Abstract
The cancer chemopreventive agent ellagic acid (EA) is a known inhibitor of glutathione S-transferases (GSTs) and possesses antiplasmodial activities in the upper-nanomolar range. In the recent drug development approach, the properties of the active site of Plasmodium falciparum GST were exploited for inhibitor design by introducing one or two additional hydroxyl groups into EA, yielding flavellagic acid (FEA) and coruleoellagic acid (CEA), respectively. Indeed, the inhibition of P. falciparum GST was improved with the increasing hydrophilicity of the planar polyaromatic ring system. Studying the effects of the two compounds on the central redox enzymes of Plasmodium revealed that glutathione reductase and thioredoxin reductase also are inhibited in the lower-micromolar range. Both compounds had strong antiplasmodial activity in the lower-nanomolar range and were particularly effective against chloroquine (CQ)-resistant P. falciparum strains. Neither FEA nor CEA showed cytotoxic effects on human cells. This was supported by negligible changes in transcript levels and enzyme activities of redox enzymes in human A549 cells upon treatment with the compounds. In Plasmodium, however, CEA treatment resulted in a marked downregulation of most antioxidant genes studied and impaired mainly the trophozoite stage of the parasites. In addition, EA, CEA, and FEA were found to strongly inhibit in vitro heme aggregation. In vitro and preliminary in vivo studies indicated that, compared to CQ, CEA is a slowly acting compound and is able to significantly improve the survival of Plasmodium berghei-infected mice. We conclude that FEA and CEA are promising antimalarial compounds that deserve to be studied further.
The polyphenolic lactone ellagic acid (EA), a naturally occurring food compound found in a variety of plant species, has been shown to possess anticarcinogenic, antioxidant, and antiinflammatory activity in bacterial and mammalian systems (23, 33). The mechanisms by which EA exerts its intracellular actions include the inhibition of DNA topoisomerases, the induction of cell cycle arrest, and the activation of apoptotic pathways. The cancer-chemopreventive properties of EA have been associated with the inhibition and long-term induction of glutathione S-transferases (GST; EC 2.5.1.18) (5, 27, 28). In addition to its chemopreventive effects in mammalian systems, EA has been shown to possess antiplasmodial activity in the upper-nanomolar range (2, 8, 37), which has been linked to the inhibition of plasmepsin II and an impairment of beta-hematin formation in the parasite (8).
The inhibition of GSTs by EA was first reported by Das et al. in 1984 (6). Later studies confirmed that plant phenols, including quercetin, purpurogallin, alizarin, and EA, differentially inhibited various GST isoenzymes purified from human tissues and rat liver (7). A more detailed study of human recombinant GST (hGST) isoenzymes recently was carried out by Hayeshi et al. (17). GSTs form a large enzyme family ubiquitously expressed in animals and plants. They are involved in the detoxification and biotransformation of hydrophobic compounds by catalyzing their conjugation with glutathione (GSH) in order to increase water solubility and facilitate excretion. The active site of GSTs is composed of two parts: the G-site, which binds reduced GSH, and the more variable H-site, which binds the second substrate (26). In contrast to most other organisms, the causative agent of malaria, Plasmodium falciparum, possesses only one GST isoenzyme, which differs structurally from all other known GST classes (13, 16). The 1.9-Å crystal structure of P. falciparum GST indicates that the G-site is highly conserved, whereas the H-site is more solvent accessible than is the case in human isoenzymes. Functional studies showed that P. falciparum GST inhibitors likely act by three different mechanisms to (i) impair general detoxification processes, (ii) reduce the antioxidant capacity of the parasites, and (iii) enhance the concentrations of parasitotoxic free heme (9, 13, 18). Taken together, the properties of P. falciparum GST render the enzyme a very promising target for antimalarial drug development.
In a recent drug development approach, we aimed at increasing and optimizing the inhibition of P. falciparum GST by derivatizing ellagic acid in order to enhance the antiplasmodial activity of this class of compounds. Based on the three-dimensional structure of the enzyme, the introduction of additional hydroxyl groups was predicted to enhance the binding of the inhibitors to the solvent-accessible active site. We synthesized flavellagic acid (FEA) and coruleoellagic acid (CEA) (14, 30, 38) (Fig. 1), which, compared to the activity of EA, indeed showed strongly improved antiplasmodial activity.
FIG. 1.
Structures of EA, FEA, and CEA.
Here, we report the comparative effects of EA, FEA, and CEA on P. falciparum in cell culture, including growth inhibition studies of parasite strains with different degrees of chloroquine (CQ) resistance, drug combination assays with clinically used antimalarials, stage specificity studies, and differential transcriptome analyses. To further understand the differential mechanism of the action of the compounds, we studied their effects on recombinant plasmodial and human redox-related enzymes as well as on enzyme activities and transcript levels in human cells.
MATERIALS AND METHODS
Drugs and chemicals.
All chemicals used were of the highest available purity and were obtained from Roth (Karlsruhe, Germany), Sigma-Aldrich (Steinheim, Germany), or Merck (Darmstadt, Germany). RPMI 1640 medium, low-glucose Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS), and antibiotics (penicillin, 100 U/ml; streptomycin, 0.1 mg/ml) were from Gibco (Paisley, United Kingdom). EA was purchased from Sigma-Aldrich (Steinheim, Germany). FEA and CEA were synthesized as described previously (14, 30, 38). Methylene blue was obtained from Roth (Karlsruhe, Germany); CQ, amodiaquine, and pyrimethamine were from Sigma-Aldrich; mefloquine was from Roche (Mannheim, Germany); artemisinin and primaquine diphosphate were from Aldrich Chemical Co. (Milwaukee, Wis.); and quinine was from Acros Organics (Geel, Belgium). Artemisinin derivatives (artemether and artesunate) were kindly provided by the Swiss Tropical Institute (Basel, Switzerland).
Kinetic studies.
To test the enzyme inhibitory effects of EA, FEA, and CEA, the spectrophotometric assays delineated below were carried out. All recombinant enzymes used were purified to homogeneity via affinity chromatography. Human thioredoxin reductase (TrxR), isolated from placenta, was >95% pure. In all reactions with isolated enzymes, the compounds to be tested were added directly to the assay before starting the reaction with the respective second substrate. P values were determined by nonparametric analysis using the Mann-Whitney U test. For GST, the median test was applied, because the distribution in the two groups differed with respect to both the median and the pattern of the distribution.
(i) Determination of GST activity.
