Abstract
The human T-cell lymphotropic virus type 1 (HTLV-1) is the cause of adult T-cell leukemia and inflammatory diseases including HTLV-1-associated myelopathy/tropical spastic paraparesis. HTLV-1 can be transmitted through sexual contact, mother-to-child transmission, and exposure to contaminated blood. Microbicides are agents that interfere with microbial infectivity at mucous membranes, and candidates are under development for use against sexually transmitted viruses such as human immunodeficiency virus type 1. We previously demonstrated that cell surface polyanionic heparan sulfate proteoglycans bind the HTLV-1 envelope glycoprotein surface subunit gp46, facilitating cell-cell and cell-free virus spread in vitro. We now show, using assays for Env-receptor binding inhibition, Env-induced cell-cell fusion, cell-cell virus spread, and pseudotype HTLV-1 infectivity, that the soluble polyanions PRO 2000 and dextran sulfate are potent inhibitors of HTLV-1 spread in vitro, with PRO 2000 being the more promising candidate. The results of these studies suggest that candidate topical microbicides may be of use in reducing HTLV-1 sexual transmission.
Human T-cell lymphotropic virus type 1 (HTLV-1), a human deltaretrovirus, is the etiological agent of adult T-cell leukemia (19, 41, 53) and various inflammatory diseases, including HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) (17, 38). HTLV-1 is transmitted through exposure to contaminated blood (37), mother-to-child transmission (46), and sexual contact (47), especially among commercial sex workers or individuals with multiple lifetime sexual partners (5, 8). In sexual transmission, the spread of infection is proposed to occur predominantly from male to female (25, 27, 36), most likely via infected T cells present in the semen. However, Belec et al. (6) detected HTLV-1 DNA in cervicovaginal secretions from 20% of HTLV-1-infected women, and higher levels of female-to-male transmission when women suffer from certain sexually transmitted infections (STIs) have been reported to occur (34, 36). An estimated 10 to 20 million people worldwide are infected (13), and HTLV-1 is endemic in southwestern Japan, Iran, Melanesia, many sub-Saharan African countries, the Caribbean basin, and South America (42). It has been suggested for individuals of Caribbean origin that the virus may be most prevalent among people with early and many sexual contacts, whereas mother-to-child transmission may be the most common route of transmission in Japan.
It is widely accepted that HTLV-1 spreads directly between infected and uninfected T cells rather than via cell-free virions (20). Recently, Igakura and colleagues described a novel mechanism of HTLV-1 cell-cell spread between T lymphocytes, via the assembly of a multimolecular complex termed a virological synapse (VS) (20). Cellular receptors proposed to be used by HTLV-1 include, among others, glucose transporter 1 (Glut-1) and the attachment receptors heparan sulfate proteoglycans and neuropillin-1 (18, 31, 40).
Condoms effectively protect against STIs, including human immunodeficiency virus type 1 (HIV-1) infection, and are assumed to be equally effective for HIV-1 and HTLV-1. However, in some cultures, condom usage between sexual partners is the exception rather than the norm. Topical microbicides are substances developed to be used as “chemical condoms” to protect against STIs, including HIV-1 (29, 30, 48). Topical microbicides may be applied vaginally or rectally in the form of creams, gels, or vaginal rings to protect the user (4) and may therefore be a valuable alternative, or addition, to condom use. Many different types of topical microbicides, which differ in their mode of action, are currently under investigation. We previously demonstrated that HTLV-1 gp46 binds negatively charged heparan sulfate proteoglycans on cell surfaces and that the soluble polyanion dextran sulfate (DexS) inhibits this interaction and reduces HTLV-1 pseudovirus transduction and syncytium formation (40). For this reason, we chose to investigate some candidate polyanionic compounds that have demonstrated in vivo efficacy against HIV-1 for their activity against HTLV-1. Soluble polyanions are negatively charged polymers which are thought to interact principally via electrostatic interactions with positively charged regions on microbial surfaces (33). Among these, PRO 2000, a naphthalene sulfonate polymer with an average molecular mass of 5 kDa and one negatively charged sulfate ion per polymer unit, dextrin 2-sulfate (D2S), a hexose polymer with three negatively charged sulfate ions per hexose unit and an average molecular mass of approximately 20 kDa, and DexS, a hexose polymer with two negatively charged sulfate ions per hexose unit and an average molecular mass of approximately 5 kDa, have shown activities against HIV-1 in vitro (14, 21, 24) and partly in vivo (45, 50, 51). Their potentials to inhibit HTLV-1 infection were investigated in this study. Dextran (Dex), a hexose polymer lacking any sulfation and hence a negative charge, with an average molecular mass of approximately 10 kDa, was used as a negative control for DexS (40). As a further potential microbicidal agent with a different mode of action, we included Pcr-400, a synthetic fusion inhibitor peptide mimicking a carboxy-terminal region of the HTLV-1 transmembrane glycoprotein and targeting the envelope structures active in fusion (39), together with the corresponding control peptide P-80. To investigate whether these agents might interfere with HTLV-1 infection, we used in vitro assays to measure the inhibition of HTLV-1-induced cell-cell fusion and cell-cell and cell-free modes of viral infection. We have shown that one of the agents currently under consideration as a potential microbicide, PRO 2000, appears to be a promising candidate.
MATERIALS AND METHODS
Cells.
The human cervical epithelial carcinoma-derived cell line HeLa and the human embryonic kidney cell line 293T (both obtained from the National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom) and 293T-GFP cells (obtained from Simona Ozden, Institut Pasteur, Paris, France, and formerly known as 293T-HTLV-1 LTR-GFP cells), which express green fluorescent protein (GFP) under the control of the HTLV-1 long terminal repeat (LTR), were maintained in Dulbecco's modified Eagle medium (DMEM; Gibco BRL, Life Technologies, Paisley, Scotland, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma Chemical Co., Poole, Dorset, United Kingdom) and 100 IU/ml penicillin-100 μg/ml streptomycin (Gibco BRL, Life Technologies, Paisley, Scotland, United Kingdom), referred to hereinafter as DMEM complete medium (DMEM-CM). The human osteosarcoma-derived CD4- and CCR5-expressing cell line Ghost Hi5 (obtained from William James, The Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom) was maintained under the same conditions, with the addition of 0.1 mg/ml hygromycin B and 1 μg/ml puromycin. C91PL and MT-2 cell lines are both human T-cell leukemia lines isolated from cord blood lymphocytes and cocultured with cells from patients with adult T-cell leukemia, and both were from the NIH AIDS Research and Reference Reagent Program, Germantown, MD. The Jurkat human T-cell line, derived from the peripheral blood of a patient with acute lymphoblastic leukemia, was obtained from the National Institute for Biological Standards and Control. T-cell lines were cultured in RPMI 1640 (Gibco, Life Technologies, Paisley, Scotland, United Kingdom) supplemented with 10% heat-inactivated FCS and 100 IU/ml penicillin-100 μg/ml streptomycin. Peripheral blood mononuclear cells were separated from the fresh blood of a healthy donor by Ficoll-Hypaque (Sigma Chemical Co., Poole, Dorset, United Kingdom) gradient centrifugation and stimulated with 10 U/ml interleukin-2 and 1 μg/ml phytohemagglutinin for 3 days. CD4+ T cells were then negatively selected from the total population by using the CD4+-T-cell isolation kit II and magnetic cell sorting according to the instructions of the manufacturer (Miltenyi Biotec, Bergisch-Gladbach, Germany).
Inhibitors, peptides, proteins, and sera.
