on Cryptococcus neoformans, Trichosporon asahii, and Aspergillus species even at a concentration of 32 μg/ml. The MICs of D75-4590 against a bacterial strain (Staphylococcus aureus 209P and E. coli NIHJ) were >32 μg/ml, and its GI50 (concentration at which 50% inhibition of growth is observed) against PC-6 (human lung cancer) cells was >16 μg/ml.
MFCs of D75-4590 against five Candida strains were >32 μg/ml, except for that with C. glabrata IFO0622. Although the MFC against C. glabrata IFO0622 was 32 μg/ml, it is possible that the fungal growth in the well for MFC determination was inhibited by drug carried over from the media of the MIC plate. To clarify the mode of action against C. glabrata, a time-kill study of D75-4590 was conducted. As shown in Fig. 3, D75-4590 showed dose-dependent growth inhibition, and it is suggested that its mode of action is fungistatic.
FIG. 3.

Effect of D75-4590 on growing cells of C. glabrata IFO0622. Cells were incubated in RPMI medium with or without D75-4590. The viable cells were counted at intervals using SDA plates. The concentrations of D75-4590 are shown.
To characterize the morphological defects, microscopic analyses of D75-4590-treated cells were performed. First, RPMI medium without serum was used for the culture to determine its effects on yeast-type growing cells. Drug-treated cells of S. cerevisiae, C. glabrata, and C. albicans were clumped; they contained more than one bud and appeared to show incomplete cell separation (Fig. 4). Next, the effect of D75-4590 on hyphal elongation of C. albicans was investigated. As indicated in Fig. 5, static incubation of cells in HFM-7 (18) induces hyphal elongation, and yeast-type cells were rarely seen after 6 h of incubation. The inhibitory effect of D75-4590 on hyphal elongation was observed at a concentration of 1 μg/ml, and yeast-type cells were predominant at a concentration of 16 μg/ml. After 24 h of incubation, cells treated without drugs formed colonies mostly consisting of yeast-type cells with many hyphae on the edges. D75-4590-treated cells also formed colonies, but hyphae on the edges were diminished. Inhibitory effects of D75-4590 on hyphal elongation were also observed when Lee's medium was used (data not shown).
FIG. 4.
Multibudding phenotype of yeasts treated with D75-4590. Growing cells of S. cerevisiae YPH500, C. glabrata IFO0622, and C. albicans ATCC 24433 were treated with D75-4590. Microscopic observation was carried out after 18 h. a, S. cerevisiae, without drug; b, S. cerevisiae, 2 μg/ml of drug; c, C. glabrata, without drug; d, C. glabrata, 2 μg/ml of drug; e, C. albicans, without drug; f, C. albicans, 2 μg/ml of drug.
FIG. 5.
Inhibitory effect of D75-4590 against hyphal elongation of C. albicans ATCC 24433. Cells were cultured in HFM-7 with or without D75-4590. Microscopic observations were carried out after 6 h of treatment with D75-4590 at concentrations of (a) 0 μg/ml, (b) 1 μg/ml, (c) 4 μg/ml, and (d) 16 μg/ml and after 24 h of treatment at concentrations of (e) 0 μg/ml, (f) 1 μg/ml, (g) 4 μg/ml, and (h) 16 μg/ml.
Biochemical study of the D75-4590 mechanism of action using C. glabrata and S. cerevisiae.
