Abstract
We evaluated the pharmacokinetics (PK) and pharmacodynamics (PD) of posaconazole (POS) in a prospective, open-label study. Twenty-five healthy adults received 14 doses of POS oral suspension (400 mg twice daily) with a high-fat meal over 8 days. Pulmonary epithelial lining fluid (ELF) and alveolar cell (AC) samples were obtained via bronchoalveolar lavage, and blood samples were collected during the 24 h after the last dose. POS concentrations were determined using liquid chromatography with tandem mass spectrometry parameters. The maximum concentrations (Cmax) (mean ± standard deviation) in plasma, ELF, and ACs were 2.08 ± 0.93, 1.86 ± 1.30, and 87.7 ± 65.0 μg/ml. The POS concentrations in plasma, ELF, and ACs did not decrease significantly, indicating slow elimination after multiple dosing. The mean concentrations of POS in plasma, ELF, and ACs were above the MIC90 (0.5 μg/ml) for Aspergillus spp. over the 12-h dosing interval and for 24 h following the last dose. Area under the curve from 0 to 12 h (AUC0-12) ratios for ELF/plasma and AC/plasma were 0.84 and 33. AUC0-24/MIC90 ratios in plasma, ELF, and AC were 87.6, 73.2, and 2,860. Nine (36%) of 25 subjects had treatment-related adverse events during the course of the study, which were all mild or moderate. We conclude that a dose of 400 mg twice daily resulted in sustained plasma, ELF, and AC concentrations above the MIC90 for Aspergillus spp. during the dosing interval. The intrapulmonary PK/PD of POS are favorable for treatment or prevention of aspergillosis, and oral POS was well tolerated in healthy adults.
Posaconazole (POS) is a triazole antifungal agent with broad-spectrum activity against Aspergillus, Cryptococcus, Candida, Histoplasma, and Blastomyces spp. and others (12, 16, 17, 19, 22, 23). The use of POS has been approved for prophylaxis of invasive aspergillosis and candidiasis in immunocompromised patients and the treatment of refractory oropharyngeal candidiasis in the United States and is undergoing clinical development for the treatment of pulmonary and disseminated mycoses.
The pharmacokinetics (PK) of POS have been extensively studied. The oral bioavailability of POS is increased significantly by divided dosing and administration with a high-fat meal (11, 13). POS is metabolized in the liver to glucuronides that are microbiologically inactive; however, POS does not have any major circulating cytochrome 450-mediated metabolites. POS is excreted primarily unchanged in feces (14). A high apparent volume of distribution of 1,774 liters suggests that POS is widely distributed in body tissues (G. Krishna and A. Sansone-Parsons, presented at the 41st American Society of Health-System Pharmacists Midyear Clinical Meeting and Exhibition, Anaheim, CA, 3 to 7 December 2006). It has a long half-life of approximately 35 h at steady state (G. Krishna and A. Sansone-Parsons, presented at the 41st American Society of Health-System Pharmacists Midyear Clinical Meeting and Exhibition, Anaheim, CA, 3 to 7 December 2006). Mild to moderate renal impairment has no significant effect on the PK parameters, and dose correction is not required for patients with renal impairment (10). Protein binding in humans is >98%. POS exhibits dose-proportional kinetics up to a daily dose of 800 mg. Increasing the dose of POS to 1,200 mg per day resulted in no further increase in the concentrations in plasma; therefore, absorption is saturated at a daily dose of 800 mg (9). Although POS is approved in the European Union and is in clinical development in the United States for the treatment of pulmonary aspergillosis, the intrapulmonary PK and pharmacodynamics (PD) of POS have not been reported.
MATERIALS AND METHODS
Study design and subjects.
This was a randomized, open-label study to measure the steady-state plasma, epithelial lining fluid (ELF), and pulmonary alveolar cell (AC) concentrations of POS. The participation of subjects was voluntary, and willingness to comply with study procedures was expressed through written informed consent. The study was conducted in the General Clinical Research Center (GCRC) at the University of California, San Francisco. Subjects were randomized to five groups of five subjects each, with bronchoscopy times at 3, 5, 8, 12, and 24 h after the final dose. The 24-h sampling period was selected to evaluate the possibility of an extended intrapulmonary half-life. Volunteers received a total of 14 doses of POS, administered as an oral suspension of 400 mg every 12 h for 8 days.
