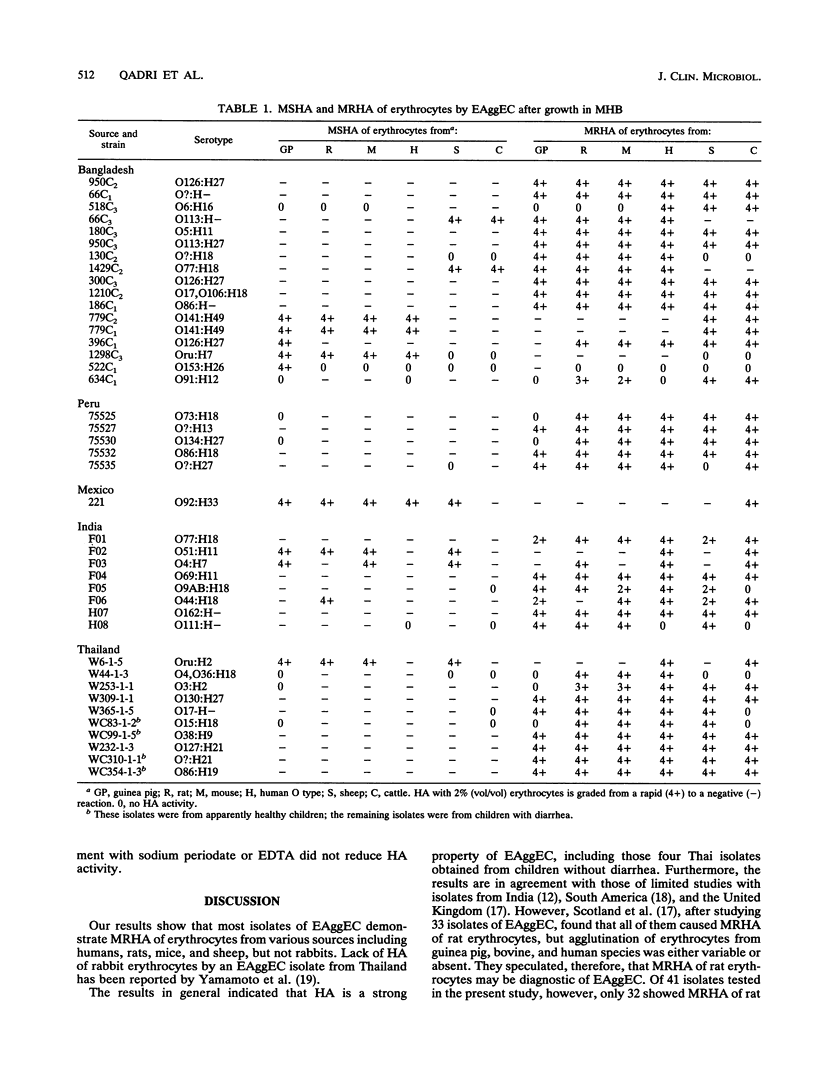
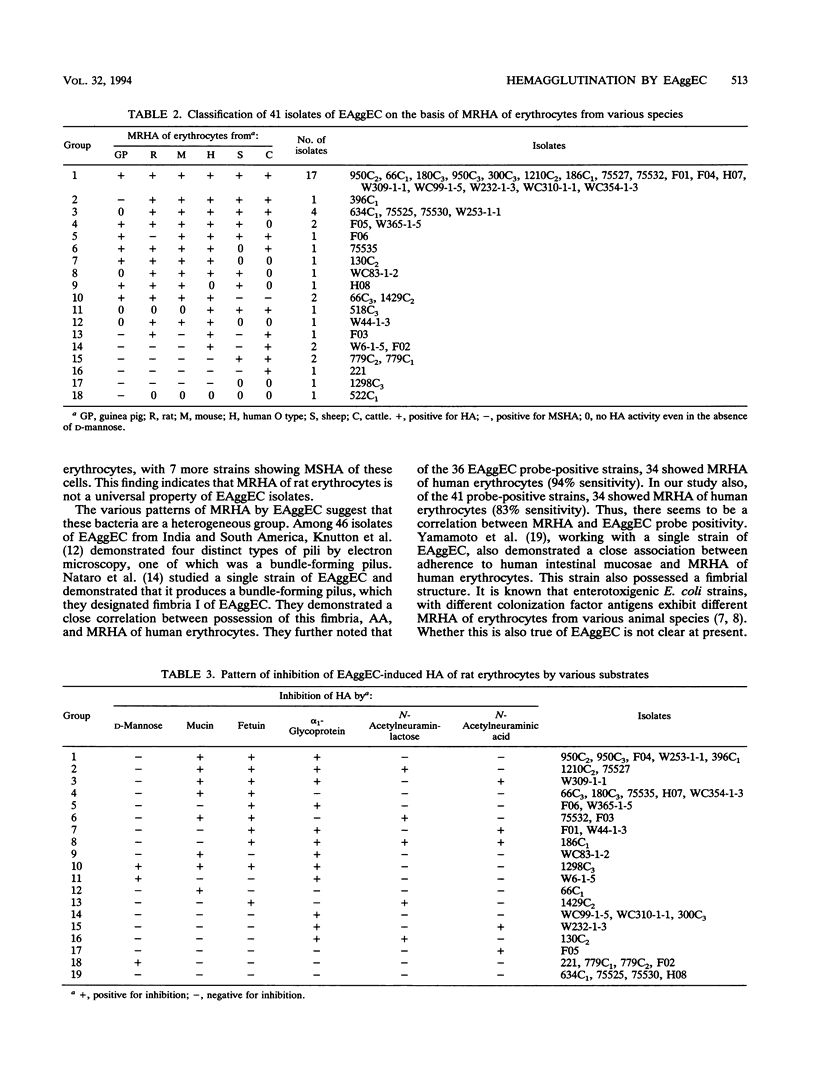
Abstract
Many intestinal bacterial pathogens possess hemagglutinating properties, which are indicative of their adhesive properties to the intestinal mucosal surface. To understand the bacteria-mucosa interaction, 41 strains of enteroaggregative Escherichia coli (EAggEC), a recently described category of diarrheagenic E. coli, isolated mostly from children with diarrhea in Bangladesh, India, Thailand, Central America, and South America were screened for mannose-sensitive hemagglutination and mannose-resistant hemagglutination of erythrocytes from humans, rats, mice, sheep, cattle, and rabbits. Some strains demonstrated mannose-sensitive hemagglutination of erythrocytes. Most isolates showed mannose-resistant hemagglutination of erythrocytes from all species except rabbits. The hemagglutination patterns could be classified into 18 groups. Studies with three selected isolates suggested that hemagglutinins are cell bound and are protein in nature. On the basis of the pattern of inhibition of hemagglutination by various chemicals, 39 isolates were classified into 19 groups. Hemagglutinations of many isolates were inhibited by sialic acid-containing compounds, suggesting that these compounds may be the receptors for these organisms on erythrocytes and possibly on the intestinal mucosa. These data indicate that strains of EAggEC are a heterogeneous group of organisms with different types of hemagglutinins or adhesins for the intestinal mucosal surface. Also, the adhesion characteristics of EAggEC strains may be too complex to be assessed by simple hemagglutination tests.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel M. A., Burness A. T. The attachment of encephalomyocarditis virus to erythrocytes from several animal species. Virology. 1977 Dec;83(2):428–432. doi: 10.1016/0042-6822(77)90189-1. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bhan M. K., Raj P., Levine M. M., Kaper J. B., Bhandari N., Srivastava R., Kumar R., Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989 Jun;159(6):1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Tello A., Navarro A., Ruiz J., Villafán H., Uribe F., Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991 Feb 2;337(8736):262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Echeverria P., Serichantalerg O., Changchawalit S., Baudry B., Levine M. M., Orskov F., Orskov I. Tissue culture-adherent Escherichia coli in infantile diarrhea. J Infect Dis. 1992 Jan;165(1):141–143. doi: 10.1093/infdis/165.1.141. