Abstract
Ibalizumab (formerly TNX-355) is a humanized monoclonal antibody that binds CD4, the primary receptor for human immunodeficiency virus type 1 (HIV-1), and inhibits the viral entry process. A phase lb multidose study of the safety, pharmacokinetics, and antiviral activity of ibalizumab was conducted with 22 HIV-1-infected patients. Nineteen patients were randomized to receive either 10 mg/kg of body weight weekly (arm A) or a 10-mg/kg loading dose followed by 6 mg/kg every 2 weeks (arm B) intravenously for 9 weeks. Three patients were assigned to receive 25 mg/kg every 2 weeks for five doses (arm C). During the study, the patients remained off other antiretrovirals or continued a stable failing regimen. Treatment with ibalizumab resulted in substantial reductions in HIV-1 RNA levels (0.5 to 1.7 log10) in 20 of 22 subjects. In most patients, HIV-1 RNA fell to nadir levels after 1 to 2 weeks of treatment and then returned to baseline despite continued treatment. Baseline viral isolates were susceptible to ibalizumab in vitro, regardless of coreceptor tropism. Emerging resistance to ibalizumab was manifested by reduced maximal percent inhibition in a single-cycle HIV infectivity assay. Resistant isolates remained CD4 dependent and were susceptible to enfuvirtide in vitro. Complete coating of CD4+ T-cell receptors was correlated with serum ibalizumab concentrations. There was no evidence of CD4+ T-cell depletion in ibalizumab-treated patients. Ibalizumab was not immunogenic, and no serious drug-related adverse effects occurred. In conclusion, ibalizumab administered either weekly or biweekly was safe and well tolerated and demonstrated antiviral activity. Further studies with ibalizumab in combination with standard antiretroviral treatments are warranted.
Since the introduction of highly active antiretroviral therapy (HAART), mortality and morbidity from human immunodeficiency virus (HIV) disease have dropped dramatically (13). Unfortunately, many patients treated with HAART regimens still fail to achieve or maintain optimal control of the infection (http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf). Viral replication in the presence of nonsuppressive drug concentrations leads to the development of antiviral drug resistance and viral rebound. Furthermore, extensive cross-resistance, overlapping toxicities, and negative drug-drug interactions among antiretrovirals limit the choices for treatment (14). Thus, there is a continued need for new antiviral agents for treating HIV infections. Such agents are most useful if they are effective against strains of HIV type 1 (HIV-1) with reduced susceptibility to current therapies, are associated with minimal side effects and drug-drug interactions, and foster adherence through convenient administration schedules. Agents targeting unique steps in the life cycle of the virus are of special interest in meeting this demand. To this end, inhibitors of HIV entry have been developed. The fusion inhibitor enfuvirtide (Fuzeon) and the CCR5 coreceptor antagonist maraviroc (Selzentry) have been approved by the U.S. Food and Drug Administration for use in treatment-experienced patients. Both have shown efficacy when combined with an optimized background regimen in clinical trials (8, 9, 11a) The safety and efficacy data from these trials support the rationale for using a virus entry inhibitor as a component of HAART.
Ibalizumab is a humanized immunoglobulin G4 monoclonal antibody that blocks HIV entry in a manner distinct from those of other entry inhibitors. Ibalizumab binds to a conformational epitope on domain 2 of the extracellular portion of CD4 (2), the primary receptor for HIV on CD4+ T cells. By blocking CD4-dependent HIV entry, ibalizumab is active against a broad spectrum of HIV isolates, including isolates from multiple clades, as well as both CCR5-tropic and CXCR4-tropic HIV isolates. Anti-CD4 monoclonal antibodies that bind domain 1 of CD4 were previously found to be immunosuppressive because of interference with major histocompatibility complex class II-mediated immune functions (4, 11). Such therapies are unsuitable for the treatment of HIV disease. With its unique specificity for domain 2 of CD4, ibalizumab inhibits postbinding steps required for viral entry and fusion without interfering with major histocompatibility complex class II-mediated immune functions and without causing immunosuppression (15). Ibalizumab was shown to safely lower plasma HIV-1 RNA levels when administered at up to 25 mg/kg of body weight as a single dose in infected patients (7). This report describes the safety, pharmacokinetics, and antiviral activity of multiple doses of ibalizumab given to patients with HIV-1 infection and evidence of ongoing viral replication.
MATERIALS AND METHODS
Study population.
HIV-1-infected patients aged 18 years or older were eligible for this study if they had stable HIV RNA levels (viral loads) of ≥5,000 copies/ml and a CD4+ cell count of 100 to 500 cells/mm3. A stable viral load was defined as a difference of ≤0.5 log10 HIV RNA copies/ml between two measurements obtained at least 48 h apart within 30 days prior to study entry. Patients were eligible if they had been receiving either a stable regimen or no therapy for ≥12 weeks and were willing to continue with their current regimen (i.e., no changes or additions) for at least 13 weeks after initiating ibalizumab study medication. Patients who had participated in previous studies with ibalizumab were not eligible for the study. Appropriate prophylaxis of opportunistic infections was provided for patients with CD4+ cell counts of less than 200/mm3. Exclusion criteria included the presence of another active infection requiring therapy, use of immunomodulating drugs or systemic chemotherapy, receipt of investigational immunomodulatory or investigational antiretroviral therapy within 12 weeks prior to enrollment, prior participation in an HIV vaccine trial, pregnancy, and breastfeeding. Female patients with childbearing potential and heterosexually active male patients were required to use effective contraception during the study. The study was approved by the institutional review board at each participating medical center, and all potential subjects gave written informed consent.
Study design.
