Abstract
The prevalences of three sulfonamide resistance genes, sul1, sul2, and sul3 and sulfachloropyridazine (SCP) resistance were determined in bacteria isolated from manured agricultural clay soils and slurry samples in the United Kingdom over a 2-year period. Slurry from tylosin-fed pigs amended with SCP and oxytetracycline was used for manuring. Isolates positive for sul genes were further screened for the presence of class 1 and 2 integrons. Phenotypic resistance to SCP was significantly higher in isolates from pig slurry and postapplication soil than in those from preapplication soil. Of 531 isolates, 23% carried sul1, 18% sul2, and 9% sul3 only. Two percent of isolates contained all three sul genes. Class 1 and class 2 integrons were identified in 5% and 11.7%, respectively, of sul-positive isolates. In previous reports, sul1 was linked to class 1 integrons, but in this study only 8% of sul1-positive isolates carried the intI1 gene. Sulfonamide-resistant pathogens, including Shigella flexneri, Aerococcus spp., and Acinetobacter baumannii, were identified in slurry-amended soil and soil leachate, suggesting a potential environmental reservoir. Sulfonamide resistance in Psychrobacter, Enterococcus, and Bacillus spp. is reported for the first time, and this study also provides the first description of the genotypes sul1, sul2, and sul3 outside the Enterobacteriaceae and in the soil environment.
Since their introduction in the 1930s, sulfonamides have been widely used in clinical and veterinary medicine to treat bacterial and protozoal infections. They act as a structural analogue of ρ-amino-benzoic acid and bind dihydropteroate synthase (DHPS), a catalytic enzyme in the folic acid biosynthesis pathway, resulting in the inhibition of dihydrofolic acid formation (26). Resistance is conferred by mutations in the DHPS gene (folP) (30) or from the acquisition of an alternative DHPS gene (sul) (18, 20, 29).
The first of the three known alternative DHPS genes, sul1, is usually located on the 3′ conserved region of a class 1 integron (25) and is frequently identified with this potentially mobile element in the slurry and soil environment (12, 22, 29). sul2 was first identified on RSF1010 in Escherichia coli and has been found on small nonconjugative resistance plasmids (20). The sul3 gene was identified during a study on sulfonamide resistance in pathogenic E. coli isolates from swine from Switzerland (18).
The prevalence of each of the sulfonamide resistance genes varies among published studies, depending on environments and bacterial species sampled. The majority of reports relate to Enterobacteriaceae isolates, specifically E. coli and Salmonella spp.
Previous investigations have screened for all three sul genes, but only Antunes et al. (2), in an investigation of Salmonella enterica strains, found all three genes. One previous study screened for all three genes in environmental isolates and soil; Heuer and Smalla (13) screened silt and loamy sand soils, known to have lower sorbance properties for antibiotics (especially for tetracyclines and sulfonamides) than clay soils (8, 10). This short-term study suggested that manure from treated pigs enhanced the spread of antibiotic resistance in bacterial communities in soil (13).
Here we report the prevalences of sulfonamide resistance genes in bacterial isolates from agricultural clay soil, where long-term (2-year) application of slurry from tylosin (TY)-fed pigs with the experimental addition of sulfachloropyridazine (SCP) and oxytetracycline (OTC) occurred. The study also aimed to investigate whether the prevalence of the sulfonamide resistance gene was due to dissemination of sulfonamide resistance in bacteria from the farm environment (manure) to indigenous soil bacteria.
MATERIALS AND METHODS
Field study.
Triplicate clay soil cores were collected at predetermined time points from an agricultural field in Lincolnshire, United Kingdom, which received an application of TY-fed-pig slurry which had been amended with SCP and OTC at concentrations of 25.58 mg liter−1 and 18.85 mg liter−1, respectively (8). The time points were as follows: preapplication; year 1, day 1 after application; year 1, day 21; year 1, day 90; year 1, day 289; year 2, day 1; year 2, day 90; and year 2, day 240. Preapplication soil cores were used as controls. No TY was detected in the slurry or soil samples preceding or following the slurry applications. SCP was detected in soil leachate at 590 μg liter−1 at day 7 postapplication, 64 μg liter−1 at day 10, and then at low levels of ≤1 μg liter−1 from day 20. SCP and OTC concentrations (365 to 1,691 μg kg−1) through the soil profile were reported previously (8). Soil leachate samples were chosen from the three highest periods of rainfall and investigated separately, from year 1, day 15; year 2, day 49; and year 2, day 164 of the study. For ease of reporting, all results from the soil leachate samples were grouped together. The liquid from each sample was centrifuged, and the pellets were resuspended in 1 ml for serial dilutions, which were carried out in triplicate. The original volume was used in calculating the numbers of bacteria per sample per milliliter.
Viable plate counts.
Counts were performed on three separate cores from slurry-amended agricultural soils from the United Kingdom at nine time points, samples of pig slurry which had been obtained from a catchment tank below TY-fed animals (pig slurry control), and three separate pig slurry samples from the slurry tank after antibiotic amendment (pig slurry amended) (8). One gram of soil from each 0- to 5-cm core or 1-ml slurry sample was resuspended in 9 ml of sterile distilled water. Serial dilutions were made and spread onto Iso-Sensitest agar (Oxoid, United Kingdom) containing different concentrations of SCP (5, 10, 25, and 50 μg ml−1; Sigma, United Kingdom), OTC (0.2, 1, 5, 10, 25, and 50 μg ml−1; Sigma), and TY (5, 10, 25, 50, and 100 μg ml−1; Sigma). All plates contained 100 μg ml−1 cycloheximide (Sigma) to inhibit growth of fungi. Plates were incubated overnight and for 5 days aerobically at 28°C. Resistance quotients (RQs) were calculated by dividing the mean count from triplicate selective plates by the mean count from triplicate nonselective plates.
