Abstract
The efficacy of two mesoionic derivatives (MI-H-H and MI-4-OCH3) was evaluated in CBA/J mice infected with Leishmania amazonensis. Treatment with these compounds demonstrated that the MI-4-OCH3 derivative and the reference drug meglumine antimoniate (Glucantime) presented significant activity relative to an untreated control. No apparent hepatic or renal toxicity due to these mesoionic compounds was found.
The World Health Organization considers leishmaniasis one of the most serious diseases worldwide caused by protozoan parasites (24). However, the control of this disease remains a problem; the available antileishmanial drugs still rely on the highly toxic pentavalent antimonials (meglumine antimoniate [Glucantime] and sodium stibogluconate [Pentostam]), which cause serious side effects and require long-term treatment (5, 19). Second-line drugs include pentamidine and amphotericin B, but these drugs have not experienced widespread use because of toxicity and cost. Recently, the oral drug miltefosine was approved for the treatment of human visceral Leishmania infections and oral fluconazole was also shown experimentally to be effective against cutaneous leishmaniasis (1). Although extensive studies of new molecules with antileishmanial activity, including natural and synthetic compounds, have been undertaken (4), the problems of drug resistance and the side effects of the chemotherapies used at present have not been solved.
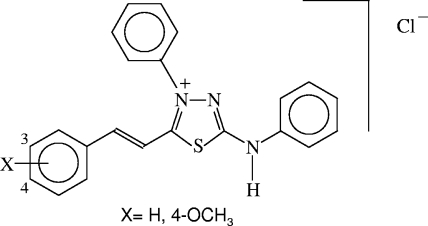
Our previous studies have proven that mesoionic derivatives of the 1,3,4-thiadiazolium-2-aminide class (Fig. 1) inhibit the in vitro growth of Leishmania amazonensis, L. brasiliensis, and L. chagasi promastigotes (6, 21). The chemistry of mesoionic rings, especially their use as masked dipoles, has been a fruitful area of research since the late 1950s. Their structures, having well-separated regions of positive and negative charge associated with a polyheteroatomic system, enable them to interact with biomolecules (14). These characteristics have been revealed by interesting biological activities including anti-inflammatory, analgesic, antibacterial, antifungal, and antitumor activities (22). In addition, this class of mesoionic compounds is known to have nitric oxide (NO)-releasing properties (11).
FIG. 1.
Chemical structures of 4-phenyl-5-(4-H- or 4-methoxy-cinnamoyl)-1,3,4-thiadiazolium-2-phenylamine mesoionic compounds.
The present study was undertaken to investigate the in vivo efficacy of two mesoionic derivatives (MI-H-H and MI-4-OCH3) in the mouse L. amazonensis cutaneous infection model. To examine the therapeutic efficacy of these mesoionic derivatives, CBA/J mice 6 to 8 weeks of age were infected subcutaneously with 1.2 × 106 promastigotes. In this experiment, MI-H-H (24 mg/kg/day), MI-4-OCH3 (22 mg/kg/day), and the reference drug meglumine antimoniate (100 mg/kg/day with 28 mg pentavalent antimonial) (2, 22) were administered by the subcutaneous route 27 days after the experimental infection at 5 doses a week for 4 weeks. Animals in the control group received the same volume of vehicle (dimethyl sulfoxide/phosphate-buffered saline). Progression of the lesion was monitored until week 12 by measurement of footpad swelling.
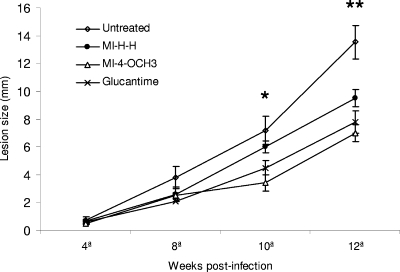
At the end of drug administration (week 8), there was a slight difference between groups of mice treated with both the test compounds and the reference drug and untreated infected mice (Fig. 2). However, at week 12 postinfection, the animals treated daily with MI-4-OCH3 or MI-H-H showed significantly reduced footpad thickness, as did those treated with meglumine antimoniate, compared with that of the control group. It is important to note that, at that time, no significant differences in lesion size were observed in the groups treated with mesoionic compounds or meglumine antimoniate.
FIG. 2.
Effects of different compounds on the development of L. amazonensis infection in several groups of CBA/J mice. Treatments were started in week 4 postinfection and continued for 4 weeks. Datum points represent the average measurements for groups of seven mice each. Lesion diameter was expressed as the thickness of the infected footpad. Bars, standard errors of the means (*, P < 0.01; **, P ≤ 0.001).