Human placenta Pi class GST was obtained from Sigma-Aldrich, and P. falciparum GST was produced as described by Harwaldt et al. (16). GST activity (in the cell-free system or cell extracts) was measured at 25°C by the rate of increase of S-(2,4-dinitrophenyl)glutathione (ɛ340 nm = 9.6 mM−1 cm−1) generated by the reaction of 0.5 mM chlorodinitrobenzene (CDNB) with 1 mM GSH in 84 mM KH2PO4, 16.6 mM K2HPO4, 1 mM EDTA, pH 6.5. The reaction with P. falciparum GST was carried out with 100 mM HEPES, 1 mM EDTA, pH 6.5 (16).
(ii) Determination of TrxR activity.
Human placenta TrxR was purified as described by Gromer et al. (15), with slight modifications. P. falciparum TrxR, P. falciparum Trx, and human thioredoxin (hTrx; TrxC73S) were recombinantly produced as described previously (20, 21). To study TrxR activity, two different assay systems were carried out at 25°C. First, 200 μM NADPH and 3 mM 5,5′-dithiobis(2-nitrobenzoate) (DTNB) were used as substrates in TrxR assay buffer (100 mM potassium phosphate, 2 mM EDTA, pH 7.4), and the production of TNB− anions (ɛ412 nm = 13.6 mM−1 cm−1) was monitored spectrophotometrically. Second, TrxR activity was determined using its physiological substrate, Trx (20 μM; for hTrx, the TrxC73S mutant, which in contrast to the wild type does not form inactive dimers, was employed), and 100 μM NADPH, and the initial change in absorbance was monitored at 340 nm (1). To measure TrxR activity in cell extracts, lysates were incubated with 3 mM DTNB in TrxR assay buffer for at least 60 s, followed by the addition of 200 μM NADPH to start the reaction. Values were corrected for background reactions (1). For 50% inhibitory concentration (IC50) determinations, assays were started with DTNB or Trx.
(iii) Determination of GR activity.
Human recombinant GSH reductase (GR) (kindly provided by Heiner Schirmer, Heidelberg, Germany) and P. falciparum GR were produced as described before (11, 29). Activity (in the cell-free system or cell extracts) was determined in GR assay buffer (20.5 mM KH2PO4, 26.5 mM K2HPO4, 1 mM EDTA, 200 mM KCl, pH 6.9) in the presence of 100 μM NADPH (ɛ340 nm = 6.22 mM−1 cm−1) and 1 mM glutathione disulfide as substrates. The change in absorbance at 340 nm was monitored spectrophotometrically at 25°C. Observed background reactions were subtracted.
(iv) Determination of GPx activity.
GSH peroxidase (GPx) activity in cell extracts was determined at 340 nm. Assays were carried out in 100 mM Tris, 0.5 mM EDTA, pH 8.0, in the presence of 1 U/ml human GR, 2 mM GSH, and 100 μM NADPH at 25°C. tert-butyl hydroperoxide (at 70 μM) was used as the substrate. Before adding the peroxide substrate, GSH, GR, NADPH, and GPx were incubated for 10 min at 25°C. Observed background reactions were subtracted.
In vitro heme aggregation assay.
The ability of the EA derivatives to interfere with heme aggregation was determined in 20 mM sodium phosphate (pH 5.2) as described by Ignatushchenko et al. (19). A 10 mM stock solution of hemin chloride in 0.1 M NaOH was prepared freshly and incubated at 37°C for at least 1 h prior to use. Inhibitors were dissolved in dimethylformamide or buffer. The time dependence of the reaction was tested in the presence of 25 μM inhibitor and 25 μM heme. After the respective incubation times at 37°C, samples were centrifuged and the absorbance was measured at 360 nm. Values of the respective control samples were subtracted.
Cultivation of Plasmodium falciparum.
CQ-sensitive (3D7-Netherlands and HB3-Honduras) and -resistant (K1-South-East Asia and Dd2-Indochina) strains of P. falciparum were grown in continuous culture as described by Trager and Jensen (36), with slight modifications. Unless otherwise stated, parasites were maintained at 1 to 10% parasitemia and 3.3% hematocrit in an RPMI 1640 culture medium supplemented with A+ erythrocytes, 0.5% lipid-rich bovine serum albumin (Albumax), 9 mM (0.16%) glucose, 0.2 mM hypoxanthine, 2.1 mM l-glutamine, and 22 μg/ml gentamicin. All incubations were carried out at 37°C in 3% O2, 3% CO2, and 94% N2. The synchronization of parasites in culture to ring stages was carried out by treatment with 5% (wt/vol) sorbitol (22). The parasites were used for the experiments delineated below.
Determination of IC50s, drug combinations, and stage specificity profiles on P. falciparum.
Isotopic drug sensitivity assays by means of the semiautomated microdilution technique (10) were employed to investigate the susceptibility of the malaria parasites to various compounds and their effects in combination with other clinically used antimalarials. The method depends on the incorporation of radioactive [3H]hypoxanthine, which is taken up by the parasite as a precursor of purine deoxynucleotides for DNA synthesis and was performed according to the modifications of Fivelman et al. (12). In 96-well microtiter plates (Nunc), a twofold serial dilution of the starting concentration of each drug to be tested was carried out. Parasites were incubated at a parasitemia of 0.25% (>70% ring forms) and 1.25% hematocrit in hypoxanthine-free medium. After 48 h, 0.5 μCi [3H]hypoxanthine was added into each well, and the plates were incubated for another 24 h. The cells of each well were harvested on a glass fiber filter (Perkin-Elmer, Rodgau-Jügesheim, Germany), washed, and dried. Their radioactivity, in counts per minute, was considered to be proportional to the respective growth of P. falciparum in the well.
In order to assess the effects of combined drugs, two compounds to be tested were applied alone and in fixed-concentration ratios of 1:1, 1:3, and 3:1 as described by Fivelman et al. (12). IC50 and IC90 values were calculated, and the fractional inhibitory concentrations (FIC) of the respective drugs were determined (12).
The stage-dependent effects of CEA and CQ were tested in vitro on synchronous cultures of the P. falciparum strain 3D7 according to Maerki et al. (25). For this purpose, seven different drug concentrations, ranging from ∼1× IC50 to ∼100× IC50, were added to ring-, trophozoite-, and schizont-stage parasites with a parasitemia of 0.15% and 5% hematocrit. After exposure for 1, 6, 12, or 24 h, the plates were washed four times with compound-free medium in order to dilute the free compound to less than 1% of its starting concentration before adding [3H]hypoxanthine and continuing as described above. Compound effects are expressed as the percentage of values for the untreated controls. The suspensions of uninfected erythrocytes were used for background subtraction.