Polyanions PRO 2000 and D2S were obtained from Indevus Pharmaceuticals Inc. (Lexington, MA) and ML Laboratories Plc (Staffordshire, United Kingdom), respectively. DexS and the corresponding control Dex were purchased from Sigma-Aldrich, United Kingdom. PRO 2000 is a naphthalene polymer sulfonate with one negatively charged sulfate molecule per polymer unit (43), D2S contains three sulfate ions per hexose (44), and DexS contains two sulfate ions per hexose unit (2). Dex lacks any sulfate ions, is not negatively charged, and served here as a control for DexS. All polyanions and Dex were dissolved in sterile 1× phosphate-buffered saline (PBS), and their molarities were calculated by assuming average molecular masses of 5,000 Da for PRO 2000 (4,000 to 6,000 Da) and DexS, 20,000 Da for D2S, and 10,000 for Dex. The gp46 fusion inhibitor peptide Pcr-400 and the corresponding control peptide P-80 used in this study were chemically synthesized and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, United Kingdom) as described previously in reference 39. HAM/TSP patient sera (HTLV-1 sera) was from Graham Taylor (Imperial College London, London, United Kingdom) and was used alongside the corresponding control, normal human serum (NHS) from a healthy donor. Soluble recombinant HTLV-1 surface glycoprotein gp46 fused to the human immunoglobulin G (IgG) Fc region (gp46-Fc; 1.3 μg/ml) was prepared as described previously (22, 40) and used in conjunction with the corresponding control, Fc (1.3 μg/ml). Fc was expressed from pMt-tpaFc in insect cells as described previously (22). The vector pMt-tpaFc was derived directly from pMtgp46-Fc (22) by deleting the gp46 coding region and fusing the leader sequence of human tissue plasminogen activator (tpa) directly in frame with the hinge and heavy-chain constant domains (CH2 and CH3) of human IgG. The tpa leader sequence allows the secretion of Fc and is removed from the mature secreted protein product by a cellular protease.
Cytotoxicity assay.
Cells were seeded and cultured exactly as in viral infectivity assays, and the compounds were titrated into the culture to give the final concentrations indicated in Fig. 1 and remained in the culture for the 3-day duration of the experiment. Cell viability was measured using a tetrazolium assay for cellular metabolic activity (CellTiter96) by following the protocol of the manufacturer (Promega, Southampton, United Kingdom).
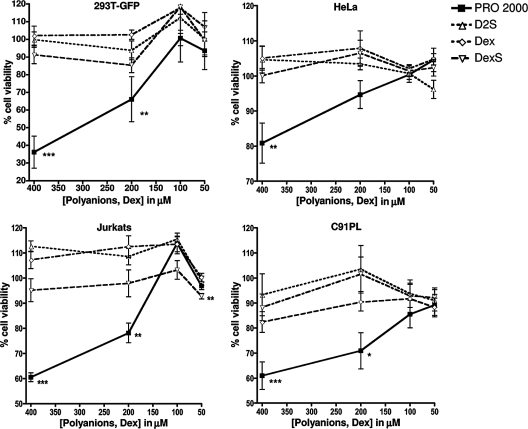
FIG. 1.
Results of the cytotoxicity assays testing the effects of high concentrations of compounds on various cell lines. Adherent cell lines 293T-GFP and HeLa and the T-cell lines Jurkat and C91PL were treated with the indicated concentrations of PRO 2000, D2S, Dex, and DexS for 3 days. Graphs represent the means of results from two independent experiments performed in triplicate, and error bars represent SEM.
Syncytium inhibition assay.
HeLa cells (1.5 × 105 cells/ml; 4.5 × 105 cells per well) were seeded into the wells of a six-well plate and transfected the following day, when cells were approximately 70% confluent, with 2 μg of the GFP-encoding plasmid pmax-GFP (Amaxa AG, Germany) either alone or together with 2 μg of pHTE-1 (a plasmid carrying the HTLV-1 envelope gene [env], described previously in reference 15) by using 3 μl of FuGene 6 (Roche Diagnostics, United Kingdom). Cells were lifted 12 to 15 h later by using PBS-2 mM EDTA, washed with PBS, and incubated either with DMEM-CM or with serial threefold dilutions of polyanions, Dex, peptides, or sera (in DMEM-CM) for 20 min at 37°C (in triplicate). The supernatant of previously seeded 60% confluent parental HeLa cells was removed and replaced by treated or untreated transfected cells. Cells were incubated at 37°C for 24 h and fixed using PBS-1% formaldehyde for 15 min at room temperature. All green fluorescent cells in at least 10 randomly selected fields per sample were enumerated by low-power microscopy. To calculate the percent inhibition of syncytium formation by inhibitors, the number of multinucleated cells in each field was first normalized with respect to the number of singly nucleated cells in that field. The number of multinucleated pmax-GFP-transfected cells was then subtracted, and the percent inhibition was calculated by using the following formula: 100 − (number for treated cotransfected cells/number for untreated cotransfected cells) × 100. Results are represented as nonlinear-fit sigmoidal dose-response curves (with variable slopes), which were used to calculate the 90 and 50% inhibitory doses (ID90 and ID50, respectively) with Prism 4 software (GraphPad Software, Inc).
Pseudovirus inhibition assay.
Pseudovirus was produced by seeding 3 ml of a concentration of 4 × 105 293T cells/ml into the wells of a six-well plate and cotransfecting the cells the following day with 1.5 μg each of vectors pHTE-1 and pNL4-3 Env− Luc+ (N. Landau, Gladstone Research Institute, CA), the latter containing the proviral backbone but lacking functional env and carrying a luciferase reporter gene inserted into the nef open reading frame (9), by using 3 μl of FuGene 6. On day 4 after transfection, recombinant virus was harvested and mixed in triplicate with serially fivefold-diluted inhibitor and the mixture was incubated at 37°C for 20 min and subsequently applied to 70% confluent Ghost cells. Controls were infected samples without inhibitor and cells without virus. After incubation for 2 days at 37°C, the supernatant was removed, cells were lysed using cell lysis buffer (Promega), and a luciferase assay was performed according to the protocol of the assay kit manufacturer (Promega). Light emission was measured using an Anthos Lucy 2 microplate luminometer (Jencons Ltd., Leighton Buzzard, United Kingdom). The percent inhibition at each concentration of each compound was calculated using the following formula: 100 − (relative light units for test well/relative light units for no-inhibitor control) × 100. Nonlinear-fit sigmoidal dose-response curves (with variable slopes) were fitted, and results are shown as the ID90 and ID50 of each inhibitor used.
Synergistic inhibition of pseudovirus infection by PRO 2000 or DexS in combination with Pcr-400 fusion inhibition peptide.
Pseudovirus production and Ghost cell transduction were carried out exactly as described for the pseudovirus inhibition assay. To test the synergistic inhibitory effects of PRO 2000 and DexS in combination with Pcr-400, the optimum starting concentration of each compound was established. Hence, serial 1.5-fold dilutions of inhibitor were applied to HTLV-1 env-pseudotyped virions (as described above). The initial concentration of each inhibitor was identified to obtain at least three datum points between 20 and 80% inhibition. Thus, the starting concentrations were defined as 100 μM for PRO 2000, 5 μM for DexS, and 0.5 μM for Pcr-400, giving combination ratios of 200 for PRO 2000 and Pcr-400 and 10 for DexS and Pcr-400. Combined compounds were also serially diluted 1.5-fold. Controls of cells without virus and infected samples without inhibitors were included, and the pseudovirus inhibition assay with the luciferase readout was performed as described above. The percent inhibition for each concentration of each drug was calculated according to the following formula: 100 − (relative light units for test well/relative light units for no-inhibitor control) × 100. ID50, ID75, and ID90 values and the synergy effects were calculated using CalcuSyn software (Biosoft). To determine the effects of drug combinations, CalcuSyn software uses the multiple-drug effect algorithm of Chou (10, 11). A dose-effect curve for each drug and combination of drugs was calculated. The program combines information on the ID50 and on the slope of the dose-effect curve to calculate the combination index (CI), with a CI of <0.9 representing synergy, a CI of 0.9 to 1.1 corresponding to additive effects, and a CI of >1.1 indicating antagonism. In this context, an additive effect means that drugs are proportionally more efficacious when combined than when used individually. Synergy is the production of a greater-than-additive effect and antagonism is the production of a smaller-than-additive effect when drugs are combined. The dose reduction index was also calculated. This index compares the dose of each drug alone with the dose of each drug in a synergistic combination: synergy between agents will result in a dose reduction for at least one of the agents.
gp46-Fc-cell receptor binding inhibition assay.