In the initial part of this phase 1, multicenter, open-label, multidose study, eligible patients (n = 19) were randomized to one of two treatment arms (arm A, n = 9, or arm B, n = 10). Enrollment in a third group (arm C, n = 3) was not randomized and began after completion of enrollment in arms A and B in order to study higher dosing of the drug. The dose regimens are illustrated in Table 1. Patients received ibalizumab via intravenous infusion (lasting approximately 1 hour) for a 9-week period starting on day 1. Those in arm A received 10 mg/kg of ibalizumab every week for a total of 10 doses, while those in arm B received a single loading dose of 10 mg/kg on day 1, followed by five maintenance doses of 6 mg/kg every 2 weeks starting at week 1 for a total of six doses of ibalizumab. Patients in arm C received 25 mg/kg of ibalizumab every 2 weeks for a total of five doses. After the last administration of ibalizumab at the week 9 visit for arms A and B and the week 8 visit for arm C, all patients were followed for safety for an additional 7 and 8 weeks, respectively.
TABLE 1.
Ibalizumab multiple-dose regimens
| Arm | Dose (mg/kg) | Dose administrationa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Week
|
||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| A | 10 | + | + | + | + | + | + | + | + | + | + |
| B | 10 | + | |||||||||
| 6 | + | + | + | + | + | ||||||
| C | 25 | + | + | + | + | + | |||||
Dose administration is indicated by a plus.
Assessment and follow-up.
Patients were assessed at screening and weeks 1, 2, 5, 10, 13, and 16. Study visits included clinical assessment, safety evaluations, and the determination of plasma HIV-1 RNA levels, CD4+ and CD8+ T-cell counts, the percentage of activated CD4+ T cells (CD4+ CD25+ cells), and the extent of CD4+ T-cell receptor coating by ibalizumab. Samples to measure drug concentrations were collected at weekly intervals throughout the dosing period, immediately before (trough) and after (peak) ibalizumab infusions. The immunogenicity of ibalizumab was also monitored by measuring the concentrations of anti-ibalizumab antibodies in predose serum samples at day 1 and again at weeks 3, 8, 11, 13, and 16 for all arms, plus weeks 24 and 32 for arm C only. Patients were removed from the study if they experienced a sustained decrease from baseline in CD4+ cell counts of ≥50% at two consecutive visits, a confirmed >0.7-log10-unit increase in HIV RNA from baseline at two consecutive visits, or adverse events (AEs) of dose-limiting toxicity. Dose-limiting toxicity was defined as hypersensitivity with severity grade 2 or higher or any AE with severity grade 3 or 4 (according to the modified National Institute of Allergy and Infectious Diseases criteria) that were considered drug related.
Plasma assays.
Plasma HIV-1 RNA levels were determined by the Amplicor HIV-1 Monitor Assay (version 1.0; Roche Molecular Systems, Branchburg, NJ). Immunophenotyping of patient samples to determine cell counts and to monitor T-cell activation was performed by Icon Laboratories (Farmingdale, NY) using standard flow cytometry methods. Immunoassays used to measure ibalizumab concentrations and anti-ibalizumab antibodies in serum were performed at Tanox, Inc. (Houston, TX). Serum samples were analyzed to determine ibalizumab levels by using a competitive enzyme immunoassay method with a lower limit of detection of 110 ng/ml. The titer of anti-ibalizumab antibodies in serum was monitored by using enzyme-linked immunosorbent assays. Measureable anti-drug antibody titers in postdose samples of ≥2 times the corresponding predose sample were considered positive and evaluated further.
Cell-coating assay.
The extent to which patient CD3+ CD4+ cells were bound by ibalizumab, termed CD4+ T-cell receptor coating, was also determined using flow cytometry (Icon Laboratories). For this, blood samples were stained with anti-CD3 and anti-CD4 antibodies to identify target cells using antibodies that did not interfere with ibalizumab binding, as determined by flow cytometry, and then incubated with phycoerythrin-conjugated ibalizumab, as described previously (7). The fluorescence intensity limits corresponding to completely uncoated or completely coated cells were determined using blood from healthy seronegative donors that was left untreated or preincubated with 100 μg/ml of unlabeled ibalizumab, respectively. Patient specimens were determined to be completely coated or uncoated when ≥90% of cells showed fluorescence intensities above these limits for their respective assays. Patient specimens in which >10% of cells showed intermediate fluorescence intensity were described as having a “partial” coating.
In vitro susceptibility.
The ibalizumab susceptibilities and coreceptor tropism of patient HIV isolates were determined at Monogram Biosciences (formerly ViroLogic) using the Phenosense HIV Entry and Trofile assays, respectively. Briefly, plasma samples were collected from patients before treatment with ibalizumab was initiated (baseline) and after administration of the last dose of ibalizumab (9 weeks). The samples were shipped on ice to Monogram Biosciences for in vitro susceptibility testing of viruses present in patient sera using single-cycle infectivity assays. These assays use recombinant viruses that express patient-derived HIV envelope proteins to evaluate the entry inhibitor drug susceptibilities and coreceptor tropism of patient viruses.
Enfuvirtide susceptibility.
Enfuvirtide-resistant envelope clones were generated to evaluate their susceptibilities to ibalizumab. The JRCSF envelope gene was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH (ARRRP) and cloned into the pCI-neo vector (Promega, Madison, WI) for mammalian cell expression. Enfuvirtide resistance-associated mutations in gp41 (G36D, V38A, and N43D) were introduced by site-directed mutagenesis (Quik Change II; Stratagene, La Jolla, CA). Archived serum samples taken from study subjects at day 1 and week 9 were obtained after study closure, and HIV envelopes were amplified by reverse transcription-PCR and cloned into pCI-neo. Single-cycle HIV infectivity assays were performed using the envelope-defective reporter (luciferase) provirus pNL4-3.Luc.E−.R− and U87 target cells expressing both CD4 and either CCR5 or CXCR4 coreceptor (ARRRP). For dose-response studies, luciferase activity was measured in triplicate wells and the values were averaged for curve fitting. Dose-response curves were fitted using the Origin Client (v7) software with a four-parameter logistic to determine values for the 50% inhibitory concentration (IC50) and the percent maximum inhibition (PMI), using the following equation: y = A + [(B − A)]/{1 + [(C/x)D]}, where A is the percent inhibition at the lowest inhibitor concentration, B is the percent inhibition at the highest inhibitor concentration, C is the inhibitor concentration at the middle of the curve, and D is the slope of the curve. Ibalizumab was produced at Tanox, Inc. (Houston, TX); enfuvirtide was dissolved in sterile water prior to use.