Bacterial isolation.
Bacterial colonies were randomly picked from nonselective and selective plates containing different concentrations of SCP, OTC, and TY and streaked until pure cultures were obtained. The numbers of isolates obtained from each antibiotic concentration at each time point varied due to differences in the resistance of the sampled population (no growth was observed at higher antibiotic concentrations in some samples) and to the loss of viability of some glycerol stocks. To overcome the variable numbers of isolates from each selective media at different time points, comparisons of sul gene prevalences were made from a subset of the data. A standardized number of isolates were randomly selected from each antibiotic selective media, SCP, TY, or OTC (11, 10, and 8 isolates, respectively), for each of the following samples: pig slurry; preapplication year 1; year 1, day 1; year 1, day 90; and year 1, day 289.
DNA extractions.
DNA was extracted using a DNeasy kit (Qiagen) according to the manufacturer's instructions from isolates grown in 5 ml of Iso-Sensitest broth (Oxoid) overnight at 28°C. PCR was performed, using 1 unit of Taq DNA polymerase and the manufacturer's buffer (Invitrogen, United Kingdom) with 4 mM MgCl2. Thirty cycles were performed at 95°C for 1 min, at varying annealing temperatures (shown in Table 1) for 1 min, and at 72°C for 1 min. The PCR products were eluted from a 1% agarose gel (Helena Biosciences, United Kingdom), using a QIAquick gel extraction kit (Qiagen). All sul-positive isolates were identified by using 16S rRNA gene sequences of approximately 800 bp in both directions. Sequencing reactions were performed with a Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) as described by the manufacturer, and electrophoresis and readout were performed on an ABI Prism 3100 genetic analyzer (Applied Biosystems).
TABLE 1.
Primer sequences used for PCR amplification and sequencing
| Gene | Primer | Sequence (5′ to 3′) | Annealing temp (°C) | Reference |
|---|---|---|---|---|
| 16S rRNAa | pA | AGAGTTTGATCCTGGCTCAG | 62 | 11 |
| pH | AAGGAGGTGATCCAGCCGCA | |||
| sul1 | sul1bF | CTTCGATGAGAGCCGGCGGC | 63 | 29 |
| sul1bR | GCAAGGCGGAAACCCGCGCC | |||
| sul2 | sul2F | TCGTCAACATAACCTCGGACAG | 60 | V. Enne |
| sul2R | GTTGCGTTTGATACCGGCAC | |||
| sul3 | sul3F | GAGCAAGATTTTTGGAATCG | 51 | 18 |
| sul3R | CATCTGCAGCTAACCTAGGGCTTTGGA | |||
| intI1 | intA | ACAGGGCAAGCTTAGTAAAGCC | 67 | 22 |
| intB | CTCGCTAGAACTTTTGGAAA | |||
| intI2 | int2F | CACGGATATGCGACAAAAAGGT | 58.5 | 32 |
| int2R | GTAGCAAACGAGTGACGAAATG | |||
| qacE | KazamF1 | GGGAATTCGCCCTACACAACAAATTGGGAGA | 50 | 14 |
| KazamR1 | TACTCGAGTTAGTGGGCACTTGCTTTGG | |||
| qacEΔ1 | KazamF2 | GGGAATTCGCCCTACACAACAAATTGGGAGA | 60 | 14 |
| KazamR2 | GCTGCAGCTGCGGTACCACTGCCACAA |
16S rRNA gene sequence from bp 8 to 1522.
Analysis of DNA sequences.
The resulting DNA sequences were edited, using BioEdit (Isis Pharmaceuticals, Inc.), and were subsequently analyzed using the BLAST program, with a sequence similarity of ≥97% used for species identification (1).
Conjugal transfers.
Pseudomonas putida UWC1 (Rifr) and E. coli K-12 CV601 (Rifr Thr− Leu− Thi−) were used as recipients in conjugal transfers which were performed according to the method of Smalla et al. (27). The recipients had a MIC of SCP of 0.5 μg ml−1. Transconjugants that had been involved in a transfer event were selected for on 50 μg ml−1 of rifampin (Sigma) and 8 μg ml−1 of SCP containing Iso-Sensitest agar plates for 48 h at 30°C. Colonies were screened by PCR to confirm sul gene transfer and disregard the possibility of spontaneous mutations. Transfer rates (transconjugants per donor) were calculated according to the method of Binh et al. (4), where the transfer event equaled the CFU ml−1 of transconjugants divided by the CFU ml−1 of recipients. This rate was then normalized by the number of positive colonies screened by PCR for the gene of interest. The limits on transfer frequencies were set by our ability to detect a single transconjugant cell but varied with experimental conditions, due to differences in the initial numbers of recipients. Control plates of donors only were included to investigate the rates of rifampin mutations; these plates were always negative.
MIC determination.
