Abstract
An engineered chitosan acetate bandage preparation (HemCon) is used as a hemostatic dressing, and its chemical structure suggests that it should also be antimicrobial. We previously showed that when a chitosan acetate bandage was applied to full-thickness excisional wounds in mice that had been infected with pathogenic bioluminescent bacteria (Pseudomonas aeruginosa, Proteus mirabilis, and Staphylococcus aureus), it was able to rapidly kill the bacteria and save the mice from developing fatal infections. Wound healing was also stimulated. In the present study, we asked whether a chitosan acetate bandage could act as a topical antimicrobial dressing when it was applied to third-degree burns in mice contaminated with two of these bacterial species (P. aeruginosa and P. mirabilis). Preliminary experiments established the length of burn time and the number of bacteria needed to produce fatal infections in untreated mice and established that the chitosan acetate bandage could adhere to the infected burn for up to 21 days. In the case of P. aeruginosa infections, the survival rate of mice treated with the chitosan acetate bandage was 73.3% (whereas the survival rate of mice treated with a nanocrystalline silver dressing was 27.3% [P = 0.0055] and that of untreated mice was 13.3% [P < 0.0002]). For P. mirabilis infections, the comparable survival rates were 66.7%, 62.5%, and 23.1% respectively. Quantitative bioluminescent signals showed that the chitosan acetate bandage effectively controlled the growth of bacteria in the burn and prevented the development of systemic sepsis, as shown by blood culture. These data suggest that chitosan acetate bandage is efficacious in preventing fatal burn infections.
Burns are one of the most common and devastating forms of trauma. Data from the National Center for Injury Prevention and Control in the United States show that approximately 2 million fires that result in 1.2 million people with burn injuries are reported each year (5). A burn injury produces a locally immunocompromised region rapidly depleted of intrinsic humoral and cellular immune components and is thus particularly susceptible to infection (14). Despite advances in therapy such as rapid excision and skin grafting, as well as the introduction of new antibiotics and topical antimicrobial preparations, infections remain leading causes of morbidity and mortality in burn patients (28, 30). In addition, the relentless worldwide increase in multidrug resistance in pathogenic bacteria has led to the present time being described as “the end of the antibiotic era” (17, 33). Therefore, there is an increasing need to develop novel and effective antimicrobial products that can combat these drug-resistant burn pathogens and be applied to potentially contaminated burns.
Chitin is a biopolymer consisting of poly-N-acetylglucosamine and is widespread in nature as a structural material, particularly in marine arthropod shells. The partial deacetylation of chitin by treatment with hot sodium hydroxide forms the more soluble polymer, chitosan. Chitosan preparations of various molecular weights and degrees of deacetylation and with further molecular derivatization patterns have attracted much attention because of their potentially beneficial biological properties (22, 44). These properties include hemostasis (34), antimicrobial activity (35), stimulation of healing (2), tissue engineering scaffolds (8), and drug delivery (1). HemCon bandage is a compressed chitosan acetate dressing that was developed as a hemostatic agent (21). It is used to stem blood flow, especially flow from severely bleeding wounds (31), and it has attracted considerable attention because of its performance during military operations in the Middle East (42).
The polycationic nature of chitosan is such that the substance possesses natural antimicrobial properties (35), and chitosan acetate could simultaneously have two highly desirable properties in a wound dressing: hemostasis and microbicidal activity. We previously demonstrated the antibacterial properties of the chitosan acetate bandage in a mouse model of highly contaminated excisional wounds (4). We used bacterial strains that were genetically engineered to stably express the lux operon, which encodes bacterial luciferase and the enzymes necessary to synthesize the luciferase substrate so that the bacteria glow in the dark (9). This allows the use of a low-light-imaging camera to monitor the progress of the infection in real time (7). Two gram-negative bacterial species (Pseudomonas aeruginosa and Proteus mirabilis) that are invasive and that can lead to the development of sepsis and a gram-positive species (Staphylococcus aureus) that leads to a more chronic localized infection were employed. A follow-up study of wound healing of infected and noninfected wounds showed that chitosan acetate optimally stimulated wound healing when it was applied to the wound for 3 days (3). In the present study, we asked whether the chitosan acetate bandage could act as a topical antimicrobial dressing when it was applied to third-degree burns in mice contaminated with fatal doses of two of these invasive bacterial species (P. aeruginosa and P. mirabilis).
MATERIALS AND METHODS
Animals.
Adult female BALB/c mice (ages, 6 to 8 weeks; weights, 17 to 21 g; Charles River Laboratories, Wilmington, MA) were used in the study. The animals were housed at one mouse per cage and were maintained on a 12-h light-12-h dark cycle with access to food and water ad libitum. All animal procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital and met the guidelines of the National Institutes of Health. The mice received buprenorphine (0.03 mg/kg of body weight subcutaneously twice a day) for 3 days after they received the burn for pain relief. The mice were euthanized according to the protocol when their condition was assessed to be moribund.
Bacterial strains and culture conditions.