CD4 dependence.
HIV envelope clones with reduced susceptibility to ibalizumab were identified by in vitro dose-response studies. Tests for CD4 dependence were conducted using human osteosarcoma cell lines (ARRRP) that were negative for CD4 expression (HOS) or that stably expressed CD4 (GHOST), both with and without CCR5 or CXCR4 expression. The receptor phenotypes and appropriate receptor expression for each cell line were confirmed by flow cytometry (data not shown). Dose-response studies with ibalizumab with or without anti-CD4 domain 1 (Q4120; Sigma) were conducted as described above. Studies with vesicular stomatitis virus G protein (VSV-G) in CD4-negative and CD4-positive HOS cells employed the pVPack-VSV-G vector (Stratagene, La Jolla, CA). Infectivity assays were performed in the same manner as susceptibility assays except that antiretroviral agents were omitted.
Statistical analysis.
Patients receiving at least one dose of the study drug were included in the analyses of safety data. Evaluation of differences between treatment arms for efficacy (pharmacodynamic endpoints) was based on two patient populations. Primary analyses were based on a modified-intent-to-treat group of patients that included randomized patients receiving study drug with a valid baseline and at least one valid measurement after receiving a dose of ibalizumab. Secondary analyses were performed on those modified-intent-to-treat patients lacking major protocol violations who received all doses of study medication. Serum drug concentrations were assessed for all patients who received one or more doses of the study drug.
Treatment group differences were summarized descriptively for demographic data, baseline disease characteristics, prior and concomitant medications, treatment compliance, physical examination findings, AEs, laboratory tests, and final disposition. Continuous variables were summarized by the number of patients without missing data, mean, standard deviation, median, and minimum and maximum values. Discrete variables were summarized by their counts and associated percentages. Two-sample Wilcoxon rank sum tests were used to assess treatment differences for measures of efficacy. Count data were transformed as needed (e.g., log10 transformation of HIV-1 RNA values). Exploratory analyses were carried out for select subgroups and for stratification of baseline factors. A graphical display of treatment effects by time was plotted for a limited number of efficacy and safety data points. Serum drug concentration data were presented using descriptive statistics by treatment group and time point. Baseline plasma HIV-1 RNA and CD4+ T-cell counts were defined as the mean of the second screening value and the day zero (preinfusion) value. Data are presented as mean change from baseline.
Pharmacokinetic modeling of subject data (arms A and C) was performed using WinNonlin (WinNonlin Pro Node version 3.2; Pharsight Corp., Mountain View, CA). A noncompartmental analysis of each subject's serum drug concentration data was performed using WinNonlin Model 202 for intravenous administration of the drug over a timed period. For a consistent evaluation of the pharmacokinetic parameters, user-defined limits were used to construct the terminal elimination lines. No serum drug concentrations determined immediately at the end of infusion were used in the determination of the terminal elimination line. The last time point included in the user-defined limit was that time point corresponding to the first visit at which the serum ibalizumab concentration fell below 1 μg/ml. For subjects in arm B, the elimination of the drug was more rapid than the interval between times at which blood samples were collected, and therefore, no estimation of terminal elimination pharmacokinetics could be made for those subjects.
RESULTS
Patient characteristics.
Of 22 HIV-1-infected patients enrolled in this study, 21 were triple-class treatment experienced, and one had not previously received antiretroviral therapy. At the time of enrollment, 6 of the 21 treatment-experienced patients were failing their treatment regimen and 15 were not receiving antiretrovirals. Thus, ibalizumab was likely to be the only active agent in the antiviral drug regimen for all patients during the course of this study. A total of 19 patients were randomized between two cohorts, A and B, to be administered ibalizumab for 9 weeks. A schematic of the three dose regimens employed in this trial is presented in Table 1. Patients in arm A received a 10-mg/kg infusion of ibalizumab every 7 days, starting on day 1, for a total of 10 doses. This regimen was selected based on the results of a single-dose phase 1 study that demonstrated antiviral efficacy and coating of CD4+ T cells with 10 mg/kg ibalizumab (7). Arms B and C were designed to vary the dose level and/or frequency of ibalizumab administration. Patients in arm B received a 10-mg/kg infusion of ibalizumab on day 1 (loading dose), followed by five maintenance doses of 6 mg/kg every 14 days starting at week 1. In arm C, three patients received infusions of 25 mg/kg of ibalizumab every 14 days through week 8, for a total of five doses. All patients were monitored for safety through week 16, at which point the study ended. Baseline cohort characteristics were comparable across the three arms (Table 2), including baseline mean log10 HIV-1 RNA levels and CD4+ cell counts.
TABLE 2.
Baseline characteristics of study populationa
| Characteristic | Value for arm:
|
||
|---|---|---|---|
| A | B | C | |
| Dose regimen (mg/kg) | 10 | 10, 6 | 25 |
| n | 9 | 10 | 3 |
| Mean age (min, max) (yr) | 39 (24, 60) | 42 (22, 52) | 41 (36, 43) |
| Gender (%) | |||
| Male | 8 (89) | 9 (90) | 2 (67) |
| Female | 1 (11) | 1 (10) | 1 (33) |
| Race | |||
| White | 7 (78) | 8 (80) | 2 (67) |
| Black | 1 (11) | 0 (0) | 1 (33) |
| Hispanic | 1 (11) | 2 (20) | 0 (0) |
| Mean wt (min, max) (kg) | 80 (60, 100) | 75 (65, 87) | 69 (58, 80) |
| Median CD4+ T-cell count/mm3 (min, max) | 294 (89, 462) | 327 (109, 458) | 338 (274, 417) |
| Median baseline HIV-1 RNA (log10 copies/ml) (range) | 4.7 (3.8-5.7) | 4.7 (3.8-5.6) | 4.9 (4.2-5.7) |
Patients not taking antiretrovirals at baseline remained off background therapy during the study. Patients taking a failing regimen at baseline continued the failing regimen during the study.
