Abstract
The two fixed-dose combinations of dihydroartemisinin and piperaquine (Artekin and Arterakine) were found to be bioinequivalent in healthy Vietnamese subjects. However, because the peak plasma concentrations and areas under the concentration-time curves of dihydroartemisinin and piperaquine were only marginally different between the two formulations, similar therapeutic efficacies are expected in the treatment of malaria infections.
Artemisinin-based combination treatments (ACTs) are now considered the best therapy for the treatment of Plasmodium falciparum malaria (13, 19). One of the most promising ACTs is the fixed-dosed dihydroartemisinin-piperaquine combination marketed as Artekin (Holleykin Pharmaceuticals Co. Ltd., China), which is well tolerated and highly effective in the treatment of P. falciparum malaria (2, 3, 8, 14). The cure rate for Artekin is typically greater than 95% following a 3-day course, with a 42-day follow-up period. Despite the availability of clinical data on the efficacy of Artekin, no pharmacokinetic data are available on dihydroartemisinin given as an oral coformulation with piperaquine and few data are available on the disposition of piperaquine following Artekin administration.
Recently, the Vietnamese Ministry of Health licensed Central Pharmaceutical Company No. 1 to produce a fixed-dose combination of dihydroartemisinin-piperaquine, marketed as Arterakine, for use in Vietnam. In the present study, we compared the pharmacokinetics of the components of Artekin and Arterakine and assessed the bioequivalence of Arterakine to the reference formulation, Artekin.
A randomized, open-label, single-dose, two-period crossover study was carried out with 24 healthy male Vietnamese subjects with a mean (± standard deviation) age of 21.0 (2.7) years and a mean weight of 59.7 (3.3) kg. Each subject received three tablets of the reference drug, Artekin (batch no. 20040201; each tablet contains 40 mg dihydroartemisinin and 320 mg piperaquine phosphate; Holleykin Pharmaceutical Co. Ltd., Guangdong, China), and three tablets of the test drug, Arterakine (batch no. 010606; each tablet contains 40 mg dihydroartemisinin and 320 mg piperaquine phosphate; Central Pharmaceutical Factory No. 1, Hanoi, Vietnam). The study was carried out within the 3-year expiration period of the medication, and the washout period between the two treatment phases was 10 weeks. The study was approved by the Review and Scientific Board of Central Military Hospital 108 and the Australian Defense Human Research Ethics Committee (ADHREC 437/06).
Each subject received the medication after an overnight fast (no food for 8 h), and the tablets were taken with 200 ml of water. Food was withheld for 4 h after drug administration. Beverages containing caffeine, alcohol, or grapefruit juice and smoking were not allowed 2 days before and after drug administration. Venous blood samples (7 ml) were collected with an indwelling cannula within 0.5 h (baseline) prior to dosing and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h after drug administration. Subsequent blood samples were collected by venipuncture at days 1, 3, 7, 14, 21, and 28 after dosing. Blood samples were centrifuged, and the separated plasma samples (3 ml) were stored at −80°C until analyzed, which was within 12 months of collection.