Polyanions and Dex were serially diluted fivefold in fluorescence-activated cell sorter wash buffer (FWB; PBS containing 1% bovine serum albumin and 0.02% NaN3) and subsequently mixed with either soluble gp46-Fc (1.3 μg/ml) or soluble control Fc (1.3 μg/ml), and the mixtures were incubated for 30 min at 4°C with gentle agitation. Controls with gp46-Fc and the Fc control protein without inhibitors were included. HeLa cells were lifted using PBS-2 mM EDTA and washed in FWB, aliquots of 2.5 × 105 cells in 25 μl were added to all wells, and the wells were incubated for 2 h at 4°C with agitation. Cells were subsequently washed twice with FWB and incubated with 10 μg/ml phycoerythrin-conjugated anti-human IgG (H+L-specific) monoclonal antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h at 4°C with agitation and then subjected to two washing steps with FWB, fixation with PBS-1% formaldehyde for 10 min, and analysis by flow cytometry with a FACSCalibur instrument (Becton Dickinson, Oxford, Oxfordshire, United Kingdom). The percent inhibition of gp46-Fc binding to the cells by the compounds was calculated using the following formula: 100 − [(number for treated gp46-Fc − number for treated Fc control)/(number for untreated gp46-Fc − number for untreated Fc control)] × 100. Results are expressed as nonlinear-fit sigmoidal dose-response curves (with variable slopes) giving ID90 and ID50 values for polyanions.
Cell-cell Tax transfer inhibition assay.
293T-GFP is a reporter cell line containing a stably introduced GFP cassette controlled by the HTLV-1 LTR: the presence of HTLV-1 Tax activates the LTR promoter, which results in GFP expression. Chronically HTLV-1-infected C91PL cells (2 × 106) were incubated with polyanions or controls for 20 to 40 min at 37°C prior to coculture for 2 days with 106 293T-GFP cells (ratio of 2:1) in a bacterial plastic tissue culture dish (Fisher Scientific, Loughborough, Leicestershire, United Kingdom) with gentle agitation. The cells were fixed with PBS-4% formaldehyde for 15 min and washed with PBS, and GFP expression was analyzed by flow cytometry. The percent inhibition of GFP expression was determined using the following formula: 100 − (geometric mean fluorescence of treated 293T-GFP cells/geometric mean fluorescence of untreated 293T-GFP cells) × 100. Error bars in figures represent the standard deviations (SD) of results for triplicates in one representative experiment. To investigate cell-cell fusion and cytoplasmic mixing, 106 C91PL cells in a solution of 200 μl of CellTracker CMRA orange dye (Molecular Probes, Leiden, The Netherlands) diluted 1:1,000 in FCS-free RPMI 1640 were incubated with the same number of 293T-GFP cells in a solution of 200 μl of 5-chloromethylfluorescein diacetate green cytoplasmic dye (CMFDA; Molecular Probes) diluted as described for CMRA. Cells were cocultured at a 1:1 ratio for 1 and 3 days. A sample of 106 cells was collected and incubated with RPMI 1640 containing 1% FCS, and cells were allowed to settle for 1 h onto a coverslip (13 mm in diameter; Scientific Laboratory Supplies, Nottingham, United Kingdom) treated with poly-l-lysine (Sigma, United Kingdom). Laser scanning confocal microscopy to evaluate the frequency of dye mixing, indicating cell fusion, was performed with a Zeiss Axioplan upright microscope.
Real-time quantitative PCR (qPCR).
Chronically infected MT-2 cells (1.5 × 106) were treated or not with PRO 2000, D2S, Dex, or peptides for 20 min at 37°C. Jurkat cells (1.5 × 106) were then added or not, and cells were cultured in 3 ml of DMEM-CM in a six-well plate for 72 h at 37°C. As a control, Jurkat cells alone or with the supernatant of MT-2 cells were cultured for the same amount of time. DNA isolation was performed using the DNeasy blood and tissue kit according to the protocol of the manufacturer (Qiagen). The following primers and probe were used to detect a sequence within HTLV-1 pol: forward and reverse primers SK 110 (5′-CCCTACAATCCAACCAGCTCAG-3′; HTLV-1 nucleotides 4758 to 4779 [GenBank accession no. J02029]) and SK 111 (5′-GTGGTGAAGCTGCCATCGGGTTTT-3′; HTLV-1 nucleotides 4943 to 4920) and a TaqMan probe with dual labeling (5′-end 6-carboxyfluorescein and 3′-end 6-carboxytetramethylrhodamine), corresponding to bp 4829 to 4858 of the HTLV-1 reference sequence (12, 26, 52). In parallel, the following primers and probe were used to detect the cellular albumin (alb) gene as an endogenous reference to determine the virus copy number per cell: forward primer Alb-S (5′-GCTGTCATCTCTTGTGGGCTGT-3′), reverse primer Alb-AS (5′-AAACTCATGGGAGCTGCTGGTT-3′), and the Alb TaqMan probe (5′ end labeled with 6-carboxyfluorescein and 3′ end labeled with 6-carboxytetramethylrhodamine; 5′-CCTGTCATGCCCACACAAATCTCTCC-3′ [described previously in reference 28]). PCR sample mixes containing 2.5 μl of template DNA and 300 and 100 nM (each) primers and probes, respectively, in a 25-μl final volume of Universal master mix (PE Applied Biosystems) were prepared in triplicate. The standard curve was obtained using a serial fivefold dilution of DNA from untreated MT-2 cells in duplicate. With the Applied Biosystems 7500 fast real-time PCR system, one run consisted of 10 min at 95°C, followed by 50 cycles of 15 s at 95°C and 60 s at 60°C. The percent inhibition of de novo synthesis of viral DNA was calculated using the following formula: 100 − [(pol readout for treated cocultured cells/alb readout for treated cocultured cells)/(pol readout for untreated cocultured cells/alb readout for untreated cocultured cells)] × 100.
Statistical analysis.
All data are means ± 1 SD from single experiments performed in triplicate or means and standard errors of the means (SEM) from multiple experiments. Significance was determined by applying the one-way analysis of variance test followed by Bonferroni's multiple-comparison test when more than two samples were compared; otherwise, an unpaired t test was performed. All statistical analysis was performed using Prism 4 software (GraphPad Software, Inc).
RESULTS
Candidate polyanion cellular toxicity.
All cell lines (Jurkat, MT-2, C91PL, HeLa, Ghost, 293T, and 293T-GFP) and activated, primary CD4+ T cells from a healthy donor were individually incubated in triplicate for 3 days with compounds at a starting concentration of fourfold the maximum concentration used in the assays described above (400 μM) and at dilutions corresponding to 200 μM, 100 μM (the maximum concentration used in the assays), and 50 μM. Figure 1 shows representative results for four of the cell types, indicating the percent cell viability compared to the viability of untreated cells. We chose to present data from two epithelial cell lines, as epithelial cells are the type most likely to encounter the microbicides at the mucosal epithelium, and two T-cell lines, one uninfected (Jurkat) and one producing HTLV-1 (C91PL). High (400 and 200 μM) concentrations of PRO 2000 resulted in significantly reduced cell viability for all cell types, whereas concentrations of PRO 2000 of 100 μM or lower resulted in no reduced viability of any cell type, with the exception of primary CD4+ T cells. None of the other compounds tested showed any significant reduction of cell viability at any concentration tested. Table 1 lists the results of one experiment (performed in triplicate) in which all cell lines tested were treated with 100 μM polyanions or Dex and 10 μM peptides for 3 days; the data are presented as the percent cell viability (±1 SD) compared to that of untreated cells. No cell type had significantly reduced viability after the treatment. By combining the results for all treated cell types per compound, the average viability levels (±SEM) were determined to be 100% (±1.1%) for PRO 2000, 99.6% (±1.5%) for D2S, 99.2% (±0.9%) for Dex, 91.4% (±1.2%) for DexS, and 100.2% (±1.6%), 99.3% (±1.1%), and 103.6% (±1.4%) for DMSO, P-80, and Pcr-400, respectively.