Antiviral response.
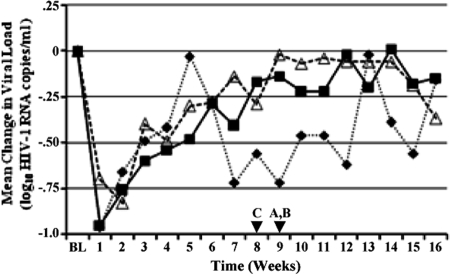
Administration of ibalizumab at all three doses used in this study exhibited anti-HIV activity, as demonstrated by transient but clinically meaningful reductions in HIV-1 RNA. At week 1 and at each visit during the infusion period, all treatment groups demonstrated reductions from baseline values in viral loads, with the largest mean reductions (nadirs) occurring at week 1 in arms A (−0.95 ± 0.33 log10 copies/ml) and C (−0.96 ± 0.61 log10 copies/ml) and at week 2 in arm B (−0.83 ± 0.43 log10 copies/ml). The mean changes in HIV-1 RNA levels over time for each cohort are shown in Fig. 1, which shows that viral loads had returned to baseline values in all three treatment arms by the end of the infusion period. After the infusion period and through the study endpoint, the viral loads for treatment groups A and B remained at baseline levels, while group C remained at or slightly below the baseline level. HIV-1 RNA levels decreased by >1.0 log10 unit in 64% of all patients across the three arms, and there were no statistically significant differences among the treatment groups in HIV-1 RNA average area under the curve minus baseline. Twenty out of 22 patients experienced viral-load reductions greater than −0.5 log10 unit/ml. The other two patients, one in arm B and one in arm C, experienced initial viral-load reductions of −0.43 and −0.37 log10 unit/ml, respectively, before their viral loads rebounded.
FIG. 1.
Mean changes in viral loads from baseline. Shown are mean HIV-1 load (log10 HIV-1 copies/ml) changes from baseline in study arms A (10 mg/kg) (filled squares), B (10 mg/kg plus 6 mg/kg) (open triangles), and C (25 mg/kg) (filled diamonds). The data were derived from weekly viral-load measurements obtained for all patients in the study. The last administration of ibalizumab (arrowheads) occurred at week 8 (arm C) or 9 (arms A and B).
Immunological response.
Patients in all three treatment arms experienced mean increases from baseline in CD4+ T-cell counts during the 9-week infusion period, with the largest mean increases occurring at week 1 (238 [range, 57 to 715] cells/mm3 for arm A and 186 [range, 12 to 449] cells/mm3 for arm B) or week 2 (129 [range, 41 to 206] cells/mm3 for arm C) (Fig. 2). The 10-mg/kg treatment group (arm A) exhibited greater mean changes from baseline in CD4+ cell counts than the other treatment groups and had increased by 112 cells/μl at the end of the treatment period. The CD4+ T-cell counts tended to decline toward the end of the treatment period, after which the CD4+ T-cell counts appeared to stabilize near the baseline for each treatment group. There were no statistically significant differences between the treatment groups with respect to CD4+ T-cell counts.
FIG. 2.
Mean changes in CD4+ counts from baseline. Shown are mean CD4+ T-cell count changes from baseline in study arms A (10 mg/kg) (filled squares), B (10 mg/kg plus 6 mg/kg) (open triangles), and C (25 mg/kg) (filled diamonds). The data were derived from weekly CD4+ T-cell counts obtained for all patients in the study. The last administration of ibalizumab (arrowheads) occurred at week 8 (arm C) or 9 (arms A and B).
CD4+ T-cell receptor coating.
The degree to which CD4+ T cells were coated with ibalizumab was evaluated in patients prior to dosing at weekly intervals throughout the study. The results are presented in Table 3. CD4+ T-cell receptors were completely coated in all patients in arm A and arm C throughout the treatment intervals, and complete coating was maintained for at least 2 weeks following the final dose infusion. In arm B, CD4+ T-cell receptors were completely coated in all but one patient through week 2, but no patients maintained complete coating of CD4+ T-cell receptors during subsequent 2-week dose intervals. Likewise, complete coating of CD4+ T cells was lost in all patients in arm B in the 2 weeks following the final infusion of 6 mg/kg. As expected, the degree to which CD4+ T-cell receptors were coated was highly dependent on the serum ibalizumab concentration. Complete coating of T-cell receptors was generally observed only when serum ibalizumab concentrations exceeded 5 μg/ml (data not shown). Serum ibalizumab concentrations between 0.5 and 5 μg/ml were generally associated with partial coating of CD4+ T cells. When serum ibalizumab concentrations fell below 0.5 μg/ml, the CD4+ T-cell receptors generally became uncoated.
TABLE 3.
CD4+ T-cell coating over time
| Visit (week no.)a | % of patients with complete CD4+ T-cell coating in each study arm:
|
||
|---|---|---|---|
| A | B | C | |
| 1 | 100 | 89 | 100 |
| 2 | 100 | 100 | 100 |
| 3 | 100 | 25 | 100 |
| 4 | 100 | 63 | 100 |
| 5 | 100 | 25 | 100 |
| 6 | 100 | 63 | 100 |
| 7 | 100 | 13 | 100 |
| 8 | 100 | 50 | 100 |
| 9 | 100 | 17 | 100 |
| 10 | 100 | 67 | 100 |
| 11 | 100 | 0 | 33 |
| 12 | 29 | 0 | 33 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
CD4+ T-cell receptor coating was evaluated prior to dosing when the visit corresponded to a dosing week.
Pharmacokinetics.
