Abstract
A highly epidemic carbapenem-resistant clone of KPC-3-producing Klebsiella pneumoniae emerged in Israel in 2006, causing a nationwide outbreak. This clone was genetically related to outbreak strains from the United States isolated in 2000 but differed in KPC-carrying plasmids. The threat of the global spread of hyperepidemic, extensively drug-resistant bacterial strains should be recognized and confronted.
KPC-type enzymes in carbapenem-resistant Klebsiella pneumoniae strains were first reported in 2001 in North Carolina (16), and until 2005, the geographical distribution of these enzymes in K. pneumoniae, with both KPC-2 and KPC-3, was limited to the eastern United States (1, 12, 15). In the New York area, KPC-producing strains have become a frequently encountered nosocomial pathogen (2, 4). The first case of KPC-producing K. pneumoniae outside the United States occurred in France, where a patient who had been hospitalized in New York carried the strain with him (9). KPC-producing K. pneumoniae strains have since been reported from Israel (6), Colombia (13), China (14), and Greece (8).
Carbapenem resistance in Klebsiella pneumoniae carrying blaKPC-2 was first observed in the Tel Aviv Sourasky Medical Center in late 2005. In February 2006, we noted a sharp increase in blaKPC-carrying, carbapenem-resistant K. pneumoniae strains in our hospital, mainly possessing blaKPC-3 (6). These extensively drug-resistant (XDR) isolates caused difficult-to-treat infections and had an adverse impact on patients' outcomes (11). Despite infection control efforts that limited the spread of KPC-2-producing clones of Klebsiella in our hospital, KPC-3-producing K. pneumoniae isolates continued to appear rapidly during 2006, in our hospital and in other hospitals throughout the country (10).
To characterize the extent of the nationwide occurrence of KPC-3-producing, carbapenem-resistant K. pneumoniae in Israel, 100 single-patient isolates collected during 2006 in eight hospitals and five chronic care centers with wide geographical distribution in the north, center, and southern parts of the country were sent to our lab for further study. Six to eight isolates were included from each institution based on their resistance to at least one carbapenem antibiotic (imipenem and/or meropenem) determined using the Vitek2 automated system (bioMerieux, Marcy l'Etoile, France) with an AST-GN09 card. The presence of blaKPC in all isolates was verified using PCRs followed by sequencing to determine the type of KPC gene (6). MICs of imipenem, meropenem, and ertapenem were determined by agar dilution according to the method of the Clinical and Laboratory Standards Institute (3). Susceptibility testing for tigecycline was performed via Etest according to the manufacturer's instructions (AB Biodisk, Solna, Sweden). Interpretive criteria for tigecycline MICs were defined based on the U.S. Food and Drug Administration breakpoint criteria for Enterobacteriaceae (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; resistant, ≥8 μg/ml). The genetic relatedness of all carbapenem-resistant K. pneumoniae strains was determined by pulsed-field gel electrophoresis (PFGE) analysis. DNA was prepared as described previously (6), and chromosomal restriction fragments obtained after SpeI or ApaI cleavage were documented and compared.
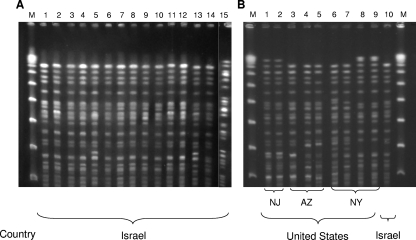
Sites of isolation included urine (n = 46), blood (n = 19), respiratory tract (n = 15), wounds (n = 15), and other (n = 5). The isolates were resistant to nearly all antimicrobial agents, including all cephalosporins, beta-lactam/beta-lactamase inhibitor combinations, trimethoprim-sulfamethoxazole, and fluoroquinolones; MICs of the carbapenems varied (Table 1). The majority of the isolates (98%) were susceptible only to gentamicin and colistin, while the other 2% were gentamicin resistant and kanamycin susceptible or resistant to both aminoglycosides. Among 20 isolates tested for tigecycline, MICs were at 1 to 3 μg/ml. PFGE analysis revealed that all isolates belonged to the same genetic clone, indistinguishable from the KPC-3-producing clone described previously in our hospital (6) (Fig. 1A). This finding suggested the nationwide spread of an epidemic carbapenem-resistant K. pneumoniae strain, designated clone Q.
TABLE 1.