TABLE 1.
Cell viability in the presence of inhibitorsa
| Cell type | % Viability ± 1 SD in the presence of:
|
||||||
|---|---|---|---|---|---|---|---|
| PRO 2000 | D2S | DexS | Dex | DMSO | P-80 | Pcr-400 | |
| HeLa | 103 ± 2 | 106 ± 3 | 103 ± 2 | 102 ± 5 | 99 ± 2 | 102 ± 5 | 99 ± 6 |
| 293T | 98 ± 3 | 95 ± 4 | 90 ± 5 | 93 ± 2 | 93 ± 2 | 99 ± 4 | 99 ± 2 |
| 293T-GFP | 104 ± 2 | 95 ± 3 | 84 ± 5 | 94 ± 3 | 104 ± 9 | 91 ± 9 | 109 ± 6 |
| Ghost | 110 ± 11 | 102 ± 3 | 90 ± 9 | 98 ± 8 | 89 ± 5 | 104 ± 13 | 105 ± 0 |
| Jurkat | 93 ± 6 | 98 ± 1 | 99 ± 1 | 100 ± 1 | 102 ± 2 | 104 ± 4 | 101 ± 4 |
| CD4+ primary cells | 93 ± 2 | 106 ± 3 | 96 ± 5 | 108 ± 4 | 108 ± 4 | 95 ± 3 | 117 ± 15 |
| C91PL | 106 ± 4 | 115 ± 10 | 93 ± 6 | 101 ± 6 | 96 ± 10 | 101 ± 3 | 101 ± 4 |
| MT-2 | 100 ± 1 | 96 ± 7 | 95 ± 2 | 97 ± 3 | 103 ± 2 | 96 ± 3 | 104 ± 1 |
Inhibitors were added to cells at 100 μM for polyanions or 10 μM for peptides, and metabolic function after 3 days was measured by the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay.
Polyanions inhibit Env-mediated cell fusion.
In this study, a variety of surrogate assays that analyze functional aspects of HTLV-1 binding, fusion, and infection have been developed and described previously. These include assays for Env-mediated syncytium formation and cell-cell virus transfer, single-cycle reporter pseudovirus assays for cell-free infectivity, and soluble Env-receptor binding assays. We expected that these assays, used alongside one another, would yield robust data sets regarding the in vitro antiviral efficacies of our selected compounds.
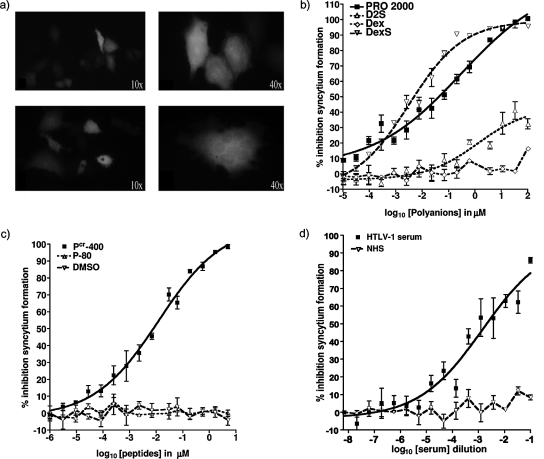
HeLa cells transfected with HTLV-1 env fuse with other HeLa cells to form multinucleated syncytia, and these syncytia can be quantified by light microscopy (40). We modified this assay by cotransfecting HeLa cells with env and a GFP-encoding plasmid: this procedure facilitated the recognition of fusion events, represented by green-fluorescing multinucleated cells (those with >3 nuclei) derived from fusion with other cells (Fig. 2a). Cells transfected with env were preincubated with serially threefold-diluted inhibitors or control compounds that were subsequently left in the culture for the duration of the assay. At the highest dose tested (100 μM), PRO 2000 inhibited cell fusion by 99.8% (±0.5%), DexS inhibited fusion by 94.5% (±0.8%), and D2S inhibited fusion by 56.7% (±11.0%), and 5 μM Pcr-400 inhibited cell fusion by 98.2% (±0.6%) (means of results ± SEM from two independent experiments). PRO 2000, DexS, and the fusion inhibition peptide Pcr-400 inhibited syncytium formation in a dose-dependent manner, giving ID90 values of 12.7 μM (±2.2 μM), 3.42 μM (±1.7 μM), and 1.22 μM (±0.3 μM), respectively, revealing DexS to be the most potent polyanionic inhibitor tested. In contrast, D2S failed to achieve 90% inhibition in both experiments and 50% inhibition in one of two experiments and was the weakest inhibitor in this assay. HAM patient sera also reduced fusion, yielding an ID50 at a reciprocal of dilution of 0.015 (±0.07). The negative control for polyanion activity (Dex), the noninhibitory peptide P-80, the peptide solvent control DMSO, and NHS all failed to interfere significantly with syncytium formation. Figure 2 shows representative results ± 1 SD from one of two experiments performed in triplicate, and Table 2 summarizes ID50 and ID90 values ± SEM obtained from two experiments.
FIG. 2.
Inhibition of HTLV-1 Env-mediated syncytium formation by polyanions. (a) Visible-light images of HeLa cells either transfected with a GFP-encoding plasmid only (top panels) or cotransfected with GFP- and HTLV-1 Env-encoding plasmids, leading to the formation of multinucleated syncytia (bottom panels). Original magnification values are indicated. (b) Percent inhibition of syncytium formation by PRO 2000, D2S, DexS, and Dex serially diluted threefold from an initial concentration of 100 μM. (c) Percent inhibition by the fusion inhibitor peptide Pcr-400, the corresponding negative control peptide P-80, and the peptide solvent agent DMSO. (d) Percent inhibition by HAM/TSP patient sera and NHS. All dilutions were performed in triplicate, and the fluorescent cells in at least 10 randomly selected microscopic fields for each sample were counted. Graphs show representative mean values from one of two independently performed experiments, and error bars represent ±1 SD.
TABLE 2.
Summary of ID90 and ID50 values
| Measurement and assay | Valuea for:
|
||||
|---|---|---|---|---|---|
| PRO 2000 | D2S | DexS | Pcr-400 | HTLV-1 sera | |
| ID90 | |||||
| Syncytium formation | 12.7 ± 2.2 | NAb | 3.4 ± 1.7 | 1.2 ± 0.3 | NA |
| gp46-Fc binding | 4.2 ± 0.5 | 5.9 ± 3.0 | 4.7 ± 0.9 | NDc | ND |
| Pseudovirus transduction | 11.5 ± 2.5 | 11.5 ± 3.2 | 8.4 ± 5.8 | 0.5 ± 0.1 | 0.00001 ± 0.0 |
| ID50 | |||||
| Syncytium formation | 0.12 ± 0.1 | 28.0 ± 8.1 | 1.83 ± 1.5 | 0.15 ± 0.1 | 0.015 ± 0.07 |
| gp46-Fc binding | 0.14 ± 0.15 | 0.01 ± 0.0 | 0.16 ± 0.1 | ND | ND |
| Pseudovirus transduction | 4.7 ± 0.6 | 9.2 ± 2.9 | 0.8 ± 0.1 | 0.04 ± 0.0 | 0.0005 ± 0.0 |
ID50 and ID90 values were calculated from the results of at least two independent replicate experiments and are the means ± SEM, with the exceptions of gp46-Fc binding assay values and the ID50 of D2S determined in the syncytium formation assay, which are the means ± SD of results from one experiment performed in triplicate, and the values for HTLV-1 sera, which are mean reciprocals of dilution ± SEM from independent replicate experiments. Values for all agents other than HTLV-1 sera are expressed in micromolars.