Subjects were required to be 21 to 55 years of age, to have a body mass index of 18 to 29 kg/m2, to be in good health based on a normal screening evaluation, and to be using contraception. The screening evaluation included a medical history and physical examination, electrocardiography, and clinical laboratory tests (complete blood count with differential, platelet count, blood urea nitrogen, serum creatinine, alkaline phosphatase, total bilirubin, albumin, aspartate aminotransferase, alanine aminotransferase, a serum pregnancy test for women of childbearing age, and urinalysis, including microscopy and screening for abuse of drugs). Subjects were excluded if they were taking any medication other than vitamins and hormonal contraceptives; had a significantly abnormal electrocardiogram (ECG); had laboratory test results significantly out of the normal range; had a positive result for drug screening or a history of substance abuse or smoking within 1 year before study enrollment; had a positive test result for human immunodeficiency virus, hepatitis C antibody, or hepatitis B surface antigen; had undergone major surgery within the 6 months preceding study enrollment; had a positive pregnancy test result or planned to be pregnant or breastfeeding within 30 days after the study; had an allergy or sensitivity to POS or other azole or triazole drugs or to lidocaine; or were participating in another clinical study or used an investigational product or drug in the 30 days preceding enrollment.
Enrolled subjects were seen for a second visit at the GCRC no more than 28 days after the screening visit. At the second visit, the subjects received their first dose of study drug, were observed for 30 min for adverse effects (AEs), and were given written instructions to take the drug after a high-fat meal every 12 h for the following 6 days. The subjects were also given a study diary to record, twice daily, the time at which they completed the high-fat meal, the time that the dose of POS was taken, and symptoms that were experienced after taking the study drug. The diary was collected and reviewed at the third visit. At visit 3, on the eighth day, the subjects received their last dose of study medication in the GCRC, the laboratory tests and electrocardiography were repeated, and blood samples were drawn for each subject at 0, 3, 5, 8, 12, and 24 h after the final dose. Each subject underwent one bronchoscopy after the last dose at a time determined by the randomization schedule. The subjects were then given a form with instructions that included body temperature monitoring and symptom reporting every 4 h for 24 h after bronchoalveolar lavage (BAL). This form was returned to the investigators by mail.
Bronchoscopy and BAL.
Standardized bronchoscopy and BAL (4-7) were performed in the GCRC at 3, 5, 8, 12, or 24 h after the administration of the last dose of POS. Topical anesthesia with lidocaine was used. As we previously reported, systemic sedation was not administered (4-7). A fiber-optic bronchoscope (FB-18BS; Pentax, Montvale, NJ) was inserted into the right middle lobe. Four 50-ml aliquots of sterile 0.9% saline were infused, and each aliquot was immediately aspirated into a trap. The average duration of bronchoscopy was approximately 4 min. The first aspirate was discarded. The aspirates from the second, third, and fourth instillations were pooled and iced. The volume of the BAL fluid was measured and recorded. A measured volume (30 ml) of the BAL fluid was immediately spun in a polypropylene tube at 400 × g for 5 min in a refrigerated (4°C) centrifuge (4K15; Sigma). The supernatants and the cell pellet were separated into two equal samples and frozen at −70°C until they were assayed. A small aliquot of the supernatant was frozen separately for urea assay.
Blood samples.
Blood was obtained for drug assay before administration of the first dose and the last dose and at the completion of BAL. Blood samples (10 ml each) were collected into EDTA salt-containing tubes and placed in ice until centrifugation. The tubes were then spun at 1,300 × g for 10 min in a Sigma 4K15 refrigerated centrifuge (4°C). The plasma was separated and frozen at at least −20°C until it was assayed.
POS assay.
The POS concentrations in plasma, BAL fluid, and ACs were assayed using sensitive and specific validated liquid chromatography with tandem mass spectrometry. Lung aspirates and cell pellets were quantitated against a human plasma calibration curve. These samples were analyzed using the human plasma method, but the extraction was different. A 100-μl sample of lung aspirate fluid was divided into aliquot portions in a separate tube. To each aliquot, 67 μl methanol was added and mixed, and a 100-μl aliquot was taken for extraction. A dilution factor of 1.67 was applied to the results. To analyze the cell pellets, 200 μl methanol was added directly to the sample tubes. The samples were vortexed, subjected to sonication for 10 min, and transferred to a centrifuge snap-cap tube. The samples were centrifuged at 13,860 × g (estimated) for 10 min. The liquid layer of the sample was then transferred to a 96-well block, and 50 μl reagent water was added to each sample before injection. The samples were analyzed using a liquid chromatography with tandem mass spectrometry method at PPD Inc. (Richmond, VA). The lower limit of quantitation for this assay was 1.00 ng/ml, and the calibration range was 1.00 to 4,000 ng/ml. For calibration standards, the accuracy (percent bias) ranged from −7.02% to 5.93%, and the precision (percentage coefficient of variation) ranged from 2.51% to 12.0%. For quality control runs (five levels: 3.00, 150, 750, 1,500, and 3,000 ng/ml), the accuracy ranged from −4.17% to 12.8% and the precision ranged from 2.29% to 14.8%.