Plasma concentrations of dihydroartemisinin were measured by liquid chromatography-tandem mass spectrometry. Briefly, to a glass tube fitted with a Teflon-lined cap were added plasma (200 μl) and alprazolam (internal standard, 50 μl of a 150-ng/ml concentration) and the contents were mixed prior to the addition of butyl chloride-ethyl acetate (9:1, vol/vol; 3 ml). The tubes were rotated for 5 min and then centrifuged (3,000 × g for 5 min). The organic layer was transferred to a clean glass tube and evaporated to dryness under a stream of air at 37°C. Chromatographic analysis was conducted with a Prominence liquid chromatography system (Shimadzu, Japan) and an HTC PAL autosampler (CTC Analytics, Switzerland) linked to an API3200 mass spectrometer (Applied Biosystems) fitted with an electrospray interface operated in the positive-ion mode. The reconstituted sample was separated on a Luna C18 high-performance liquid chromatography column (50 by 2.0 mm [inside diameter], 3-μm particle size; Phenomenex) preceded by a Luna C18 guard cartridge with an acetonitrile-ammonium acetate gradient at a flow rate of 0.4 ml/min. The peak area ratios of the NH+ adduct of dihydroartemisinin (product at m/z 267.2 from the parent ion at m/z 302.2) to the internal standard (product at m/z 281.2 from the parent ion at m/z 309.2) were calculated for each sample from the measured peak areas obtained by selected reaction monitoring. The retention times of the internal standard and dihydroartemisinin were 2.0 and 2.2 min, respectively. Quadratic regression of the concentration data (range, 1 to 1,000 ng/ml) with 1/concentration2 (χ2) weighting yielded a correlation coefficient of >0.994 for dihydroartemisinin. The lower limit of quantification was 1 ng/ml with 0.2 ml of plasma. The overall precision of analysis for dihydroartemisinin, as defined by the percent coefficient of variation of spiked samples was 6.3% at 1 ng/ml, 5.7% at 20 ng/ml, 4.6% at 200 ng/ml, and 6.6% at 750 ng/ml. The corresponding inaccuracy values were −1.3%, −1.5%, −0.7%, and 3.2%. Plasma concentrations of piperaquine were measured by a validated high-performance liquid chromatography method (10). The precision of the assay was 10.3% at 10 ng/ml, 6.8% at 100 ng/ml, and 6.7% at 500 ng/ml. The corresponding inaccuracy values were 11%, 3%, and 1%. The lower limit of quantification of piperaquine was 5 ng/ml with 0.5 ml of plasma.
Pharmacokinetic parameters (peak concentration [Cmax], time to reach maximum concentration [Tmax], area under the concentration-time curve from 0 h to the last data point [AUC0→last] and from the last data point to infinity [AUClast→∞], terminal half-life [t1/2], apparent oral clearance [CL/F], and apparent volume of distribution [V/F]) were determined from the plasma concentration-time data by noncompartmental methods (5). The pharmacokinetic parameters of dihydroartemisinin and piperaquine were calculated from the nominal potency of the tablets and tested parametrically with the paired t test for statistically significant differences between the two formulations. Schuirmann's two one-sided t test procedure was used to calculate the 90% confidence interval (CI) from the log-transformed pharmacokinetic data (15). Treatments were considered bioequivalent if the 90% CI for mean point estimators (ratio of test/reference) for the Cmax and AUC of dihydroartemisinin and piperaquine fell within the acceptance range of 80 to 125% for log-transformed values (4). The effects of formulation, period, and sequence effects were studied by analyses of variance (ANOVA) of the log-transformed data.
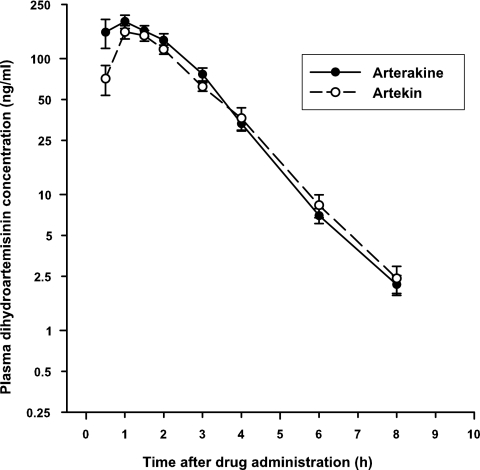
The mean plasma concentration-time profiles of dihydroartemisinin after a single oral dose of Arterakine and Artekin are shown in Fig. 1, and the pharmacokinetics of dihydroartemisinin are summarized in Table 1.The geometric mean Cmax of dihydroartemisinin was higher and was achieved marginally faster after Arterakine (Cmax, 198 ng/ml; mean Tmax, 1.0 h) than after Artekin (Cmax, 159 ng/ml; Tmax, 1.5 h) administration. The AUC0→last of dihydroartemisinin was higher after Arterakine than after Artekin administration, with geometric mean values of 442 and 366 ng·h/ml, respectively, but the difference was not significant (P > 0.05). Dihydroartemisinin was rapidly eliminated, with a t1/2 of about 1 h for the two formulations. There was a significant difference in the CL/F (4.95 versus 5.87 liters/h/kg; 95% CI, −1.72, −0.12; P = 0.03) and V/F of dihydroartemisinin (7.00 versus 8.02 liters/kg; 95% CI, −3.10, −0.13; P = 0.03) following the administration of the two formulations. Overall, the pharmacokinetics of dihydroartemisinin after Arterakine or Artekin administration were comparable to values obtained in healthy Vietnamese and Thai subjects given dihydroartemisinin alone (9, 11). Thus, piperaquine does not appear to alter the pharmacokinetics of dihydroartemisinin when given as a coformulation.