Preparation of parasite cDNA for transcriptome studies.
In order to assess the mRNA levels of parasites after CEA treatment, P. falciparum trophozoites of the strain 3D7 were exposed to 126 nM CEA, which corresponds to ∼3× IC50. After 12, 15, and 18 h, respectively, the parasites of the treated and untreated control plates were harvested by suspending the red cells for 10 min at 37°C in a 20-fold volume of saponin lysis buffer containing 7 mM K2HPO4, 1 mM NaH2PO4, 11 mM NaHCO3, 58 mM KCl, 56 mM NaCl, 1 mM MgCl2, 14 mM glucose, and 0.02% saponin, pH 7.4. The saponin lysis was repeated twice before washing the parasites with phosphate-buffered saline (PBS) and disrupting them by freezing and thawing them three times in liquid nitrogen. For extracting total RNA, the NucleoSpin RNA/protein kit (Macherey-Nagel, Düren, Germany) was used. RNA extracts were treated with concentrated DNase I (RNase-free; MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturer's instructions to remove genomic parasite DNA contaminants, and the removal was confirmed by PCR. Aliquots of 800 ng of each sample were reversely transcribed to cDNA using anchored oligo(dT) primers (Reverse-iT MAX 1st strand synthesis kit; Abgene, Hamburg, Germany).
Cultivation of human A549 cells.
Nonsmall cell lung cancer A549 cells were provided by the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). A549 cells were cultivated in DMEM with 10% FCS and with penicillin and streptomycin at 37°C, 5% CO2. Cells were subcultured, and 2.5 × 105 cells were seeded in 25-cm2 wells. When the attached cells reached 40 to 45% confluence, cells were treated with 166 or 662 μM EA, 158 or 315 μM FEA, or 5 or 24 μM CEA. The dosages of the different compounds were chosen on the basis of preliminary inhibitory tests (screenings on 96-well microtiter plates; less than 10% cell death in the culture after 24 h of incubation). Control cells were treated with less than 5% DMSO. After a 24-h treatment, cells were washed three times with cold PBS and subsequently trypsinized. Medium containing fetal bovine serum was used to deactivate the trypsin, followed by centrifugation at 400 × g and 4°C for 4 min. Cells were resuspended in PBS, and the numbers of viable and nonviable cells were counted by measuring trypan blue exclusion using a hemocytometer. For enzymatic studies, harvested cells were disrupted by sonication and centrifuged. For real-time PCR, total RNA was isolated with the Qiagen RNA miniprep kit (Qiagen, Hilden, Germany), and a DNA digestion step using RNase-free DNase (MBI Fermentas) was performed. Samples of 800 ng of total RNA were reversely transcribed to cDNA (Reverse-iT RTase blend; Abgene) using poly(dT)15 primers (Roche Diagnostics, Mannheim, Germany).
Quantitative real-time PCR.
The QuantiTect SYBR green PCR kit (Qiagen; for human samples) and SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich; for Plasmodium samples) were used in the real-time PCR approach on a Rotor-Gene 3000 real-time PCR cycler (Corbett Research, Sydney, Australia). The required primers (Table 1) were designed in our laboratory or obtained from the real-time PCR database (RTPrimerDB Real Time PCR Primer and Probe Database; http://medgen.ugent.be/rtprimerdb/). All primers used were tested previously in a normal PCR to ensure their target gene specificity. The Rotor-Gene 6.0 software was used to analyze the PCR results and to determine cycle threshold values. Data collected was based on the threshold cycle and the reaction efficiency for target genes and reference genes in treated cells and untreated control cells. Relative quantification was carried out by applying the efficiency-corrected ΔΔCT method (32). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S rRNA were used as internal reference genes for human samples, whereas P. falciparum lactate dehydrogenase (LDH) and 18S rRNA were used for Plasmodium samples. The specificity of PCR results was confirmed by melting-curve analysis, and a no-template control was always included. In our experiments, each real-time PCR run was carried out in quadruplet.
TABLE 1.
Oligonucleotide primers for transcripts studied by quantitative real-time PCR
| Target organism and type of gene | Gene product | GenBank accession no. | Sequence (5′→3′)
|
|
|---|---|---|---|---|
| Sense | Antisense | |||
| Human | ||||
| Housekeeping genes | GAPDH | NM_002046 | GTGGTCTCCTCTGACTTCAACA | CTGTAGCCAAATTCGTTGTCATAC |
| 18S rRNA | M10098 | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | |
| Redox-related genes | GPx1 | NM_000581 | GTTCGAGCCCAACTTCATGCT | ATCGTTGCGACACACCGGAG |
| GR | NM_000637 | AGACCTATTCAACGAGCTTTACC | CCTGCAGCATTTCATCACACC | |
| GSTpi | NM_000852 | GGAGACCTCACCCTGTACCA | GGGCAGTGCCTTCACATAGT | |
| TrxR1 | NM_003330 | TGCCACTGGTGAAAGACCAC | CAAGAAATCCAGCGCACTCC | |
| Plasmodium | ||||
| Housekeeping genes | LDH | XM_001349953 | AAACCCAGTAGATGTTATGGTAC | CATTTTATTTCCATGAGCACCTAC |
| 18S rRNA | M19172 | AGGCTGCACGCGTGCTACAC | ACAATTCATCATATCTTTCAATCGG | |
| Redox-related and | EF | XM_001350074 | TAATGCCATGCCACATTTCTGG | TACCTCTGAATAACCAGACACC |
| other genes | GCS | XM_001352024 | GAAGGATTATTAAATCAATCAGCC | TTTATATGGATCGACAAAAACACC |
| GR | XM_001348329 | CGTGTGTCAACGTTGGATGTG | CCCTTCTCTCTACCAACAGAG | |
| Grx1 | XM_001351103 | GAGAACATCATTGCTGTATTTGC | TTTAAGTATGCTTGAATATTAGCC | |
| GS | XM_001351643 | GGAGGGAAAAATAATTTACATGG | GCGATGGTTTCTTTTTCTGTTG | |
| GST | XM_001348324 | CCTACCTTGGAATAGAATATACAG | ATTCATTCAATTCACTTTCACCAC | |
| HSP | XM_001352014 | AGAGGTATAAATCCTGATGAAGC | GGTTATCTTGATATGTTGAAAAGG | |
| RP | XM_001349626 | GTTTTAGAGAGCTTCCAGCAGG | TTTAACCACTCGCACCGATGG | |
| SOD1 | XM_001349339 | AAACACATGATGCTGGTAATC | GCAAAGTTCCAATTTACTAGG | |
| Sel1 | XM_001348170 | TTAAGACCCTACTTGGACAATC | TCAGGTACTTCCCAAGCAGG | |
| TPx1 | XM_001348506 | TCGACAAGCAAGGTGTTGTTC | TCTGATGGTTTCATGGCTACC | |
| TrxR | XM_001352073 | ATGGGTTAGCCAAGTTAAAAGAC | CTGGTATATGTGGTCTACATCC | |
| UCE | XM_001350238 | CAGGCGCTATATGTTTGGACG | CTTCTCTTGGCTCTAATTTAGGC | |
RESULTS
Inhibition of recombinant redox enzymes.
