Abstract
Nephrotoxicity and ototoxicity have historically been documented as relatively rare complications of vancomycin monotherapy. Recent reports have linked aggressive vancomycin dosing strategies to significant risks of nephrotoxicity. We evaluated the rate of high-frequency hearing loss detected by audiometry for patients on vancomycin therapy. For this purpose, we used retrospective case-control analysis of audiometry results for patients on vancomycin therapy for whom baseline and follow-up exams were available. Analysis of 89 patients for whom audiograms were performed after an average of 27 days of vancomycin therapy showed a 12% rate of high-frequency hearing loss, with a trend in univariate analysis toward a higher rate with advanced age. The mean of the highest vancomycin trough levels for both patients with worsening audiograms and those without worsening audiograms was 19 mg/liter. Regression tree modeling demonstrated that for patients <53 years old, the rate of high-frequency hearing loss detected by audiogram was 0%, while for patients >53 years old, the incidence was 19% (P = 0.008). We conclude that a significant rate of high-frequency hearing loss in older patients receiving vancomycin monotherapy was detected by audiometry. While these data urge caution against continued indiscriminate vancomycin dose escalation to treat infections caused by Staphylococcus aureus strains for which vancomycin MICs are 2 mg/liter, further prospective studies are needed to determine the clinical significance and reversibility of these effects.
Vancomycin has been available to clinicians for 50 years, but its use has increased dramatically in the past 2 decades to keep pace with the increase in methicillin-resistant Staphylococcus aureus (MRSA) infections seen in both community and health care settings (6, 7). In addition, recent increases in vancomycin MICs among clinical MRSA isolates (12, 16, 18) have prompted many clinicians to use higher-dose therapy, although data supporting positive outcomes with higher doses are lacking (4). On the contrary, several reports have emerged suggesting a higher incidence of nephrotoxicity with high-dose vancomycin therapy (5, 8).
Ototoxicity is a less common adverse event previously documented with vancomycin therapy (2, 13, 17). While considered less severe because, unlike renal failure, it has not been linked to inferior clinical outcomes and antibiotic treatment failure (11), ototoxicity from antibiotics has potential quality-of-life implications. It is also less likely to be caused by factors encountered among hospitalized patients, such as those for nephrotoxicity. Given that there is no contemporary evaluation of the ototoxicity of vancomycin in a setting of aggressive dosing, we sought to evaluate the ototoxic effects of vancomycin by using audiometry.
MATERIALS AND METHODS
Patient selection.
All patients with anticipated long-term (>14-day) vancomycin therapy at Westchester Medical Center are routinely referred for baseline and follow-up audiometry. All patients on therapy between June 2005 and December 2006 who received a baseline (within 3 days of the initiation of vancomycin therapy) and a follow-up audiogram were included in the analysis. After approval of the study by the appropriate institutional review board, patients were identified through records of audiograms in the audiology department. Patients were excluded if they had a documented perforated tympanic membrane, had hearing aids, or had only one (baseline) audiogram without a follow-up study. A certified audiologist recorded findings for both ears either as normal or as showing mild, moderate, severe, or profound sensorineural hearing loss, ranked in order of increasing severity based on the sound intensity required for hearing, as follows: normal, <25 dB; mild, 25 to 40 dB; moderate, 40 to 70 dB; severe, 70 to 90 dB; profound, >90 dB.
Data extraction.
Once patients were identified, medical records were reviewed for standardized data extraction per protocol, including age, race, gender, baseline serum creatinine level, serum creatinine level obtained during vancomycin therapy, indication for vancomycin therapy, vancomycin dose, all available vancomycin trough levels during therapy, duration of therapy, duration of therapy at the time of the follow-up audiogram, administration of potentially ototoxic concomitant medications, and results of baseline and follow-up audiograms as described above.
Data analysis.
For data analysis, dependent variables were an abnormal baseline audiogram and a follow-up audiogram worse than the baseline. Independent variables were age, gender, race, highest vancomycin trough level recorded during therapy, duration of vancomycin therapy, and duration of vancomycin therapy until the follow-up audiogram.