The percentage of unbound drug in plasma was calculated from the following formula: unbound fraction = 0.015 × total concentration. Unbound-drug concentrations in ELF and ACs were not calculated because the extent of protein binding in these compartments is unknown.
The BAL supernatant was assayed for the urea concentration using a modified enzymatic assay (Infinity BUN reagent, enzymatic determination no. 63-UV; Sigma Diagnostics, St. Louis, MO). The assay was linear throughout the range of urea concentrations present in the BAL samples (data not shown).
The volume of ELF in BAL fluid, the concentration of antibiotics in the ELF, and the concentration of antibiotics in ACs were derived using methods and calculations that we have previously reported (4-7).
PK and PD analyses.
The PK and PD analyses were performed using the Pharsight knowledge base server version 2.0.1 with WinNonlin version 4.0.1 (Pharsight Corporation, Cary, NC). The plasma concentration-time data set for each individual was used to calculate the maximum observed concentration (Cmax), the time to the maximum concentration (Tmax), the area under the concentration-time curve over the dosing interval (0 to 12 h) (AUC0-12), the trough concentration immediately before the last dose (Cmin), and the terminal-phase half-life (t1/2). For ELF and AC, the individual subject concentrations at each collection time point were averaged, and the mean data were used to calculate the Cmax, Tmax, AUC, Cmin, and t1/2.
The analysis was performed using a model-independent method. For each subject, the terminal-phase rate constant (k) was calculated as the negative of the slope of the log-linear terminal portion of the plasma concentration-time curve using linear regression. The AUC0-∞ was calculated using the linear trapezoidal method and was extrapolated to infinity. The t1/2 was calculated from 0.693/k, and the value was not reported because the extrapolated portion of the AUC was greater than 25% of the AUC0-∞.
The Cmax, AUC0-12, and concentration-time data were used to calculate the PD parameters: Cmax/MIC90 ratio, AUC0-12/MIC90 ratio, and time above MIC90 (T > MIC90) in plasma, ELF, and ACs. The PD parameters in plasma were derived from the free (i.e., unbound) drug concentrations, as well as from total (i.e., unbound plus bound) drug concentrations. The MIC90 values for Aspergillus spp. were obtained from the recent literature (19).
RESULTS
Twenty-five subjects were enrolled in the study. The mean age (± standard deviation [SD]) of the 25 subjects was 30.4 ± 7.9 years; 15 were men, and 10 were women; and 17 were white, 4 were Asian, 5 were Hispanic, 2 were multiracial, and 1 was African American. The mean weight (± SD) of the subjects was 66.11 kg ± 10.0 kg.
Of the 25 subjects enrolled in the study, 21 completed BAL. Twenty of the subjects who completed BAL received 14 doses, and one received 13 doses. Of the four subjects who did not complete the study, two withdrew for reasons unrelated to the study drug and two were withdrawn from the study because of AEs possibly related to the study drug. This resulted in the following BAL schedule: three subjects 3 h after the last dose, four subjects at 5 h, four subjects at 8 h, five subjects at 12 h, and five subjects at 24 h.
The 14 doses of oral POS (400 mg) taken every 12 h over 8 days were safe and well tolerated. By protocol-set criteria, nine (36%) of 25 subjects had treatment-related AEs during the course of the study, which were all mild or moderate. Three subjects experienced nausea associated with the study drug, three subjects experienced fatigue, two subjects experienced headache, and two subjects reported diarrhea. The study drug had no clinically relevant effect on blood chemistry, hematology, urinalysis, vital signs, or ECGs, including corrected QT intervals.