FEA and CEA were tested in direct comparison to EA on isolated human and plasmodial GST, GR, and TrxR (Table 2). EA inhibited most human and plasmodial enzymes, with similar IC50s. However, P. falciparum TrxR in the DTNB assay was more efficiently inhibited than its human counterpart. In contrast to TrxR, which is reduced via the C-terminal redox center of TrxR, the low-molecular-weight substrate DTNB can be reduced both at the N-terminal and the C-terminal active sites. The inhibition of the enzymes by FEA was more pronounced for most plasmodial enzymes tested than for their human counterparts (P = 0.088 for GS, P = 0.200 for GR, P = 0.100 for TrxR [Trx], and P = 0.100 for TrxR [DTNB]). CEA, however, was more active on the human enzymes, with the exception of P. falciparum GST, which was five times more potently inhibited than hGST, with IC50s of 7.0 and 35.1 μM, respectively.
TABLE 2.
IC50s of EA, FEA, and CEA on isolated P. falciparum and human redox enzymes
| Inhibitor | IC50 (μM)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GST
|
GR
|
TrxR (Trx assay)
|
TrxR (DTNB assay)
|
|||||
| P. falciparum | Human | P. falciparum | Human | P. falciparum | Human | P. falciparum | Human | |
| EA | 74.4 ± 6.1 | 77.1 ± 4.4 | 27.6 ± 2.8 | 22.8 ± 3.2 | 66.7 ± 3.2 | 78.3 ± 5.1 | 137.8 ± 17.3 | 275.3 ± 35.0 |
| FEA | 29.3 ± 5.4 | 35.7 ± 3.9 | 16.3 ± 1.8 | 26.7 ± 5.8 | 21.7 ± 2.9 | 41.7 ± 3.9 | 35.3 ± 6.7 | 51.7 ± 2.9 |
| CEA | 7.0 ± 2.1 | 35.1 ± 4.1 | 14.6 ± 4.2 | 7.6 ± 1.2 | 6.8 ± 0.3 | 3.0 ± 0.6 | 23.8 ± 4.8 | 4.2 ± 1.1 |
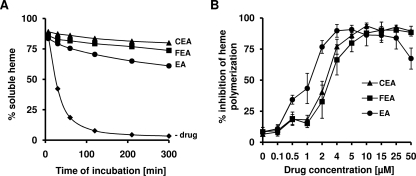
Inhibition of heme aggregation.
Under the assay conditions described above (19), heme aggregation occurred spontaneously in the absence of inhibitors and was more than 90% complete after 2 h. In the presence of any of the three polyaromatic compounds (25 μM), however, this reaction was markedly inhibited, as shown in Fig. 2A. After 300 min, CEA showed significantly stronger effects on heme aggregation than EA (P = 0.003; determined using the Games-Howell test). IC50s determined for the various compounds were 1.2 for EA, 2.2 for CEA, and 2.6 for FEA (Fig. 2B). In contrast, up to 20 equivalents of CQ (500 μM) had no inhibitory effect on heme aggregation (data not shown), which also was observed by Ignatushchenko et al. (19) and is due to a coprecipitation of CQ with the heme aggregate.
FIG. 2.
(A) In vitro heme aggregation in the absence (−drug) and presence of EA, FEA, or CEA. Heme and inhibitors were added in equimolar concentrations of 25 μM. Values are the means ± standard deviations from two independent experiments. (B) IC50s of the inhibition of heme aggregation as determined for EA, FEA, and CEA. The initial heme concentration was 25 μM. At a concentration of 50 μM, EA precipitated.
Inhibition of P. falciparum growth in vitro.
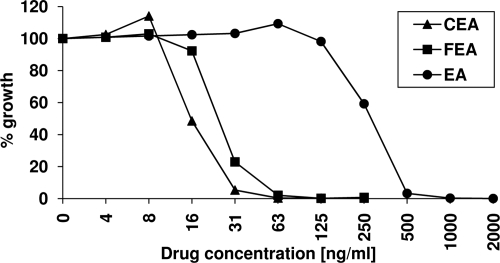
The growth-inhibitory effects of EA, FEA, and CEA were determined in vitro on two CQ-sensitive and two CQ-resistant P. falciparum strains and are summarized in Table 3. The CQ-resistant strain Dd2 showed the highest sensitivity to EA treatment, with an IC50 of 351 nM. All other strains required approximately three times higher inhibitor concentrations for similar growth inhibition. FEA and CEA inhibited parasite growth more effectively than EA, with IC50s in the lower-nanomolar range. CEA treatment resulted in a further reduction of the IC50s by around 50% compared to that of FEA (except for strain K1). The effects of FEA and CEA on CQ-sensitive and -resistant strains were comparable. Dose-response curves for the three compounds on strain 3D7 are shown in Fig. 3.
TABLE 3.
IC50s of EA, FEA, CEA, and CQ on a human cell line and on P. falciparum strains with various degrees of CQ resistance
| Inhibitor | IC50 (nM)
|
||||
|---|---|---|---|---|---|
|
P. falciparuma
|
Mammalian
|
||||
| 3D7 | HB3 | Dd2 | K1 | L6 | |
| EA | 819 ± 142 | 1,325 ± 102 | 351 ± 55 | 970 ± 58 | NDb |
| FEA | 94 ± 32 | 138 ± 32 | 186 ± 33 | 95 ± 11 | 21 × 103 |
| CEA | 42 ± 5.5 | 72 ± 19 | 116 ± 12 | 86 ± 25 | 94 × 103 |
| CQ | 8.6 ± 0.4 | 16 ± 1.5 | 93 ± 8.7 | 163 ± 7.0 | ND |
Results are mean values and standard deviations. Data have been obtained from at least four independent experiments.
ND, not determined.
FIG. 3.