Univariate analysis of nonparametric continuous and ordinal data was performed using Kruskal-Wallis analysis of variance, and categorical data were analyzed using the chi-square or Fisher exact test when appropriate. Multivariate analysis was performed with logistic regression and regression tree-based modeling. All statistical procedures were performed with Systat 11 (Systat Software Inc., Point Richmond, CA).
RESULTS
A total of 150 patients had baseline audiograms during the study period. An additional 82 patients who were eligible for audiograms due to an anticipated duration of vancomycin therapy of >14 days did not receive audiograms, either because patients refused or because their clinical state did not allow it (e.g., compromised mentation or noisy intensive-care unit background). Of the patients who had initial baseline audiograms, 35 (23.3%) did not have follow-up studies, 15 (10%) had hearing aids, and 11 (7.3%) had perforated tympanic membranes, leaving 89 patients (59.3%) for inclusion in this analysis. The study patients ranged in age from 16 to 86 years (mean, 59 yrs) and were 63% male. Forty-eight percent were white, 16% were Hispanic, and 12% were black.
Of the 89 patients evaluated, 78 (88%) had no changes detected when the follow-up audiogram was compared to the baseline study, and 11 (12%) had a worse result on the follow-up study. Univariate analysis of demographic data on the patients based on audiogram results (Table 1) showed no major differences other than a trend for patients who had worsening audiograms on vancomycin therapy to be older. There was no difference in vancomycin exposure (including the daily dose, the duration of therapy, the duration of vancomycin therapy at the time of the follow-up audiogram, and the mean of the highest vancomycin trough level documented on therapy) between the 78 patients who had no audiogram changes and those with worsening audiograms (Table 2). In univariate analysis, abnormal baseline audiograms showed a trend toward a higher frequency of worsening follow-up audiograms (P = 0.149), but this was colinear with age.
TABLE 1.
Characteristics of study patients based on change in audiogram during vancomycin therapy
| Patient group (n)a | Mean ± SD (median) age (yrs) | No. (%):
|
Mean ± SD (median) peak serum creatinine level (mg/dl) on therapy | ||
|---|---|---|---|---|---|
| Male | African-American | With ESRD, on HDb | |||
| All (89) | 59 ± 17 (60) | 56 (63) | 11 (12) | 9 (10) | 1.9 ± 1.7 (1.2) |
| No change in audiogram (78) | 58 ± 18 (58) | 50 (64) | 10 (13) | 8 (10) | 1.9 ± 1.6 (1.3) |
| Worsening audiogram (11) | 67 ± 9 (66) | 6 (55) | 1 (9) | 1 (9) | 1.8 ± 2.1 (0.9) |
None of the variables measured showed significant differences between the patient groups (P values, 0.141 for age, 0.539 for gender, 0.725 for race, 0.904 for ESRD, and 0.663 for peak serum creatinine level).
ESRD, end-stage renal disease; HD, hemodialysis.
TABLE 2.
Vancomycin exposure based on changes in audiogram results during vancomycin therapya
| Patient group (n)b | Daily vancomycin dose (g) | Highest vancomycin trough level (mg/liter) | Duration of vancomycin therapy (days)
|
|
|---|---|---|---|---|
| Total | Prior to follow-up audiogram | |||
| All (89) | 1.7 ± 0.6 (2.0) | 19 ± 7 (17) | 28 ± 14 (28) | 27 ± 23 (21) |
| No change in audiogram (78) | 1.7 ± 0.7 (2.0) | 19 ± 7 (17) | 28 ± 15 (28) | 28 ± 25 (21) |
| Worsening audiogram (11) | 1.8 ± 0.6 (2.0) | 20 ± 8 (20) | 25 ± 15 (20) | 25 ± 9 (25) |
Data are means ± standard deviations (medians).
None of the variables measured showed significant differences between the patient groups (P values, 0.575 for the daily vancomycin dose, 0.524 for the highest vancomycin trough level, 0.712 for the total duration of vancomycin therapy, and 0.477 for the duration of vancomycin therapy prior to the follow-up audiogram).
The clinical details of the 11 patients who had worsening audiograms are listed in Table 3. Of these patients, three were on concomitant ototoxic medications (one on gentamicin and two on furosemide), but this frequency was not significantly different than that for the group of patients without audiogram changes.