FIG. 1.
Mean (standard error of the mean) plasma dihydroartemisinin concentration-time profiles following the administration of a single oral dose of three Arterakine (•) or Artekin (○) tablets to 24 healthy Vietnamese subjects.
TABLE 1.
Pharmacokinetic parameters of dihydroartemisinin and piperaquine in 24 healthy Vietnamese subjects following the administration of a single oral dose of 120 mg dihydroartemisinin and 960 mg piperaquine in the form of three Arterakine or three Artekin tablets (reference formulation)a
| Pharmacokinetic parameter | Dihydroartemisinin
|
Piperaquine
|
||
|---|---|---|---|---|
| Arterakine | Artekin | Arterakine | Artekin | |
| Cmax (ng/ml) | 198 (48-745) [240 ± 165] | 159 (65-359) [176 ± 81] | 232 (86-691) [276 ± 174] | 204 (78-841) [237 ± 159] |
| Tmax (h) | 1.0 (0.5-3.0) [1.2 ± 0.7] | 1.5 (0.5-3.0) [1.4 ± 0.6] | 3.0 (1.5-8.0) [3.1 ± 1.6] | 3.0 (1.0-10.0) [3.3 ± 1.9] |
| AUC0-last (ng · h/ml) | 442 (199-938) [482 ± 211] | 366 (180-761) [395 ± 157] | 13,431 (8,219-26,314) [13,984 ± 4,301] | 11,988 (5,361-24,514) [12,515 ± 3,809] |
| AUC0-∞ (ng · h/ml) | 444 (200-940) [484 ± 211] | 370 (182-716) [398 ± 159] | 23,308 (13,770-34,304) [24,168 ± 6,537]b | 19,929 (7,980-31,858) [20,942 ± 6,179] |
| AUCextrap (%)c | 0.5 ± 0.2 | 0.9 ± 0.7 | 41.5 ± 10.8 | 38.6 ± 12.8 |
| t1/2 (h) | 0.98 (0.73-1.28) [1.00 ± 0.21] | 1.01 (0.72-1.66) [1.05 ± 0.29] | 613 (273-1065) [647 ± 206] | 589 (221-1249) [643 ± 260] |
| CL/F (liters/h/kg) | 4.54 (2.28-10.34) [4.95 ± 2.10]b | 5.45 (2.49-11.02) [5.87 ± 2.31] | 0.40 (0.25-0.66) [0.42 ± 0.12]b | 0.47 (0.28-1.14) [0.50 ± 0.20] |
| V/F (liters/kg) | 6.31 (2.84-18.30) [7.00 ± 3.38]b | 7.97 (4.75-19.55) [8.02 ± 3.75] | 353 (135-627) [377 ± 125] | 394 (107-814) [428 ± 170] |
Data are presented as the geometric mean (range) [mean ± standard deviation].
Significant difference (paired t test, P < 0.05) from the reference formulation.
AUCextrap (%) = [(AUC0→∞ − AUC0→last)/AUC0→∞] × 100.