Since it was screened by our assay system focusing on the cell wall, it is expected that D75-4590 acts on the cell wall. To obtain information on which components are inhibited, the effects of D75-4590 on the incorporation of radioactive precursors into various macromolecules in growing cells were examined and compared with the effects of aculeacin A (β-1,3-glucan synthesis inhibitor). First, we compared the effects of D75-4590 and aculeacin A on the cell wall components of S. cerevisiae AY-10 (Fig. 6a and b). Aculeacin A strongly reduced the radioactivity in the fraction of β-1,3-glucan and weakly reduced that of β-1,6-glucan. The reduction in the β-1,6-glucan fraction may be a consequence of the inhibition of β-1,3-glucan synthesis, because loss of β-1,3-glucan most likely results in the release of β-1,6-glucan from a cell. In contrast, D75-4590 markedly reduced the radioactivity in the fraction of β-1,6-glucan with only slight or no reduction in the fractions of other cell wall components. A significant reduction was observed at a concentration of 0.078 μg/ml, which is much lower than its MIC-0 value. D75-4590 showed no significant effects on the radioactivity in the fractions of RNA, protein, or sterol at the concentrations tested (Fig. 6b). A selective reduction in the β-1,6-glucan fraction at a lower concentration of MIC-0 was also observed in the experiment using C. glabrata IFO0622 (Fig. 6c).
FIG. 6.
Effects of D75-4590 and aculeacin A on the incorporation of radioactive precursors into macromolecules in growing cells of S. cerevisiae AY-10 and C. glabrata IFO0622. Growing cells in RPMI medium were treated with or without drug in the presence of radioactive glucose, leucine, uridine, or acetic acid. β-1,3-Glucan, β-1,6-glucan, chitin, mannan, RNA, protein, and sterol fractions were prepared by the methods described in Materials and Methods. The percent changes of the incorporated radioactivities by drug treatment for 1 h (upper panels) or 3 h (lower panels) at each concentration tested are displayed. (a) S. cerevisiae AY-10 treated with aculeacin A. (b) S. cerevisiae AY-10 treated with D75-4590. (c) C. glabrata IFO0622 treated with D75-4590.
Reduction of radioactivity in the fraction of β-1,6-glucan can be explained by either inhibition of β-1,6-glucan synthesis or the release of cell wall protein with β-1,6-glucan from the cells. If D75-4590 inhibits β-1,6-glucan synthesis, the released proteins are expected to have a small or no β-1,6-glucan moiety. To determine the reason for the reduction, the amounts of β-1,6-glucan attached to proteins in the culture medium with or without drug treatment were compared by Western analysis. Anti-β-1,6-glucan antiserum detected β-1,6-glucan attached to proteins in the medium without drug. The reaction was diminished when cells were treated with D75-4590 in a dose-dependent manner (Fig. 7). Since significant reduction was observed at a lower concentration than its MIC-0 value, it is unlikely that the reduction is caused by a growth defect. These results suggest that D75-4590 most likely inhibits β-1,6-glucan synthesis.
FIG. 7.

Western analysis of proteins secreted from S. cerevisiae AY-10 treated with D75-4590. Cells were treated with D75-4590 for 6 h, and the extracellular medium was concentrated and used for Western blot analysis with anti-pustulan (β-1,6-glucan) antiserum. Concentrations of D75-4590 added were as follows: 1, 20 μg/ml; 2, 10 μg/ml; 3, 5 μg/ml; 4, 2.5 μg/ml; 5, 1.25 μg/ml; 6, 0.625 μg/ml; 7, 0.313 μg/ml; and 8, 0 μg/ml. M, marker.
Genetic study of the D75-4590 mechanism of action using S. cerevisiae.