TABLE 3.
Characteristics of patients with audiogram changes during vancomycin therapya
| Age (yr) | Sex | Race | Serum creatinine concn (mg/dl) | Range of VAN trough levels (mg/liter) | VAN indication | Days of VAN at time of follow-up audiogram | Audiogram result
|
VAN 24-h dose (durationb) | Other ototoxins | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||||||
| 71 | M | White | 0.5-0.9 | 10.9-34.9 | Osteomyelitis | 40 | Mod to severe bilat SNHL | Severe bilat SNHL | 1 g q12h (40 days) | None |
| 59 | F | White | 0.5 | 7.9-12.3 | MRSA bacteremia | 35 | Normal | R, no change; L, severe SNHL | 1 g q12h (35 days) | None |
| 74 | M | Hispanic | 0.9 | 8.8-9.8 | Osteomyelitis | 17 | Mild bilat SNHL | Severe bilat SNHL | 1.5 g q12h (17 days) | None |
| 74 | F | White | 4.0-4.9 | 22-23 | Osteomyelitis | 16 | Mild to mod R SNHL | Mod to severe R SNHL | By serum concns (16 days) | Gentamicin, 6 days |
| 59 | F | White | 0.9 | 20-21 | Sternal wound infection | 35 | Mild to mod bilat SNHL | Mod to severe bilat SNHL | 750 mg q12h (35 days) | None |
| 83 | M | White | 1.2 | 10-15 | Soft tissue infection | 15 | Mild to mod bilat SNHL | Severe bilat SNHL | 1 g q24h (15 days) | None |
| 66 | F | White | 0.5 | 2.5-18.1 | Osteomyelitis | 27 | Mild bilat SNHL | Severe bilat SNHL | 1 g q12h (27 days) | None |
| 60 | M | Black | 1.3-1.7 | 15 | MRSA pneumonia | 20 | Mild bilat SNHL | Mod bilat SNHL | 1 g q24h (20 days) | None |
| 71 | M | White | HD | 15.1-20.1 | MRSA bacteremia | 25 | Mod to severe bilat SNHL | Profound bilat SNHL | By serum concns (25 days) | None |
| 53 | M | Hispanic | 0.7-1.1 | 19-31 | Soft tissue infection | 21 | Normal | R, normal; L, mod SNHL | 1 g q12h (21 days) | Furosemidec |
| 65 | F | Black | 0.8 | 15-22 | MRSA soft tissue bacteremia | 25 | Mild bilat SNHL | Mod bilat SNHL | 1 g q12h (25 days) | Furosemidec |
Abbreviations: M, male; F, female; VAN, vancomycin; mod, moderate; bilat, bilateral; R, right; L, left; SNHL, sensorineural hearing loss; q12h, every 12 h.
Duration of vancomycin therapy before follow-up audiogram.
Standing-order baseline medication of indefinite duration, not new.
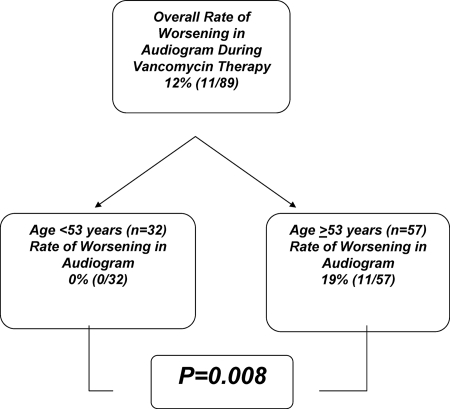
Using regression tree modeling, we identified the age of 53 years as strongly predictive of the frequency of changes in vancomycin ototoxicity (Fig. 1). None of the 32 patients under the age of 53 developed changes in audiogram results on vancomycin therapy, whereas 11 of 57 (19%) patients ≥53 years old developed audiogram-documented vancomycin ototoxicity (P = 0.008).
FIG. 1.
Regression tree model analysis of vancomycin ototoxicity risk by age.