MICs and antibiotic resistance breakpoints were determined on Iso-Sensitest agar plates, using an agar dilution method (21). The inoculum (100 μl) was adjusted to an optical density at 600 nm of 0.4 for each isolate to ensure consistency in the determination of the MIC. The antibiotics (Sigma) tested were streptomycin (at a concentration of 16 μg ml−1), ampicillin (16 μg ml−1), kanamycin (16 μg ml−1), chloramphenicol (16 μg ml−1), tetracycline (8 μg ml−1), trimethoprim (16 μg ml−1), neomycin (8 μg ml−1), and nalidixic acid (16 μg ml−1). Breakpoints were selected on the basis of identifying mechanisms of resistance that were likely clinically relevant. MIC determinations for SCP were performed, using the following concentrations: 0, 1, 2.5, 5, 25, 50, and 100 μg ml−1.
Statistical analysis.
RQs and prevalences were compared, using a chi-square test for the comparison of two proportions (from independent samples). Statistical analyses were performed, using MedCalc for Windows, version 9.3.7.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS AND DISCUSSION
Antibiotic resistance.
RQs calculated from SCP plate counts are shown for each sample in Fig. 1. Using a chi-square test for the comparison of two proportions (from independent samples), RQs for culturable bacteria were compared among samples and at different time points. SCP resistance was significantly higher in pig slurry than in preapplication soil, with selections of 10, 25, and 50 μg ml−1 (P < 0.0001). Resistance was also significantly higher at day 1 in postapplication soils than in preapplication soils (P < 0.0001) and remained higher at day 289 (P < 0.0001). This was not the case in day 21 and day 90 samples, possibly due to a patchy distribution of slurry and uneven retention of antibiotic residues. Clay soils are characterized by a network of cracks and fissures that allow localized mobilization of rainfall, dissolved compounds, and suspended particles. The resistance observed at day 289 cannot be attributed to continued selective pressure exerted by SCP after slurry application, as SCP was quickly washed out of the soil due to its high mobility and low Kd (15), where Kd is the sorption coefficient (28). Thus, the persistence of the resistant phenotype is likely due to the survival of bacteria carrying resistance determinants or transfer of the resistance gene to indigenous bacteria rather than selective pressure exerted by SCP in the environment.
FIG. 1.
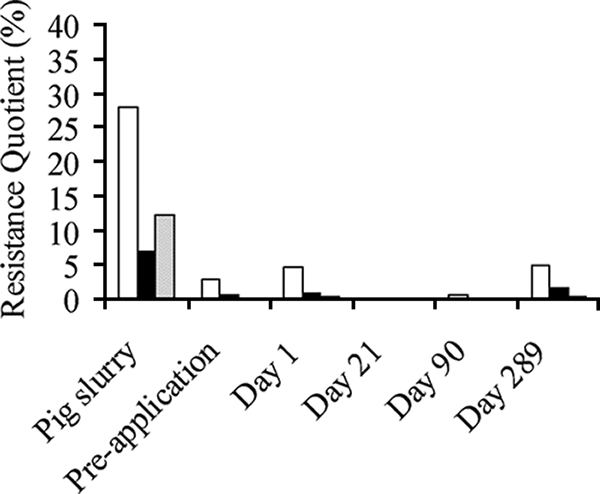
SCP RQ values for soil and slurry samples collected over year 1; 10 μg ml−1, white bars; 25 μg ml−1, black bars; 50 μg ml−1, gray bars.
sul gene prevalence.
All 531 bacterial isolates collected in this study were screened by PCR for sul1, sul2, and sul3. The most common genotypes were those of single genes; sul1 had the highest prevalence, followed by sul2 and subsequently by sul3 (Table 2). A total of 67% (n = 358) of the isolates collected were PCR positive for one or more sulfonamide resistance genes, and 17.5% (n = 93) of these carried combinations of the three genes, sul2 and sul3 being the most frequent. The genotypes of sul1, sul2, and sul3 occurred in 2.3% (n = 12) of isolates.
TABLE 2.
Summary of total numbers of isolates collected per sample during the study and number positive for each sul genotype
| Sample site and time of sampling | No. of isolates with indicated sul genotype(s):
|
No. of isolates analyzed from each sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| sul ve | sul1 | sul2 | sul3 | sul1 sul2 | sul1 sul3 | sul2 sul3 | sul1 sul2 sul3 | ||
| PS (amended) | 52 | 7 | 18 | 10 | 4 | 0 | 11 | 2 | 78 |
| PS (control) | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 18 |
| Preapp (soil) | 53 | 21 | 13 | 7 | 6 | 0 | 0 | 6 | 79 |
| 1,1 (soil) | 53 | 6 | 14 | 12 | 7 | 1 | 12 | 1 | 71 |
| 1,21 (soil) | 9 | 6 | 1 | 0 | 2 | 0 | 0 | 0 | 20 |
| 1,90 (soil) | 15 | 13 | 0 | 0 | 1 | 0 | 1 | 0 | 39 |
| 1, 289 (soil) | 23 | 6 | 8 | 6 | 3 | 0 | 0 | 0 | 41 |
| 2,1 (soil) | 37 | 6 | 21 | 3 | 3 | 0 | 4 | 0 | 45 |
| 2,21 (soil) | 35 | 13 | 7 | 6 | 3 | 1 | 3 | 2 | 39 |
| 2,90 (soil) | 36 | 17 | 4 | 1 | 6 | 0 | 7 | 1 | 41 |
| 2,240 (soil) | 19 | 16 | 1 | 1 | 0 | 0 | 1 | 0 | 29 |
| Soil leachate | 22 | 8 | 7 | 2 | 1 | 2 | 2 | 0 | 31 |
| Total | 358 | 120 | 95 | 50 | 36 | 4 | 41 | 12 | 531 |
a Sample sites were as follows: PS (amended), pig slurry amended with 25.58 mg liter−1 SCP and 18.85 mg liter−1 OTC; PS (control), unamended pig slurry; preapp (soil), soil cores from year 1 before slurry application; 1,1 (soil), soil cores from year 1, day 1 time point; 1,289 (soil), soil cores from year 1, day 289 time point; 2,1 (soil), soil cores from year 2, day 1 time point; soil leachate (three combined samples collected over the sample period from large rainfall events).