Dose-response curves for EA, FEA, and CEA on the P. falciparum strain 3D7. Parasites were grown as described in Materials and Methods in the presence of various drug concentrations and are compared to results for untreated controls.
Drug combinations.
Several drug combinations of EA and derivates with clinically used antimalarials were tested on the P. falciparum strain K1 in vitro (data not shown). All combinations studied, including those with artemisinins (artemisinin, artemether and artesunate) and quinolines (amodiaquine, CQ, mefloquine, primaquine, and quinine) as well as with pyrimethamine and methylene blue, were antagonistic (with FIC50 values between 1.0 and 2.3).
Pharmacodynamic studies.
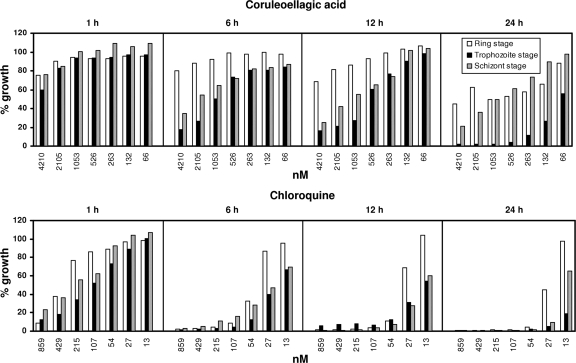
Stage specificity and the speed of action were tested for CEA (Fig. 4). After 1 h of exposure to CEA, the parasite growth of all stages was only slightly affected. After 6 h, trophozoites and schizonts were most affected, whereas ring forms were more resistant to CEA. These effects became more obvious after 12 h of exposure. After 24 h, a stage-specific effect of CEA became particularly evident on the trophozoite stage of the parasite. At CEA concentrations higher than 526 nM (∼12× IC50; added to the trophozoite stage), parasite growth was reduced by more than 95% after 24 h. Under the same conditions, less inhibition was observed for the ring (38 to 55%) and schizont (39 to 79%) stages.
FIG. 4.
Stage-dependent effects of CEA and CQ (∼1× to ∼100× the IC50) on [3H]hypoxanthine incorporation in synchronous cultures of P. falciparum strain 3D7. Compounds were added for 1, 6, 12, or 24 h. After the removal of the compounds, parasites were incubated for another 24 h in the presence of [3H]hypoxanthine. Compound effects are expressed as the percentage of the growth of the respective development stage relative to that of the untreated control. All experiments were carried out in triplicate.
After 1 h of exposure to CQ, all parasite stages were affected similarly, with a strong inhibition (approximately 10 to 20% growth) at the highest CQ concentration. From 6 h onwards, parasite growth was strongly inhibited at low concentrations; however, ring forms were less sensitive than trophozoites and schizonts. The data indicate that CEA is a slowly acting compound compared to the speed of CQ's activity, and it acts mainly on the trophozoite stage of the parasite.
Changes of transcript levels in P. falciparum caused by CEA.
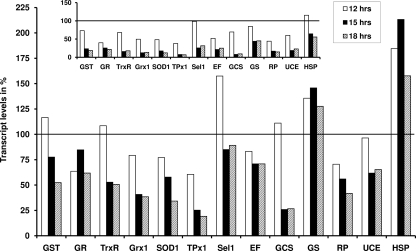
As shown in Fig. 5, transcript levels of a set of selected P. falciparum genes were significantly altered after treating the trophozoites of strain 3D7 with CEA (∼3× IC50) for 12, 15, and 18 h.
FIG. 5.
Influence of CEA on transcript levels in P. falciparum. Trophozoites of the strain 3D7 were exposed to 126 nM CEA, which corresponds to ∼3× the IC50. After an incubation time of 12, 15, and 18 h, respectively, parasites were harvested. After the extraction of RNA and transcription to cDNA, transcript levels were analyzed by quantitative real-time PCR. All values were determined in quadruplet, and data were quantified with the ΔΔCT method (32) using 18S rRNA as the reference gene. Results are presented as percentages (a value of 100% means that the respective gene is not regulated). The inset shows that when values were normalized for LDH as the reference gene, very similar results were obtained.
A number of antioxidant enzymes of the parasite, including GST, GR, TrxR, glutaredoxin (Grx1), superoxide dismutase (SOD1), and thioredoxin peroxidase (TPx1), showed a decrease in mRNA levels during the time period investigated. After an incubation time of 12 h, transcript levels for GST and TrxR were almost unchanged or only slightly upregulated (when normalized against 18S rRNA), whereas the transcript levels of the other antioxidant enzymes studied were lower than 75% (a value of 100% indicates that the respective gene is not regulated compared to levels for an untreated control and when values were normalized for the reference gene). Further incubation of the parasites with CEA resulted in a steady downregulation of transcripts for these genes to levels between 62 (GR) and 19% (TPx1) after 18 h. For selenoprotein 1 (Sel1), a very recently identified selenium-containing enzyme with potential antioxidant activity (24), we observed an upregulation after 12 h (∼160%), which normalized again after 15 and 18 h.
Two genes involved in GSH synthesis, namely gamma-glutamylcysteine synthetase (GCS) and GSH synthetase (GS), were altered differently by CEA. GCS mRNA was relatively stable after 12 h (111%) and strongly downregulated to approximately 25% after 15 and 18 h. GS, however, showed a positive trend for all three time points (between 128 and 146%).
We investigated the effect of CEA on four other genes that are not involved in antioxidant processes, including elongation factor 1-gamma, which shows high sequence similarities with GSTs, the second largest subunit of DNA-directed RNA polymerase II (RP), ubiquitin-conjugating enzyme (UCE), and a heat shock protein (HSP). Elongation factor 1-gamma and UCE mRNA levels changed similarly and seemed to be relatively stable compared to those of RP transcripts, which continuously decreased from 71 to 42%. An upregulation for HSP transcripts was particularly evident (between 158 and 213%).
The regulation of transcript levels was comparable and even more pronounced when using LDH instead of 18S rRNA as the reference gene (Fig. 5, inset).
Effect of EA, FEA, and CEA on enzyme activities and transcript levels of redox enzymes in A549 cells.
The effect of the three polyphenolic compounds on enzyme activities and transcript levels in human A549 cells was determined after a 24-h incubation period with the compounds. The results are summarized in Table 4.
TABLE 4.