In evaluating the relationships among renal dysfunction, hearing loss, and age, we did not find a relationship between a rise in the serum creatinine level during vancomycin therapy and a worsening audiogram. Four of 11 patients (36%) with worsening audiograms showed rises in serum creatinine levels, compared to 25 of 78 patients (32%) without audiogram changes (P = 0.775 by Pearson's chi-square test). When we examined the data for patients ≥53 years old, we also failed to find a relationship between a rise in the serum creatinine level and a worsening audiogram. In this subgroup, serum creatinine levels increased in 16 of 46 patients (35%) without audiogram changes (P = 0.921 by Pearson's chi-square test).
DISCUSSION
Increasing vancomycin MICs within the microbiologically susceptible range among clinical MRSA isolates have been associated with inferior outcomes for vancomycin treatment of pneumonia and bacteremia (3, 9, 11, 14, 15). While all these studies were retrospective, with differences in methodologies, they have consistently found lower vancomycin treatment success for infections with vancomycin-susceptible MRSA strains with MICs of 2 mg/liter than for cases where the vancomycin MIC was <2 mg/liter (3, 9, 11, 14, 15). Previous data suggest that vancomycin activity is dependent on patient exposure, defined by the ratio of the area under the concentration-time curve to the MIC (AUC/MIC), with an optimal ratio of >400 documented for the treatment of S. aureus pneumonia (10). This has led to attempts to deal with infections with S. aureus (particularly MRSA) strains with higher vancomycin MICs by using higher vancomycin doses, aiming at trough levels of 15 to 20 mg/liter (1). To date, however, the available data fail to suggest a clinical benefit for this approach (3, 4). This would be consistent with the AUC/MIC ratio of 400, which would require trough levels of >20 mg/liter for MRSA strains with vancomycin MICs of 2 mg/liter. Many clinicians are hesitant to exceed 20 mg/liter.
At the same time, evidence is mounting in the literature that higher vancomycin doses may be associated with significant nephrotoxicity, an adverse event anticipated when vancomycin is used concomitantly with an aminoglycoside but not with monotherapy (5, 8). These investigations have been limited by confounders that make the study of vancomycin nephrotoxicity very difficult in a hospital setting, where many other risks of renal failure are present. A more recent, rigorous study argues that vancomycin elevates the nephrotoxicity risk but limits this claim to vancomycin doses of >4 g per day, a dose that most clinicians rarely use (8).
Ototoxicity is a dose-dependent adverse event previously documented with vancomycin but has not been reevaluated in the contemporary setting of aggressive vancomycin dosing (2, 13, 17). Furthermore, unlike renal toxicity, ototoxicity is unlikely to occur as a result of other clinical factors present in the hospital environment and therefore would represent a “cleaner” evaluation of vancomycin toxicity with higher doses.
This retrospective study of 89 patients who had baseline audiograms and follow-up audiograms after approximately 27 days of vancomycin therapy demonstrated a 12% risk of ototoxicity. These patients had doses of vancomycin administered by clinicians to achieve trough levels of 10 to 20 mg/liter, with the highest trough level documented on therapy averaging 19 mg/liter. An abnormal baseline audiogram was associated with a trend toward a risk in ototoxicity by univariate analysis, a finding that was colinear with age. What was exceptionally interesting was the significant age-related risk of vancomycin ototoxicity found by regression tree modeling, with a 0% risk at an age of <53 years and a 19% risk at ≥53 years (P = 0.008). Therefore, these data suggest that high-dose vancomycin therapy may pose a significant ototoxic risk to middle-aged and elderly patients.
This study should be interpreted with caution and should be used merely as a starting point for further investigation, for several reasons. The study was small and retrospective, with a sample of patients that was not randomly chosen. Patients were excluded if follow-up audiograms were not done, and reasons (which could be potential confounders) were not documented in the charts. Given the retrospective nature of the study, data on potentially important parameters such as vancomycin trough levels and serum creatinine measurements were not available at the same level of detail for all patients. Long-term follow-up to determine the clinical significance and reversibility of the audiogram changes was lacking. The lack of long-term follow-up also did not allow for the detection of ototoxicity after the discontinuation of vancomycin, a phenomenon that may occur with aminoglycosides. None of the data acquired from these audiograms altered the treatment plans of these patients. Further potential technical limitations include the fact that the audiogram evaluations were not performed by a single member of the audiology department. While testing is standardized, variations based on operator differences are possible. The performance of audiograms requires significant patient cooperation, which can be limited in an inpatient setting by background noise and by underlying illness and medications with effects on cognitive function and perception. These limitations would be most hampering in the evaluation of patients in the intensive care unit, who many clinicians would agree are at very high risk for ototoxicity through high-dose diuretic therapy, concomitant aminoglycoside use, and hypotension. Finally, this study did not evaluate a baseline group of patients who did not receive vancomycin.