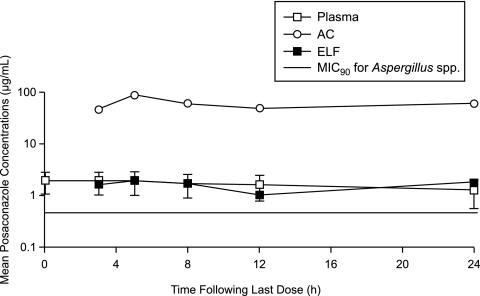
The recovery of ACs from the BAL fluid in the different time groups ranged from 7.1 × 107 ± 3.1 × 107 to 1.2 × 107 ± 5.5 × 107. The volume of ELF recovered ranged from 0.8 ± 0.1 to 1.0 ± 0.3 ml. None of the differences between groups in cell recovery or ELF volume were statistically significant (data not shown). The concentrations of POS in plasma, ACs, and ELF and the AC/plasma and ELF/plasma ratios at the time of BAL are summarized in Table 1. The ELF/plasma and AC/plasma ratios ranged from 0.589 to 1.08 and 27.3 to 44.3, respectively. The T > MIC90 (mean ± SD) in plasma was 22.3 ± 5.34 h. In 2 of the 21 subjects, individual plasma concentrations were not greater than the MIC90 for Aspergillus spp. (Fig. 1). However, the concentration of POS in ACs in these subjects was substantially greater (17- and 25-fold, respectively) than the MIC90. The T > MIC90 values in ELF and ACs were 24 h and 24 h, respectively. The free Cmax/MIC90 and total Cmax/MIC90 ratios in plasma were 0.06 and 4.15, respectively.
TABLE 1.
POS concentrations in plasma, ACs, and ELF at the time of BAL
| Time of BAL (h) | n | Plasma concna (μg/ml) | AC concnb (μg/ml) | AC/plasma ratio | ELF concnb (μg/ml) | ELF/plasma ratio |
|---|---|---|---|---|---|---|
| 3 | 3 | 1.93 (47) | 46.2 ± 26.3 | 34.5 ± 25.8 | 1.66 ± 1.05 | 1.08 ± 0.47 |
| 5 | 4 | 1.93 (48) | 87.7 ± 65.0 | 44.3 ± 44.2 | 1.86 ± 1.30 | 0.75 ± 0.38 |
| 8 | 4 | 1.73 (48) | 60.9 ± 32.3 | 32.5 ± 14.0 | 1.69 ± 0.82 | 0.95 ± 0.20 |
| 12 | 5 | 1.62 (52) | 49.0 ± 49.7 | 27.3 ± 18.0 | 1.02 ± 0.97 | 0.59 ± 0.35 |
| 24 | 5 | 1.28 (56) | 60.7 ± 49.2 | 34.9 ± 18.6 | 1.80 ± 1.71 | 0.92 ± 0.65 |
Mean values (percent coefficient of variation) are shown.
Mean values ± SD are shown.
FIG. 1.
Mean POS concentrations in plasma, ELF, and ACs. Shown are concentration-time profiles for POS on day 8 after healthy subjects received POS (400 mg; 40-mg/ml oral suspension) every 12 h with food (10 min after eating a high-fat meal) for a total of 14 doses. Note that only the plasma data has error bars (± SD), as the data were obtained from multiple subjects at the same time. The ELF and AC data were obtained as one data value per subject. The MIC90 value for Aspergillus spp. was obtained from the recent literature (19).
The median Tmax in plasma was 3.6 h, and the mean Cmax and AUC0-12 in plasma were 2.08 ± 0.93 μg/ml and 21.9 μg·h/ml (Table 2), respectively, and are comparable to the exposure reported previously (9). In plasma, ELF, and ACs, the drug concentrations did not decrease significantly, indicating slow elimination of POS from these compartments (Fig. 1) after dosing to steady state.
TABLE 2.
Plasma, ELF, and AC PK/PDa parameters
| Compartment | Cmin (μg/ml) | Cmaxb (μg/ml) | Cmax/MIC90 ratio | Tmax (h) | AUC0-12 (μg·h/ml) | AUC0-24/MIC90 ratioc |
|---|---|---|---|---|---|---|
| Plasma | 1.54 | 2.08 ± 0.93 | 4.15 | 3.6 | 21.9 | 87.6 |
| ELF | 1.02 | 1.86 ± 1.30 | 3.72 | 5.0 | 18.3 | 73.2 |
| AC | 46.2 | 87.7 ± 65.0 | 175 | 5.0 | 715 | 2,860 |
The parameters were derived using total (i.e., unbound plus bound) drug concentrations (see the text).
Mean values ± SD are shown.
Based on the AUC0-24, which was twice the AUC0-12.
