Abstract
Imipenem plasma concentrations were analyzed in 57 febrile neutropenic patients using the NONMEM program. The recommended 2-g/day regimen achieved coverage of the most common bacteria (MIC90 = 1 mg/liter) during the whole dosing interval in only 53% of patients. This goal was achieved in 90% of patients with 3 g/day.
Imipenem dosing recommendations (2 g/day) are based on studies in healthy volunteers (8). Pharmacokinetic parameters differ significantly in critically ill patients, and information in febrile neutropenic cancer patients is lacking (4, 7, 15). The pharmacodynamic parameter best predicting the in vivo antibacterial activity of beta-lactam antibiotics is the time during which plasma concentrations are above the MIC of the causative pathogen (t>MIC). For imipenem, a t>MIC during variable proportions of the dosing interval is required to achieve bactericidal activity in neutropenic animal models (3, 8). In vitro/in vivo analyses have suggested that an optimal bactericidal activity of beta-lactams is obtained at concentrations exceeding the MIC throughout the dosing interval (2, 9). In potentially life-threatening infections, such as febrile neutropenia, some experts thus recommend to maintain antibiotic concentrations above the MIC over 66 to 100% of the dosing interval (8, 16).
The aim of this study was to assess the population pharmacokinetics of imipenem in febrile neutropenic cancer patients in order to optimize dosing recommendations.
Consecutive imipenem plasma concentrations (159; 86 troughs and 73 peaks) in 57 febrile neutropenic patients with hematological malignancies (median, 2 samples per patient; range, 1 to 10) were retrospectively analyzed. Imipenem was prescribed according to the recommended dosing schedules for febrile neutropenia (500 mg infused over 30 min every 6 h) and adjusted to the calculated glomerular filtration rate (GFR; derived from age, sex, total body weight, and serum creatinine according to the Cockroft-Gault formula).
Blood samples were drawn for trough and peak imipenem measurements around the same dose, 10 min before and at a median of 2 h (range, 0.5 to 4 h) after the start of the infusion. Blood was drawn, processed, and frozen within 1 h after sampling. Free-circulating imipenem plasma concentrations were measured by a high-performance liquid chromatography (HPLC) method validated according to international guidelines: analytical range, 0.25 to 200 mg/liter; intra-/interassay accuracy and precision, <5% (6).
The NONMEM computer program (version VI; GloboMax, Hanover, MD) was used for the pharmacokinetic analysis. Several models were applied and compared for their ability to describe the observations. Simulations were obtained from NONMEM based on the final population model for groups of 1,000 virtual patients, with given covariates receiving different imipenem dosing regimens. The therapeutic objective was to maintain imipenem blood concentrations over the MIC during the whole dosing interval (i.e., trough>MIC). The targeted MIC was 1 mg/liter (MIC90 of the most frequent gram-positive and -negative bacteria in febrile neutropenic patients according to the EUCAST database [5]). Simulations with various dosing regimens were performed to determine the percentage of patients achieving this therapeutic goal.
Demographic characteristics and imipenem dosing schedules are summarized in Table 1. Blood samples for imipenem measurement were drawn after reaching steady state at a median of 3 days (range, 1 to 9) after the start of therapy or the last change in dosing schedule. The median imipenem peak and trough plasma concentrations were 6.6 mg/liter (range, 0.9 to 14) and 0.9 mg/liter (range, 0.25 to 5), respectively. The simplest population pharmacokinetic model (one compartment, no covariate) indicated an average V of 34.1 liters, an average clearance (CL) value of 16.0 liters/h with 26% proportional interpatient variability, and a proportional residual variability of 54%. Introducing a second compartment or testing alternative variability models did not result in a significant decrease of the objective function (ΔOF). The definition of V as a multiple of body weight improved the model (ΔOF = −16.4), as did the assignment of GFR as an explanatory factor for CL (ΔOF = −8.8). The final population model estimates are summarized in Table 2. Post-hoc individual estimates of the pharmacokinetic parameters averaged 35.7 liters (range, 17.6 to 66.0) for V, 16.2 liters/h (range, 8.4 to 25.1) for CL, and 1.43 h (range, 0.8 to 2.7) for the elimination half-life.
TABLE 1.