All genotypes were present in all samples, with the exception of the sul2 sul3 genotype, which appeared to originate from the amended slurry and was present only in isolates collected from amended soil in year 1 postapplication, appearing again in isolates from year 2 after a second slurry application. Preapplication soil cores displayed a high number of sul-containing isolates (Table 2), possibly because of repeated pig slurry applications over the previous decade. While these previous slurry applications were not known to have included sulfonamides, they may have been used therapeutically.
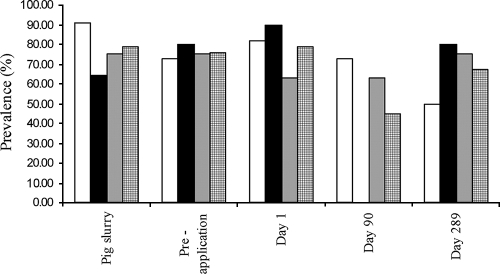
When sul gene prevalences were compared over time in a subset of the data that included bacteria isolated on the same selective media, there were no significant differences in prevalences when all sul genes were considered together (Fig. 2).
FIG. 2.
Prevalences of sul gene-bearing bacteria, isolated on TY (white bars), SCP (black bars), OTC (gray-stippled bars), and all selective plates (cross-hatched bars). For TY, there were 11 isolates at each time point, for SCP, 10 isolates, and for OTC, 8 isolates.
Characterization of isolates.
All sul-positive isolates were identified by 16S rRNA gene typing, and the presence of class 1 and 2 integrons was determined. Seventeen genera, including opportunistic pathogens and indigenous soil bacteria, were identified, as shown in Table 3. The most prevalent sul-positive species isolated in this study were Acinetobacter spp., which were collected from all soil/slurry samples. Acinetobacter spp. were reported to have developed resistance to a large number of antibiotic groups, including the sulfonamides, making them a serious problem in hospitals (5, 9, 31). A. baumannii and other species of Acinetobacter have previously been identified in diverse environments, now including an agricultural soil environment, a potential hot spot of gene acquisition from the vast gene pool found in soil and rhizosphere bacteria (16, 17, 19, 23). This is the first report of sul3 in Acinetobacter. Table 3 demonstrates that Acinetobacter spp. carrying sul genes were present in the soil for up to a year after slurry application, as were Arthrobacter, Bacillus, Carnobacterium, and Pseudomonas spp. Agrobacterium and Stenotrophomonas spp. carrying sul genes were detected at 90 days post-slurry application. In contrast, Aerococcus, Brevibacterium, Comamonas, Corynebacterium, Planococcus, Providencia, Psychrobacter, and Wiessella spp. were present in pig slurry or amended soil only immediately after slurry application; these bacteria are therefore more likely to enter the human population via the food chain than by environmental-transport routes. Enterococcus and Shigella spp. were found only in pig slurry and soil leachate samples, suggesting that they were quickly washed out of the soil into ground water and drainage systems by rain.
TABLE 3.
Summary of prevalences and total numbers of sul-positive bacterial species isolated, their sul genotypes, and samples from which they were collected
| Genusa | No. (percentage) of sul-positive isolates | Source of isolateb | sul genotype | No. of sul-positive isolates |
|---|---|---|---|---|
| Acinetobacter | 127 (35.7) | PS, PSC, all soil samples, SL | sul1 | 20 |
| sul2 | 30 | |||
| sul3 | 31 | |||
| sul1 sul2 | 13 | |||
| sul1 sul3 | 3 | |||
| sul2 sul3 | 21 | |||
| sul1 sul2 sul3 | 7 | |||
| Aerococcus | 10 (2.8) | PS; 1,1 | sul1 | 2 |
| sul2 | 8 | |||
| Agrobacterium | 2 (0.6) | 2,90 | sul2 sul3 | 2 |
| Arthrobacter | 16 (4.5) | PSC; PS; 1P; 1,90; 1,289; 2,21 | sul1 | 6 |
| sul2 | 4 | |||
| sul3 | 1 | |||
| sul1 sul2 | 5 | |||
| Bacillus | 29 (8.2) | PSC; 1P; 1,289; 2,21; 2,240 | sul1 | 7 |
| sul2 | 8 | |||
| sul3 | 9 | |||
| sul1 sul2 | 1 | |||
| sul2 sul3 | 3 | |||
| sul1 sul2 sul3 | 1 | |||
| Brevibacterium | 1 (0.3) | PS, PSC | sul2 sul3 | 1 |
| Carnobacterium | 3 (0.8) | 1,289 | sul1 | 3 |
| Comamonas | 3 (0.8) | 2,1 | sul2 | 3 |
| Corynebacterium | 3 (0.8) | 2,1 | sul2 | 3 |
| Enterococcus | 10 (2.8) | PS, PSC | sul2 | 4 |
| sul3 | 3 | |||
| sul2 sul3 | 3 | |||
| Planococcus | 1 (0.3) | PSC | sul1 sul2 | 1 |
| Providencia | 3 (0.8) | 2,1 | sul3 | 3 |
| Pseudomonas | 88 (24.7) | PS; all soil samples; 1P; 1,1; 1,21; 2,90 | sul1 | 64 |
| sul2 | 12 | |||
| sul3 | 3 | |||
| sul1 sul2 | 5 | |||
| sul2 sul3 | 4 | |||
| Psychrobacter | 51 (14.3) | PS; 1P; 1,1 | sul1 | 13 |
| sul2 | 18 | |||
| sul1 sul2 | 10 | |||
| sul1 sul3 | 1 | |||
| sul2 sul3 | 5 | |||
| sul1 sul2 sul3 | 4 | |||
| Shigella | 3 (0.8) | SL | sul2 | 3 |
| Stenotrophomonas | 5 (1.4) | 2,21; 2,90 | sul1 | 1 |
| sul2 | 1 | |||
| sul3 | 1 | |||
| sul2 sul3 | 2 | |||
| Weissella | 3 (0.8) | PSC | sul1 sul2 | 3 |
As identified by 16S DNA.