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque S. M., Haider K., Rahman M. M., Abdul Alim A. R., Baqui A. H., Ahmad Q. S., Hossain K. M., Albert M. J. Evaluation of a DNA probe to identify enteroaggregative Escherichia coli from children with diarrhoea in Bangladesh. J Diarrhoeal Dis Res. 1992 Mar;10(1):31–34. [PubMed] [Google Scholar]
- Haider K., Faruque S. M., Shahid N. S., Albert M. J., Nahar S., Malek A., Tzipori S., Alam A. N. Enteroaggregative Escherichia coli infections in Bangladeshi children: clinical and microbiological features. J Diarrhoeal Dis Res. 1991 Dec;9(4):318–322. [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Shaw R. K., Bhan M. K., Smith H. R., McConnell M. M., Cheasty T., Williams P. H., Baldwin T. J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992 May;60(5):2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Deng Y., Maneval D. R., German A. L., Martin W. C., Levine M. M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992 Jun;60(6):2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F., Haq S., Hossain S. A., Ciznar I., Tzipori S. The association of haemagglutination and adhesion with lipopolysaccharide of Shigella dysenteriae serotype 1. J Med Microbiol. 1991 May;34(5):259–264. doi: 10.1099/00222615-34-5-259. [DOI] [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979 Jul 25;254(14):6724–6731. [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Said B., Willshaw G. A., Cheasty T., Rowe B. Identification of enteropathogenic Escherichia coli isolated in Britain as enteroaggregative or as members of a subclass of attaching-and-effacing E. coli not hybridising with the EPEC adherence-factor probe. J Med Microbiol. 1991 Nov;35(5):278–283. doi: 10.1099/00222615-35-5-278. [DOI] [PubMed] [Google Scholar]
- Vial P. A., Robins-Browne R., Lior H., Prado V., Kaper J. B., Nataro J. P., Maneval D., Elsayed A., Levine M. M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988 Jul;158(1):70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Endo S., Yokota T., Echeverria P. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect Immun. 1991 Oct;59(10):3722–3739. doi: 10.1128/iai.59.10.3722-3739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