Carbapenem-resistant K. pneumoniae isolates included in this binational study
| Country | Yr of isolation | No. of isolates | MIC (μg/ml)a
|
||
|---|---|---|---|---|---|
| Imipenem | Meropenem | Ertapenem | |||
| Israel | 2006-2008 | 100 | 16, 32 (<0.25-128) | 64, 128 (2-256) | 128, 256 (<0.5->256) |
| United Statesb | 2000-2006 | 26 | 4, 64 (<0.25-128) | 8, 64 (<0.5-256) | 16, 128 (<0.25->256) |
MIC50, MIC90 (MIC range).
Outbreak isolates from various states, including New York, Delaware, Maryland, Pennsylvania, New Jersey, and Arizona.
FIG. 1.
(A) PFGE (SpeI restricted) of 14 representative KPC-3-producing, carbapenem-resistant K. pneumoniae isolates from various Israeli hospitals (lanes 1 to 14) demonstrates a nationwide epidemic clone different from a KPC-2-producing clone reported previously in our hospital (6) (lane 15). (B) Genetic relatedness of the predominant Israeli epidemic clone (lane 10) with nine KPC-3-producing K. pneumoniae isolates involved in outbreaks in the United States, from New Jersey (NJ; lanes 1 and 2), Arizona (AZ; lanes 3 to 5), and New York (NY; lanes 6 to 9). M, lambda ladder (New England Biolabs).
PCR and sequencing revealed the presence of blaKPC-3 in all isolates.
When the Israeli outbreak started, the only country in which KPC-producing K. pneumoniae had been reported was the United States. Therefore, we compared the hyperepidemic strain isolated in Israel to a collection of 26 KPC-producing, carbapenem-resistant K. pneumoniae outbreak isolates from the United States. These U.S. isolates originated from patients with diverse infections who were hospitalized in five different states between 2000 and 2006 (Table 1).
Testing for genetic relatedness between the Israeli hyperepidemic XDR clone and the American isolates revealed that nine (35%) of the 26 KPC-3-producing U.S. isolates had PFGE profiles identical or highly similar to each other (Fig. 1B). These nine isolates represented outbreaks that occurred in a New York medical center in 2000 (15) and in New Jersey and Arizona in 2006. Four of these nine isolates were indistinguishable by PFGE from the Israeli epidemic clone, and the remaining five isolates were closely related to that clone (with differences of up to three bands). The other 17 isolates, representing an outbreak during 2004 in a New York medical center and clinical isolates identified in Delaware, Maryland, New Jersey, and Pennsylvania during 2006, differed from the common PFGE type by more than seven bands.
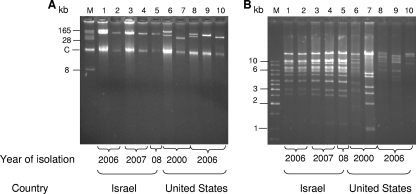
Plasmid analysis was performed to determine whether the highly genetically related Israeli-American K. pneumoniae clones carried the same KPC-3-encoding plasmid. Ten isolates were analyzed: five isolates from Israel (from 2006 to 2008) and five isolates from the United States (from 2000 to 2006). Plasmid DNA was purified using a NucleoBond PC100 plasmid Midi kit (Macherey-Nagel GmbH, Duren, Germany), and transformation was performed into Escherichia coli GeneHogs (Invitrogene Corp., Dorset, United Kingdom). Transformants were selected on Luria-Broth agar plates with ampicillin (100 μg/ml), and selected colonies were subjected to blaKPC PCR screening to confirm the acquisition of a KPC-encoding plasmid. KPC-encoding plasmids were purified from 10 transformants originating from the 10 genetically related Israeli and American isolates (Fig. 2A). All five Israeli isolates carried the same plasmid with an apparent molecular mass of 100 kb. The genetically related U.S. isolates carried various plasmids ranging in size from 165 kb to 38 kb, which differed from the typical KPC-3-encoding Israeli plasmid (Fig. 2B).
FIG. 2.
A comparison of blaKPC-3-encoding plasmids (A) and their EcoRI restriction patterns (B) from five Israeli K. pneumoniae isolates (lanes 1 to 5 in each panel) and five of the nine genetically related K. pneumoniae U.S. isolates (lanes 6 to 10 in each panel). Analysis was performed on plasmids isolated from E. coli GeneHogs transformants carrying the blaKPC-3-encoding plasmids. Lanes 1 to 5, representative Israeli isolates from 2006 to 2008; lanes 6 to 10, representative U.S. isolates from 2000 and 2006; lanes 6 and 7, two isolates from a New York medical center; lanes 8 to 10, one isolate from New Jersey and two isolates from Arizona. For panel A, lane M is a BAC-Tracker supercoiled DNA ladder (Epicentre Biotechnologies); for panel B, lane M is a GeneRuler 1-kb DNA ladder (Fermentas Life Sciences).