Measurable serum ibalizumab concentrations were maintained throughout each dosing interval in the 10-mg/kg (arm A) and 25-mg/kg (arm C) treatment groups (Table 4). Accumulation of ibalizumab was evident from increasing trough values when the drug was administered weekly at a dose of 10 mg/kg and to a lesser extent when it was administered every 2 weeks at a dose of 25 mg/kg, though there was significant (110%) variability in serum drug concentrations later in the dosing period among the three patients in arm C. There was no evidence of drug accumulation in the sera of patients administered 6 mg/kg every 2 weeks (arm B), and ibalizumab was rapidly cleared from the systemic circulation following the final intravenous infusion.
TABLE 4.
Ibalizumab serum concentrations
| Sample | Concn (μg/ml) in arm:
|
||
|---|---|---|---|
| A | B | C | |
| Postinfusion, day 1 (peak) | 280 ± 69 | 215 ± 51 | 429 ± 195 |
| Preinfusion, week 1 (trough) | 48 ± 14 | 31 ± 12 | 96 ± 31 |
| Preinfusion, week 9a (trough) | 138 ± 58 | 0.2 ± 0.1 | 96 ± 106 |
| Postinfusion, week 9a (peak) | 411 ± 122 | 108 ± 33 | 564 ± 267 |
Week 8 in arm C.
Serum drug concentration data for arms A and C were sufficient to provide an estimate of the pharmacokinetics of ibalizumab following the final dose of the drug (Table 5). The elimination half-life of ibalizumab in this study was estimated to be 3 to 3.5 days on average and ranged from 1.8 to 5.1 days across both arms. For subjects in arm B, the rapid drop in serum ibalizumab concentrations and the lack of multiple collection points in the first week following the final dose precluded any estimation of terminal elimination pharmacokinetics. While systemic exposure appeared to be dose dependent, the actual dose linearity was difficult to evaluate given the difference in dose regimens for arms A and B and the limited number of patients in arm C.
TABLE 5.
Ibalizumab pharmacokinetic-parameter estimates for dose arms A and Ca
| Arm | Dose (mg/kg) | Cmax (μg/ml) | AUCall (μg·day/ml) | t1/2 (days) | VDss (ml/kg) | CLss (ml/day/kg) |
|---|---|---|---|---|---|---|
| A | 10 | 402 ± 117 (206-627) | 3,604 ± 1,530 (1,523-5,910) | 3.3 ± 0.9 (2.3-5.0) | 44 ± 21 (18-78) | 5.7 ± 2.3 (3.6-11) |
| C | 25 | 564 ± 267 (378-870) | 4,941 ± 4,433 (2,250-10,058) | 3.1 ± 1.8 (1.8-5.1) | 50 ± 20 (27-64) | 8.8 ± 4.7 (3.5-12) |
Mean ± standard deviation (minimum-maximum) from noncompartmental analysis (see Materials and Methods). The parameters are as follows: Cmax, maximum concentration of drug in serum; AUC, area under the concentration-time curve; t1/2, half-life; VDss, volume of distribution at steady state; CLss, clearance at steady state.
Safety.
Ibalizumab was well tolerated during the study. Of 22 patients, 20 received all scheduled administrations of the study drug. The most frequent treatment-emergent AEs reported by subjects were headache (two subjects in each treatment arm), nausea (two subjects in arm A and one subject in arm B), and productive cough (three subjects in arm B). There were no intravenous-fusion-related AEs and no infusion site reactions. Ten patients reported at least one AE that was considered to be related to study drug administration. All were mild to moderate in severity, and the only drug-related AE that was reported by more than one patient was headache (n = 4); the numbers of patients and events were similar across the treatment arms. There were no drug-related laboratory abnormalities reported, CD4+ T-cell depletion did not occur, and there were no infections reported. One patient (arm A) was withdrawn from the study after receiving the seventh dose due to a protocol violation. A second patient was withdrawn because of a serious AE (SAE) 7 days after receiving the first dose of the study drug; this event was considered unrelated to the study drug. There were a total of four SAEs, but none were attributed to the study medication.
Patient samples were monitored continuously for the presence of anti-ibalizumab antibodies. Low levels of anti-ibalizumab antibodies (0.1 to 1 μg/ml) were detected in three patients at week 3 of the infusion period, after which the levels declined and became undetectable. Anti-ibalizumab antibodies at levels near the assay background (<0.1 μg/ml) were also detected in samples drawn during study treatment from four additional patients, who also had detectable antibodies in samples taken at the study baseline prior to ibalizumab administration. There was no correlation between the presence of anti-ibalizumab antibodies and serum ibalizumab concentrations, and the significance of this apparent low-level anti-ibalizumab reactivity is unclear.
Baseline susceptibility to ibalizumab and development of resistance.
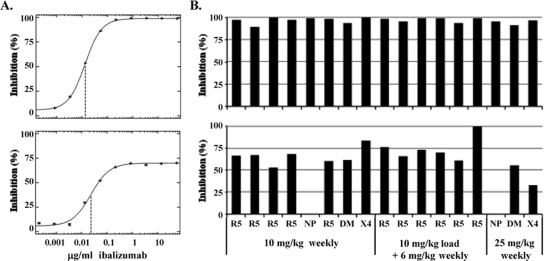
A rapid drop in the viral load was observed after ibalizumab administration in the majority of patients in all dose cohorts. To confirm that patient viruses were susceptible to ibalizumab at study entry, in vitro susceptibility testing was performed. Results were obtained for 17 baseline isolates (see Materials and Methods), and all 17 were susceptible to ibalizumab, with IC50s in the range of 0.02 to 0.16 μg/ml (Fig. 3A). Thirteen of these isolates exhibited tropism for the CCR5 coreceptor only, three exhibited dual-mixed tropism (for both CCR5 and CXCR4 coreceptors), and one exhibited tropism for the CXCR4 receptor only. During the 9-week treatment period, there was a return to baseline viral-RNA levels in most patients despite continued administration of ibalizumab and partial (arm B) or complete (arms A and C) coating of CD4+ T-cell receptors. In vitro susceptibility to ibalizumab was tested for samples obtained at week 9 (Fig. 3A). Thirteen of the 14 posttreatment samples tested demonstrated reduced susceptibility to ibalizumab relative to baseline samples. In each case, a plateau in the dose-response curve was observed such that the PMI was in the range of 33 to 83% compared to PMI values of 89 to 99% observed at baseline (Fig. 3B). The virus from one patient in arm B remained fully susceptible to ibalizumab (99% PMI) in vitro at week 9 (at which point this patient's viral load was still reduced from baseline by 1.1 log10 copies/ml). The concentrations at which maximal inhibition was achieved were similar for both baseline and week 9 samples and are likely to represent the concentrations required for saturation of CD4 receptors on target cells. Thus, for ibalizumab, a reduction in susceptibility was manifested as a reduction in the PMI, rather than a shift in the IC50. No correlation between the level of PMI and the initial virologic response to therapy for individual patients was detected, but there was a temporal association between reduced PMI and the increase in the viral load after 2 to 3 weeks (data not shown).