The P. aeruginosa strain that we employed was ATCC 19660 (strain 180), which causes septicemia after intraperitoneal injection (37) and which has been shown to be invasive in mice with skin burns (25). The P. mirabilis strain that we used was ATCC 51393. This strain has been reported to cause burn infections and sepsis in rats that receive scald wounds to 30% of their body surface area (26). The stable bioluminescent variants of these strains (strains Xen 44 and Xen 5, respectively) carried the entire bacterial lux operon integrated in their chromosomes for stable luciferase expression, which allowed them to be used for bioluminescent imaging (strains Xen 44 and Xen 5 were kind donations from Xenogen Inc, Alameda, CA) (36). The bacteria were grown in brain heart infusion (BHI) medium supplemented with 50 μg ml−1 kanamycin in an orbital incubator (37°C; 100 rpm) to an optical density of 0.6 to 0.8 at 600 nm, which corresponds to 108 cells/ml (mid-log phase). This suspension was centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in PBS at the same density. The luminescence of 100-μl aliquots of the bacterial suspensions was routinely measured in 96-well black-sided plates by use of a Victor-2 1420 multilabel plate reader (EG&G Wallac).
Ex vivo-isolated cultures were obtained as follows. Blood samples for culture for isolation of the bacteria were taken from the mice and streaked on agar plates. The plates were incubated at 37°C until colonies were generated. The plates were then stored at 4°C as a stock culture on an agar plate for a maximum of 1 week. Experimental inocula were made from the stock culture by transferring a single colony to 3 ml BHI solution and then incubating the culture at 37°C for 24 h. Further blood samples from dying or dead mice were available to renew the agar plate, the contents of which was stirred at 4°C.
Chitosan acetate bandage and topical silver-releasing dressing.
The chitosan acetate (HemCon) bandage was prepared by HemCon Medical Technologies, Inc. (Portland, OR), by methods fully described by McCarthy et al. (S. J. McCarthy, K. W. Gregory, and J. W. Morgan, U.S. patent application 20050147656). Briefly, sponges (74% chitosan, 9% moisture, 17% acetic acid by weight) were prepared by freeze-drying dilute aqueous acetic acid solutions of chitosan with a fractional degree of deacetylation of 81% ± 2%, a number average molecular mass (gel permeation chromatography with polyethylene oxide standards) of 75 ± 10 kDa, and a polydispersity of 5 ± 1 (Primex, Iceland) in a Teflon-coated aluminum mold (110 mm in width by 110 mm in length by 20 mm in depth) at room temperature. The resultant sponges were compressed, annealed, and gamma irradiated to yield sterile, dissolution-resistant, and adhesive dressings. The chitosan acetate bandage pieces used for the experiment (100 mm by 100 mm by 1.0 mm) were cut from the stock pieces, with the chitosan sponge having a density close to 0.2 g cm−3.
We also employed a clinically approved topical silver-releasing bandage (Acticoat-7 dressing; Smith & Nephew Healthcare Ltd., Largo, FL). The nanocrystalline silver dressing consisted of two layers of a fine silver-coated mesh of high-density polyethylene enclosing an inner core consisting of two layers of an apertured, nonwoven fabric made from rayon and polyester. Between the two layers of nonwoven fabric was an additional layer of the silver-coated polyethylene mesh. All five layers were ultrasonically welded together in order to maintain the integrity of the dressing while it was in use.
Burn injury and bacterial infection.
The backs of the mice were shaved and depilated with Nair (Carter-Wallace Inc, New York, NY). On the next day, the mice were anesthetized with intraperitoneal injections of a ketamine-xylazine cocktail, and burns were created by applying two preheated (92 to 95°C) brass blocks (10 mm by 10 mm; Small Parts, Inc., Miami, FL) to the opposing sides of an elevated skin fold on the dorsal surface of the mice (38) for 10, 30, or 60 s (all were nonlethal, full-thickness, third-degree burns). The combined brass block area was 20 mm by 10 mm, which gave a total area of 200 mm2 and which corresponded to 5% of the total body surface area (13). Immediately after the creation of the burn, the mice were resuscitated with intraperitoneal injections of 0.5 ml sterile saline (Phoenix Scientific Inc., St. Joseph, MO).
Bacterial infection took place as described by Ha and Jin (15). Five minutes after the creation of the burn (to allow the burn to cool down), a suspension (40 μl) of bacteria in sterile PBS was inoculated onto the surface of each burn with a pipette tip and was then smeared onto the burn surface with an inoculating loop.
Treatment of burn infection.
Chitosan acetate bandages or silver dressings were applied to the infected burns 15 min after the application of the bacteria. This allowed the bacteria sufficient time to bind to the burned tissue. The chitosan acetate bandages were moistened with sodium acetate buffer (100 mM, pH 4.5) before application, while the silver dressing was moistened with Milli-Q water. The silver dressing was attached to the burns by gluing the dressing edges to the skin surrounding the burns with Dermabond cyanoacrylate adhesive (Ethicon Inc., Piscataway, NJ). The chitosan acetate bandages adhering to the burns were then moistened daily with 100 μl of 50 mM sodium acetate buffer, and the silver dressings were moistened with 100 μl of Milli-Q water.
Bioluminescence imaging.
The low-light-imaging system (Hamamatsu Photonics KK, Bridgewater, NJ) used in this study has been described elsewhere in detail (16). In short, it consists of an intensified charge-coupled-device camera mounted in a light-tight specimen chamber and fitted with a light-emitting diode, a setup that allowed a background gray-scale image of the entire mouse to be captured. In the photon-counting mode, an image of the light emitted from the bacteria was captured by using an integration time of 2 min and the maximum setting on the image-intensifier control module. By use of ARGUS software (Hamamatsu Photonics KK, Bridgewater, NJ), the luminescence image was presented as a false-color image superimposed on top of the gray-scale reference image. The image-processing component of the software calculated the total pixel values from the luminescence images of the infected wound area. Before they underwent bioluminescence imaging, the mice were anesthetized with intraperitoneal injections of 20 μl of a ketamine-xylazine (10:1) cocktail.