As many genes are involved in β-1,6-glucan synthesis, the target enzymes of D75-4590 are unpredictable. Although β-1,6-glucan polymer is thought to be fungus specific, not all the genes involved in β-1,6-glucan synthesis are fungus specific. To determine the primary target of D75-4590, we attempted a genetic approach using S. cerevisiae. Several resistant mutants were generated by means of UV irradiation (see Materials and Methods). Twenty-eight colonies were selected, and their susceptibilities to D75-4590 and other known antifungal compounds were compared (Table 2). There were four patterns in their susceptibility to these compounds. Because AY-10-R15 acquired high-level resistance only to D75-4590, we speculated that it could have a mutation in the target enzyme of D75-4590. Genomic libraries of AY-10-R15 were transformed to S. cerevisiae AY-10, and resistant transformants were selected on YNB agar containing D75-4590 (64 μg/ml). Two kinds of plasmid (pRS416-13 and pRS416-31) were recovered from them, each having a 5- and 3-kbp insert of yeast genomic DNA, respectively. Genetic analysis of these inserts revealed that pRS416-13 contains a 5,252-bp fragment of chromosome III which contains only a single ORF of ADP1 and that pRS416-31 contains a 3,164-bp fragment of chromosome XVI which contains only a single ORF of KRE6. It has been reported that Adp1p is a putative ATP-dependent permease of the ABC transporter family of proteins (7) and that Kre6p is a putative β-1,6-glucan synthase (38). Comparison of the DNA sequence of KRE6 of pRS416-31 with that of the wild type indicated a T-to-A transition at position 1654 of its ORF, resulting in an amino acid transition from Phe to Ile at position 552. The conferring of resistance to D75-4590 by retransformation of these plasmids into S. cerevisiae AY-10 was confirmed (Table 3). Furthermore, we introduced a resistant mutation of KRE6 into the chromosomal DNA of S. cerevisiae AY-10c (SKN1 null mutant), yielding S. cerevisiae CY-2a. It was found to be highly resistant to D75-4590, which indicates that the mutation at position 1654 of KRE6 is solely responsible for the resistance (Table 2).
TABLE 2.
Susceptibilities of S. cerevisiae mutants and the parental strain to various antifungal compoundsa
| Strain | MIC-0 (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| D75-4590 | AMB | 5FC | TM | AC | CW | |
| AY-10 | 2 | 0.25 | 2 | 0.5 | 0.5 | 2 |
| AY-10-R4 | 8 | 0.0625 | 2 | 1 | 0.125 | 4 |
| AY-10-R11 | 8 | 0.125 | 4 | 0.5 | 0.25 | 4 |
| AY-10-R15 | >64 | 0.25 | 4 | 0.5 | 0.5 | 2 |
| AY-10-R16 | 4 | 0.25 | 2 | 1 | 0.25 | 4 |
| CY-1a | 8 | NT | NT | NT | NT | NT |
| CY-2a | >64 | NT | NT | NT | NT | NT |
Abbreviations: AMB, amphotericin B; 5FC, 5-flucytosine; TM, tunicamycin; AC, aculeacin A; CW, calcofluor white; NT, not tested.
TABLE 3.
Susceptibilities of S. cerevisiae AY-10, transformed with or without plasmid, to various antifungal compoundsa
| Plasmid | Cloned gene | MIC-0 (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| D75-4590 | AMB | 5FC | TM | AC | CW | ||
| None | 2 | 0.25 | 2 | 0.5 | 0.5 | 2 | |
| pRS416 | 2 | 0.25 | 2 | 0.5 | 0.5 | 2 | |
| pRS416-13 | ADP1 | 4 | 0.125 | 4 | 0.5 | 0.25 | 8 |
| pRS416-31 | KRE6 | 32 | 0.25 | 2 | 0.5 | 0.5 | 2 |
Abbreviations: AMB, amphotericin B; 5FC, 5-flucytosine; TM, tunicamycin; AC, aculeacin A; CW, calcofluor white.
Homology search of KRE6 among other species.
A homology search in DDBJ, GenBank, and EMBL revealed the existence of KRE6 homologues in many fungal species (C. albicans, C. glabrata, C. tropicalis, Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus terreus, Aspergillus clavatus, and Pneumocystis carinii). We also have cloned some parts of the KRE6 homologues of Candida krusei and C. parapsilosis (data not shown). High homologies are observed among all of these homologues at the C-terminal regions of the transmembrane domains but not at the N-terminal regions. No mammalian gene having significant homology to KRE6 was found. These data confirm that KRE6 is fungus specific and is conserved among various fungi. Interestingly, the amino acid at position 552 in S. cerevisiae, where we have found a resistant mutation, was conserved among Candida species but not among Aspergillus species (Fig. 8).