NA (not applicable) indicates that 90% inhibition was not obtained.
ND, not determined.
DexS and PRO 2000 inhibit cell-cell transfer of HTLV-1 Tax.
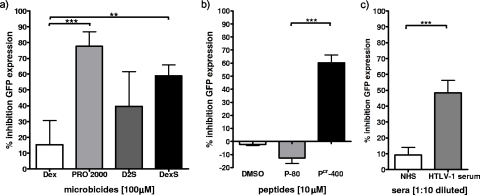
293T cells stably expressing a GFP cassette integrated downstream of the HTLV-1 LTR were used to investigate the transfer of HTLV-1 Tax from infected C91PL cells after coculture. The presence of Tax in the reporter cell line indicated Tax transfer following cell-cell fusion, the cell-cell spread of HTLV-1 via a VS without syncytium formation, or contributions from both mechanisms. To investigate which mechanism was operating, we labeled C91PL effector cells with red cytoplasmic dye and 293T target cells with green cytoplasmic dye. Coculture for up to 3 days resulted in ∼1% obvious cell fusion as measured by counting syncytia. After 1 day of coculture, cytoplasmic dye transfer from effector cells to 6.53% of target cells and from target cells to ∼1% of effector cells was observed (data not shown). This result suggests the predominance of the unidirectional cell-cell spread of HTLV-1 from effector to target cells rather than cell-cell fusion at this time point. Three days of coculture led to an increase in dye mixing, with approximately 9% of both cell types containing both dyes, implying cytoplasmic exchange via cell-cell fusion. Considering that <5% of the total population of reporter cells was positive for GFP expression after 3 days of coculture, it can be assumed that both cell-cell transfer of virus and cell-cell fusion contribute to the GFP signal but that the dominant mechanism of Tax transfer after 3 days is probably cell-cell fusion. To determine inhibitor efficacy, GFP expression in 293T target cells was quantified by flow cytometry. The treatment of C91PL cells with PRO 2000, DexS, Pcr-400, or HAM patient sera prior to and during coculture with the target cells significantly reduced GFP expression compared to that in the no-inhibitor controls (Fig. 3). At 100 μM, PRO 2000 resulted in the inhibition of GFP expression by 77.7% (±9.1%), DexS inhibited expression by 59.0% (±6.9%), and Pcr-400 inhibited expression by 60.3% (±5.8%). In contrast, D2S inhibited GFP expression by only 39.6% (±9.0%), which is not significant compared to the results for the Dex control. Tenfold-diluted HAM/TSP sera (HTLV-1-positive sera) inhibited expression significantly compared to NHS (Fig. 3c). The results presented are means ± SEM from three independently performed experiments.
FIG. 3.
Inhibition of cell-cell Tax transfer. The reporter cell line 293T-GFP, expressing GFP in the presence of HTLV-1 Tax, was cocultured for 72 h with HTLV-1-infected C91PL cells previously treated (or not) with 100 μM polyanion, 10 μM peptide, or 1:10 diluted sera. GFP expression was assayed by flow cytometry. (a) Results for PRO 2000, D2S, and DexS compared to those for the control compound Dex at 100 μM. (b) Results for the fusion inhibitor peptide Pcr-400 compared to those for the control peptide P-80 at 10 μM and the solvent DMSO. (c) Results for HAM/TSP patient sera compared to those for NHS at a 1/10 dilution. Data are means of results from three independent experiments, and error bars represent SEM. **, P < 0.01; ***, P < 0.001.
Measurement of cell-to-cell HTLV-1 spread by qPCR.
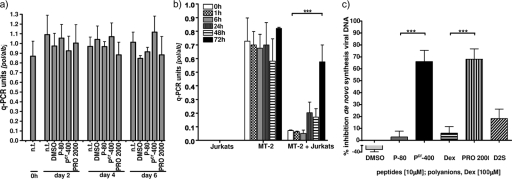
Although used in the past by others, the assays described above have incorporated non-T-cell lines to investigate HTLV-1 cell-cell spread, and readouts based upon Tax transfer may represent predominantly cell-cell fusion rather than the productive spread of virus into target cells. To quantify the transfer of HTLV-1 to T cells, we established a sensitive assay to investigate early HTLV-1 cell-cell spread using real-time qPCR to detect de novo reverse transcription products within target cells. New viral DNA was detected by specific primers binding within the pol sequence of HTLV-1, which produces a late transcript that therefore represents the synthesis of near-full-length provirus. The simultaneous detection of cellular sequences of the alb housekeeping gene allows the normalization of the viral DNA level per cell. To exclude cell-cell fusion as a mode of viral genome transfer, cells were monitored visually: no evidence of syncytium formation was observed over the 3 days of the assay. We initially investigated whether MT-2 cells were able to reinfect themselves with HTLV-1 by culturing the cells over a 6-day period with or without inhibitors of infection (10 μM Pcr-400 or 100 μM PRO 2000). No significant difference in the signal with or without inhibitor was observed. We assume that the viral genome is passed to daughter cells at each division, as the ratio of housekeeping alb gene sequences to viral pol sequences did not vary over time. The fact that there was no obvious increase in the relative quantity of the viral genome implies a lack of reinfection, and the fact that the inhibitors did not reduce the relative level of pol sequences is in line with this conclusion (Fig. 4a). In contrast, when MT-2 cells were cocultured with Jurkat cells, there was a time-dependent increase in viral DNA over 3 days (Fig. 4b). No reverse-transcribed pol was detected when Jurkat cells were incubated alone (Fig. 4b) or with supernatant from MT-2 cells for the same period of time (data not shown). The pol/alb copy number ratio in the mixed culture of Jurkat and MT-2 cells was less than 50% of the pol/alb copy number ratio in the culture of MT-2 cells only, possibly due to an increase in the number of alb gene copies in the MT-2 cell line, a decrease in the number in the Jurkat line, or both, events not uncommon in transformed cell lines in which chromosomes may be lost or duplicated. We selected day 3 after the mixing of MT-2 and Jurkat cells as a time when the signal was robustly higher than the baseline (P < 0.001). The addition of 100 μM PRO 2000 or 10 μM Pcr-400 prior to the coculture over this period reduced the de novo synthesis of viral DNA by 68.1% (±8.5%) or 66.1% (±9.4%), respectively, which was significant (P < 0.001) in both cases (Fig. 4c). In contrast, D2S did not significantly inhibit the cell-cell transfer of the viral genome (18.3% ± 7.9%; P > 0.05). DexS could not be tested in this assay, as unlike the other agents, it interfered with the PCR.
FIG. 4.
Inhibition of cell-cell transfer of HTLV-1 as detected by qPCR. (a) MT-2 cells were treated or not treated (n.t.) with 100 μM PRO 2000, 10 μM peptide, or the corresponding amount of DMSO as a control for up to 6 days. Immediately and 2, 4, and 6 days after the addition of compounds, cells were harvested, DNA was extracted, and a qPCR was performed to detect viral pol and cellular alb sequences. Values are viral genome numbers per albumin gene copies per cell. (b) MT-2 or Jurkat cells were cultured alone or cocultured in equal numbers for up to 72 h. (c) MT-2 cells were treated with inhibitors or control compounds for 10 min prior to and during coculture with Jurkat cells for 72 h. Data in panels a and b are means ± 1 SD of representative results from one experiment performed in triplicate, and data in panel c are the means of results from three independent experiments performed in triplicate; error bars represent the SEM. ***, P < 0.001 as determined by one-way analysis of variance followed by Bonferroni's multiple-comparison posttest.
Polyanions reduce gp46-Fc binding to cellular receptors.