Preliminary tests of transmissivity of chitosan acetate or silver with respect to bioluminescence.
In order to be able to interpret the bioluminescence images of the burns when they were covered with the chitosan acetate or the silver dressings, we tested the transmissivities of the dressings with respect to the bioluminescent signal emitted by the bacterial cells. A 2-ml suspension of luminescent P. aeruginosa or P. mirabilis containing 2 × 109 cells was evenly spread onto the surface of a 60-mm-diameter BHI agar plate, and the plate was incubated at 37°C for 24 h to achieve a strong luminescent signal. Bioluminescence images were obtained before and after the chitosan acetate or the silver dressing was each placed on the surface of an agar plate. The transmissivities were determined by obtaining the ratio of the bioluminescent value of the agar plate with the dressing to that of the plate without the dressing. The transmissivity measurements were performed in triplicate with three different pieces of chitosan acetate or silver dressing. We assumed that the transmissivities of the dressings were constant throughout the experiment.
Mouse follow-up.
During the experiment, the mice underwent bioluminescence imaging immediately after the bacteria were added, immediately after application of the dressing, and at 24-h intervals thereafter. Blood samples were withdrawn daily from the orbital plexus and were streaked onto BHI agar plates for determination of the presence of bacteria in the blood of the mice. The mice were also monitored daily for weight, wound healing, and survival. When a mouse died, 3 ml sterile saline was injected into the abdominal cavity of the mouse and then 1 ml was withdrawn and cultured on BHI agar plates to determine whether bacteria were present within the peritoneum. Blood samples were also taken from the heart, which was removed from each dead mouse, and were streaked on BHI agar plates. Bioluminescent colonies were identified as P. aeruginosa, while nonbioluminescent colonies were identified as Escherichia coli by use of a commercial identification kit (BBL E/NF Crystal; BD Biosciences) and by biochemical characterization as oxidase negative and indole positive. When bacteria were detected in the bloodstream, approximately 20 μl blood was taken from each mouse. Therefore, the lower limit of detection of bacteria was >50 CFU/ml blood (i.e., 1 CFU/20 μl). When bacteria were detected in the peritoneum, approximately 3 ml saline was injected into the abdominal cavity and 1 ml liquid was subsequently withdrawn. As a result, the lower limit of detection was 1 CFU/ml liquid, or in the region of 10 CFU freely suspended bacteria in the peritoneal cavity.
Statistics.
Data points are given as means ± standard errors of the means. Differences among means were analyzed for statistical significance by one-way analysis of variance. Survival curves were compared by use of the Kaplan-Meier log-rank test. P values of <0.05 were considered statistically significant.
RESULTS
Initial experiments with infected mouse burns.
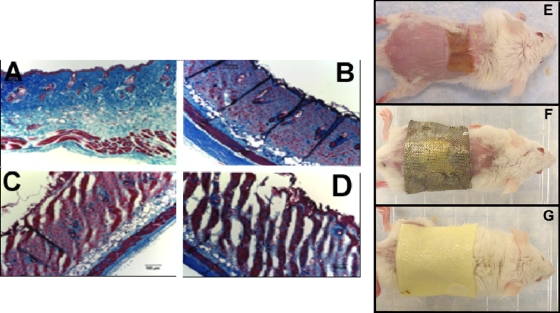
Our initial experiments with burns on the backs of mice in which P. aeruginosa had been inoculated yielded confusing results. We first used a 10-s burn and added 107 or 108 cells. At first, most of the mice died of sepsis, but as time progressed, the mice did not die as frequently and later did not die at all. At first, there was an active local infection in the burn, as determined by bioluminescence imaging, but as the experiment progressed, the strength and the duration of the bioluminescence signal in the burn decreased. We increased the time of the burn from 10 s to 30 to 60 s. The rationale for adjusting the burn time is that the bacteria may have faced some difficulty in penetrating or adhering to the surface of the 10-s burn and the creation of more severe damage to the burned skin may enhance the ability of the bacteria to cause a robust infection in the burn that could then progress to sepsis. Figure 1A to D shows Masson trichrome-stained histological sections of healthy mouse skin and skin that was burned for 10, 30, and 60 s. As can be seen, the 30- and 60-s burns have channels in the dermis that were not formed in the 10-s burns, and we believed that bacteria applied to the surface could more easily penetrate these channels, thus gaining a foothold in the burn in order to multiply. Some reports in the literature involve the injection of bacteria into or beneath the burned tissue rather than their application to the surface (6, 19). Since the chitosan acetate bandage is a superficial antimicrobial dressing, we preferred to use the more natural mode of surface contamination.
FIG. 1.
Masson's trichrome histological sections of healthy BALB/c mouse skin (A) and mouse skin with burns produced by the application of a heated brass block for 20 s (B), 40 s (C), and 60 s (D). Successively greater channels that reach through the dermis and that allow penetration of bacteria into the burns are produced in the 40- and 60-s burns. (E to G) Photographs of representative mice with no dressing on the burn (E), a silver dressing on the burn (F), and a chitosan acetate bandage on the burn (G).