Characteristics of patients and imipenem therapy courses
| Parameter | Value |
|---|---|
| Patients | |
| Total | 57 |
| Gender (men/women) | 44 (77.2%)/13 (22.8%) |
| Median age [yr (range)] | 58 (17-78) |
| Underlying disease | |
| Acute myeloid leukemia | 37 (64.9%) |
| Acute lymphoblastic leukemia | 3 (5.3%) |
| Multiple myeloma | 4 (7%) |
| Lymphoma | 5 (8.8%) |
| Other | 8 (14%) |
| Chemotherapy courses | |
| Total | 69 |
| Induction/consolidation for acute leukemia | 33 (47.8%)/19 (27.5%) |
| Autologous stem cell transplantation | 10 (14.5%) |
| Other | 7 (10.1%) |
| Imipenem therapy courses | |
| Total | 69 |
| Median daily dose [g/day (range)] | 2 (0.75-4) |
| Determinants of pharmacokinetics | |
| Median body wt [kg (range)] | 73 (41-135) |
| Median serum creatinine [μmol/liter (range)] | 68 (29-235) |
| Median GFR [ml/min (range)] | 105 (38-285) |
TABLE 2.
Final population pharmacokinetic model for imipenem in febrile neutropenic patients
| Parametera | Mean estimate ± SEb |
|---|---|
| Nonrenal CL (liters/h) | 10.7 ± 1.7 (17% ± 6%) |
| Renal CL (liters/h per | |
| 100 ml/min GFR) | 4.79 ± 1.4 |
| V (liters per 70 kg BW) | 33.5 ± 4.0 |
| Residual error | (59% ± 7%) |
CL, imipenem clearance, defined as CLnonrenal + CLrenal·GFR/100 (ml/min); V, imipenem volume of distribution, defined as V·BW/70 (kg); GFR, glomerular filtration rate according to the Cockroft-Gault formula; BW, body weight.
Values in parentheses are the coefficient of variation (CV) ± SE. The CV is taken as the square root of the variance of Gaussian errors (the antilog of which is multiplied by the total CL or predicted concentration, respectively; SE are added to/subtracted from variance and expressed in the CV scale as the difference of square roots).
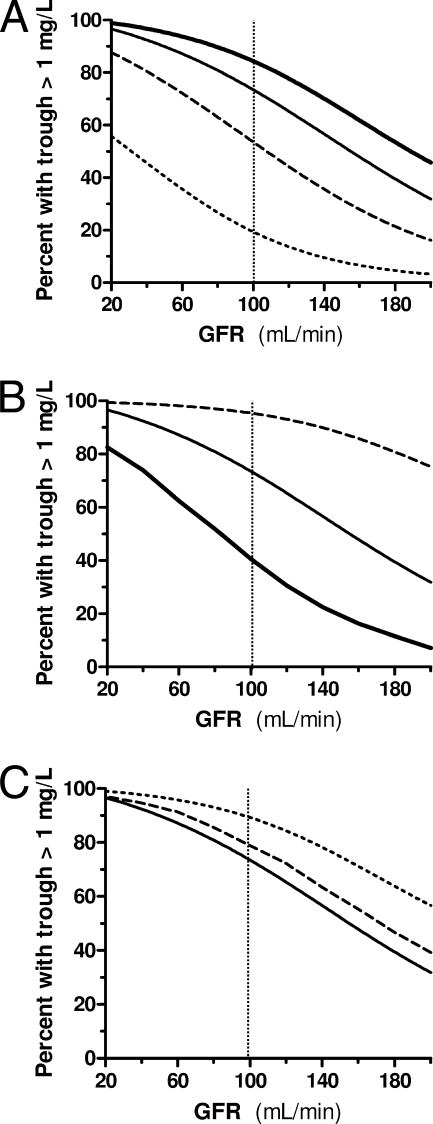
According to the simulations, the recommended 500-mg, four times per day regimen achieved a MIC90 coverage over the whole dosing interval in only 53% of patients with 70 kg weight and 100 ml/min GFR (Fig. 1A). Shorter dosing intervals with the 500-mg unitary dose (4 instead of 6 h) or higher unitary doses (750 instead of 500 mg) with longer infusion times (120 instead of 30 min) all achieved the goal of MIC90 coverage during the whole dosing interval in 90% of patients (Fig. 1). Considering a MIC90 of 2 mg/liter (e.g., Pseudomonas aeruginosa and enterococci), the 500-mg every 6 h, 500-mg every 4 h, and 750-mg over 120 min every 6 h regimens ensured coverage in 18%, 73%, and 58% of patients, respectively; 6 g/day was required to reach coverage in 90% of cases.