Sample sites were as follows: pig slurry amended with 25.58 mg liter−1 SCP and 18.85 mg liter−1 OTC (PS); unamended pig slurry (PSC); soil cores from year 1 before slurry application (1P); soil cores from year 1, day 1 time point (1,1); soil cores from year 1, day 289 time point (1,289); soil cores from year 2, day 1 time point (2,1); soil leachate (SL; three combined samples collected over the sample period from large rainfall events).
Table 4 displays a number of representative isolates from the main genotypes and all isolates containing the three sul genes. A surviving enteric isolate and human pathogen, identified as Shigella flexneri (C506), with 96% similarity, was isolated from a soil leachate sample and contained sul2 and intI1 with a multiple-resistance phenotype. The sul2 and sul3 genotype was found in Acinetobacter lwoffii, Enterococcus sulfureus, and Aerococcus viridans isolates, all pig-associated pathogens/commensals entering the soil through the slurry applications. Isolates PGS21 and PGS22 from the antibiotic-amended slurry were both identified as Aerococcus viridians with 99% nucleotide similarity (16S rRNA gene, 800 bp), and a number of Psychrobacter spp. were also identified (Table 3). The Psychrobacter sp. isolates were resistant to tetracycline, streptomycin, chloramphenicol, trimethoprim, and nalidixic acid, with calculated SCP MICs of between 5 and 16 mg liter−1. The isolation of Psychrobacter spp. was unusual, as they are commonly isolated from cold marine environments and sediments (6, 7, 24). BLAST analysis (1) of the newly sequenced Psychrobacter genomes (www.jgi.doe.gov) did not reveal any sulfonamide resistance genes.
TABLE 4.
Characterization of a number of cultured bacterial isolates encoding different sul genotypes
| Isolate | Bacterial sourcea | 16S rRNA gene identification | % BLASTb similarity | sul gene | Class of integronc | Antibiotic resistance phenotype of isolatese | MIC of SCP (mg liter−1) |
|---|---|---|---|---|---|---|---|
| C237 | 1, Preapp (soil) | Bacillus sphaericus | 98 | 1 | Smr Tmpr Nalr | 1 | |
| C422 | 2,21 (soil) | Stenotrophomonas maltophilia | 98 | 1 | 2 | Smr | 1 |
| C131 | 2,240 (soil) | Pseudomonas fluorescens | 97 | 1 | Tetr | 32 | |
| C3 | Pig slurry | Acinetobacter lwoffi | 99 | 1 | 2 | NG | 1 |
| PGS22 | Pig slurry | Aerococcus viridians | 99 | 2 | 1d | Smr Cmr Tetr Nmr Nalr | 8 |
| C506 | Soil leachate | Shigella flexneri | 99 | 2 | 1d | Smr Cmr Tetr Tmpr Nmr Nalr Kmr Ampr | 8 |
| C701 | 1, Preapp (soil) | Pseudomonas lini | 99 | 2 | 2 | Smr Ampr Tmpr Nalr | 1 |
| C439 | 2,90 (Soil) | Acinetobacter sp. N2 | 97 | 2 | 2 | Smr Tetr Tmpr Nalr | 16 |
| C5 | Pig slurry | Pseudomonas borealis | 99 | 3 | 2 | Cmr Tetr | 32 |
| PGS48 | Pig slurry | Enterococcus hirae | 97 | 3 | Smr Tetr | 4 | |
| C167 | 2,1 (soil) | Providencia stuart2 | 97 | 3 | Smr Tetr | 6 | |
| C2 | Pig slurry | Acinetobacter lwoffi | 97 | 3 | Smr Cmr Tetr Tmpr Nalr | 5 | |
| C231 | 1,21 (soil) | Pseudomonas putida | 97 | 1 + 2 | 1d | Ampr Cmr Tetr Nmr Nalr | 8 |
| C361 | 1,289 (soil) | Arthrobacter arilaitensis | 99 | 1 + 2 | 1d | Smr Cmr Tetr Nmr Nalr | 5 |
| PGS49 | Pig slurry | Acinetobacter sp. An9 | 99 | 1 + 2 | 2 | Smr Nalr | 16 |
| C410 | 1,1 (soil) | Psychrobacter ikaite | 98 | 1 + 2 | 2 | Tetr | 16 |
| PGS47 | Pig slurry | Acinetobacter lwoffi | 97 | 2 + 3 | 2 | Smr Nalr | 16 |
| PGS61 | Pig slurry | Enterococcus sulfureus | 97 | 2 + 3 | Smr Tetr | 4 | |
| PGS21 | Pig slurry | Aerococcus viridans | 99 | 2 + 3 | 2 | Cmr Tetr | 32 |
| C15 | Pig slurry | Acinetobacter lwoffi | 99 | 1 + 2 + 3 | 2 | Smr Cmr Tetr Tmpr Nmr Nalr | 5 |
| C20 | Pig slurry | Psychrobacter ikaite | 98 | 1 + 2 + 3 | Smr Cmr Tetr Tmpr Nalr | 5 | |
| C711 | 1, Preapp (soil) | Psychrobacter sp. DY9-2 | 97 | 1 + 2 + 3 | Smr Tetr Tmpr Nalr | 8 | |