FIG. 8.
Comparison of partial sequences of Kre6p homologues from various fungal species. The amino acid residues are shown in single-letter amino acid code. The region near the position of the residue (indicated by an arrow) that, when mutated, confers D75-4590 resistance in S. cerevisiae is displayed in the alignment. Abbreviations: SC, S. cerevisiae; CG, C. glabrata; CA, C. albicans; CK, C. krusei; CP, C. parapsilosis; AF, A. fumigatus; AT, A. terreus; AC, A. clavatus.
DISCUSSION
Our findings in biochemical and genetic studies strongly suggest that D75-4590 is a specific inhibitor of β-1,6-glucan synthesis and that its primary target is Kre6p. Many reports have shown that a reduction of β-1,6-glucan leads to partial defects in cell separation, resulting in a multibudding phenotype (24, 25, 29, 38). Lussier et al. reported that homozygous null disruptants of C. albicans KRE9, which are involved in β-1,6-glucan synthesis, failed to form hyphae (24). These phenotypic features were also observed in D75-4590-treated cells, which supports our conclusion that D75-4590 is a β-1,6-glucan inhibitor.
Kre6p is a predicted type II membrane protein localized on the endoplasmic reticulum or Golgi apparatus (30, 38). Kre6p and its functional homologue Skn1p are both present in S. cerevisiae (37). Although neither KRE6 nor SKN1 is essential; disruption of SKN1 has little effect on growth, and disruption of both genes results in extremely slow growth or lethality (37, 38). Since D75-4590 showed a complete growth inhibitory effect against S. cerevisiae, it is likely to inhibit both Kre6p and Skn1p. The essentiality of β-1,6-glucan for normal growth is predicted in Candida species as well (24, 25, 29). Our results showing that a mutation providing resistance against D75-4590 is found in the C terminus of Kre6p indicate that the binding site of D75-4590 is likely to be in its predicted C-terminal luminal domain. Since amino acid sequences in the C-terminal domain are highly conserved, D75-4590 may inhibit the Kre6p of various Candida species. It did actually show activities against various Candida species; however, incomplete inhibition (trailing phenomenon) was observed in some species, including the major pathogen C. albicans. One of the possible explanations is that D75-4590 inhibits only some of the Kre6p homologues in C. albicans. The inhibitory effects of D75-4590 on each Kre6p homologue of C. albicans are under investigation.
It was demonstrated that the cell wall of filamentous fungi, such as Aspergillus species, contains no β-1,6-glucan polymer (10), whereas Kre6p homologues have been found in A. fumigatus, A. terreus, and A. clavatus. The functions of these homologues in Aspergillus species are not well understood, and Henry et al. have suggested that partial silencing of KRE6 expression makes A. fumigatus more susceptible to Congo red (15), which seems to indicate that Kre6p has a role in the cell wall construction in A. fumigatus. To our regret, we did not find any inhibitory or morphological effect of D75-4590 against Aspergillus species, at least under light microscopic observation. One of the possibilities is that amino acid differences contribute to the resistance to D75-4590 in this species. We found a resistant mutation in S. cerevisiae resulting in an amino acid transition from Phe to Ile at position 552. The Kre6p of Aspergillus species has Tyr at a corresponding position, which may be responsible for the resistance (Fig. 8). If D75-4590 or its derivatives inhibit Kre6p of Aspergillus species, analysis of cells treated with the compound may shed light on the role of Kre6p in Aspergillus species. Further work is needed to understand the effect of Kre6p inhibition in Aspergillus species.