Effect of EA and its analogues on transcripts and activities of selected redox enzymes in A549 cells after 24 h of treatmenta
| Inhibitor and concn (μM) | TrxR
|
GST
|
GR
|
GPx
|
||||
|---|---|---|---|---|---|---|---|---|
| qRT-PCRb | Enzyme activityc | qRT-PCR | Enzyme activity | qRT-PCR | Enzyme activity | qRT-PCR | Enzyme activity | |
| EA | ||||||||
| 166 | 115 | 82 ± 7 | 122 | 109 ± 7 | 152 | 87 ± 11 | 109 | 86 ± 18 |
| 662 | 131 | 77 ± 9 | 131 | 101 ± 7 | 125 | 85 ± 15 | 150 | 125 ± 20 |
| FEA | ||||||||
| 158 | 97 | 91 ± 12 | 115 | 99 ± 8 | 105 | 87 ± 12 | 107 | 122 ± 22 |
| 315 | 61 | 115 ± 18 | 164 | 121 ± 12 | 70 | 119 ± 16 | 77 | 112 ± 19 |
| CEA | ||||||||
| 5 | 76 | 42 ± 10 | 68 | 101 ± 6 | 77 | 94 ± 6 | 51 | 85 ± 15 |
| 24 | 73 | 45 ± 9 | 84 | 106 ± 9 | 84 | 103 ± 8 | 71 | 78 ± 17 |
All values are given as percentages of the values for untreated controls.
All values given for quantitative real-time PCR (qRT-PCR) in this table were normalized for GAPDH. However, for all transcripts the same trends were observed when normalizing for 18S rRNA (data not shown).
TrxR activity in cell extracts was measured with DTNB as the substrate.
In vivo efficacy and cytotoxicity.
In addition to the in vitro studies, CEA was tested in a murine Plasmodium berghei model (31). The compound was applied subcutaneously for 4 days postinfection at a concentration of 10 mg/kg of body weight. After treatment, parasitemia did not differ significantly between treated (n = 13; parasitemia of 84 to 93% of control levels on day 4) and control mice (n = 13). However, a clear reduction of absolute parasitemia, down to 30 and 50% on days 7 and 9, respectively, was achieved (P < 0.05 for both days, as determined using the Fisher's exact test; in untreated mice, parasitemia reaches values of >90%). Furthermore, the life span of CEA-treated mice was clearly extended by about 50% (9.5 and 6.4 days for CEA-treated mice and the mean survival for control mice; P < 0.05, as determined by nonparametric analysis using the Mann-Whitney U test).
The cytotoxicity of FEA and CEA was tested in vitro on L6 cells, revealing that both substances are around three orders of magnitude less toxic than the reference drug podophyllotoxin (IC50s for podophyllotoxin, 0.008 μg/ml; FEA, 6.8 μg/ml; and CEA, 31.4 μg/ml). Furthermore, the effect of CEA on human A549 cells was determined by trypan blue exclusion after a 24-h incubation time. Concentrations up to 2 μM did not impair cell numbers. To reduce cell numbers by 50%, 60 μM CEA was required (data not shown). This concentration is three orders of magnitude higher than the IC50 for P. falciparum.
DISCUSSION
The phytochemical EA is a known inhibitor of GSTs and previously has been shown to possess antiplasmodial activity (2, 6, 8, 37). To study the potential of EA-derived compounds as antimalarial leads, we assessed the effects of EA, FEA, and CEA on plasmodial and mammalian cell systems, redox-related enzymes, and transcripts.
P. falciparum GST represents a novel GST isoform, and its H-site differs significantly from those of its human counterparts (13). In contrast to all other GSTs, P. falciparum GST contains only five residues following helix α8, which is too short to form a wall (Mu or Pi class) or an α-helix (alpha class). This leads to a more solvent-accessible H-site in P. falciparum GST, which suggests that the substrate spectrum of P. falciparum GST is broader, includes amphiphilic compounds, and is accessible to amphiphilic inhibitors that are not able to enter the H-site of the human isoforms. Based on these considerations, one or two additional hydroxyl groups were inserted into EA, resulting in FEA and CEA, respectively (Fig. 1). CEA was found to be more effective on P. falciparum GST than on hGST. The inhibition of the plasmodial enzyme increased significantly with increasing hydrophilicity, indicating that the structural properties of the active site of P. falciparum GST can be exploited for rational drug design using these planar polyaromatic ring systems (Table 2).
It had been shown previously that EA inhibits the growth of CQ-sensitive and -resistant malarial parasites in vitro, with an IC50 in the range of 200 to 500 nM (2, 37). In order to compare the inhibitory effect of EA to those of FEA and CEA, we tested all compounds on four P. falciparum strains with various degrees of CQ resistance. The previously reported antiplasmodial effect of EA could be reproduced, yielding IC50s between ∼350 and ∼1,300 nM (Table 3).
The introduction of one additional hydroxyl group into EA (yielding FEA) increased antiplasmodial activity by around one order of magnitude. The addition of a second hydroxyl group, yielding CEA, led to a further reduction of the IC50 by around 50%. Thus, the two derivatives had much more potent antiplasmodial activity than EA (Table 3). These data support the notion that FEA and CEA are promising antimalarial leads that are particularly effective against CQ-resistant P. falciparum strains, as indicated by IC50s of 95 and 86 nM, respectively, on the resistant strain K1 (Table 3). Under the same conditions, CQ was active at an IC50 of 163 nM.
A comparison of the effects of the inhibitors on recombinant P. falciparum GST and P. falciparum in cell culture suggests that all three compounds are likely to have additional targets in the parasite. The activity of a specific inhibitor with only one target usually decreases by one order of magnitude from enzyme to cell culture. For our compounds, however, the effects on cell culture were about two orders of magnitude stronger (IC50 around 100 nM) than the inhibition of P. falciparum GST (IC50 around 10 μM). Studying other Plasmodium enzymes indeed demonstrated that P. falciparum GR and P. falciparum TrxR were effectively inhibited by the substances, with IC50s in the same range as that determined for P. falciparum GST (Table 2). As shown previously, EA furthermore inhibits the formation of beta-hematin, and plasmepsin II has been suggested as a target for EA (8). Since multiple drug targets reduce the risk of resistance development, this mode of action is not a principle hindrance for further lead optimization.
The malaria pigment, also called hemozoin, is composed of an ordered arrangement of reciprocal heme dimers (4). Interestingly, in vitro heme aggregation assays revealed that EA, as well as the two structural derivatives, may exert some of their antiplasmodial activity by forming soluble complexes with free heme in the parasite's digestive vacuole, thereby blocking the formation of hemozoin (Fig. 2A and B). In the time-dependent assay (Fig. 2A), CEA showed the strongest effects. Similar results and IC50s in the lower-micromolar range were obtained by Ignatushchenko et al. (19) for xanthones. Xanthones are, like EA and derivatives, planar aromatic systems, and the authors suggest that the ability to inhibit heme aggregation represents the mode of the antiplasmodial action of these compounds.