| C712 | 1, Preapp (soil) | Psychrobacter frigidicola | 96 | 1 + 2 + 3 | Smr Tetr Tmpr Nalr | 8 | |
| C713 | 1, Preapp (soil) | Psychrobacter ikaite | 97 | 1 + 2 + 3 | 2 | Smr Cmr Tetr Tmpr Nalr | 8 |
| C35 | 1, Preapp (soil) | Acinetobacter calcoaceticus | 97 | 1 + 2 + 3 | Smr Cmr Tetr Tmpr Nalr | 8 | |
| C36 | 1, Preapp (soil) | Acinetobacter calcoaceticus | 97 | 1 + 2 + 3 | Smr Cmr Tetr Tmpr Nalr | 8 | |
| C37 | 1, Preapp (soil) | Acinetobacter lwoffi | 97 | 1 + 2 + 3 | Smr Cmr Tetr Tmpr Nalr | 8 | |
| C44 | 1,1 (soil) | Acinetobacter rhizosphaerae | 98 | 1 + 2 + 3 | Smr Cmr Tetr Tmpr Nmr Nalr | 5 | |
| C141 | 2,21 (soil) | Acinetobacter lwoffi | 98 | 1 + 2 + 3 | Smr Kmr Cmr Tetr TmprNmr Nalr Ampr | 6 | |
| C328 | 2,21 (soil) | Bacillus psychrodurans | 98 | 1 + 2 + 3 | Smr Kmr Cmr Tetr Nmr Nalr | 8 | |
| C442 | 2,90 (soil) | Acinetobacter baumannii | 99 | 1 + 2 + 3 | Cmr Tetr Nalr | 8 |
1, Preapp (soil), soil cores from year 1 before slurry application; 1,1, soil cores from year 1, day 1 time point; 1,289, soil cores from year 1, day 289 time point; 2,1, soil cores from year 2, day 1 time point.
Percent nucleotide similarity of an approximately 800-bp 16S rRNA gene sequence to bacterial strains submitted to databases and searched using the BLAST program (1).
qacEΔ1/qacE was screened for in all intI1-positive isolates.
qacEΔ1 detected.
Antibiotic resistance breakpoints: Smr, resistance to 16 μg ml−1 streptomycin; Ampr, 16 μg ml−1 ampicillin; Kmr, 16 μg ml−1 kanamycin; Cmr, 16 μg ml−1 chloramphenicol; Tetr, 8 μg ml−1 tetracycline; Tmpr, 16 μg ml−1, trimethoprim; Nmr, 8 μg ml−1 neomycin; Nalr, 16 μg ml−1, nalidixic acid. NG, no growth.
Isolate C361, identified with 99% nucleotide similarity to the 16S rRNA gene sequence of Arthrobacter arilaitensis, was resistant to five antibiotics, including streptomycin, chloramphenicol, tetracycline, neomycin, and nalidixic acid, as well as to a low MIC of SCP of 5 mg l−1.
Class 1 and class 2 integron carriage.
Screening of the sul-positive isolates (n = 358) revealed that 5.0% (n = 18) carried class 1 integrons and 11.7% (n = 42) carried class 2 integrons (positive for the intI1 and intI2 genes, respectively). Of 173 sul-negative isolates, 8.7% (n = 15) carried intI1 genes, and 5.2% (n = 9) carried intI2 genes. There was no significant difference in intI1 prevalences between sul-positive and sul-negative isolates (chi-square test, 0.5; P = 0.5), whereas the prevalence of intI2 was significantly higher in sul-positive isolates (chi-square test, 57.6; P < 0.0001). Given the association of sul1 with class 1 integrons and the fact that there was no known link between class 2 integrons and sul gene carriage, the observed prevalences of intI1 and intI2 in sul-positive and -negative populations were surprising. Only 8.1% (n = 11) of sul1-positive isolates carried the intI1 gene, whereas 9.4% (n = 12) were observed to carry the intI2 gene (data not shown). Interestingly, no isolates were positive for both sul3 and intI1 (data not shown). Of the sul2 isolates, 17.9% (n = 18) and 1.1% (n = 2), respectively, carried an intI2 or intI1 gene (data not shown). A low frequency of sul1-positive isolates contained intI1, despite sul1 having only been found adjacent to qacEΔ1 in the 3′ conserved region of class 1 integrons (3). This finding indicates that sul1 is likely to be situated on non-class 1 integron mobile elements in most sul1-positive isolates identified in this study. Only one other published investigation has reported the prevalence of class 1 integrons in sulfonamide-resistant isolates from the environment, but it involved a brief temporal study in a different soil type (13).
Isolates carrying the three known sul genes.