FIG. 3.
Ibalizumab susceptibilities of patient HIV isolates at baseline and week 9. Shown are results from Phenosense HIV Entry and Trofile assays performed at Monogram Biosciences. (A) Representative dose-response curves used to determine in vitro susceptibilities to ibalizumab for patient HIV isolates taken at baseline (upper graph) and week 9 after the dosing interval (lower graph); the IC50 is indicated by a dashed vertical line. (B) PMIs of all patient HIV isolates from baseline (top) and week 9 (bottom) patient samples. Target cells for the HIV Entry assay dually expressed CCR5 and CXCR4 coreceptors. Virus coreceptor tropism (Trofile) is indicated as R5 (CCR5 tropic), DM (dual-mixed tropic), or X4 (CXCR4 tropic). Two R5 isolates at baseline could not be phenotyped at week 9 (NP). Samples are grouped by dose regimen: 10 mg/kg weekly (arm A), 10 mg/kg weekly plus 6 mg/kg biweekly (arm B), and 25 mg/kg weekly (arm C).
Lack of enfuvirtide cross-resistance.
At the time of the study, enfuvirtide was the only approved entry inhibitor available for the treatment of HIV infection. To examine the potential for cross-resistance with enfuvirtide, mutations in the HIV envelope protein that are associated with clinical resistance to enfuvirtide (G36D, V38A, and N43D) were introduced into the cloned JRCSF envelope gene by site-directed mutagenesis. These cloned enfuvirtide-resistant envelopes were then used to construct pseudotyped viruses and tested for susceptibility in single-round infectivity assays. The in vitro enfuvirtide IC50s for the G36D, V38A, and N43D mutants increased by 11-fold, 32-fold, and 22-fold, respectively. In contrast, the ibalizumab IC50s for the three mutants did not change significantly from that of wild-type JRCSF, and the corresponding PMI values of 92 to 97% were also similar to that of wild-type JRCSF (95%). These results indicate that enfuvirtide-resistant HIV remains susceptible to ibalizumab. Initial results with pooled virus isolates from one patient, taken at baseline and week 9, showed no change in enfuvirtide susceptibility despite significantly reduced susceptibility to ibalizumab at week 9 (PMI = 73%). Similar results demonstrating the enfuvirtide susceptibility of ibalizumab-resistant HIV isolates have been observed in ongoing clinical trials (unpublished data).
CD4 dependence of ibalizumab-resistant HIV.
Because ibalizumab blocks CD4-dependent HIV entry, in vitro infectivity studies were conducted to characterize the CD4 dependence of patient HIV isolates with reduced susceptibility to ibalizumab. Pseudotyped reporter viruses were prepared with cloned HIV envelopes derived from week 9 serum samples of patients with ibalizumab-resistant virus infections. The dependence of these viruses on CD4 for HIV entry was assessed by (i) blocking gp120 attachment to domain 1 of CD4 with a monoclonal antibody and (ii) testing infectivity on target cells expressing appropriate coreceptors but lacking CD4. Saturating levels of ibalizumab inhibited a maximum of 15 to 30% infectivity, depending on the clone, for three CCR5-tropic and one CXCR4-tropic envelopes. In contrast, a monoclonal antibody that blocked gp120 binding to domain 1 of CD4 (Q4120) completely inhibited infection by ibalizumab-resistant viruses, suggesting a continued requirement for CD4-mediated entry (data not shown). Essentially identical results were observed when the dose-response study was conducted in the presence of a saturating amount of ibalizumab (10 μg/ml), ruling out the possibility that ibalizumab binding conferred an atypical binding interaction between resistant gp120 and CD4. In addition, paired cell lines expressing similar levels of the CCR5 or CXCR4 coreceptor with or without CD4 (see Materials and Methods) were tested for infectivity with ibalizumab-resistant envelope clones. A CD4-dependent HIV envelope (JRCSF) and CD4-independent reporter virus (pseudotyped with VSV G protein) were used as comparators. Target cells lacking CD4 were completely resistant to infection by ibalizumab-resistant viruses to a sensitivity of <0.1%, similar to the JRCSF envelope, whereas the VSV-G reporter virus exhibited the same level of infectivity regardless of the presence of CD4 on the host cells (data not shown).
