Abstract
The activity of vancomycin against heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) and non-hVISA isolates, using an in vitro pharmacodynamic model, was reduced in the presence of a high inoculum amount (108 CFU/ml). A high bacterial load of >105 CFU/ml persisted for all strains with doses up to 5 g every 12 h against high inoculum amounts. No change in the vancomycin MIC was detected in any isolate at a moderate inoculum amount (106 CFU/ml), and bactericidal activity occurred only against the non-hVISA isolate (time to 99% kill, 7.5 h; P = 0.001).
The treatment of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) infections with vancomycin presents many therapeutic challenges. Patients with hVISA bacteremia have significantly higher rates of morbidity and high-bacterial-load infections than patients with non-hVISA multidrug-resistant S. aureus (MRSA). In addition, patients with hVISA bacteremia have been documented to have low initial vancomycin concentrations (<10 mg/liter) (1). The rate of vancomycin failure in serious infections associated with high bacterial loads is supported by the reduced vancomycin activity against high inoculum amounts in experimental in vitro and in vivo pharmacodynamic (PD) models (6, 7). Although low vancomycin concentrations have been associated with hVISA, the optimal treatment regimen with vancomycin remains undetermined. A vancomycin area under the concentration-time curve (AUC)/MIC ratio of ≥400 (free drug concentration [ƒ] AUC/MIC of ≥180) has been associated with improved clinical and microbiological responses against standard MRSA (3, 9). The purpose of this study was to identify bacterial kill and reduction of susceptibility for high and moderate inoculum amounts of hVISA and non-hVISA isolates, utilizing a range of vancomycin regimens in an in vitro PD model.
(A portion of this work was presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17-20 September 2007 [9a].)
Three S. aureus strains were employed: (i) Mu3, the first isolate with hVISA characteristics to be described, from Japan (4); (ii) MRSA 1629, a clinical hVISA isolate; and (iii) MRSA 3286, a clinical non-hVISA strain. Vancomycin was purchased from Sigma. Mueller-Hinton broth (Difco, Detroit, MI) supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium was used for all susceptibility and in vitro PD model assessments. MICs and minimum bactericidal concentrations were determined by broth microdilution or E-test according to Clinical and Laboratory Standards Institute methods (2). The MIC of vancomycin with the high inoculum amount was determined as previously described (6). The detection of hVISA was determined by population analysis-AUC profile and macrodilution E-test methodology (10).
A previously described in vitro pharmacokinetic (PK) and PD (PK/PD) model was utilized for all simulations, with amounts of 106 (moderate) and 108 (high) CFU/ml of inoculum (6). Vancomycin was evaluated by using a range for the ƒAUC/MIC over 0 to 24 h of 105 to 799 (750 to 5,000 mg every 12 h). The development of resistance was evaluated every 24 h by microtiter and E-test with a 0.5 McFarland standard of (i) samples directly from the model and (ii) subsequent bacterial growth on vancomycin-screening plates containing three and six times the organism's MIC. Vancomycin PKs were determined by using a fluorescence polarization immunoassay (Abbott Diagnostics TDx) and PK Analyst software (version 1.10; MicroMath Scientific Software) as previously described (6). Changes in CFU/ml at 24, 48, and 72 h were compared by two-way analysis of variance with Tukey's post hoc test. A P value of ≤0.05 was considered significant. (SPSS version 14; SPSS, Inc.).
Susceptibility results for all isolates at the moderate inoculum amount are listed in Table 1. The MICs for the high inoculum amount of MRSA 3286, hVISA 1629, and Mu3 were 4, 4, and 8, respectively. The PK values of the simulated dosing regimens shown in Table 1 were comparable to predicted values.
TABLE 1.
Pharmacokinetic and pharmacodynamic parameters of vancomycin dosing regimensa
| Isolate | Dose (mg/12 h) | fAUC/MIC | fTrough (mg/liter) | MIC at indicated time point (h)
|
|||
|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | ||||
| Mu3 | 750 | 105 | 3 | 2 | 3 | 4 to 8 | 8 |
| 1,000 | 168 | 4 | 2 | 2 to 3 | 3 to 8 | 8 | |
| 1,500 | 252 | 7 | 2 | 3 | 3 to 4 | 4 to 6 | |
| 2,000 | 269 | 11 | 2 | 3 | 3 to 4 | 4 to 6 | |
| 2,250 | 317 | 12 | 2 | 2 | 2 to 3 | 2 to 3 | |
| 2,500 | 374 | 16 | 2 | 2 | 2 | 2 | |
| 5,000 | 799 | 26 | 2 | 2 | 2 | 2 | |
| 1629 | 750 | 105 | 3 | 2 | 2 | 3 | 3 to 4 |
| 1,000 | 138 | 3 | 2 | 2 | 2 to 3 | 3 | |
| 1,500 | 248 | 7 | 2 | 2 | 2 | 3 | |
| 1,750 | 258 | 10 | 2 | 4 | 3 to 4 | 4 | |
| 2,000 | 271 | 12 | 2 | 2 | 2 | 2 | |
| 4,000 | 644 | 22 | 2 | 2 | 2 | 2 | |
| 3286 | 1,000 | 552 | 3 | 0.5 | 0.5 | 0.5 | 0.5 |
| 1,500 | 992 | 7 | 0.5 | 0.5 | 0.5 | 0.5 | |
| 2,000 | 1,084 | 12 | 0.5 | 0.5 | 0.5 | 0.5 | |
| 4,000 | 2,576 | 22 | 0.5 | 0.5 | 0.5 | 0.5 | |
Pharmacokinetic and pharmacodynamic parameters of vancomycin dosing regimens ranged from 750 to 5,000 mg every 12 h in the in vitro pharmacodynamic model against the two hVISA isolates Mu3 and 1629 and one non-hVISA strain (3286); results using a high inoculum amount (108 CFU/ml) are shown. Each regimen is listed with the corresponding MIC changes throughout the model.