In summary, we have documented a significant risk of vancomycin ototoxicity for patients ≥53 years of age. These findings should provide an additional caution against the use of indiscriminately higher doses of vancomycin to chase increasing vancomycin MICs for MRSA strains causing serious infections, such as pneumonia or bacteremia, in older patients. A prospective study evaluating vancomycin ototoxicity in older patients, with standardized data acquisition and audiology testing at a single center by a single operator, and longer follow-up to determine quality-of-life and other clinically meaningful effects and the reversibility of any changes that occur on vancomycin therapy, is warranted.
Acknowledgments
This study was not funded.
A.F. has no financial disclosures. P.A.M. is currently employed by Cubist Pharmaceuticals. G.S. has received research funding from Cubist and Pfizer Pharmaceuticals; has served as a consultant for Cubist, Ortho-McNeil, and Pfizer Pharmaceuticals; and has received speaking honoraria from Cubist, Pfizer, and Wyeth Pharmaceuticals.
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.American Thoracic Society and Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 2.Brummett, R. E., and K. E. Fox. 1989. Vancomycin- and erythromycin-induced hearing loss in humans. Antimicrob. Agents Chemother. 33:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chua, T., C. L. Moore, M. B. Perri, S. M. Donabedian, W. Masch, D. Vager, S. L. Davis, K. Lulek, B. Zimnicki, and M. J. Zervos. 2008. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in urban Detroit. J. Clin. Microbiol. 46:2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidayat, L. K., D. I. Hsu, R. Quist, K. A. Shriner, and A. Wong-Beringer. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138-2144. [DOI] [PubMed] [Google Scholar]
- 5.Jeffres, M. N., W. Isakow, J. A. Dohert, S. T. Micek, and M. H. Kollef. 2007. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with heathcare-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin. Ther. 29:1107-1115. [DOI] [PubMed] [Google Scholar]
- 6.Kirst, H. A., D. G. Thompson, and T. I. Nicas. 1998. Historical yearly usage of vancomycin. Antimicrob. Agents Chemother. 42:1303-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine, D. P. 2006. Vancomycin: a history. Clin. Infect. Dis. 42:S5-S12. [DOI] [PubMed] [Google Scholar]
- 8.Lodise, T. P., B. Lomaestro, J. Graves, and G. L. Drusano. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodise, T. P., J. Graves, A. Evans, E. Graffunder, M. Helmecke, B. M. Lomaestro, and K. Stellrecht. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed]
- 10.Moise, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 11.Moise, P. A., G. Sakoulas, G. M. Eliopoulos, J. J. Schentag, A. Forrest, and R. C. Moellering, Jr. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin. Infect. Dis. 38:1700-1705. [DOI] [PubMed] [Google Scholar]
- 12.Rhee, K. Y., D. F. Gardiner, and M. Charles. 2005. Decreasing in vitro susceptibility of clinical Staphylococcus aureus isolates to vancomycin at the New York Hospital: quantitative testing redux. Clin. Infect. Dis. 40:1705-1706. [DOI] [PubMed] [Google Scholar]
- 13.Rybak, M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42:S35-S39. [DOI] [PubMed] [Google Scholar]
- 14.Sakoulas, G., P. A. Moise, J. J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortego, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 15:193-200. [DOI] [PubMed] [Google Scholar]
- 16.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood islolates from 2001-05. J. Antimicrob. Chemother. 60:788-794. [DOI] [PubMed] [Google Scholar]
- 17.Traber, P. G., and D. P. Levine. 1981. Vancomycin ototoxicity in patient with normal renal function. Ann. Intern. Med. 95:458-460. [DOI] [PubMed] [Google Scholar]
- 18.Wang, G., J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2006. Increasing vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 44:3883-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]