Although growth inhibitory effects of D75-4590 are not potent enough in some species to expect in vivo effects, additional effects in vivo can be expected for β-1,6-glucan inhibitors. Cell wall proteins, such as Hwp1p and Als1p, are thought to play important roles in the process of fungal pathogenesis, such as adhesion, hyphal elongation, and biofilm production (1, 12, 33, 41). Most of these proteins are attached to the cell wall via β-1,6-glucan. Although these proteins are not essential for the growth of C. albicans, some of their null mutants have been shown to be avirulent in an animal model. Therefore, a β-1,6-glucan inhibitor is expected to remove these proteins from the cell and reduce the pathogenesis of C. albicans as a result. In fact, there are several reports suggesting that C. albicans mutants with a reduced level of β-1,6-glucan are avirulent (16, 45). Our study clearly shows a potent inhibitory effect of D75-4590 against hyphal elongation as well.
As far as we know, this is the first report of a β-1,6-glucan synthase inhibitor. Since KRE6 is conserved in various fungi, while mammalian cells have neither KRE6 nor β-1,6-glucan polymer, D75-4590 is a promising lead compound for new antifungal agents. However, as its physicochemical profile is not ideal for a drug, the efficacy of D75-4590 in animal models is not promising. Nevertheless, more potent derivatives with ideal physicochemical profiles would be effective. In addition, it is advantageous from the viewpoint of oral administration and chemical modification because it is a small molecule. Derivatization and in vivo studies are now in progress.
Acknowledgments
We thank T. Ohtani for his valuable comments and review of the manuscript.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Albrecht, A., A. Felk, I. Pichova, J. R. Naglik, M. Schaller, P. Groot, D. MacCallum, F. C. Odds, W. Schäfer, F. Klis, M. Monod, and B. Hube. 2006. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J. Biol. Chem. 281:688-694. [DOI] [PubMed] [Google Scholar]
- 2.Arendrup, M. C., K. Fuursted, B. Gahrn-Hansen, I. M. Jensen, J. D. Knudsen, B. Lundgren, H. C. Schonheyder, and M. Tvede. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 43:4434-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blot, S., and K. Vandewoude. 2004. Management of invasive candidiasis in critically ill patients. Drugs 64:2159-2175. [DOI] [PubMed] [Google Scholar]
- 4.Boone, C., S. S. Sommer, A. Hensel, and H. Bussey. 1990. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J. Cell Biol. 110:1833-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cid, V. J., A. Duran, F. Rey, M. P. Snyder, C. Nombela, and M. Sanchez. 1995. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59:345-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey, K. G., A. Szekely, E. M. Johnson, and D. W. Warnock. 1998. Comparison of new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J. Antimicrob. Chemother. 42:439-444. [DOI] [PubMed] [Google Scholar]
- 7.Decottignies, A., and A. Goffeau. 1997. Complete inventory of the yeast ABC proteins. Nat. Genet. 15:137-145. [DOI] [PubMed] [Google Scholar]
- 8.Drew, R. 2006. Potential role of aerosolized amphotericin B formulations in the prevention and adjunctive treatment of invasive fungal infections. Int. J. Antimicrob. Agents 1(Suppl.):36-44. [DOI] [PubMed] [Google Scholar]
- 9.Enoch, D. A., H. A. Ludlam, and N. M. Brown. 2006. Invasive fungal infections: a review of epidemiology and management options. J. Med. Microbiol. 55:809-818. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine, T., C. Simenel, G. Dubreucq, O. Adam, M. Delepierre, J. Lemoine, C. E. Vorgias, M. Diaquin, and J. P. Latge. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:27594-27607. [DOI] [PubMed] [Google Scholar]
- 11.Frieman, M. B., and B. P. Cormack. 2003. The omega-site sequence of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae can determine distribution between the membrane and the cell wall. Mol. Microbiol. 50:883-896. [DOI] [PubMed] [Google Scholar]