In order to evaluate the toxicity of these compounds in mice, body weight was determined and samples of blood were taken at different times during compound administration from the tails of both uninfected and infected mice left untreated or treated. The total number of leukocytes was estimated by counting in a Neubauer chamber. The sera collected were assayed colorimetrically for alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine with commercial kits (Labtest Diagnostica, Brazil). No apparent signs of drug toxicity, weight loss, or lymphocyte, monocyte, or neutrophil alterations were observed in any experiment, and AST, ALT, and creatinine concentrations showed no apparent hepatic or renal toxicity after the treatment with mesoionic compounds (Table 1), compared with uninfected mice left untreated or treated with these compounds (data not shown).
TABLE 1.
Hematological values and toxicological aspects for uninfected mice left untreated or treated with mesoionic compounds at week 4 of treatment
| Mice | White blood cell count (103/μl) | Lymphocytes (%) | Monocytes (%) | Neutrophils (%) | Creatinine level (dg/ml) | AST activity (U/ml) | ALT activity (U/ml) | Body wt (g) |
|---|---|---|---|---|---|---|---|---|
| Untreated | 14 | 75 | 5 | 19 | 2.81 ± 0.28 | 51 ± 6.8 | 52 ± 6.2 | 25 |
| MI-4-OCH3 treated | 12 | 69 | 5 | 25 | 2.56 ± 0.48 | 53 ± 5.8 | 57 ± 6.2 | 27 |
| MI-H-H treated | 12 | 70 | 5 | 24 | 2.84 ± 0.49 | 48 ± 5.6 | 58 ± 8.5 | 26 |
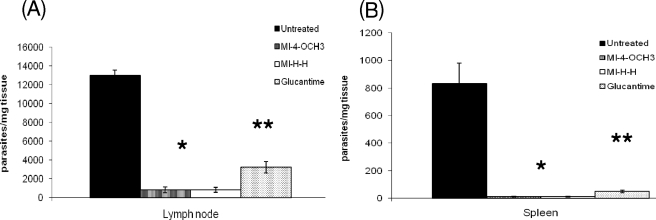
At 4 weeks after the end of treatment (week 12 of infection), the animals were killed and their popliteal lymph nodes and spleens were aseptically removed, weighed, and then homogenized in Schneider's medium supplemented with 10% fetal calf serum. Briefly, under sterile conditions, eight serial dilutions (1:10) were prepared and distributed in 96-well microtiter plates in triplicate. After incubation at 26°C, the wells were examined with an inverted microscope. The final titer was the last dilution at which the well contained at least one parasite (Fig. 3) (3). The parasite loads in both the popliteal lymph nodes and spleens of animals treated with the mesoionic derivatives or meglumine antimoniate were significantly reduced compared to those of untreated control animals (Fig. 3; P ≤ 0.0001). However, the reduction of the parasite loads in both organs after mesoionic derivative treatment was greater than that observed after meglumine antimoniate treatment (P ≤ 0.001).
FIG. 3.
Effects of treatment with mesoionic compounds (MI-4-OCH3, MI-H-H) and meglumine antimoniate on lymph node (A) and on spleen (B) parasite numbers. CBA/J mice were inoculated with L. amazonensis promastigotes and treated with the mesoionic compounds for 4 weeks. The popliteal lymph nodes and spleens of seven animals were then removed, and parasite numbers were estimated by the limiting-dilution technique (*, P < 0.0001; **, P < 0.001).
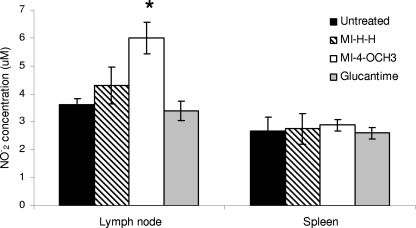
In order to elucidate possible NO induction in infected CBA/J mice, we measured the concentrations of nitrites present in the supernatant of the lymph node and spleen cell cultures as described by Green et al. (10). The results are expressed as micromolar concentrations of NO2 based on a standard curve derived from known concentrations of sodium nitrite (NaNO2) dissolved in cell culture medium. We observed a significant increase in NO production in lymph node cell culture supernatants of infected mice after treatment with MI-4-OCH3 compared to those of untreated mice (Fig. 4). These results could suggest that mesoionic derivatives modulate infection by L. amazonensis in vivo, activating mechanisms that positively affect the host's capacity to eliminate the parasites from infected cells, thus controlling parasite dissemination. Further studies to evaluate this phenomenon would be interesting.
FIG. 4.
NO production in suspensions of lymph nodes and spleens from four groups of CBA/J mice. NO production was assayed by measuring the nitrite concentrations present in the supernatant of the lymph node and spleen cells cultures of all four groups at the end of the experimental period. The results were read in a spectrophotometer (μQuant) at 550 nm (*, P ≤ 0.01).