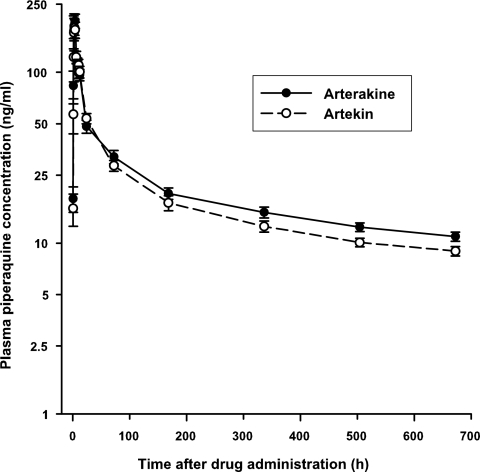
The mean plasma concentration-time profiles and pharmacokinetics of piperaquine are presented in Fig. 2 and Table 1, respectively. The geometric mean Cmax (232 versus 204 ng/ml) and AUC0→last (13,431 versus 11,988 ng · h/ml) of piperaquine were higher after Arterakine than after Artekin administration, but the difference was not significant. After adjusting for dose and weight differences, the Cmax of piperaquine was markedly higher in the healthy Vietnamese subjects after the administration of both ACTs than in healthy Caucasians administered piperaquine alone (1, 16). Ethnic differences and differences in tablet excipients and dissolution behavior may account for the variations in piperaquine concentrations achieved after the administration of piperaquine alone and the two coformulations used in the present study. The elimination of piperaquine (based on measurements from day 7 forward) was lengthy, with an estimated mean t1/2 of about 645 h (26.8 days) in the healthy subjects, which is in good agreement with values obtained in healthy Vietnamese (6, 12) and Caucasian subjects (16, 18) and in malaria patients (7, 17). The mean CL/Fs of piperaquine after Arterakine and Artekin administration of 0.42 and 0.50 liter/h/kg, respectively, were lower than previously reported in healthy Vietnamese (0.74 liter/h/kg [12]) and Caucasian subjects (1.14 liters/h/kg [16]) given piperaquine alone. A large V/F of piperaquine was estimated after Arterakine (377 ± 125 liter/kg) and Artekin (428 ± 170 liter/kg) administration, which is consistent with the high values reported in healthy subjects (6, 12, 16) and malaria patients (7, 17).
FIG. 2.
Mean (standard error of the mean) plasma piperaquine concentration-time profiles following the administration of a single oral dose of three Arterakine (•) or Artekin (○) tablets to 24 healthy Vietnamese subjects.
Statistical evaluation of the pharmacokinetic data for dihydroartemisinin and piperaquine showed bioinequivalence of Arterakine and Artekin for the Cmax, AUC0→last, and AUC0→∞ of dihydroartemisinin and the Cmax and AUC0→∞ of piperaquine within the predetermined acceptance range of 80 to 125% at the 90% CI (Table 2). The results of the ANOVA found that formulation, period, and sequence had no statistically significant effects on the Cmax of dihydroartemisinin and piperaquine. However, there was a significant effect of formulation on the AUC0→last of dihydroartemisinin (P = 0.01) and piperaquine (P = 0.04). Similarly, there was a significant effect of period on the AUC0→last of dihydroartemisinin (P = 0.02) and piperaquine (P = 0.03), which may be a random occurrence but is unlikely to be of importance given the balanced study design. The sequence had no significant effect on the AUC0→last of either drug.
TABLE 2.
Parametric 90% CIs for the mean pharmacokinetic properties of dihydroartemisinin and piperaquine after the administration of a single oral dose of Arterakine and Artekin (reference formulation)
| Drug and parameter | Arterakine/Artekin point estimator (%) | 90% CIa |
|---|---|---|
| Dihydroartemisinin | ||
| Cmax | 136.4 | 100.6-152.8b |
| AUC0-last | 122.0 | 105.4-137.3b |
| AUC0-∞ | 121.6 | 105.0-137.3b |
| Cmax/AUC0-∞ | 105.9 | 92.9-114.8 |
| t1/2 | 95.2 | 88.7-105.3 |
| Piperaquine | ||
| Cmax | 116.5 | 91.3-142.0b |
| AUC0-last | 111.7 | 101.6-123.6 |
| AUC0-∞ | 115.4 | 106.5-128.5b |
| Cmax/AUC0-∞ | 97.7 | 78.3-121.3 |
| t1/2 | 100.6 | 86.4-125.2b |
Determined by using log-transformed data.
Value falls outside the range of 80 to 125%.
The mean drug contents based on five tablets of Arterakine were 103.3% ± 4.4% for dihydroartemisinin and 100.6% ± 1.2% for piperaquine. The corresponding contents in the Artekin tablets were 95.3% ± 8.3% and 98.8% ± 2.9%. Although the lower contents of dihydroartemisinin (8%) and piperaquine (1.8%) in the Artekin than in the Arterakine tablets would have contributed to the bioinequivalence of the two formulations, after adjustment for drug content, the Cmax and AUC0→∞ of both drugs were still outside the acceptance range of 80 to 125% (data not shown).
In conclusion, the pharmacokinetic parameters of dihydroartemisinin and piperaquine and the values obtained were consistent with previous studies in which the drugs were given alone. Because the bioavailability of dihydroartemisinin and piperaquine was only marginally higher after Arterakine than after Artekin administration, the two formulations are expected to result in similar therapeutic efficacies in the treatment of malaria infections.