As expected, the P. aeruginosa cells proved to be more virulent and deadly when they were applied to these longer-duration burns that contained channels, with fatalities occurring when 108 bacteria were applied. We then isolated a single colony of bioluminescent P. aeruginosa from an agar plate onto which blood from one of these mice that died of sepsis had been streaked. When this colony was grown in liquid BHI medium, it proved to be much more virulent than the previously used bacterial isolate that had been passaged in vitro for some time. For P. aeruginosa infections, we tested three inoculum densities (107, 108, and 109) on 10- and 60-s burns. For inocula of 108 and 109 CFU on 60-s burns, the rate of survival of nontreated mice (n = 15) was 0% within 4 weeks and the sepsis rate was 100%. For an inoculum of 107 CFU on 10-s burns, the survival rate and the sepsis rate were 13.3% and 100%, respectively (n = 15). The rate of survival of mice with noninfected 60-s burns (n = 9) was 100%. The combination of a 107-CFU inoculum and a 10-s burn was selected as representing fatal infections in the infected burn model used in the study. A total of 107 CFU in a 50-μl PBS suspension had an average bioluminescence value of 13,860 and a standard error of 2,000.
We concluded that serial passage of P. aeruginosa in vitro had selected for less invasive and virulent phenotypes in a gradual but progressive manner. With periodic isolation (every few weeks) from the blood of mice that developed sepsis, the virulence could be maintained and the experiments could be made much more reproducible.
We also studied burn infections caused by P. mirabilis strain ATCC 51393. Mice that received 108 cells on a 10-s burn sometimes died, but we found that the most reliable rate of sepsis development was when 108 cells were inoculated onto a 30-s burn. In vivo passage was not used for P. mirabilis, as changes in virulence were not observed during the course of our experiments.
P. aeruginosa burn infections treated with chitosan acetate bandage or silver dressing.
Burn infections were induced by the inoculation of 107 P. aeruginosa cells isolated in vivo onto 10-s burns. In contrast to human third-degree burns, mouse third-degree burns have a dry texture, irrespective of whether they have been contaminated or infected with bacteria. It was therefore necessary to regularly moisten both the chitosan acetate and the silver bandages to allow the active antimicrobial ingredient to percolate into the burned tissue. In order to not compromise the activity of the nanocrystalline silver by using buffers, we used pure water to do this. We had previously shown that the pH 4.5 acetate buffer used to moisten chitosan acetate does not by itself have an antibacterial effect on P. aeruginosa in the short term (hours) (4). The chitosan acetate bandage adhered extremely well to the surface of the burn when the piece of bandage had previously been moistened with acetate buffer to render it flexible. The normally flexible silver bandage adhered well when it was glued to the healthy skin with the clinically approved cyanoacrylate skin adhesive (Dermabond). The median adhesion time of the chitosan acetate bandages was 16 days (range, 11 to 21 days), while the glued silver dressing remained adherent throughout the entire course of the experiment. We used pieces of chitosan acetate bandage (Fig. 1G) and silver dressing (>30 mm by 30 mm; Fig. 1F) that were significantly larger than the burn (≈20 mm by 10 mm; Fig. 1E) because the bacteria sometimes spread laterally into the skin beyond the burned area, as observed by bioluminescence imaging.
Figure 2 shows successive bioluminescence images (at the same bit range of 3) from day 0 to day 3 postinfection of an untreated burn, a silver dressing-treated burn, and a chitosan acetate bandage-treated burn. In the untreated burn, the bacteria multiplied approximately 100-fold from day 0 to day 3, while in both the silver dressing- and chitosan acetate bandage-treated burns, a decrease in the bioluminescence signal was seen at day 1, as the dressing immediately quenched the light. There was a detectable increase in the signal from the silver dressing-treated burn on days 2 and 3 (compared to that on day 1) that was not seen in the chitosan acetate-treated burn. The inset in Fig. 2 showing a silver-treated burn on day 3 was taken after the silver dressing was removed from a dead mouse and shows vigorous bacterial proliferation beneath the dressing. There was an almost equally strong increase in bioluminescence when the chitosan acetate bandage was removed from a mouse that had died, even though it had been treated with the chitosan acetate bandage (data not shown), suggesting that a failure to control the infection locally correlated with death from sepsis.
FIG. 2.
Representative successive bioluminescence images from days 0 to 3 (at the same bit range of 3) of mice with P. aeruginosa-infected burns with no treatment, silver dressing treatment, and chitosan acetate bandage treatment. The inset of the image for day 3 with a burn with a silver dressing shows the bioluminescence signal after the dressing was removed from a dead mouse.
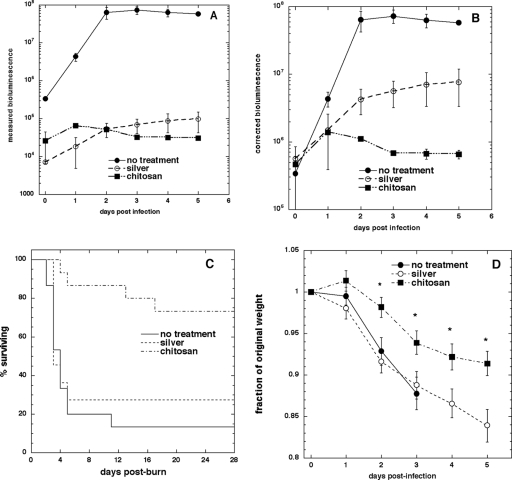
The transmissivity test (Table 1) showed that only between 1.14 and 1.25% of the bioluminescent light was transmitted through the silver dressing, while the transmissivity of chitosan acetate was higher, at between 2.9 and 3.3%. The time courses of the mean measured bioluminescence values are shown in Fig. 3A, and those after correction for optical quenching by the bandage are shown in Fig. 3B. The mean bioluminescence value in untreated burns increased over 100 times, while the increase in silver-treated burns was only 10-fold. The bioluminescence signal decreased slightly after the first day in chitosan acetate-treated burns.