Twelve isolates positive for the three sul genes were identified by 16S rRNA gene typing as members of the genera Psychrobacter, Acinetobacter, and Bacillus (Table 4). Of these, 10 isolates were cultured from agricultural soils which had undergone long-term applications of slurry from TY-fed pigs. Two isolates, Acinetobacter lwoffii (C15) and Psychrobacter ikaite (C20), were recovered from the antibiotic-amended slurry. The 12 isolates were negative for intI1, but one, Psychrobacter ikaite (C713), contained intI2. The 12 isolates displayed phenotypes with multiple-antibiotic resistance, with resistance to between three and eight antibiotics, including nalidixic acid, tetracycline, trimethoprim, and neomycin (Table 4). MIC tests indicated that the presence of the three sulfonamide resistance genes conferred only a low level of resistance to SCP of between 5 to 8 mg liter−1.
Conjugal transfers.
Conjugal transfers were performed with 11 of the 12 strains that contained, simultaneously, sul1, sul2, and sul3 and had P. putida or E. coli recipients (isolate C36 failed to grow). The transfer rates of these three genes are shown in Table 5. It was observed that in most isolates, sul1 and sul2 were transferred at different rates, indicating their presence on different mobile elements. The exception to separate transfers of sul1 and sul2 was an Acinetobacter sp. (C141) from which both genes transferred at a frequency of 3.44 × 10−3 transconjugants per donor cell into P. putida recipients, an equal transfer rate suggesting that the two genes are physically linked. sul3 was not observed to transfer into any of the recipients used in this study. In all cases where genes transferred into P. putida, transfer also took place into E. coli, but at a lower frequency. The absence of transfer in a number of isolates may have been due to the carriage of sul genes on nonconjugative plasmids or on the chromosome, whereas in the Bacillus sp. (C328), the failure to transfer may have been due to the presence of a gram-positive specific mobile element.
TABLE 5.
Conjugal transfer rates of the sul1, sul2, and sul3 genes from the 12 bacterial host isolates carrying the three known sul genes into either an E. coli K-12 CV601 or P. putida UWC1 recipient
| Donor isolatea | Transfer rates of indicated sul genes for recipients E. coli and P. putidac:
|
|||||
|---|---|---|---|---|---|---|
|
sul1
|
sul2
|
sul3
|
||||
| E. coli | P. putida | E.coli | P. putida | E. coli | P. putida | |
| Pseudomonas sp. DY9-2 (C711) | 4.3 × 10−4 | 7.3 × 10−3 | ND | ND | ND | ND |
| P. frigidicola (C712) | ND | ND | ND | ND | ND | ND |
| P. ikaiteb (C713) | 6.5 × 10−5 | 1.9 × 10−3 | ND | ND | ND | ND |
| A. lwoffi (C15) | 2.6 × 10−4 | 7.5 × 10−3 | 7.2 × 10−5 | 2.5 × 10−3 | ND | ND |
| P. ikaite (C20) | ND | ND | ND | ND | ND | ND |
| A. calcoaceticus (C35) | 9.5 × 10−4 | 1.1 × 10−3 | 7.5 × 10−4 | 1.9 × 10−3 | ND | ND |
| A. lwoffi (C37) | ND | ND | ND | ND | ND | ND |
| A. rhizosphaera (C44) | 9.0 × 10−4 | 2.4 × 10−2 | 2.5 × 10−4 | 7.1 × 10−2 | ND | ND |
| A. lwoffi (C141) | 4.2 × 10−4 | 3.4 × 10−3 | 4.2 × 10−4 | 3.4 × 10−3 | ND | ND |
| B. psychrodurans (C328) | ND | ND | ND | ND | ND | ND |
| A. baumannii (C442) | 3.5 × 10−4 | 1.7 × 10−3 | ND | ND | ND | ND |
Isolate A. calcoaceticus (C36) failed to grow when tested for transfer.
This isolate carried an intI2 gene.
Number of transconjugants per donor. ND, not detected.
Acknowledgments
We thank K. Smalla (BBA, Braunschweig, Germany) for the exogenous strains, V. Enne (Bristol, United Kingdom) for the sul2 primers and sul2 gene-containing vector, and V. Perreten (University of Bern, Switzerland) for the sul3 gene-containing vector.
This work was funded in part by an educational grant from Wyeth Pharmaceutical Company, a BBSRC CASE studentship, and NERC grant NER/A/S/2000/01253.