The Cmax values in ELF and ACs were 1.86 ± 1.30 μg/ml and 87.7 ± 65.0 μg/ml, and both occurred at a Tmax of 5 h (Table 2). The AUC0-12 in ELF and ACs were 18.3 and 715 μg·h/ml. While POS concentrations were similar in plasma and ELF, they were substantially higher in ACs. The AUC0-24/MIC90 ratios in plasma, ELF, and ACs were 43.8, 36.6, and 1,430, respectively. This observation is consistent with the lipophilic nature and high membrane permeability of POS. POS concentrations in ACs were severalfold greater than the MIC90 of Aspergillus spp. for the dosing interval of 12 h and for up to 24 h after the last dose.
DISCUSSION
We previously reported the intrapulmonary PK/PD of itraconazole (ITC) in healthy human subjects (5). In that study, ITC was administered in a dose of 200 mg every 12 h for 5 days. The ITC and hydroxyitraconazole (OH-IT) Cmax values (mean ± SD) in plasma, ELF, and ACs were 2.1 ± 0.8 and 3.3 ± 1.0 μg/ml, 0.5 ± 0.7 and 1.0 ± 0.9 μg/ml, and 5.5 ± 2.9 and 6.6 ± 3.1 μg/ml, respectively. The ITC and OH-IT AUC0-24 values for plasma, ELF, and ACs were 34.4 and 60.2 μg·h/ml, 7.4 and 18.9 μg·h/ml, and 101 and 134 μg·h/ml, respectively. In the current study, we administered 400 mg POS every 12 h for 7 days. The Cmax values (mean ± SD) for POS in plasma, ELF, and ACs were 2.08 ± 0.93, 1.86 ± 1.30, and 87.7 ± 65.0 μg/ml. The AUC0-12 values for POS in plasma, ELF, and ACs were 21.9, 18.3, and 715 μg·h/ml. Thus, while the Cmax values of ITC and POS in plasma and ELF were roughly comparable, the Cmax of POS in AC was 16-fold greater than the Cmax of ITC in ACs and 7-fold greater than the Cmax values for ITC and OH-IT in ACs combined.
This differential penetration into ACs was also reflected in the AUC values. The AUC0-24 for POS in ACs was approximately 28-fold greater than the AUC0-24 for ITC in ACs and approximately 12-fold greater than the AUC0-24 for ITC and OH-IT combined.
In a study of 12 lung transplant recipients who received at least six oral doses of voriconazole as antifungal prophylaxis before undergoing surveillance bronchoscopy, ELF/plasma ratios ranged from 2 to 28 (2). AC concentrations were not measured (2). In our study, the ELF/plasma ratios were less variable and ranged from 0.67 to 1.17; the AC/plasma ratios ranged from 31.1 to 42.2. Methodological differences may account for the differences observed in ELF penetration in these studies. The subjects in the former study were lung transplant recipients; ours were healthy volunteers. The current study was performed in a clinical research unit using standardized procedures. There also may be true biological differences among the azoles in the penetration into ELF and ACs. A comparison of POS ELF and AC levels in our study with previously published data for ITC (5) may be clinically more relevant because both the POS and the ITC studies were conducted with healthy subjects, used the same standard methods, and reported levels in ELF and ACs.
These observations have clinical significance because it has been demonstrated that the conidia of Aspergillus spp. are ingested by alveolar macrophages in the early phase of infection (20, 21). In an immunosuppressed-rabbit model and in children with AIDS, the antiphagocytic function of alveolar macrophages was impaired (3, 18). The ability of POS to inhibit Candida spp. is concentration dependent and is best correlated with the AUC/MIC ratio (1), and a similar relationship may exist for Aspergillus spp. Thus, greater AC drug concentrations are likely to be important in preventing or treating Aspergillus pulmonary infection in humans. This is also supported by the results in animal models and humans, in which the efficacy of POS has been superior to that of ITC (1, 8, 15).
In a neutropenic-rabbit model, a sustained total concentration of 1.0 μg/ml in plasma was judged to be the minimally effective concentration (15). In our study of humans, a total ELF concentration greater than 1.0 μg/ml and a total AC concentration greater than 40 μg/ml were maintained for the dosing interval of 12 h and for 24 h after the last dose. The total AUC0-12/MIC90 ratios for Aspergillus spp. in plasma, ELF, and ACs were 43.8, 36.6, and 1,430, respectively. The total AUC/MIC ratio is a more appropriate PK/PD parameter for POS since, in a prospective, randomized trial in neutropenic patients, POS was more effective than fluconazole or ITC for prophylaxis, prevention of invasive fungal infections, and increased overall survival (8). The high AUC/MIC ratio in the current study provides additional PK/PD support for the clinical observations that the efficacy of POS is superior to that of ITC.