TABLE 1.
Transmissivity test of chitosan acetate bandage and silver dressing with respect to bioluminescence
| Dressing | % Transmissivitya
|
|
|---|---|---|
| P. aeruginosa | P. mirabilis | |
| None | 100.00 | 100.00 |
| Chitosan acetate | 3.29 ± 0.66 | 2.9 ± 0.74 |
| Silver | 1.25 ± 0.16 | 1.14 ± 0.23 |
The values for the chitosan acetate and silver dressings are the means ± SDs of triplicate determinations.
FIG. 3.
(A) Time course of mean measured bioluminescence signals from mice with infected burns over days 0 to 5; (B) time course of bioluminescence signals as in panel A but corrected for optical quenching by the bandage; (C) survival curves for mice with P. aeruginosa-infected burns treated with chitosan acetate bandage (n = 15), silver dressing (n = 11), or no treatment (n = 15); (D) time courses of mean weight loss of mice over 5 days postinfection.
At 4 weeks postinfection, the rates of survival of the chitosan acetate bandage-treated group (n = 15), the silver dressing-treated group (n = 11), and the untreated group (n = 15) were 73.3%, 27.3%, and 13.3%, respectively (Fig. 3C). The survival curves were found to be significantly different between the chitosan acetate bandage-treated group and the untreated group (P < 0.0002) and between the chitosan acetate bandage-treated group and the silver dressing-treated group (P = 0.0055). In all three groups of animals, most of the fatalities (22 of 25) occurred between 2 and 6 days postinfection. Figure 3D compares the mean weight loss of mice in the different groups over the 5 days postinfection, when most of the fatalities occurred. The chitosan acetate-treated group lost significantly less weight than the other two groups at time points between 2 and 5 days postinfection (P < 0.05).
For all surviving mice, no bioluminescent P. aeruginosa colonies were observed at any time point in the blood cultures. For all the mice that died, P. aeruginosa was found in the blood cultures the day before the fatalities occurred. Interestingly, a nonluminescent bacterium identified as Escherichia coli was observed in the blood cultures of 37% (15 of 41) of mice with burns and infections (but not in the blood cultures of mice with burns alone [0 of 6 mice]) during the initial several days postinfection (Table 2).
TABLE 2.
Incidence of bacteria in the bloodstream of mice with infected burns
| Group | No. of mice with bloodstream infection/total no. of mice
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burn alone
|
Burn + infection
|
Burn, infection, silver
|
Burn, infection, chitosan acetate
|
|||||||||||||
|
P. aeruginosa infections
|
P. mirabilis infections
|
P. aeruginosa infections
|
P. mirabilis infections
|
P. aeruginosa infections
|
P. mirabilis infections
|
P. aeruginosa infections
|
P. mirabilis infections
|
|||||||||
| E. coli | P. aeruginosa | E. coli | P. mirabilis | E. coli | P. aeruginosa | E. coli | P. mirabilis | E. coli | P. aeruginosa | E. coli | P. mirabilis | E. coli | P. aeruginosa | E. coli | P. mirabilis | |
| Alive | 0/6 | 0/6 | 0/6 | 0/6 | 1/2 | 0/2 | 0/0 | 0/0 | 1/3 | 0/3 | 1/5 | 0/5 | 4/11 | 0/11 | 2/8 | 0/8 |
| Dead | 0/0 | 0/0 | 0/0 | 0/0 | 4/13 | 13/13 | 3/13 | 13/13 | 5/8 | 8/8 | 1/3 | 3/3 | 0/4 | 4/4 | 1/4 | 4/4 |
| Total | 0/6 | 0/6 | 0/6 | 0/6 | 5/15 | 13/15 | 3/13 | 13/13 | 6/11 | 8/11 | 2/8 | 3/8 | 4/15 | 4/15 | 3/12 | 4/12 |
In the surviving chitosan acetate bandage-treated mice (n = 11), the bandage peeled off from the burns at day 16 ± 5 postinfection. The mean burn area at the time of bandage loss was 133.6 ± 41 mm2 (66.8% of the initial burn area). The bioluminescence values of the chitosan acetate-treated burns at the time of bandage loss ranged from 1.9 × 104 to 3.2 × 105. Two of 15 untreated mice in the study survived. The bioluminescence values at day 16 postinfection were 8.5 × 104 and 1.1 × 105, respectively. Three of 11 silver-treated mice survived. As the silver dressing was attached to the burns by gluing the dressing edges to the skin surrounding the burns, the dressing stayed on the burns for the whole observation period. At day 16 postinfection, no luminescence could be detected from the surface of the sliver dressing on the burns. In two of the mice, the dressing was replaced on day 11, and the bioluminescence values of the burns were measured to be 1.4 × 105 and 7.6 × 105, respectively, before the application of new silver dressings. In most of the burns, the bioluminescence increased somewhat after the loss of chitosan acetate and then began to decrease. The complete disappearance of bioluminescence occurred at day 24 ± 3 postinfection. Complete reepithelialization of the burns was observed at day 27 ± 3 postinfection.