FIG. 1.
Percentage of simulated patients with different GFR levels in whom imipenem plasma concentrations covered the MIC90 of the most frequent pathogens (i.e., 1 mg/liter) over the whole dosing interval. The vertical dotted line indicates the standard GFR at 100 ml/min. (A) Different dosing schedules infused over 30 min: 1,000 mg every 6 h (thick line), 750 mg every 6 h (plain line), 500 mg every 6 h (dashed line), and 250 mg every 6 h (dotted line). (B) Different dosing schedules of a 3-g daily dose infused over 30 min are represented: 1,000 mg every 8 h (thick line), 750 mg every 6 h (plain line), and 500 mg every 4 h (dashed line). (C) Different infusion times of a 750-mg every 6 h dosing schedule are represented: 30 min (plain line), 60 min (dashed line), and 120 min (dotted line).
These observations are consistent with previous reports highlighting the difficulty in achieving therapeutic concentrations with aminoglycosides or glycopeptides in febrile neutropenic cancer patients, who display increased volume of distribution and/or renal clearance (11, 19). The need for a MIC coverage during 100% of the dosing interval is a matter of debate. For beta-lactams, it has been demonstrated that the stationary concentration (concentration at which killing equals growth) is close to the MIC and that regrowth occurs when the concentration falls below the MIC (9). Bactericidal activity against resistant strains and a higher probability of in vivo microbiological success have been reported with t>MICs of 90 to 100% (1, 2, 14). Such pharmacodynamic observations provide a rationale for using continuous antibiotic infusions (10, 12, 13). A MIC coverage during 100% of the antibiotic dosing interval is thus an appropriate goal in life-threatening bacterial infections occurring in febrile neutropenic patients.
The MIC90 of the most common pathogens in febrile neutropenic patients that was used in this model (1 mg/liter) might have overestimated the target trough concentration in some patients. Conversely, higher trough concentrations (e.g., 2 to 4 mg/liter) may be needed to ensure appropriate coverage of pathogens with higher MICs, such as Pseudomonas aeruginosa or Enterococcus faecalis/Enterococcus faecium. Using such high MIC thresholds resulted in an inappropriate MIC coverage in a higher proportion of patients receiving recommended dosing regimens. However, recent epidemiological data have shown that these pathogens account for less than 5% of all febrile neutropenic episodes, suggesting that targeting a MIC threshold of 1 mg/liter may be appropriate for the majority of cases (17).
Adverse events such as nausea/vomiting or neurological toxicity occurring with imipenem doses of 4 g/day have been a concern in the past. However, a prolonged infusion time may improve gastrointestinal tolerance, and high-dose imipenem therapy, if appropriately adjusted to renal function (3 to 4 g/day per 100 ml/min GFR), is rarely associated with neurological adverse events (18).
This analysis has several limitations. The lack of complete pharmacokinetic profiles and the inaccuracy of retrospectively collected data may have contributed to the large intraindividual variability in this model. The calculated GFR might have overestimated the renal clearance because of the decrease in creatinine production in severely ill patients. In addition, imipenem plasma concentration measurements were not performed on a routine basis and were requested by the attending physicians for cases of suspected toxicity, lack of response to therapy, or impaired renal function, which may have been a source of a selection bias.
In conclusion, this pharmacokinetic analysis suggests that the recommended imipenem dosage (2 g/day) is insufficient for an appropriate coverage of the most common bacterial pathogens in febrile neutropenic patients. Intermittent imipenem regimens with higher doses (either 500 mg every 4 h or 750 mg in 2-h infusions every 6 h) may optimize drug exposure in this life-threatening condition.
Acknowledgments
We thank Astra-Zeneca and Merck Sharp & Dohme-Chibret for unrestricted grant support for development of HPLC analytical methods, data collection, and analysis.