Transfer of the KPC-3-encoding plasmids to the susceptible E. coli GeneHogs recipient strain raised the MICs of extended-spectrum cephalosporins and aztreonam by more than 60-fold, rendering resistance. MICs of carbapenems, although increased (from 0.012 μg/ml to 0.016 to 0.5 μg/ml for meropenem and ertapenem, and from 0.094 μg/ml to 0.125 to 1.0 μg/ml for imipenem), were not in the resistant range. This observation may be due to the presence of additional mechanisms of carbapenem resistance in these strains, such as porin alterations (17).
Our finding of the occurrence of closely related K. pneumoniae strains carrying blaKPC-3 on different plasmids in two continents is intriguing.
This report on the international occurrence of a clone of XDR blaKPC-3-producing K. pneumoniae, which has caused outbreaks in the United States and Israel, emphasizes the potential for transmission of highly resistant, gram-negative pathogens that are difficult, and in some cases impossible, to treat with currently available antimicrobial agents. This situation is exacerbated by the lack of novel agents in the antimicrobial pipeline for treating these pathogens. Our finding of similar strains in the United States and Israel raises the possibility of a clonal XDR strain of K. pneumoniae, akin to what has been described for the 300.0114 strain of methicillin-resistant Staphylococcus aureus (5) and the NAP1/027 strain of Clostridium difficile (7). This issue will require further investigation, but it may help inform future studies directed at characterizing the molecular mechanisms, environmental factors, and selection pressures which promote the spread of this XDR hyperepidemic strain. In the meantime, currently established infection control measures, such as hand hygiene, isolation precautions, and judicious antimicrobial use, should be employed throughout the world to limit the emergence and transmission of KPC-producing organisms.
Acknowledgments
We thank Keren Strauss-Robinson for her technical assistance. This study was supported in part by a grant from the Public Committee for the Designation of Estate Funds Ministry of Justice State of Israel.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 2.Bratu, S., D. Landman, R. Haag, R. Rocco, A. Eramo, M. Alam, and J. Quale. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City. Arch. Intern. Med. 165:1430-1435. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute/NCCLS. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI/NCCLS M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Desphande, L. M., R. N. Jones, T. R. Fritsche, and H. S. Sader. 2006. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000-2004). Microb. Drug Resist. 12:223-230. [DOI] [PubMed] [Google Scholar]
- 5.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 6.Leavitt, A., S. Navon-Venezia, I. Chmelnitsky, M. J. Schwaber, and Y. Carmeli. 2007. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob. Agents Chemother. 51:3026-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 8.Naas, T., P. G. Cuzon, M. V. Villegas, M. F. Lartigue, and P. Nordmann. 2008. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52:1257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naas, T., P. Nordmann, G. Vedel, and C. Poyart. 2005. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob. Agents Chemother. 49:4423-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samra, Z., O. Ofir, Y. Lishtzinsky, L. Mador-Shapiro, and J. Bishara. 2007. Outbreak of carbapenem-resistant Klebsiella pneumoniae producing KPC-3 in a tertiary medical centre in Israel. Int. J. Antimicrob. Agents 30:525-529. [DOI] [PubMed] [Google Scholar]
- 11.Schwaber, M. J., S. Klarfeld-Lidji, S. Navon-Venezia, D. Sc h wartz, A. Leavitt, and Y. Carmeli. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith Moland, E., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 13.Villegas, M. V., K. Lolans, A. Correa, C. J. Suarez, J. A. Lopez, M. Vallejo, J. P. Quinn, and the Colombian Nosocomial Resistance Study Group. 2006. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob. Agents Chemother. 50:2880-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei, Z. Q., X. X. Du, Y. S. Yu, P. Shen, Y. G. Chen, and L. J. Li. 2007. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob. Agents Chemother. 51:763-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M. F. I. Papelou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing ß-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang, R., L. Yang, J. C. Cai, H. W. Zhou, and G. X. Chen. 2008. High-level carbapenem resistance in a Citrobacter freundii clinical isolate is due to a combination of KPC-2 production and decreased porin expression. J. Med. Microbiol. 57:332-337. [DOI] [PubMed] [Google Scholar]