In addition, in the current study, POS (400 mg) taken every 12 h, administered orally, over 8 days had no clinically relevant effect on blood chemistry, hematology, urinalysis, vital signs, or ECGs, including corrected QT intervals in healthy volunteers.
A limitation of the current study is that its 24-hour sampling interval was short in comparison with the long half-life of POS in ACs, plasma, and ELF. This resulted in a flat concentration-time curve, which made it impossible to calculate the POS half-life in any compartment. There are also differences between the PK determined in this study and those in other literature. We feel this is most likely a result of within-study variability (11). A final limitation is that the randomization schedule did not allow study dropouts to be replaced, resulting in fewer subjects in some time groups than in others. We consider it unlikely that this last limitation affected our results, because the groups were comparable and there was a flat concentration-time profile in all groups.
We conclude that (i) an oral dosing regimen of POS (400 mg) every 12 h resulted in sustained plasma, ELF, and AC concentrations above the MIC90 for Aspergillus spp. during the entire 12-h dosing interval; (ii) the high intrapulmonary AUC0-12/MIC90 ratio observed in this study is favorable for the treatment or prevention of aspergillosis; and (iii) oral POS was well tolerated in healthy adults.
Acknowledgments
Financial support for this study was provided by Schering-Plough, Kenilworth, NJ, and by funds provided by the National Institutes of Health, Rockville, MD, grant MO1RR00079 (General Clinical Research Center) at the University of California, San Francisco.
We thank Darshana Malavade, Schering-Plough, for her help with bioanalytical aspects of this study.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capitano, B., B. A. Potoski, S. Husain, S. Zhang, D. L. Paterson, S. M. Studer, K. R. McCurry, and R. Venkataramanan. 2006. Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 50:1878-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chilvers, E. R., C. L. Spreadbury, and J. Cohen. 1989. Bronchoalveolar lavage in an immunosuppressed rabbit model of invasive pulmonary aspergillosis. Mycopathologia 108:163-171. [DOI] [PubMed] [Google Scholar]
- 4.Conte, J. E., Jr., J. A. Golden, M. G. Kelly, and E. Zurlinden. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523-529. [DOI] [PubMed] [Google Scholar]
- 5.Conte, J. E., Jr., J. A. Golden, J. Kipps, M. McIver, and E. Zurlinden. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte, J. E., Jr., J. A. Golden, M. McIver, E. Little, and E. Zurlinden. 2007. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int. J. Antimicrob. Agents 30:422-427. [DOI] [PubMed] [Google Scholar]
- 7.Conte, J. E., Jr., J. A. Golden, M. McIver, and E. Zurlinden. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int. J. Antimicrob. Agents 28:114-121. [DOI] [PubMed] [Google Scholar]
- 8.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y.-T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 9.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney, R., A. Sansone, W. Smith, T. Marbury, P. Statkevich, M. Martinho, M. Laughlin, and S. Swan. 2005. Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J. Clin. Pharmacol. 45:185-192. [DOI] [PubMed] [Google Scholar]
- 11.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2003. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 14.Krieter, P., B. Flannery, T. Musick, M. Gohdes, M. Martinho, and R. Courtney. 2004. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob. Agents Chemother. 48:3543-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, S. Piscitelli, M. Candelario, A. Field-Ridley, N. Avila, J. Bacher, and T. J. Walsh. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., S. A. Messera, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS Global Antifungal Surveillance Program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 17.Ramani, R., and V. Chaturvedi. 2007. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia 163:315-319. [DOI] [PubMed] [Google Scholar]
- 18.Roilides, E., A. Holmes, C. Blake, P. A. Pizzo, and T. J. Walsh. 1993. Defective antifungal activity of monocyte-derived macrophages from human immunodeficiency virus-infected children against Aspergillus fumigatus. J. Infect. Dis. 168:1562-1565. [DOI] [PubMed] [Google Scholar]
- 19.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen, J. X., G. Krishna, and R. N. Hayes. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J. Pharm. Biomed. Anal. 43:228-236. [DOI] [PubMed] [Google Scholar]
- 21.Waldorf, A. R. 1989. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 47:243-271. [PubMed] [Google Scholar]
- 22.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
- 23.Wheat, L. J., P. Connolly, M. Smedema, M. Durkin, E. Brizendine, P. Mann, R. Patel, P. M. McNicholas, and M. Goldman. 2006. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J. Antimicrob. Chemother. 57:1235-1239. [DOI] [PubMed] [Google Scholar]