Acknowledgments
This study was carried out under the auspices of the Vietnam Australia Defense Malaria Project, a defense cooperation between the Vietnam People's Army and the Australian Defense Force. We thank the Vietnam People's Army Department of Military Medicine for supporting this study and the financial sponsor, the Australian Defense Force International Policy Division.
We thank Ric Price for providing the Artekin tablets, Le Ngoc Anh for administrative-logistic support, and Chu Xuan Anh for coordinating staff in the collection and processing of blood samples. We are most grateful to Steve Wallis for performing the ANOVA and to G. Dennis Shanks and Bob Cooper for comments on the manuscript.
The opinions expressed in this report are ours and do not necessarily reflect those of the Defense Health Service or any extant Australian Defense Force policy.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Ahmed, T., P. Sharma, A. Gautam, B. Varshney, M. Kothari, S. Ganguly, J. J. Moehrle, J. Paliwal, N. Saha, and V. Batra. 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J. Clin. Pharmacol. 48:166-175. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, E. A., R. McGready, R. Hutagalung, L. Phaiphun, T. Slight, S. Proux, K. L. Thwai, M. Barends, S. Looareesuwan, N. J. White, and F. Nosten. 2005. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41:425-432. [DOI] [PubMed] [Google Scholar]
- 3.Denis, M. B., T. M. Davis, S. Hewitt, S. Incardona, K. Nimol, T. Fandeur, Y. Poravuth, C. Lim, and D. Socheat. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1469-1476. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. 2003. Guidance for industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. Food and Drug Administration, Washington, DC.
- 5.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics. Marcel Dekker Inc., New York, NY.
- 6.Hai, T. N., S. F. Hietala, N. Van Huong, and M. Ashton. 2008. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 107:145-149. [DOI] [PubMed] [Google Scholar]
- 7.Hung, T. Y., T. M. Davis, K. F. Ilett, H. Karunajeewa, S. Hewitt, M. B. Denis, C. Lim, and D. Socheat. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karunajeewa, H. A., K. F. Ilett, I. Mueller, P. Siba, I. Law, M. Page-Sharp, E. Lin, J. Lammey, K. T. Batty, and T. M. Davis. 2008. Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le, N. H., K. Na-Bangchang, T. D. Le, K. A. Thrinh, and J. Karbwang. 1999. Pharmacokinetics of a single oral dose of dihydroartemisinin in Vietnamese healthy volunteers. Southeast Asian J. Trop. Med. Public Health 30:11-16. [PubMed] [Google Scholar]
- 10.Lindegårdh, N., N. J. White, and N. P. Day. 2005. High throughput assay for the determination of piperaquine in plasma. J. Pharm. Biomed. Anal. 39:601-605. [DOI] [PubMed] [Google Scholar]
- 11.Na-Bangchang, K., S. Krudsood, U. Silachamroon, P. Molunto, O. Tasanor, K. Chalermrut, N. Tangpukdee, O. Matangkasombut, S. Kano, and S. Looareesuwan. 2004. The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J. Trop. Med. Public Health 35:575-582. [PubMed] [Google Scholar]
- 12.Nguyen, T. C., N. Q. Nguyen, X. T. Nguyen, D. Bui, T. Travers, and M. D. Edstein. 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79:620-623. [PubMed] [Google Scholar]
- 13.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181-192. [PubMed] [Google Scholar]
- 14.Ratcliff, A., H. Siswantoro, E. Kenangalem, R. Maristela, R. M. Wuwung, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuirmann, D. J. 1987. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 15:657-680. [DOI] [PubMed] [Google Scholar]
- 16.Sim, I. K., T. M. Davis, and K. F. Ilett. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49:2407-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarning, J., E. A. Ashley, N. Lindegardh, K. Stepniewska, L. Phaiphun, N. P. Day, R. McGready, M. Ashton, F. Nosten, and N. J. White. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob. Agents Chemother. 52:1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarning, J., N. Lindegardh, A. Annerberg, T. Singtoroj, N. P. Day, M. Ashton, and N. J. White. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob. Agents Chemother. 49:5127-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White, N. J. 2006. Malaria—time to act. N. Engl. J. Med. 355:1956-1957. [DOI] [PubMed] [Google Scholar]