P. mirabilis burn infections treated with chitosan acetate or silver.
Burn infections were induced by the inoculation of 108 P. mirabilis cells onto 30-s burns. Figure 4A shows representative bioluminescent images of P. mirabilis infections in an untreated burn, a silver dressing-treated burn, and a chitosan acetate bandage-treated burn. In these experiments the bandage pieces used were smaller than those used for the P. aeruginosa-infected burns, and it can be seen that bacterial luminescence is visible around the edges of the applied bandage for both the silver and the chitosan acetate bandages.
FIG. 4.
(A) Representative bioluminescence images (at the same bit range of 3) of P. mirabilis-infected burns with no treatment, silver dressing treatment, and chitosan acetate bandage treatment; (B) survival curves for mice with P. mirabilis-infected burns a chitosan acetate bandage treatment (n = 12), a silver dressing treatment (n = 8), and no treatment (n = 13). The survival curves were significantly different between the chitosan acetate bandage-treated or the silver dressing-treated group and the untreated-group (P < 0.0002) but not between the chitosan acetate bandage-treated group and the silver dressing-treated group (P = 0.93).
For the chitosan acetate bandage-treated group (n = 12), the silver dressing-treated group (n = 8), and the untreated group (n = 13), the survival rates within 4 weeks postinfection were 66.7%, 62.5%, and 0%, respectively (Fig. 4B). Most fatalities (18 of 20) occurred before day 6 postinfection. The survival curves were found to be significantly different between the chitosan acetate-treated group and the untreated group (P < 0.0002) but not between the chitosan acetate-treated group and the silver-treated group (P = 0.93).
Blood cultures were carried out for all mice that died following P. mirabilis burn infection and confirmed that the mice that died had bioluminescent bacteria in their bloodstreams. Nonbioluminescent E. coli was found in the bloodstreams of both surviving and dead mice at a frequency (roughly 25%) comparable to that for the mice with P. aeruginosa burn infections (Table 2).
DISCUSSION
This study has shown that the chitosan acetate bandage can act as an effective topical antimicrobial dressing for infected or contaminated burns. There have been many reports of the development of animal models of infected thermal burns with both mice and rats (29, 39, 40). As mentioned above, it was necessary to apply the bacteria to the surface of the burn in order to test the effects of the antimicrobial dressings. The main determinants of the severity of the burn infection and whether the rodents develop sepsis and die are as follows: the number of bacteria applied to the burn, the virulence of the particular strain, the size of the burn expressed as a percentage of the total body surface area, whether the bacteria are applied to the surface or injected into or beneath the burn, and the length of time that the heated object or liquid is in contact with the skin. The loss of invasiveness and virulence in P. aeruginosa after repeated in vitro culture can be attributed to its extended genotypic and phenotypic variation (11, 41). The use of longer burn times does produce more mechanical damage to the dermis, and this damage may allow less invasive bacteria a better chance to gain a foothold in the tissue.
In P. aeruginosa infections, the mice in the chitosan acetate bandage-treated group demonstrated a higher rate of survival than the rates for the mice in the other two groups. This implies the rapid killing of the bacteria in the burns by the chitosan acetate bandage, which prevented the bacteria from proliferating in the burn and subsequently invading the mouse tissue, gaining access to the bloodstream, and causing death from sepsis. Chitosan acetate kills bacteria through disruption of the cell membrane caused by the electrostatic interaction between cationic NH3+ groups of chitosan acetate and the anionic phospholipid components of cell membranes (23). In a previous study (4) that looked at the application of chitosan acetate bandages to infected excisional wounds, we included a nonantimicrobial bandage made of alginate as a control. We found that occlusion of the wound by a dressing that could not kill bacteria actually encouraged bacterial growth, presumably by preserving the bacteria from drying out and dying from dehydration. Although we did not include that control in the present studies, a similar result could be anticipated.
Among the mice with P. mirabilis infections, the survival rates were almost equal between the two treatment groups (mice treated with chitosan acetate and silver dressings), while no treatment gave approximately the same rate of fatality as that among mice infected with P. aeruginosa. This difference between the two species of pathogens (in mice with P. aeruginosa infections, the chitosan acetate dressing was much more effective than the silver dressing) probably reflects the fact that P. mirabilis relies on its remarkable motility or swarming ability for its virulence (18, 27), while P. aeruginosa possesses an array of enzymes such as multiple proteases that are designed to facilitate tissue invasion and that have been correlated with virulence in many disease models, including burn infections (32, 43). If it is accepted that chitosan acetate kills bacteria much faster than silver does, then it could be argued that the P. aeruginosa cells in the burn infection need to be killed faster than P. mirabilis cells to prevent the development of sepsis. A recent paper (20) described a study that compared the in vitro sensitivities of several bacteria (including Proteus and Pseudomonas) to silver dressings (including the topical silver-releasing bandage). Both these bacterial species were among the most sensitive of all the species tested. Therefore, we do not believe that the relatively poor performance of the silver dressing was a reflection only of the silver sensitivity of the bacterial species tested.