From studies concerning structure-activity relationships, especially those based on the nature of the group at position 4 of the phenyl ring (Fig. 1), the compounds with the best in vitro activity were selected as promising drug candidates (6). Of the two compounds selected, the mesoionic with a 4-OCH3 substituent was more effective than the one with no substitution (MI-H-H). MI-4-OCH3 administered subcutaneously to mice for 4 weeks controlled the infection induced by L. amazonensis in the paws, resulting in lesions smaller than those seen in mice treated with the reference drug (meglumine antimoniate) or in control animals (P < 0.001).
This study also demonstrates that both mesoionic derivative treatment decreased parasite loads (P < 0.0001) in the regional popliteal lymph nodes and in the spleen, which suggests that there is control of infection progression and that dissemination is limited. Because of the mesoionic treatment, the lymph node and spleen weights also decreased compared to those of untreated control mice. This could be correlated with the decreasing parasite loads in these groups. No renal or hepatic alterations occurred, as evidenced by normal levels of creatinine, AST, and ALT in infected mice treated with mesoionic compounds and meglumine antimoniate. The toxicity of meglumine antimoniate was also evaluated by Henao et al. (12) in the cutaneous leishmaniasis hamster model, and no hazard to the animals was observed (7). Moreover, increased NO production was observed in lymph node cell culture supernatants of infected mice treated with MI-4-OCH3 (P < 0.01). The function of NO in the leishmanicidal activity of activated macrophages has been demonstrated both in vitro and in vivo (13, 15-18). The in vitro experiments done by our group clearly revealed that mesoionic derivatives can modulate macrophage infection by NO released by L. amazonensis (data not shown). In contrast, the in vitro production of NO by L. amazonensis alone (8, 9) was decreased by MI-4-OCH3 and MI-3-OCH3 addition (23).
Furthermore, NO production by macrophages alone does not fully explain the inhibitory effect of mesoionic compounds on lesions induced by L. amazonensis in vivo. Thus, while macrophages are one of the main sources of NO, this radical may also be released by other cells involved in the infection process, including Leishmania parasites (8). Cytokines and other mediators released from activated cells that modify macrophage functions underscore the complexity of the process.
It is already known that meglumine antimoniate is usually parenterally administered and that it irritates the intestinal mucosa, causing a low absorption rate in the gastrointestinal tract, and for that reason this drug could not be used by the oral route (20). However, mesoionic derivatives should be tested orally in future research. These compounds are able to interact with biomolecules; although the compounds are internally charged, they are neutral overall and therefore can cross biological membranes in vivo (6). These properties could allow other routes of treatment with mesoionic derivatives, meaning advantages over antimonials.
Given these considerations, further studies are necessary to elucidate the mechanism of action of mesoionic compounds in the defense of the organisms against infection, creating new perspectives for the investigation of other mediators.
In conclusion, the lack of apparent toxicity of this compound, as attested by the blood and serum pathology of treated mice and its protective in vivo effect during murine leishmaniasis encourage further studies of mesoionic derivatives such as MI-OCH3 as new antileishmanial drugs and as modifiers of the immunological response to combat infections with intracellular pathogens, as well as tissue histology.