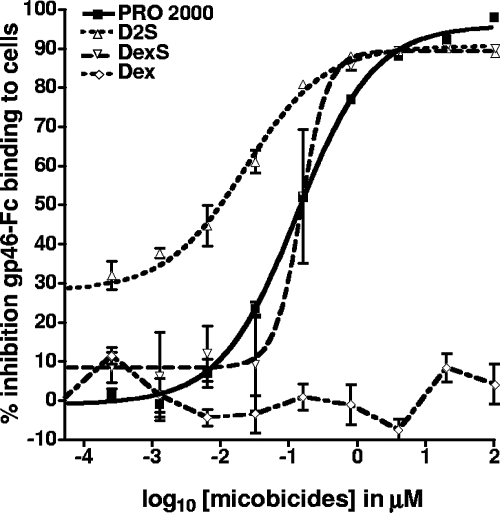
The Env protein gp46 mediates viral entry by interaction with a cellular receptor(s). A product comprising soluble recombinant gp46 fused to the Fc portion of human IgG was developed to study the interactions of gp46 with target cells and their potential receptor(s) (23, 40). gp46-Fc was treated or not with polyanions and control reagents and subsequently incubated with HTLV-1-susceptible target cells. Cell-bound gp46-Fc was detected via a fluorochrome-conjugated anti-Fc reagent and analyzed by flow cytometry, and the results were compared to the signal obtained with the Fc control protein alone (Fig. 5). The treatment of gp46-Fc with PRO 2000, D2S, and DexS significantly inhibited the binding of soluble gp46 to cells, with PRO 2000 inhibiting most efficiently, exhibiting an ID90 of 4.2 μM (±0.5 μM), followed by DexS and D2S, exhibiting ID90s of 4.7 μM (±0.9 μM) and 5.9 μM (±3 μM), respectively (Table 2). Interestingly, at the ID50, D2S was the most potent compound, but at the ID90, it was less potent than the other polyanions. At the highest concentration tested for all compounds (100 μM), the levels of inhibition of gp46 binding were as follows: PRO 2000, 98% (±0.7%); DexS, 90.1% (±1.1%); and D2S, 89% (±0.1%). We were unable to analyze patient sera for activity as a result of the high background labeling detected by the secondary anti-human Fc reagent. Fusion peptide activity was not studied, as this was not predicted to interfere with receptor attachment (39).
FIG. 5.
Inhibition of gp46 binding to cellular receptors by polyanions. Soluble recombinant gp46-Fc fusion protein or negative control soluble Fc protein was preincubated with compounds before application to detached HeLa cells. After 2 h of incubation, cells were washed to remove unbound gp46-Fc or Fc control protein. Cells were stained with an anti-Fc phycoerythrin-conjugated antibody and analyzed by flow cytometry. Results are expressed as the percent inhibition of gp46-Fc binding and are from one representative experiment performed in triplicate, and error bars represent ±1 SD.
Inhibition of HTLV-1 Env-pseudotyped virion transduction.
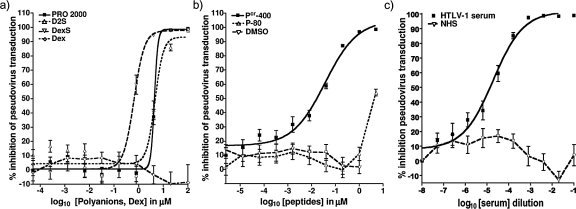
HTLV-1 Env-pseudotyped virions are expected to mimic the fusion and entry properties of cell-free virions, contain a backbone encoding luc which allows a sensitive and quantitative indication of target cell transduction, and deliver a single-cycle assay readout. Pseudotyped virions treated with polyanions (100 μM), Pcr-400 (10 μM), and HAM patient sera (1:10 dilution) had significantly reduced transduction efficiency, with PRO 2000, D2S, and DexS inhibiting by 99.1% (±0.2%), 98% (±0.3%), and 98.4% (±0.6%) respectively (Fig. 6a). In contrast, and in agreement with our previous data, controls Dex, P-80, DMSO, and NHS failed to significantly reduce pseudovirus transduction, independent of the concentration used. The inhibition effect was dose dependent, as shown in Fig. 6b and c, yielding ID50 and ID90 values as reported in Table 2. As before, DexS was the most potent, with PRO 2000 and D2S yielding similar inhibition profiles at the ID50s and ID90s.
FIG. 6.
Inhibition of infection with pseudotyped recombinant HTLV-1 by individual compounds. Pseudovirus produced in 293T cells bearing HTLV-1 Env and encoding Luc was incubated with serially fivefold-diluted compounds for 20 min before application to target Ghost cells. Cells were lysed after 3 days, and the luciferase activity was measured. (a) Effects of polyanions and control Dex; (b) effects of the test peptide Pcr-400, control peptide P-80, and control solvent DMSO; and (c) effects of HAM/TSP patient sera and NHS. Datum points represent the means of results from six independent experiments each performed in triplicate, and error bars represent SEM.
Synergy between inhibitors.
Synergy in vitro between polyanions and other potential microbicides in the context of HIV-1 infection has been described previously (16) and is of interest because synergy can increase the relative potencies of active agents at given doses and combinations of agents may reduce the risk of encountering viruses resistant to microbicidal action. Here, we tested the synergy effects of compounds inhibiting HTLV-1 infection by combining the most promising inhibitors, PRO 2000 and DexS, with the fusion inhibitor peptide Pcr-400. To initially identify an appropriate starting concentration for each agent, all compounds were individually serially diluted 1.5-fold and tested as in the pseudovirus inhibition assay described above to identify at least three datum points between 20 and 80% inhibition. The single-cycle pseudovirus assay was chosen to measure synergy as it best represents the instantaneous inhibition of viral infectivity, the most accurate measure for this type of analysis (1). Optimal starting concentrations were identified to be 100 μM for PRO 2000, 5 μM for DexS, and 0.5 μM for Pcr-400. Combined compounds were then serially diluted 1.5-fold into the assay mixtures, and the results were processed using CalcuSyn software to define the CI. The CI indicates whether the combined compounds act in a synergisitic (CI values of <0.9), additive (CI values of 0.9 to 1.1), or antagonistic (CI values of >1.1) manner compared to the action of each compound used individually. The combination of PRO 2000 with Pcr-400 resulted in mean CI values ± SEM reflecting mild synergy at the ID50s (0.84 ± 0.02), ID75s (0.75 ± 0.08), and ID90s (0.74 ± 0.07). In contrast, DexS with Pcr-400 gave CI values indicating additive activities at the ID50s (1.16 ± 0.01), ID75s (1.02 ± 0.01), and ID90s (0.98 ± 0.01) (Table 3).
TABLE 3.
CIs for PRO 2000 and DexS in combination with Pcr-400
| Inhibitor combination | CIa at:
|
||
|---|---|---|---|
| ID50 | ID75 | ID90 | |
| PRO 2000-Pcr-400 | 0.84 ± 0.02 | 0.75 ± 0.08 | 0.74 ± 0.07 |
| DexS-Pcr-400 | 1.16 ± 0.01 | 1.02 ± 0.01 | 0.98 ± 0.01 |
CIs of <0.9 indicate synergy; CIs of 0.9 to 1.1 indicate addition; CIs of >1.1 indicate antagonism. Values are means ± SEM derived from three replicate experiments.