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Antunes, P., J. Machado, J. C. Sousa, and L. Peixe. 2005. Dissemination of sulfonamide resistance genes (sul1, sul2, and sul3) in Portuguese Salmonella enterica strains and relation with integrons. Antimicrob. Agents Chemother. 49:836-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, P. M. 1999. Integrons and gene cassettes: a genetic construction kit for bacteria. J. Antimicrob. Chemother. 43:1-4. [PubMed] [Google Scholar]
- 4.Binh, C. T., H. Heuer, M. Kaupenjohann, and K. Smalla. 2008. Piggery manure used for soil fertilization is a reservoir for transferable antibiotic resistance plasmids. FEMS Microbiol. Ecol. 66:25-37. [DOI] [PubMed] [Google Scholar]
- 5.Böerlin, P., S. Eugster, F. Gaschen, R. Straub, and P. Schawalder. 2001. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet. Microbiol. 82:347-359. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, J. P., S. A. McCammon, M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman, J. P., J. Cavanagh, J. J. Austin, and K. Sanderson. 1996. Novel Psychrobacter species from Antarctic ornithogenic soils. Int. J. Syst. Bacteriol. 46:841-848. [DOI] [PubMed] [Google Scholar]
- 8.Boxall, A. B., P. Blackwell, R. Cavallo, P. Kay, and J. Tolls. 2002. The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol. Lett. 131:19-28. [DOI] [PubMed] [Google Scholar]
- 9.Chastre, J. 2003. Infections due to Acinetobacter baumannii in the ICU. Semin. Respir. Crit. Care Med. 24:69-78. [DOI] [PubMed] [Google Scholar]
- 10.De Liguoro, M., V. Cibin, F. Capolongo, B. Halling-Sørensen, and C. Montesissa. 2003. Use of oxytetracycline and tylosin in intensive calf farming: evaluation of transfer to manure and soil. Chemosphere 52:203-212. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Böttger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra, B., E. Junker, A. Schröeter, B. Malorny, S. Lehmann, and R. Helmuth. 2003. Phenotypic and genotypic characterization of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J. Antimicrob. Chemother. 52:489-492. [DOI] [PubMed] [Google Scholar]
- 13.Heuer, H., and K. Smalla. 2007. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 9:657. [DOI] [PubMed] [Google Scholar]
- 14.Kazama, H., H. Hamashima, M. Sasatsu, and T. Arai. 1998. Distribution of the antiseptic-resistance gene qacEΔ1 in gram-positive bacteria. FEMS Microbiol. Lett. 165:295-299. [DOI] [PubMed] [Google Scholar]
- 15.Loke, M. L., J. Tjornelund, and B. Halling-Sorensen. 2002. Determination of the distribution coefficient (logKd) of oxytetracycline, tylosin A, olaquindox and metronidazole in manure. Chemosphere 48:351-361. [DOI] [PubMed] [Google Scholar]
- 16.Menezes Bento, F., F. A. de Oliveira Camargo, B. C. Okeke, and W. T. Frankenberger, Jr. 2005. Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microb. Res. 160:. [DOI] [PubMed]
- 17.Messi, P., E. Guerrieri, and M. Bondi. 2005. Antibiotic resistance and antibacterial activity in heterotrophic bacteria of mineral water origin. Sci. Total Environ. 346:213-219. [DOI] [PubMed] [Google Scholar]
- 18.Perreten, V., and P. Böerlin. 2003. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 47:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen, A., L. Guardabassi, A. Dalsgäard, and J. E. Olsen. 2000. Class I integrons containing a dhfrI trimethoprim resistance gene cassette in aquatic Acinetobacter spp. FEMS Microbiol. Lett. 182:73-76. [DOI] [PubMed] [Google Scholar]
- 20.Rådström, P., and G. Swedberg. 1988. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob. Agents Chemother. 32:1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds, R., J. Shackcloth, D. Felmingham, and A. MacGowan. 2003. Antimicrobial susceptibility of lower respiratory tract pathogens in Great Britain and Ireland 1999-2001 related to demographic and geographical factors: the BSAC Respiratory Resistance Surveillance Programme. J. Antimicrob. Chemother. 52:931-943. [DOI] [PubMed] [Google Scholar]
- 22.Rosser, S. J., and H. K. Young. 1999. Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J. Antimicrob. Chemother. 44:11-18. [DOI] [PubMed] [Google Scholar]
- 23.Sarma, P. M., D. Bhattacharya, S. Krishnan, and B. Lal. 2004. Assessment of intra-species diversity among strains of Acinetobacter baumannii isolated from sites contaminated with petroleum hydrocarbons. Can. J. Microbiol. 50:405-414. [DOI] [PubMed] [Google Scholar]
- 24.Shivaji, S., G. S. Reddy, K. Suresh, P. Gupta, S. Chintalapati, P. Schumann, E. Stackebrandt, and G. I. Matsumoto. 2005. Psychrobacter vallis sp. nov. and Psychrobacter aquaticus sp. nov., from Antarctica. Int. J. Syst. Evol. Microbiol. 55:757-762. [DOI] [PubMed] [Google Scholar]
- 25.Sköld, O. 1976. R-factor-mediated resistance to sulfonamides by a plasmid-borne, drug-resistant dihydropteroate synthase. Antimicrob. Agents Chemother. 9:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sköld, O. 2000. Sulfonamide resistance: mechanisms and trends. Drug Resist. Updates 3:155-160. [DOI] [PubMed] [Google Scholar]
- 27.Smalla, K., H. Heuer, A. Götz, D. Niemeyer, E. Krogerrecklenfort, and E. Tietze. 2000. Exogenous isolation of antibiotic resistance plasmids from piggery manure slurries reveals a high prevalence and diversity of IncQ-like plasmids. Appl. Environ. Microbiol. 66:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuer-Lauridsen, F., M. Birkved, L. P. Hansen, H. C. Lutzhoft, and B. Halling-Sorensen. 2000. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 40:783-793. [DOI] [PubMed] [Google Scholar]
- 29.Sundström, L., P. Rädström, G. Swedberg, and O. Sköld. 1988. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol. Gen. Genet. 213:191-201. [DOI] [PubMed] [Google Scholar]
- 30.Swedberg, G., C. Fermer, and O. Sköld. 1993. Point mutations in the dihydropteroate synthase gene causing sulfonamide resistance. Adv. Exp. Med. Biol. 338:555-558. [DOI] [PubMed] [Google Scholar]
- 31.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]
- 32.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]