DISCUSSION
In a previous study, single doses of ibalizumab in antiretroviral treatment-experienced patients with HIV infection resulted in dose-related HIV load reductions. In the current study, three different multiple-dose regimens of the antibody were tested in a similar patient population over a 9-week dosing period. All three dose regimens exhibited anti-HIV-1 activity, as demonstrated by decreased HIV loads and increased CD4+ cell counts (absolute and as a percent of total T lymphocytes). The antiviral effects of each regimen were similar and were correlated with complete coating of T cells with CD4 by ibalizumab early during the dosing period. In this group of patients, many harboring multiple-drug-resistant HIV-1, plasma HIV-1 RNA reductions were observed in the absence of other active antiretrovirals. Viral loads did return to baseline levels despite continued treatment, and this rebound was concomitant with the development of reduced susceptibility to ibalizumab in vitro. Data from in vitro assays indicated that viruses from all patients were susceptible to ibalizumab before treatment, regardless of coreceptor tropism. This finding is consistent with those of previous studies demonstrating that ibalizumab blocked infection of a diverse group of laboratory and primary HIV-1 isolates, including those with mutations conferring resistance to currently available reverse transcriptase inhibitors and protease inhibitors (TaiMed Biologics, Inc., data not shown). After 9 weeks of essential monotherapy with ibalizumab, viruses from nearly all patients exhibited reduced susceptibility in vitro. This finding is consistent with those for other antiretrovirals when administered as a single agent or when added alone to a failing background regimen (3).
Plasma HIV-1 RNA levels were reduced by 0.5 to 1.7 log10 copies/ml in 20 of 22 patients administered ibalizumab, and HIV-1 RNA decreased by >1.0 log10 copy/ml in 13 of these patients. These reductions are similar in magnitude to the maximal responses observed after single-dose administrations of ibalizumab in an earlier clinical trial (7). Although a placebo group was not included in the present study, the reductions of viral loads were clinically significant and strongly linked to improved immunological responses. Viral-load nadirs occurred within the first 1 to 2 weeks for a large majority of patients, followed by a gradual return to near-baseline levels. The maximal average viral-load reduction within each arm was concurrent with the achievement of complete (100%) coating of T-cells with CD4 by ibalizumab. Viral rebound did not result from loss of binding or displacement of the ibalizumab, as complete coating of CD4+ T cells was observed throughout the dosing intervals in patients administered 10 mg/kg every 7 days for 9 weeks (arm A) or 25 mg/kg every 14 days for 8 weeks (arm C). Complete coating in arm B was not achieved after week 2.
Serum ibalizumab levels were maintained in arms A and C, with evidence of drug accumulation, but not in arm B (a 10-mg/kg initial dose followed by 6 mg/kg administered every 2 weeks), consistent with the cell-coating data described above. Though ibalizumab was not entirely cleared from the blood by the time the first 6-mg/kg dose was given in arm B, it was generally cleared prior to the administration of each subsequent 6-mg/kg dose. The initial 10-mg/kg (loading) dose administered to this group probably accounts for the initial high level of cell coating and the corresponding viral-load reduction. Subsequent 6-mg/kg doses were insufficient to maintain serum ibalizumab levels and complete coating of CD4 molecules in these patients. Evidence of drug accumulation in arms A and C is consistent with a dose-dependent, capacity-limited (saturable) elimination mechanism for ibalizumab. This was also indicated by the pharmacokinetic analysis in a previous study of single, escalating dose administrations in HIV-infected patients (7). This mode of clearance has been observed with other anti-CD4 antibodies, both in clinical studies and in preclinical studies in nonhuman primates (1, 5). Anti-CD4 monoclonal antibody administration has been shown to modulate the CD4 receptor from the surface of the T cell, possibly via internalization and/or shedding of CD4 from the cell surface (5, 12). Thus, it is likely that the elimination of ibalizumab is driven partly by binding to the CD4 receptor, internalization, and degradation, not by the normal clearance mechanisms of an immunoglobulin G molecule, where the half-life is on the order of 2 to 3 weeks (10, 16).
Overall, ibalizumab was well tolerated in this study. Treatment-emergent AEs that were considered related to the study drug were mild to moderate in severity. They included headache, nausea, and productive cough, with only headache reported by more than one patient. There were no SAEs related to the study drug. Moreover, there were no notable changes from baseline in laboratory values or vital sign measurements, clinically significant electrocardiogram findings, or ophthalmologic findings during the infusion and postinfusion periods. Patient adherence to the study visits suggested that repeated intravenous infusion was not prohibitive for this patient population over the study period. The incidence of anti-drug antibodies with chronic administration of humanized monoclonal antibodies is generally quite low (6). Low levels of anti-ibalizumab antibodies were detected in a small number of patients during the course of the study. While the significance of these anti-ibalizumab antibodies remains unclear, they were not associated with lower serum ibalizumab levels, drug-related SAEs, or impaired clinical responses. These results suggest a low risk of immunogenicity to ibalizumab even after repeated administration at the doses tested in the study.
The development of antiviral drug resistance and the potential for cross-resistance with existing agents are significant concerns with the use of new antiretroviral agents. When phenotypic assessments of HIV isolates taken from week 9 samples were compared to baseline assessments, reduced susceptibility was characterized by a shift in the PMI. This is similar to the phenotype observed for maraviroc, the recently approved CCR5-specific coreceptor antagonist, for which resistance also develops through changes in the HIV envelope (17). Reduced susceptibility in viral isolates treated with agents that bind to HIV targets, such as protease and reverse transcriptase, is typically manifested by shifts in the IC50. As an entry inhibitor with a unique mechanism of action, it is unlikely that ibalizumab will exhibit cross-resistance with other antiretroviral agents. Enfuvirtide resistance develops through changes in the gp41 domain of HIV envelope, and these changes did not alter susceptibility to ibalizumab. Similarly, initial results indicate that ibalizumab-resistant HIV retains susceptibility to enfuvirtide. Studies are currently planned to further investigate the susceptibility of ibalizumab-resistant HIV to both enfuvirtide and maraviroc. To further investigate the mechanism of resistance development to ibalizumab, we demonstrated that HIV with reduced susceptibility to ibalizumab remains CD4 dependent. As ibalizumab does not block the binding of HIV gp120 to CD4 but rather inhibits postattachment steps in HIV entry, it does not exert selective pressure against CD4 binding. It is likely, then, that resistant isolates will emerge to evade the downstream blockade and not through the use of an alternative primary receptor. Genotypic analysis of the week 9 isolates did not reveal signature mutations that are diagnostic of emerging ibalizumab resistance, but studies are ongoing to better understand genotypic changes related to resistance development.