The authors have no conflicts of interest.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Alou, L., L. Aguilar, D. Sevillano, M. J. Gimenez, O. Echeverria, M. L. Gomez-Lus, and J. Prieto. 2005. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J. Antimicrob. Chemother. 55:209-213. [DOI] [PubMed] [Google Scholar]
- 2.Ariano, R. E., A. Nyhlen, J. P. Donnelly, D. S. Sitar, G. K. Harding, and S. A. Zelenitsky. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39:32-38. [DOI] [PubMed] [Google Scholar]
- 3.Drusano, G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36:S42-S50. [DOI] [PubMed] [Google Scholar]
- 4.Drusano, G. L., K. I. Plaisance, A. Forrest, C. Bustamante, A. Devlin, H. C. Standiford, and J. C. Wade. 1987. Steady-state pharmacokinetics of imipenem in febrile neutropenic cancer patients. Antimicrob. Agents Chemother. 31:1420-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2008. Antimicrobial wild type distributions of microorganisms. http://www.srga.org/eucastwt/WT_EUCAST.htm. Accessed 4 September 2008.
- 6.Giannoni, E., P. Moreillon, J. Cotting, A. Moessinger, J. Bille, L. Decosterd, G. Zanetti, P. Majcherczyk, and D. Bugnon. 2006. Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob. Agents Chemother. 50:2563-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janmohamed, R. M., M. J. Leyland, J. Kelly, and I. Farrell. 1990. Pharmacokinetics of imipenem/cilastatin in neutropenic patients with haematological malignancies. J. Antimicrob. Chemother. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 8.Mouton, J. W., D. J. Touzw, A. M. Horrevorts, and A. A. Vinks. 2000. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin. Pharmacokinet. 39:185-201. [DOI] [PubMed] [Google Scholar]
- 9.Mouton, J. W., and A. A. Vinks. 2005. Pharmacokinetic/pharmacodynamic modelling of antibacterials in vitro and in vivo using bacterial growth and kill kinetics: the minimum inhibitory concentration versus stationary concentration. Clin. Pharmacokinet. 44:201-210. [DOI] [PubMed] [Google Scholar]
- 10.Mouton, J. W., and A. A. Vinks. 2007. Continuous infusion of beta-lactams. Curr. Opin. Crit. Care 13:598-606. [DOI] [PubMed] [Google Scholar]
- 11.Pea, F., P. Viale, A. Candoni, F. Pavan, L. Pagani, D. Damiani, M. Casini, and M. Furlanut. 2004. Teicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosages. Clin. Pharmacokinet. 43:405-415. [DOI] [PubMed] [Google Scholar]
- 12.Pea, F., P. Viale, D. Damiani, F. Pavan, F. Cristini, R. Fanin, and M. Furlanut. 2005. Ceftazidime in acute myeloid leukemia patients with febrile neutropenia: helpfulness of continuous intravenous infusion in maximizing pharmacodynamic exposure. Antimicrob. Agents Chemother. 49:3550-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakka, S. G., A. K. Glauner, J. B. Bulitta, M. Kinzig-Schippers, W. Pfister, G. L. Drusano, and F. Sorgel. 2007. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob. Agents Chemother. 51:3304-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam, V. H., P. S. McKinnon, R. L. Akins, M. J. Rybak, and G. L. Drusano. 2002. Pharmacodynamics of cefepime in patients with gram-negative infections. J. Antimicrob. Chemother. 50:425-428. [DOI] [PubMed] [Google Scholar]
- 15.Tegeder, I., A. Schmidtko, L. Brautigam, A. Kirschbaum, G. Geisslinger, and J. Lotsch. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325-333. [DOI] [PubMed] [Google Scholar]
- 16.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 17.Viscoli, C., A. Cometta, W. V. Kern, R. Bock, M. Paesmans, F. Crokaert, M. P. Glauser, and T. Calandra. 2006. Piperacillin-tazobactam monotherapy in high-risk febrile and neutropenic cancer patients. Clin. Microbiol. Infect. 12:212-216. [DOI] [PubMed] [Google Scholar]
- 18.Zajac, B. A., M. A. Fisher, G. A. Gibson, and R. R. MacGregor. 1985. Safety and efficacy of high-dose treatment with imipenem-cilastatin in seriously ill patients. Antimicrob. Agents Chemother. 27:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitany, R. G., N. S. El Saghir, C. R. Santhosh-Kumar, and M. A. Sigmon. 1990. Increased aminoglycoside dosage requirements in hematologic malignancy. Antimicrob. Agents Chemother. 34:702-708. [DOI] [PMC free article] [PubMed] [Google Scholar]