- 12.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y. C. Chen, S. W. French, J. E. Culter, S. G. Filler, and J. E. Edwards, Jr. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed] [Google Scholar]
- 13.Gaughran, J. P., M. H. Lai, D. R. Kirsch, and S. J. Silverman. 1994. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J. Bacteriol. 176:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry, C., I. Mouya, and J. P. Latge. 2007. Testing the efficacy of RNA interference constructs in Aspergillus fumigatus. Curr. Genet. 51:277-284. [DOI] [PubMed] [Google Scholar]
- 16.Herrero, A. B., P. Magnelli, M. K. Mansour, S. M. Levitz, H. Bussey, and C. Abeijon. 2004. KRE5 gene null mutant strains of Candida albicans are avirulent and have altered cell wall composition and hypha formation properties. Eukaryot. Cell 3:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson, R. P. 2003. The global epidemiology of invasive Candida infections—is the tide turning? J. Hosp. Infect. 55:159-168. [DOI] [PubMed] [Google Scholar]
- 18.Imanishi, Y., K. Yokoyama, and K. Nishimura. 2004. Inductions of germ tube and hyphal formations are controlled by mRNA synthesis inhibitor in Candida albicans. Jpn. J. Med. Mycol. 45:113-119. [DOI] [PubMed] [Google Scholar]
- 19.Kapteyn, J. C., L. L. Hoyer, J. E. Hecht, W. H. Muller, A. Andel, A. J. Verkleij, M. Makarow, H. Van Den Ende, and F. M. Klis. 2000. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 35:601-611. [DOI] [PubMed] [Google Scholar]
- 20.Klis, F. M. 1994. Cell wall assembly in yeast. Yeast 10:851-869. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz, M. B., and C. M. Douglas. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79-86. [DOI] [PubMed] [Google Scholar]
- 22.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, C. F., R. C. Montijn, J. L. Brown, F. M. Klis, J. Kurjan, H. Bussey, and P. N. Lipke. 1995. Glycosyl phosphatidylinositol-dependent cross-linking of alpha-agglutinin and beta 1,6-glucan in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 128:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lussier, M., A. M. Sdicu, S. Shahinian, and H. Bussey. 1998. The Candida albicans KRE9 gene is required for cell wall beta-1, 6-glucan synthesis and is essential for growth on glucose. Proc. Natl. Acad. Sci. USA 95:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mio, T., T. Yamada-Okabe, T. Yabe, T. Nakajima, M. Arisawa, and H. Yamada-Okabe. 1997. Isolation of the Candida albicans homologs of Saccharomyces cerevisiae KRE6 and SKN1: expression and physiological function. J. Bacteriol. 179:2363-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsui, I., E. Kumazawa, Y. Hirota, M. Aonuma, M. Sugimori, S. Ohsuki, K. Uoto, A. Ejima, H. Terasawa, and K. Sato. 1995. A new water-soluble camptothecin derivative, DX-8951f, exhibits potent antitumor activity against human tumors in vitro and in vivo. Jpn. J. Cancer Res. 86:776-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montijin, R. C., E. Vink, W. H. Müller, A. J. Verkleij, H. van den ende, B. Henrissat, and F. M. Klis. 1999. Localization of synthesis of β-1,6-glucan in Saccharomyces cerevisiae. J. Bacteriol. 181:7414-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagahashi, S., M. Lussier, and H. Bussey. 1998. Isolation of Candida glabrata homologs of the Saccharomyces cerevisiae KRE9 and KNH1 genes and their involvement in cell wall beta-1,6-glucan synthesis. J. Bacteriol. 180:5020-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamata, K., T. Kurita, M. S. A. Bhuiyan, K. Sato, Y. Noda, and K. Yoda. 2007. KEG1/YFR042w encodes a novel Kre6-binding endoplasmic reticulum membrane protein responsible for β-1,6-glucan synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 282:34315-34324. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard documents M27-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 32.National Committee for Clinical Laboratory Standards. 1990. Method for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 2nd ed. Approved standard documents M7-A2. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 33.Nobile, C. J., J. E. Nett, D. R. Andes, and A. P. Mitchell. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5:1604-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onishi, J., M. Meinz, J. Thompson, J. Curotto, S. Dreikorn, M. Rosenbach, C. Douglas, G. Abruzzo, A. Flattery, L. Kong, A. Cabello, F. Vicente, F. Pelaez, M. T. Diez, I. Martin, G. Bills, R. Giacobbe, A. Dombrowski, R. Schwartz, S. Morris, G. Harris, A. Tsipouras, K. Wilson, and M. B. Krutz. 2000. Discovery of novel antifungal (1,3)-beta-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 44:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapp, R. P. 2004. Changing strategies for the management of invasive fungal infections. Pharmacotherapy 2:4S-28S. [PubMed] [Google Scholar]
- 36.Revankar, S. G., W. R. Kirkpatrick, R. K. Mcatee, A. W. Fotherqill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roemer, T., S. Delaney, and H. Bussey. 1993. SKN1 and KRE6 define a pair of functional homologs encoding putative membrane proteins involved in beta-glucan synthesis. Mol. Cell. Biol. 13:4039-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roemer, T., G. Paravicini, M. A. Payton, and H. Bussey. 1994. Characterization of the yeast (1→6)-beta-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J. Cell Biol. 127:567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahinian, S., and H. Bussey. 2000. Beta-1,6-glucan synthesis in Saccharomyces cerevisiae. Mol. Microbiol. 35:477-489. [DOI] [PubMed] [Google Scholar]
- 40.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstrom. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]
- 42.Sudoh, M., T. Yamazaki, K. Masubuchi, M. Taniguchi, N. Shimma, M. Arisawa, and H. Yamada-Okabe. 2000. Identification of a novel inhibitor specific to the fungal chitin synthase. Inhibition of chitin synthase 1 arrests the cell growth, but inhibition of chitin synthase 1 and 2 is lethal in the pathogenic fungus Candida albicans. J. Biol. Chem. 275:32901-32905. [DOI] [PubMed] [Google Scholar]
- 43.Tiballi, R. N., X. He, L. T. Zarins, S. G. Revankar, and C. A. Kauffman. 1995. Use of a colorimetric system for yeast susceptibility testing. J. Clin. Microbiol. 33:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traxler, P., J. Gruner, and J. A. Auden. 1977. Papulacandins, a new family of antibiotics with antifungal activity. I. Fermentation, isolation, chemical and biological characterization of papulacandins A, B, C, D and E. J. Antibiot. 30:289-296. [DOI] [PubMed] [Google Scholar]
- 45.Umeyama, T., A. Kaneko, H. Watanabe, A. Hirai, Y. Uehara, M. Niimi, and M. Azuma. 2006. Deletion of the CaBIG1 gene reduces β-1,6-glucan synthesis, filamentation, adhesion, and virulence in Candida albicans. Infect. Immun. 74:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Berkel, M. A. A., L. H. P. Caro, R. C. Montijn, and F. M. Klis. 1994. Glucosylation of chimeric proteins in the cell wall of Saccharomyces cerevisiae. FEBS Lett. 349:135-138. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Varrt, J. M., R. Biesebeke, J. W. Chapman, H. Y. Toschka, F. M. Klis, and C. T. Verrips. 1997. Comparison of cell wall proteins of Saccharomyces cerevisiae as anchors for cell surface expression of heterologous proteins. Appl. Environ. Microbiol. 63:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiebe, V., and M. Karriker. 2005. Therapy of systemic fungal infections: a pharmacologic perspective. Clin. Tech. Small Anim. Pract. 20:250-257. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi, H., T. Hiratani, K. Iwata, and Y. Yamamoto. 1982. Studies on the mechanism of antifungal action of aculeacin A. J. Antibiot. 35:210-219. [DOI] [PubMed] [Google Scholar]