Entry inhibitor therapy can be an additional option for HIV patients with ongoing viral replication. Ibalizumab has demonstrated safety and antiviral activity in this multiple-dose study of HIV-infected patients. This study further supports the strategy of blocking HIV entry to reduce the viral burden and preserve immune system function. Follow-up studies of several dosing regimens of ibalizumab in combination with other active agents for longer duration are planned.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Anderson, D., K. Chambers, N. Hanna, J. Leonard, M. Reff, R. Newman, J. Baldoni, D. Dunleavy, M. Reddy, R. Sweet, and A. Truneh. 1997. A primatized MAb to human CD4 causes receptor modulation without marked reduction in CD4+ T-cells in chimpanzees: in vitro and in vivo characterization of MAb (IDEC-CE9.1) to human CD4. Clin.l Immunol. Immunopathol. 84:73-84. [DOI] [PubMed] [Google Scholar]
- 2.Burkly, L. C., D. Olson, R. Shapiro, G. Winkler, J. J. Rosa, D. W. Thomas, C. Williams, and P. Chisholm. 1992. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody: dissecting the basis for its inhibitory effect on HIV-induced cell fusion. Immunology 149:1779-1787. [PubMed] [Google Scholar]
- 3.DeGruttola, V., L. Dix, R. D'Aquila, D. Holder, A. Phillips, M. Ait-Khaled, J. Baxter, P. Clevenbergh, S. Hammer, R. Harrigan, D. Katzenstein, R. Lanier, M. Miller, M. Para, S. Yerly, A. Zolopa, J. Murray, A. Patick, V. Miller, S. Castillo, L. Pedneault, and J. Mellors. 2000. The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir. Ther. 5:41-48. [DOI] [PubMed] [Google Scholar]
- 4.Delmonico, F. L., R. W. Knowles, R. B. Colvin, D. E. Cavender, T. Kawai, M. Bedle, D. Stroka, R. I. Preffer, C. Haug, and A. B. Cosimi. 1993. Immunosuppression of cynomolgus renal allograft recipients with humanized OKT4A monoclonal antibodies. Transplant. Proc. 25:784-785. [PubMed] [Google Scholar]
- 5.Fishwild, D. M., D. V. Hudson, U. Deshpande, and A. H. C. Kung. 1999. Differential effects of administration of a human anti-CD4 monoclonal antibody, HM6G, in nonhuman primates. Clin. Immunol. 92:138-152. [DOI] [PubMed] [Google Scholar]
- 6.Koren, E., L. A. Zuckerman, and A. R. Mire-Sluis. 2002. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 3:349-360. [DOI] [PubMed] [Google Scholar]
- 7.Kuritzkes, D. R., J. Jacobson, W. G. Powderly, E. Godofsky, E. DeJesus, F. Haas, K. A. Reimann, J. L. Larson, P. O. Yarbough, V. Curt, and W. R. Shanahan, Jr. 2004. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J. Infect. Dis. 189:286-291. [DOI] [PubMed] [Google Scholar]
- 8.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. Montaner, E. J. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, Jr., J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, and M. Salgo for the TORO 1 Study Group. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H.-J. Stellbrink, J.-F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, and M. Salgo for the TORO 2 Study Group. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186-2195. [DOI] [PubMed] [Google Scholar]
- 10.LoBuglio, A. F., R. H. Wheeler, J. Trang, A. Haynes, K. Rogers, E. B. Harvey, L. Sun, J. Ghrayeb, and M. B. Khazaeli. 1989. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc. Natl. Acad. Sci. USA 86:4220-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkenschlager, M., D. Buck, P. C. L. Beverley, and Q. J. Sattentau. 1990. Functional epitope analysis of the human CD4 molecule. J. Immunol. 145:2839-2845. [PubMed] [Google Scholar]
- 11a.M. Nelson, G. Fätkenheuer, I. Konourina, A. Lazzarin, N. Clumeck, A. Horban, M. Tawadrous, J. Sullivan, H. Mayer, and E. van der Ryst. 2007. Efficacy and safety of maraviroc plus optimized background therapy in viremic, ART-experienced patients infected with CCR5-tropic HIV-1 in Europe, Australia, and North America: 24-week results. Abstr. 14th Conf. Retrovir. Opportunistic Infect., abstr. 104aLB.
- 12.Newman, R., K. Hariharan, M. Reff, D. R. Anderson, G. Braslawsky, D. Santoro, N. Hanna, P. J. Bugelski, M. Brigham-Burke, C. Crysler, R. C. Gagnon, P. Dal Monte, M. L. Doyle, P. C. Hensley, M. P. Reddy, R. W. Sweet, and A. Truneh. 2001. Modification of the Fc region of a primatized IgG antibody to human CD4 retains its ability to modulate CD4 receptors but does not deplete CD4+ T-cells in chimpanzees. Clin. Immunol. 98:164-174. [DOI] [PubMed] [Google Scholar]
- 13.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Homberg, and HOPS Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2008. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, p.1-128. Department of Health and Human Services, Washington, DC.
- 15.Reimann, K. A., L. C. Burkly, B. Burus, B. C. D. Waite, C. I. Lord, and N. L. Letvin. 1993. In vivo administration to rhesus monkeys of a CD4-specific monoclonal antibody capable of blocking AIDS virus replication. AIDS Res. Hum. Retrovir. 9:199-207. [DOI] [PubMed] [Google Scholar]
- 16.Waldman, T. A., and W. Strober. 1969. Metabolism of immunoglobulins. Prog. Allergy 13:1-110. [DOI] [PubMed] [Google Scholar]
- 17.Westby, M., C. Smith-Burchnell, J. Mori, M. Lewis, M. Mosley, M. Stockdale, P. Dorr, G. Ciaramella, and M. Perros. 2007. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J. Virol. 81:2359-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]