DISCUSSION
Male-to-female transmission of HTLV-1 appears to be substantially more efficient (with a frequency of 60.8%) than the opposite route (with a frequency of 0.4%) (25), indicating that women are particularly at risk (25, 34, 35). This finding highlights the importance of developing strategies to prevent or reduce the sexual transmission of HTLV-1 and enable women to protect themselves by methods independent of, or in addition to, condom use. Here, we have focused on soluble polyanions currently being tested as potential topical microbicides against HIV-1 infection and have shown in five different in vitro assays encompassing HTLV-1-mediated cell-cell fusion, the cell-cell transmission of HTLV-1 Tax, the cell-cell spread of virus, cell-free pseudovirus transduction, and soluble Env-receptor binding analysis that these agents inhibit HTLV-1 transmission. Each of these assays is, of course, a surrogate for true in vivo virus spread, and each involves its own artifacts relating to the target or the effector cell type and the mechanism and mode of spread. However, the fact that at least two polyanions (PRO 2000 and DexS) exhibited substantial inhibition in all assays gives confidence that certain polyanions and particularly PRO 2000 as a candidate microbicide may have activity against HTLV-1 transmission in an in vivo setting and early virus spread within the host. Moreover, in assays in which ID50 values were obtained (the fusion assay, pseudotype assay, and gp46-Fc binding assay), PRO 2000 gave values within the range that can be safely applied to humans in vivo (32, 49) (Table 1). This result is despite our finding that at concentrations above 100 μM, PRO 2000 was toxic to the cells tested. We assume that PRO 2000 may have increased toxicity in tissue culture compared to toxicity in vivo. Nevertheless, our toxicity data suggest that caution should be exercised in using PRO 2000 at high concentrations. DexS was also highly active in our assays but has not so far been tested as a microbicide candidate in vivo. This situation is due to its anticoagulant activities, making it unsafe for use in humans (3). The fusion inhibition peptide Pcr-400 also displayed significant inhibition in all assays in which it was tested and showed increased activity when combined with PRO 2000 and DexS. These results set the scene for the further study of polyanions, peptide inhibitors, and combinations thereof as topical microbicides.
The mechanisms underlying the mild synergy observed between PRO 2000 and the fusion inhibitor peptide are currently unclear and require further investigation. HTLV-1 antiserum, which contains high-titer anti-Env neutralizing antibodies, was also a potent inhibitor in most of the assays. This indicates that neutralizing antibodies in patient sera may play an important role in protection from HTLV-1 transmission, particularly if they are present at high titers in vaginal secretions (6, 7). D2S potently inhibited transduction by cell-free pseudovirus, whereas it inhibited Env-mediated cell-cell fusion to a limited extent. Despite the greater overall negative charge of D2S than of PRO 2000 and DexS (with three sulfate ions per hexose unit compared to one and two sulfate ions per unit, respectively) (see also Materials and Methods), D2S was found not to significantly inhibit HTLV-1 cell-cell dissemination, which is the main mode of virus spread, and is therefore likely to be of less interest as an inhibitor of this virus. The limited potency of D2S in assays for HTLV-1 cell-cell spread may be due to the high average molecular mass of D2S (20 kDa), imposing steric constraints in gaining access to viral Env within the spatially limited environment of the VS.
In conclusion, with D2S failing to cause substantial inhibition in a cell-cell environment and DexS being unsafe for use in humans, we suggest that both PRO 2000, an agent currently in phase III efficacy trials for protection against HIV-1 transmission, and the fusion inhibition peptide Pcr-400 warrant further testing, either alone or in combination with other HTLV-1 inhibitors, as vaginal microbicides. Other inhibitors based on polyanionic compounds or fusion inhibitors may also warrant investigation in the context of the inhibition of HTLV-1 sexual transmission.
Acknowledgments
We thank the following persons: Simona Ozden, Institut Pasteur, Paris, France, for 293T-GFP cells (formerly known as 293T-HTLV-1 LTR-GFP cells); Graham Taylor, St. Mary's Hospital, Imperial College London, London, United Kingdom, for providing HAM/TSP patient sera and for constructive comments on the manuscript; Al Profy, Indevus Pharmaceuticals Inc., Lexington, MA, for the supply of PRO 2000; Elizabeth Peers, ML Laboratories Plc, Staffordshire, United Kingdom, for providing D2S; William James, The Sir William Dunn School of Pathology, Oxford, for Ghost Hi5 cells; and Amin Mogghadam, Neil Sheppard, and Katherine Gantlett, The Sir William Dunn School of Pathology, Oxford, for help with statistics and general discussions.
We thank the European Microbicide Project “EMPRO” (European Community grant LSHP-CT-2003-503558) and the Microbicide Development Programme (MDP), MRC, United Kingdom (grant G0400453) for funding this study. We thank Fondation Dormeur for the generous donation of equipment used in this study. The contribution of D.W.B. was supported by a grant from the Leukemia Research Fund (LRF 05062).
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Anderson, R. A., K. A. Feathergill, X. H. Diao, M. D. Cooper, R. Kirkpatrick, B. C. Herold, G. F. Doncel, C. J. Chany, D. P. Waller, W. F. Rencher, and L. J. Zaneveld. 2002. Preclinical evaluation of sodium cellulose sulfate (Ushercell) as a contraceptive antimicrobial agent. J. Androl. 23:426-438. [PubMed] [Google Scholar]
- 2.Baba, M., R. Pauwels, J. Balzarini, J. Arnout, J. Desmyter, and E. De Clercq. 1988. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 85:6132-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, M., R. Snoeck, R. Pauwels, and E. de Clercq. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini, J., Y. Van Herrewege, K. Vermeire, G. Vanham, and D. Schols. 2007. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol. Pharmacol. 71:3-11. [DOI] [PubMed] [Google Scholar]
- 5.Bartholomew, C., W. C. Saxinger, J. W. Clark, M. Gail, A. Dudgeon, B. Mahabir, B. Hull-Drysdale, F. Cleghorn, R. C. Gallo, and W. A. Blattner. 1987. Transmission of HTLV-I and HIV among homosexual men in Trinidad. JAMA 257:2604-2608. [PubMed] [Google Scholar]
- 6.Belec, L., M. C. Georges-Courbot, A. Georges, A. S. Mohamed, D. Londos-Gagliardi, M. C. Hallouin, H. Hocini, and B. Guillemain. 1996. Cervicovaginal synthesis of IgG antibodies to the immunodominant 175-199 domain of the surface glycoprotein gp46 of human T-cell leukemia virus type I. J. Med. Virol. 50:42-49. [DOI] [PubMed] [Google Scholar]
- 7.Belec, L., A. Jean Georges, M. C. Hallouin, A. Si Mohamed, L. Morand-Joubert, and M. C. Georges-Courbot. 1996. Human T-lymphotropic virus type I excretion and specific antibody response in paired saliva and cervicovaginal secretions. AIDS Res. Hum. Retrovir. 12:157-167. [DOI] [PubMed] [Google Scholar]
- 8.Belza, M. J. 2004. Prevalence of HIV, HTLV-I and HTLV-II among female sex workers in Spain, 2000-2001. Eur. J. Epidemiol. 19:279-282. [DOI] [PubMed] [Google Scholar]
- 9.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, T. C. 1976. Derivation and properties of Michaelis-Menten type and Hill type equations for reference ligands. J. Theor. Biol. 59:253-276. [DOI] [PubMed] [Google Scholar]
- 11.Chou, T. C., and P. Talalay. 1981. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur. J. Biochem. 115:207-216. [DOI] [PubMed] [Google Scholar]
- 12.Dehee, A., R. Cesaire, N. Desire, A. Lezin, O. Bourdonne, O. Bera, Y. Plumelle, D. Smadja, and J. C. Nicolas. 2002. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J. Virol. Methods 102:37-51. [DOI] [PubMed] [Google Scholar]