Drug combination assays revealed no synergistic action for the polyphenolic compounds with either of the antimalarials tested. Rather, EA, FEA, and CEA acted antagonistically in vitro. Whether CEA, FEA, or their potential derivatives could be successful in combination therapy with known antimalarials in vivo remains to be studied.
The potential of CEA and FEA as antimalarial lead compounds is substantiated by their low cytotoxicity in human cells. At low-nanomolar CEA concentrations, which would be required for the inhibition of P. falciparum growth in vitro, the number of human A549 cells undergoing cell death is marginal. These data are further supported by the fact that EA and related compounds have even been recommended as cancer-chemopreventive agents in humans (33).
The impact of this finding also is reflected in transcript levels and enzyme activities of A549 cells after treatment with EA and its analogues (Table 4). Two different concentrations of each compound were applied on the basis of preliminary inhibitory tests at which less than 10% cell death in the culture occurred after 24 h of incubation. The cellular effects caused by EA, FEA, and CEA were negligible considering the much lower inhibitor concentrations required for antiplasmodial activity. The inhibition (about 60%) of human TrxR activity by CEA should be mentioned (Table 4); it occurred at an inhibitor concentration near the IC50 on the isolated enzyme (Table 2).
All aspects delineated above feature CEA as the most potent of the compounds tested. In vivo studies in a P. berghei mouse model indicated that CEA is able to improve the survival of infected animals. The slow decrease of parasitemia matches the in vitro data on stage specificity, which demonstrate that CEA is a slowly acting compound. CQ, which is known to be a fast-acting antimalarial, was assessed in parallel. An exposure time of ≥1 h at a high compound concentration range was sufficient to achieve substantial growth inhibition of all parasite stages. Our stage dependency data are in good agreement with other studies using similar techniques and reporting that trophozoite and schizont stages are considerably more sensitive to CQ than ring stages (25, 34). CEA, in contrast, was found to be strongly stage specific for trophozoites, for which a long exposure time seemed to be more critical than a high compound concentration (Fig. 4). Thus, CEA is a promising candidate for use in combination therapy with the fast-acting drugs that now are applied in order to prevent and circumvent drug resistance (35). The fact that CEA showed the highest activity against parasites in the trophozoite stage, when hemoglobin catabolism is maximal, is consistent with the observation that CEA inhibits heme aggregation.
As rapidly growing and multiplying cells, malarial parasites rely on functional and efficient antioxidant systems for survival (3). Transcriptional studies revealed that CEA-treated parasites possess impaired redox capacities, since the mRNA levels of several antioxidant enzymes, including GST, GR, and TrxR, were remarkably downregulated (Fig. 5). It is unlikely that this downregulation of redox-active genes is simply a cell death-related event: (i) the genes were downregulated compared to the levels of two independent reference genes (LDH and 18S rRNA), and (ii) other genes (for HSP, GS, and Sel1) showed stable transcription or even upregulation. Furthermore, to rule out that the observed downregulation of redox genes is simply an effect of any toxic agent, we determined the effects of inducers of oxidative stress (20 μM paraquat) or nitrosative stress (10 μM sodium nitroprusside) on TrxR, TPx1, and Sel1 transcript levels in P. falciparum 3D7. After an incubation time of 15 h, all three genes were clearly upregulated using the same reference gene (18S rRNA) and, thus, followed a trend opposite that observed for CEA (data not shown).
In summary, the two compounds structurally related to EA show improved antiplasmodial activity. Combined with their low cytotoxicity, they represent promising antimalarial lead compounds (international patent application no. PCT/EP2006/060707) that might be particularly effective against CQ-resistant P. falciparum.
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (Be 1540/10-1).
We thank Elisabeth Fischer and Anette Netsch for their excellent technical assistance in cell culture as well as Anne Röseler, Monique Akoachere, and Marina Fischer for their support.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Ahmadi, R., S. Urig, M. Hartmann, B. M. Helmke, S. Koncarevic, B. Allenberger, C. Kienhoefer, M. Neher, H. H. Steiner, A. Unterberg, C. Herold-Mende, and K. Becker. 2006. Antiglioma activity of 2,2′:6′,2″-terpyridineplatinum(II) complexes in a rat model—effects on cellular redox metabolism. Free Radic. Biol. Med. 40:763-778. [DOI] [PubMed] [Google Scholar]
- 2.Banzouzi, J. T., R. Prado, H. Menan, A. Valentin, C. Roumestan, M. Mallie, Y. Pelissier, and Y. Blache. 2002. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. J. Ethnopharmacol. 81:399-401. [DOI] [PubMed] [Google Scholar]
- 3.Becker, K., L. Tilley, J. L. Vennerstrom, D. Roberts, S. Rogerson, and H. Ginsburg. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol. 34:163-189. [DOI] [PubMed] [Google Scholar]
- 4.Bohle, D. S., A. D. Kosar, and P. W. Stephens. 2002. Phase homogeneity and crystal morphology of the malaria pigment beta-hematin. Acta Crystallogr. D Biol. Crystallogr. 58:1752-1756. [DOI] [PubMed] [Google Scholar]
- 5.Constantinou, A., G. D. Stoner, R. Mehta, K. Rao, C. Runyan, and R. Moon. 1995. The dietary anticancer agent ellagic acid is a potent inhibitor of DNA topoisomerases in vitro. Nutr. Cancer 23:121-130. [DOI] [PubMed] [Google Scholar]
- 6.Das, M., D. R. Bickers, and H. Mukhtar. 1984. Plant phenols as in vitro inhibitors of glutathione S-transferase(s). Biochem. Biophys. Res. Commun. 120:427-433. [DOI] [PubMed] [Google Scholar]
- 7.Das, M., S. V. Singh, H. Mukhtar, and Y. C. Awasthi. 1986. Differential inhibition of rat and human glutathione S-transferase isoenzymes by plant phenols. Biochem. Biophys. Res. Commun. 141:1170-1176. [DOI] [PubMed] [Google Scholar]