E. coli was observed in the bloodstreams of 37% of the P. aeruginosa-infected, burned mice, while mice with noninfected burns did not show the presence of E. coli in the bloodstream. We therefore believe that the presence of E. coli in the bloodstream arose by translocation from the large intestine induced by the combination of the third-degree burn and the superimposed infection, which caused a deficiency of the gut barrier (10, 12). Previous studies have also shown that infectious insults combined with thermal burns in small-animal models promoted bacterial translocation from the gut (24).
One limitation of this study was the relatively short time between the burn and the infection (5 min) and between the infection and the application of the antimicrobial bandage (15 min). It would be unrealistic to expect these time intervals to apply clinically. However clinical burn infections start with very much lower numbers (many orders of magnitude less) of CFU, and in addition, the bacteria in clinical infections have not been selected according to their virulence. Further studies would be needed to determine whether the chitosan acetate bandage is effective in controlling more established infections from a much smaller initial inoculum that are allowed to develop over a more extended time period.
In conclusion, we have demonstrated that the chitosan acetate bandage performs better than the clinically approved nanocrystalline silver bandage when it is topically applied to third-degree burns heavily contaminated with an aggressive and invasive strain of P. aeruginosa. In the case of the virulent but less invasive organism P. mirabilis, the effectiveness of the two dressings was equal. During the care of combat casualties, heavy bacterial contamination of serious burns is a distinct possibility.
Acknowledgments
This work was supported in part by HemCon Medical Technologies, Inc., and the U.S. National Institutes of Health (grant R01 AI050875 to M.R.H.).
We thank Xenogen Corporation and Kevin P. Francis for the generous gift of stable bioluminescent bacteria. We thank HemCon Medical Technologies, Inc., for supplying samples of the HemCon bandage. We are grateful to Victoria Hamrahi for assistance with microbial identification. We are grateful to Christopher H. Contag, Tayyaba Hasan, Albert T. McManus, and William P. Wiesmann for helpful advice and discussion.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Aksungur, P., A. Sungur, S. Unal, A. B. Iskit, C. A. Squier, and S. Senel. 2004. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J. Control. Release 98:269-279. [DOI] [PubMed] [Google Scholar]
- 2.Azad, A. K., N. Sermsintham, S. Chandrkrachang, and W. F. Stevens. 2004. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J. Biomed. Mater. Res. B Appl. Biomater. 69:216-222. [DOI] [PubMed] [Google Scholar]
- 3.Burkatovskaya, M., A. P. Castano, T. N. Demidova-Rice, G. P. Tegos, and M. R. Hamblin. 2008. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 16:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkatovskaya, M., G. P. Tegos, E. Swietlik, T. N. Demidova, A. P. Castano, and M. R. Hamblin. 2006. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 27:4157-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church, D., S. Elsayed, O. Reid, B. Winston, and R. Lindsay. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale, R. M., G. Schnell, and J. P. Wong. 2004. Therapeutic efficacy of “nubiotics” against burn wound infection by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demidova, T. N., F. Gad, T. Zahra, K. P. Francis, and M. R. Hamblin. 2005. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J. Photochem. Photobiol. B 81:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Martino, A., M. Sittinger, and M. V. Risbud. 2005. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983-5990. [DOI] [PubMed] [Google Scholar]
- 9.Doyle, T. C., S. M. Burns, and C. H. Contag. 2004. In vivo bioluminescence imaging for integrated studies of infection. Cell. Microbiol. 6:303-317. [DOI] [PubMed] [Google Scholar]
- 10.Eaves-Pyles, T., and J. W. Alexander. 2001. Comparison of translocation of different types of microorganisms from the intestinal tract of burned mice. Shock 16:148-152. [DOI] [PubMed] [Google Scholar]
- 11.Foweraker, J. E., C. R. Laughton, D. F. Brown, and D. Bilton. 2005. Phenotypic variability of Pseudomonas aeruginosa in sputa from patients with acute infective exacerbation of cystic fibrosis and its impact on the validity of antimicrobial susceptibility testing. J. Antimicrob. Chemother. 55:921-927. [DOI] [PubMed] [Google Scholar]
- 12.Gianotti, L., J. W. Alexander, T. Pyles, L. James, and G. F. Babcock. 1993. Relationship between extent of burn injury and magnitude of microbial translocation from the intestine. J. Burn Care Rehabil. 14:336-342. [DOI] [PubMed] [Google Scholar]
- 13.Gilpin, D. A. 1996. Calculation of a new Meeh constant and experimental determination of burn size. Burns 22:607-611. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield, E., and A. T. McManus. 1997. Infectious complications: prevention and strategies for their control. Nurs. Clin. N. Am. 32:297-309. [PubMed] [Google Scholar]
- 15.Ha, U., and S. Jin. 1999. Expression of the soxR gene of Pseudomonas aeruginosa is inducible during infection of burn wounds in mice and is required to cause efficient bacteremia. Infect. Immun. 67:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblin, M. R., D. A. O'Donnell, N. Murthy, C. H. Contag, and T. Hasan. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75:51-57. [DOI] [PubMed] [Google Scholar]