- 13.de The, G., and M. Kazanji. 1996. An HTLV-I/II vaccine: from animal models to clinical trials? J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S191-S198. [DOI] [PubMed] [Google Scholar]
- 14.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dokhelar, M. C., H. Pickford, J. Sodroski, and W. A. Haseltine. 1989. HTLV-I p27rex regulates gag and env protein expression. J. Acquir. Immune Defic. Syndr. 2:431-440. [PubMed] [Google Scholar]
- 16.Gantlett, K. E., J. N. Weber, and Q. J. Sattentau. 2007. Synergistic inhibition of HIV-1 infection by combinations of soluble polyanions with other potential microbicides. Antivir. Res. 75:188-197. [DOI] [PubMed] [Google Scholar]
- 17.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 18.Ghez, D., Y. Lepelletier, S. Lambert, J. M. Fourneau, V. Blot, S. Janvier, B. Arnulf, P. M. van Endert, N. Heveker, C. Pique, and O. Hermine. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 80:6844-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 21.Ito, M., M. Baba, A. Sato, R. Pauwels, E. De Clercq, and S. Shigeta. 1987. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 7:361-367. [DOI] [PubMed] [Google Scholar]
- 22.Jassal, S. R., M. D. Lairmore, A. J. Leigh-Brown, and D. W. Brighty. 2001. Soluble recombinant HTLV-1 surface glycoprotein competitively inhibits syncytia formation and viral infection of cells. Virus Res. 78:17-34. [DOI] [PubMed] [Google Scholar]
- 23.Jassal, S. R., R. G. Pohler, and D. W. Brighty. 2001. Human T-cell leukemia virus type 1 receptor expression among syncytium-resistant cell lines revealed by a novel surface glycoprotein-immunoadhesin. J. Virol. 75:8317-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javan, C. M., N. J. Gooderham, R. J. Edwards, D. S. Davies, and S. Shaunak. 1997. Anti-HIV type 1 activity of sulfated derivatives of dextrin against primary viral isolates of HIV type 1 in lymphocytes and monocyte-derived macrophages. AIDS Res. Hum. Retrovir. 13:875-880. [DOI] [PubMed] [Google Scholar]
- 25.Kajiyama, W., S. Kashiwagi, H. Ikematsu, J. Hayashi, H. Nomura, and K. Okochi. 1986. Intrafamilial transmission of adult T cell leukemia virus. J. Infect. Dis. 154:851-857. [DOI] [PubMed] [Google Scholar]
- 26.Kwok, S., D. Kellogg, G. Ehrlich, B. Poiesz, S. Bhagavati, and J. J. Sninsky. 1988. Characterization of a sequence of human T cell leukemia virus type I from a patient with chronic progressive myelopathy. J. Infect. Dis. 158:1193-1197. [DOI] [PubMed] [Google Scholar]
- 27.Larsen, O., S. Andersson, Z. da Silva, K. Hedegaard, A. Sandstrom, A. Naucler, F. Dias, M. Melbye, and P. Aaby. 2000. Prevalences of HTLV-1 infection and associated risk determinants in an urban population in Guinea-Bissau, West Africa. J. Acquir. Immune Defic. Syndr. 25:157-163. [DOI] [PubMed] [Google Scholar]
- 28.Laurendeau, I., M. Bahuau, N. Vodovar, C. Larramendy, M. Olivi, I. Bieche, M. Vidaud, and D. Vidaud. 1999. TaqMan PCR-based gene dosage assay for predictive testing in individuals from a cancer family with INK4 locus haploinsufficiency. Clin. Chem. 45:982-986. [PubMed] [Google Scholar]
- 29.Lederman, M. M., R. E. Offord, and O. Hartley. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371-382. [DOI] [PubMed] [Google Scholar]
- 30.Madan, R. P., M. J. Keller, and B. C. Herold. 2006. Prioritizing prevention of HIV and sexually transmitted infections: first-generation vaginal microbicides. Curr. Opin. Infect. Dis. 19:49-54. [DOI] [PubMed] [Google Scholar]
- 31.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 32.Mayer, K. H., S. A. Karim, C. Kelly, L. Maslankowski, H. Rees, A. T. Profy, J. Day, J. Welch, and Z. Rosenberg. 2003. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS 17:321-329. [DOI] [PubMed] [Google Scholar]
- 33.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy, E. L., J. P. Figueroa, W. N. Gibbs, A. Brathwaite, M. Holding-Cobham, D. Waters, B. Cranston, B. Hanchard, and W. A. Blattner. 1989. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann. Intern. Med. 111:555-560. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, E. L., R. Wilks, B. Hanchard, B. Cranston, J. P. Figueroa, W. N. Gibbs, J. Murphy, and W. A. Blattner. 1996. A case-control study of risk factors for seropositivity to human T-lymphotropic virus type I (HTLV-I) in Jamaica. Int. J. Epidemiol. 25:1083-1089. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima, K., S. Kashiwagi, W. Kajiyama, M. Hirata, J. Hayashi, A. Noguchi, K. Urabe, K. Minami, and Y. Maeda. 1995. Sexual transmission of human T-lymphotropic virus type I among female prostitutes and among patients with sexually transmitted diseases in Fukuoka, Kyushu, Japan. Am. J. Epidemiol. 141:305-311. [DOI] [PubMed] [Google Scholar]
- 37.Okochi, K., H. Sato, and Y. Hinuma. 1984. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46:245-253. [DOI] [PubMed] [Google Scholar]
- 38.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 39.Pinon, J. D., S. M. Kelly, N. C. Price, J. U. Flanagan, and D. W. Brighty. 2003. An antiviral peptide targets a coiled-coil domain of the human T-cell leukemia virus envelope glycoprotein. J. Virol. 77:3281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinon, J. D., P. J. Klasse, S. R. Jassal, S. Welson, J. Weber, D. W. Brighty, and Q. J. Sattentau. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 77:9922-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proietti, F. A., A. B. Carneiro-Proietti, B. C. Catalan-Soares, and E. L. Murphy. 2005. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24:6058-6068. [DOI] [PubMed] [Google Scholar]
- 43.Rusconi, S., M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Singh, K. J. Willis, M. S. Osburne, A. T. Profy, J. C. Jenson, and M. S. Hirsch. 1996. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 40:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaunak, S., N. J. Gooderham, R. J. Edwards, N. Payvandi, C. M. Javan, N. Baggett, J. MacDermot, J. N. Weber, and D. S. Davies. 1994. Infection by HIV-1 blocked by binding of dextrin 2-sulphate to the cell surface of activated human peripheral blood mononuclear cells and cultured T-cells. Br. J. Pharmacol. 113:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaunak, S., M. Thornton, S. John, I. Teo, E. Peers, P. Mason, T. Krausz, and D. S. Davies. 1998. Reduction of the viral load of HIV-1 after the intraperitoneal administration of dextrin 2-sulphate in patients with AIDS. AIDS 12:399-409. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama, H., H. Doi, K. Yamaguchi, Y. Tsuji, T. Miyamoto, and S. Hino. 1986. Significance of postnatal mother-to-child transmission of human T-lymphotropic virus type-I on the development of adult T-cell leukemia/lymphoma. J. Med. Virol. 20:253-260. [DOI] [PubMed] [Google Scholar]
- 47.Tajima, K., S. Tominaga, and T. Suchi. 1986. Malignant lymphomas in Japan: epidemiological analysis on adult T-cell leukemia/lymphoma. Hematol. Oncol. 4:31-44. [DOI] [PubMed] [Google Scholar]
- 48.Van Damme, L. 2004. Clinical microbicide research: an overview. Trop. Med. Int. Health 9:1290-1296. [DOI] [PubMed] [Google Scholar]
- 49.Van Damme, L., A. Wright, K. Depraetere, I. Rosenstein, V. Vandersmissen, L. Poulter, M. McKinlay, E. Van Dyck, J. Weber, A. Profy, M. Laga, and V. Kitchen. 2000. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex. Transm. Infect. 76:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber, J., K. Desai, and J. Darbyshire. 2005. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber, J., A. Nunn, T. O'Connor, D. Jeffries, V. Kitchen, S. McCormack, J. Stott, N. Almond, A. Stone, and J. Darbyshire. 2001. ‘Chemical condoms’ for the prevention of HIV infection: evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS 15:1563-1568. [DOI] [PubMed] [Google Scholar]
- 52.Yakova, M., A. Lezin, F. Dantin, G. Lagathu, S. Olindo, G. Jean-Baptiste, S. Arfi, and R. Cesaire. 2005. Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]