- 8.Dell'Agli, M., S. Parapini, N. Basilico, L. Verotta, D. Taramelli, C. Berry, and E. Bosisio. 2003. In vitro studies on the mechanism of action of two compounds with antiplasmodial activity: ellagic acid and 3,4,5-trimethoxyphenyl(6′-O-aalloyl)-beta-D-glucopyranoside. Planta Med. 69:162-164. [DOI] [PubMed] [Google Scholar]
- 9.Deponte, M., and K. Becker. 2005. Glutathione S-transferase from malarial parasites: structural and functional aspects. Methods Enzymol. 401:241-253. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Färber, P. M., L. D. Arscott, C. H. Williams, Jr., K. Becker, and R. H. Schirmer. 1998. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 422:311-314. [DOI] [PubMed] [Google Scholar]
- 12.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz-Wolf, K., A. Becker, S. Rahlfs, P. Harwaldt, R. H. Schirmer, W. Kabsch, and K. Becker. 2003. X-ray structure of glutathione S-transferase from the malarial parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 100:13821-13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geevananda, Y. A., P. Gunawardana, N. S. Kumar, and M. U. S. Sultanbawa. 1979. Three hydroxy ellagic acid methyl ethers, chrysophanol and scopoletin from Shorea worthingtonii and Vatica obscura. Phytochemistry 18:1017-1019. [Google Scholar]
- 15.Gromer, S., L. D. Arscott, C. H. Williams, Jr., R. H. Schirmer, and K. Becker. 1998. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 273:20096-20101. [DOI] [PubMed] [Google Scholar]
- 16.Harwaldt, P., S. Rahlfs, and K. Becker. 2002. Glutathione S-transferase of the malarial parasite Plasmodium falciparum: characterization of a potential drug target. Biol. Chem. 383:821-830. [DOI] [PubMed] [Google Scholar]
- 17.Hayeshi, R., I. Mutingwende, W. Mavengere, V. Masiyanise, and S. Mukanganyama. 2007. The inhibition of human glutathione S-transferases activity by plant polyphenolic compounds ellagic acid and curcumin. Food Chem. Toxicol. 45:286-295. [DOI] [PubMed] [Google Scholar]
- 18.Hiller, N., K. Fritz-Wolf, M. Deponte, W. Wende, H. Zimmermann, and K. Becker. 2006. Plasmodium falciparum glutathione S-transferase—structural and mechanistic studies on ligand binding and enzyme inhibition. Protein Sci. 15:281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignatushchenko, M. V., R. W. Winter, H. P. Bachinger, D. J. Hinrichs, and M. K. Riscoe. 1997. Xanthones as antimalarial agents; studies of a possible mode of action. FEBS Lett. 409:67-73. [DOI] [PubMed] [Google Scholar]
- 20.Irmler, A., A. Bechthold, E. Davioud-Charvet, V. Hofmann, R. Réau, S. Gromer, R. H. Schirmer, and K. Becker. 2002. Disulfide reductases—current developments, p. 803-815. In S. Chapman, R. Perham, and N. Scrutton (ed.), Flavins and flavoproteins, vol. 14. Walter de Gruyter, New York, NY. [Google Scholar]
- 21.Kanzok, S. M., R. H. Schirmer, I. Turbachova, R. Iozef, and K. Becker. 2000. The thioredoxin system of the malaria parasite Plasmodium falciparum. Glutathione reduction revisited. J. Biol. Chem. 275:40180-40186. [DOI] [PubMed] [Google Scholar]
- 22.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 23.Loarca-Piña, G., P. A. Kuzmicky, E. G. de Mejia, and N. Y. Kado. 1998. Inhibitory effects of ellagic acid on the direct-acting mutagenicity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat. Res. 398:183-187. [DOI] [PubMed] [Google Scholar]
- 24.Lobanov, A. V., C. Delgado, S. Rahlfs, S. V. Novoselov, G. V. Kryukov, S. Gromer, D. L. Hatfield, K. Becker, and V. N. Gladyshev. 2006. The Plasmodium selenoproteome. Nucleic Acids Res. 34:496-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maerki, S., R. Brun, S. A. Charman, A. Dorn, H. Matile, and S. Wittlin. 2006. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J. Antimicrob. Chemother. 58:52-58. [DOI] [PubMed] [Google Scholar]
- 26.Mannervik, B., and U. H. Danielson. 1988. Glutathione transferases—structure and catalytic activity. CRC Crit. Rev. Biochem. 23:283-337. [DOI] [PubMed] [Google Scholar]
- 27.Narayanan, B. A., O. Geoffroy, M. C. Willingham, G. G. Re, and D. W. Nixon. 1999. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 136:215-221. [DOI] [PubMed] [Google Scholar]
- 28.Nijhoff, W. A., and W. H. Peters. 1994. Quantification of induction of rat oesophageal, gastric and pancreatic glutathione and glutathione S-transferases by dietary anticarcinogens. Carcinogenesis 15:1769-1772. [DOI] [PubMed] [Google Scholar]
- 29.Nordhoff, A., U. S. Bucheler, D. Werner, and R. H. Schirmer. 1993. Folding of the four domains and dimerization are impaired by the Gly446→Glu exchange in human glutathione reductase. Implications for the design of antiparasitic drugs. Biochemistry 32:4060-4066. [DOI] [PubMed] [Google Scholar]
- 30.Perkin, A. G. 1911. Some Oxidation Products of the hydroxybenxoic acids. J. Chem. Soc. 99:1442-1450. [Google Scholar]
- 31.Peters, W. 1987. Chemotherapy and drug resistance in malaria, vol. 1. Academic Press, London, United Kingdom.
- 32.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoner, G. D., and H. Mukhtar. 1995. Polyphenols as cancer chemopreventive agents. J. Cell Biochem. Suppl. 22:169-180. [DOI] [PubMed] [Google Scholar]
- 34.ter Kuile, F., N. J. White, P. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76:85-95. [DOI] [PubMed] [Google Scholar]
- 35.Tilley, L., T. M. Davis, and P. G. Bray. 2006. Prospects for the treatment of drug-resistant malaria parasites. Future Microbiol. 1:127-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 37.Verotta, L., M. Dell'Agli, A. Giolito, M. Guerrini, P. Cabalion, and E. Bosisio. 2001. In vitro antiplasmodial activity of extracts of Tristaniopsis species and identification of the active constituents: ellagic acid and 3,4,5-trimethoxyphenyl-(6′-O-galloyl)-O-beta-D-glucopyranoside. J. Nat. Prod. 64:603-607. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann, H., J. Billard, H. Gutman, E. J. Wachtel, R. Poupko, and Z. Luz. 1992. Thermotropic liquid crystals derived from benzopyranobenzopyran-dione. Preparation and physical properties. Liquid Crystals 12:245-262. [Google Scholar]