Acknowledgments
This work was supported by grants from CNPq, CAPES, PDTIS, and FIOCRUZ and fellowships from CNPq and CAPES.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Alrajhi, A. A., E. B. De Vol, and J. H. Maguire. 2002. Fluconazole for the treatment of cutaneous leishmaniasis. N. Engl. J. Med. 346:891-895. [DOI] [PubMed] [Google Scholar]
- 2.Barão, S. C., and S. Giorgio. 2003. Efficacy of 8-bromoguanosine against murine cutaneous leishmaniasis induced with Leishmania amazonensis. Chemotherapy 49:159-162. [DOI] [PubMed] [Google Scholar]
- 3.Bertho, A. L., M. A. Santiago, and S. G. Coutinho. 1994. An experimental model of the production of metastases in murine cutaneous leishmaniasis. J. Parasitol. 80:93-99. [PubMed] [Google Scholar]
- 4.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L., K. Seifert, and V. Yardley. 2006. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 123:399-410. [PubMed] [Google Scholar]
- 6.da Silva, E. F., M. M. Canto-Cavalheiro, V. R. Braz, L. Cysne-Finkelstein, L. L. Leon, and A. Echevarria. 2002. Synthesis and biological evaluation of new 1,3,4-thiadiazolium-2-phenylamine derivatives against Leishmania amazonensis promastigotes and amastigotes. Eur. J. Med. Chem. 37:979-984. [DOI] [PubMed] [Google Scholar]
- 7.Deps, P. D., M. C. Vianna, A. Falqueto, and R. Dietze. 2000. Evaluation of the efficacy and toxicity of N-methyl-glucamine vs BP88 sodium stibogluconate in the treatment of localized cutaneous leishmaniasis. Rev. Soc. Bras. Med. Trop. 33:535-543. [DOI] [PubMed] [Google Scholar]
- 8.Géigel, L. F., and L. L. Leon. 2003. Cyclic 3′-5′ guanosine monophosphate-dependent activity in Leishmania amazonensis. Mem. Inst. Oswaldo Cruz 98:499-500. [DOI] [PubMed] [Google Scholar]
- 9.Genestra, M. S., W. J. Souza, L. Cysne-Finkelstein, and L. L. Leon. 2003. Comparative analysis of nitric oxide production by Leishmania sp. Med. Microbiol. Immunol. 192:217-223. [DOI] [PubMed] [Google Scholar]
- 10.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 126:131. [DOI] [PubMed] [Google Scholar]
- 11.Gryglewski, R. J., E. Marcinkiewicz, J. Robak, Z. Michalska, and J. Madej. 2002. Mesoionic oxatriazoles (MOTA): NO-donating characteristics and pharmacology. Curr. Pharm. Des. 8:167-176. [DOI] [PubMed] [Google Scholar]
- 12.Henao, H. H., Y. Osorio, N. G. Saravia, A. Gomes, and B. Travi. 2004. Efficacy and toxicity of pentavalent antimonials (Glucantime and Pentostam) in an American cutaneous leishmaniasis animal model: luminometry application. Biomedica 24:393-402. [PubMed] [Google Scholar]
- 13.Kavoosi, G., S. K. Ardestani, A. Kariminia, and Z. Tavakoli. 2006. Production of nitric oxide by murine macrophages induced by lipophosphoglycan of Leishmania major. Korean J. Parasitol. 44:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kier, L. B., and E. B. Roche. 1967. Medicinal chemistry of the mesoionic compounds. J. Pharm. Sci. 56:149-168. [DOI] [PubMed] [Google Scholar]
- 15.Lemos de Souza, V., J. A. Souza, T. M. C. Silva, and P. S. T. Veras. 2000. Different Leishmania species determine distinct profiles of immune and histopathological responses in CBA mice. Microbes Infect. 2:1807-1815. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., C. A. Hunter, and J. P. Farrel. 1999. Anti-TGF-β treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J. Immunol. 162:974-979. [PubMed] [Google Scholar]
- 17.Liew, F. Y., L. Yun, and S. Millott. 1990. Tumor necrosis factor α synergizes with INT-γ in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 18.Liew, F. Y., Y. Li, D. Moss, C. Parkinson, M. V. Rogers, and S. Moncada. 1991. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur. J. Immunol. 21:3009-3014. [DOI] [PubMed] [Google Scholar]
- 19.Ouellette, M., J. Drummelsmith, and B. Papadopoulou. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updates 7:257-266. [DOI] [PubMed] [Google Scholar]
- 20.Pintado, V., and R. Lopez-Velez. 2001. HIV-associated visceral leishmaniasis. Clin. Microbiol. Infect. 7:291-300. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues, R. F., E. F. da Silva, A. Echevarria, R. Fajardo-Bonin, V. F. Amaral, L. L. Leon, and M. M. Canto-Cavalheiro. 2007. A comparative study of mesoionic compounds in Leishmania sp and toxicity evaluation. Eur. J. Med. Chem. 42:1039-1043. [DOI] [PubMed] [Google Scholar]
- 22.Senff-Ribeiro, A., A. Echevarria, E. F. da Silva, S. S. Veiga, and M. B. M. Oliveira. 2003. Effect of a new 1,3,4-thiadiazolium mesoionics compound (MI-D) on B16-F10 murine melanoma. Melanoma Res. 13:465-471. [DOI] [PubMed] [Google Scholar]
- 23.Soares-Bezerra, R. J., E. F. da Silva, A. Echevarria, L. Gomes-da-Silva, L. Cysne-Finkelstein, F. P. Monteiro, L. L. Leon, and M. Genestra. 2008. Effect of mesoionic 4-phenyl-5-(cinnamoyl)-1,3,4-thiadiazolium-2-phenylamine chloride derivative salts on the activities of the nitric oxide synthase and arginase of Leishmania amazonensis. J. Enzyme Inhib. Med. Chem. 23:328-333. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1999. Tropical disease research: progress in international research, 1997-1998. World Health Organization, Geneva, Switzerland.