- 17.Harrison, J. W., and T. A. Svec. 1998. The beginning of the end of the antibiotic era? Part I. The problem: abuse of the “miracle drugs.” Quintessence Int. 29:151-162. [PubMed] [Google Scholar]
- 18.Harshey, R. M. 1994. Bees aren't the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13:389-394. [DOI] [PubMed] [Google Scholar]
- 19.Holder, I. A., and A. N. Neely. 1989. Pseudomonas elastase acts as a virulence factor in burned hosts by Hageman factor-dependent activation of the host kinin cascade. Infect. Immun. 57:3345-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip, M., S. L. Lui, V. K. Poon, I. Lung, and A. Burd. 2006. Antimicrobial activities of silver dressings: an in vitro comparison. J. Med. Microbiol. 55:59-63. [DOI] [PubMed] [Google Scholar]
- 21.Kheirabadi, B. S., E. M. Acheson, R. Deguzman, J. L. Sondeen, K. L. Ryan, A. Delgado, E. J. Dick, Jr., and J. B. Holcomb. 2005. Hemostatic efficacy of two advanced dressings in an aortic hemorrhage model in swine. J. Trauma 59:25-34. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, M. N., R. A. Muzzarelli, C. Muzzarelli, H. Sashiwa, and A. J. Domb. 2004. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104:6017-6084. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H., Y. Du, X. Wang, and L. Sun. 2004. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 95:147-155. [DOI] [PubMed] [Google Scholar]
- 24.Manson, W. L., J. M. Coenen, H. J. Klasen, and E. H. Horwitz. 1992. Intestinal bacterial translocation in experimentally burned mice with wounds colonized by Pseudomonas aeruginosa. J. Trauma 33:654-658. [DOI] [PubMed] [Google Scholar]
- 25.Markley, K., and E. Smallman. 1968. Protection by vaccination against Pseudomonas infection after thermal injury. J. Bacteriol. 96:867-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McManus, A. T., C. G. McLeod, Jr., and A. D. Mason, Jr. 1982. Experimental Proteus mirabilis burn surface infection. Arch. Surg. 117:187-191. [DOI] [PubMed] [Google Scholar]
- 27.Mobley, H. L., and R. Belas. 1995. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 3:280-284. [DOI] [PubMed] [Google Scholar]
- 28.Murray, C. K. 2007. Infections in burns. J. Trauma 62:S73. [DOI] [PubMed] [Google Scholar]
- 29.Neely, A. N., I. A. Holder, and G. D. Warden. 1999. Then and now: studies using a burned mouse model reflect trends in burn research over the past 25 years. Burns 25:603-609. [DOI] [PubMed] [Google Scholar]
- 30.Neely, A. N., R. G. Miller, and I. A. Holder. 1994. Proteolytic activity and fatal gram-negative sepsis in burned mice: effect of exogenous proteinase inhibition. Infect. Immun. 62:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuffer, M. C., J. McDivitt, D. Rose, K. King, C. C. Cloonan, and J. S. Vayer. 2004. Hemostatic dressings for the first responder: a review. Mil. Med. 169:716-720. [DOI] [PubMed] [Google Scholar]
- 32.Pavlovskis, O. R., and B. Wretlind. 1979. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect. Immun. 24:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poole, M. D. 1993. Are we facing the end of the antibiotic era? Ear Nose Throat J. 72:433. [PubMed] [Google Scholar]
- 34.Pusateri, A. E., S. J. McCarthy, K. W. Gregory, R. A. Harris, L. Cardenas, A. T. McManus, and C. W. Goodwin, Jr. 2003. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J. Trauma 54:177-182. [DOI] [PubMed] [Google Scholar]
- 35.Rabea, E. I., M. E. Badawy, C. V. Stevens, G. Smagghe, and W. Steurbaut. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457-1465. [DOI] [PubMed] [Google Scholar]
- 36.Rocchetta, H. L., C. J. Boylan, J. W. Foley, P. W. Iversen, D. L. LeTourneau, C. L. McMillian, P. R. Contag, D. E. Jenkins, and T. R. Parr, Jr. 2001. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob. Agents Chemother. 45:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal, S. M. 1967. Local and systemic therapy of Pseudomonas septicemia in burned mice. Ann. Surg. 165:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens, E. J., C. M. Ryan, J. S. Friedberg, R. L. Barnhill, M. L. Yarmush, and R. G. Tompkins. 1994. A quantitative model of invasive Pseudomonas infection in burn injury. J. Burn Care Rehabil. 15:232-235. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson, J. M., R. L. Gamelli, and R. Shankar. 2003. A mouse model of burn wounding and sepsis. Methods Mol. Med. 78:95-105. [DOI] [PubMed] [Google Scholar]
- 40.Walker, H. L., A. D. Mason, Jr., and G. L. Raulston. 1964. Surface infection with Pseudomonas aeruginosa. Ann. Surg. 160:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb, J. S., M. Lau, and S. Kjelleberg. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:8066-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedmore, I., J. G. McManus, A. E. Pusateri, and J. B. Holcomb. 2006. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J. Trauma 60:655-658. [DOI] [PubMed] [Google Scholar]
- 43.Yagci, A., Y. Tuc, and G. Soyletir. 2002. Elastase and alkaline protease production by Pseudomonas aeruginosa strains: comparison of two procedures. New Microbiol. 25:223-229. [PubMed] [Google Scholar]
- 44.Yilmaz, E. 2004. Chitosan: a versatile biomaterial. Adv. Exp. Med. Biol. 553:59-68. [DOI] [PubMed] [Google Scholar]