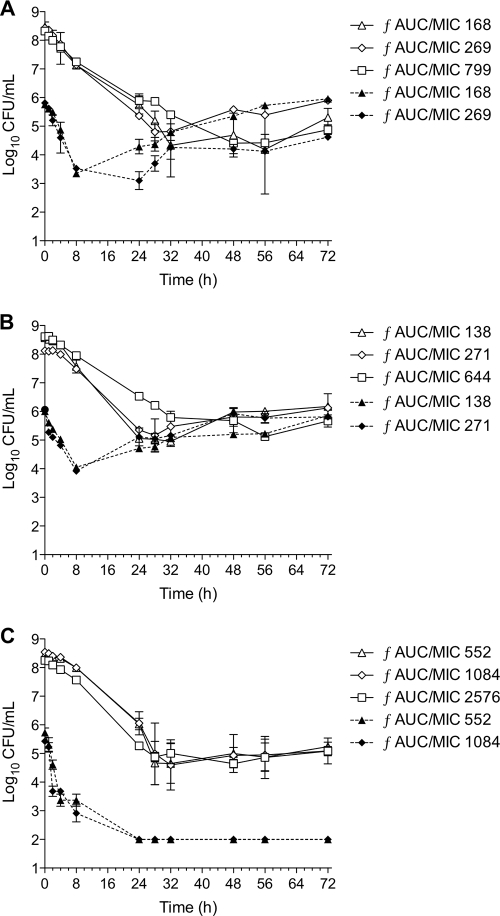
The activities of vancomycin against all three isolates at the different inoculum amounts for a range of dosing regimens over 72 h are displayed in Fig. 1. With the high inoculum amount of all isolates, bactericidal activity at 24 h occurred in the majority of dosing regimens regardless of the organism tested. However, the additional killing effect beyond 24 h of exposure was minimal even at doses as high as 5 g every 12 h (ƒAUC/MIC, 799). A high bacterial load of approximately 105 organisms remained by the end of the 72 h for all strains.
FIG. 1.
In vitro PD model activities for a range of vancomycin dosing simulations with from 1,000 to 5,000 mg every 12 h against high and moderate inoculum amounts of hVISA isolates Mu3 (A) and 1629 (B) and non-hVISA isolate 3286 (C) over 72 h.
The impact of hVISA expression on vancomycin activity was more evident with the moderate inoculum amount. Rapid bactericidal activity was achieved in MRSA 3286 within 8 h using vancomycin doses of 1,000 to 2,000 mg every 12 h (ƒAUC/MIC, 552 to 1,084). The time to achieve bactericidal activity with this strain was significantly more rapid and sustained with the moderate inoculum amount than with the high inoculum amount (7.5 versus 27.8 h; P < 0.001). In addition, an increased time to 99.9% kill and regrowth of ≥1 log were demonstrated for both of the hVISA strains (P = 0.001). There were no MIC changes detected for any test strain with the moderate inoculum amount (Table 1).
With the high inoculum amount, doses ranging from 750 to 2,000 mg every 12 h resulted in decreased susceptibility of the hVISA isolates. Although no resistance was detected with doses from 2.25 to 5 g every 12 h (ƒAUC/MIC, 799) for the hVISA isolates, these regimens had minimal activity in overall bacterial-load reduction. Vancomycin exposure of the non-hVISA 3286 did not result in susceptibility changes beyond the baseline.
The hVISA isolate Mu3 was discovered to be genetically related to Mu50, the first VISA, which is from Japan, and therefore represents an intermediate step from vancomycin-susceptible S. aureus to VISA (4). In our in vitro model study with Mu3, we were able to reproduce VISA-like phenotypic characteristics with dosing regimens ranging from 750 to 2,250 mg every 12 h (ƒAUC/MIC, 105 to 317) against a simulated high-bacterial-load infection, resulting in up to a fourfold change in the MIC. No MIC changes were found with the non-hVISA clinical isolate. In addition, MIC increases in hVISA only occurred with the high-bacterial-load simulations. We attribute this to the 1,000-times-greater organism burden with the high-bacterial-load simulation, resulting in an increased probability of the expression of a heterogeneous population.
The vancomycin killing activities in our in vitro PK/PD model appeared to be significantly affected by the inoculum amount and consistent with the results of prior studies (3, 6, 7). The moderate inoculum amount of our non-hVISA strain resulted in more-rapid and -sustained bactericidal activity. The antimicrobial activity in the first 24 h differed between the hVISA isolates in high and low inoculum amounts, but similar organism burdens remained after 72 h of vancomycin therapy. This suggests that a similar bacterial population survived the effects of vancomycin exposure and that tolerance may play a role in regrowth. The clinical reports of hVISA infections would support this theory since these clinical failures have corresponded to high-bacterial-load infections along with complicated and persistent bacteremia (1, 5, 8).
Overall, we demonstrated that vancomycin at doses ranging from 750 to 2,250 mg every 12 h (ƒAUC/MIC, 105 to 317) had poor activity against clinical strains of hVISA in an in vitro PK/PD model and resulted in a large organism burden with reduced vancomycin susceptibility after exposure to a high-bacterial-load simulation. The presence of hVISA and a high bacterial load both affect vancomycin activity, and patients with either of these circumstances may be at increased risk of treatment failure.
Acknowledgments
There was no external funding of any kind supporting this work.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Charles, P. G., P. B. Ward, P. D. Johnson, B. P. Howden, and M. L. Grayson. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38:448-451. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. M100-S16. Clinical Laboratory Standards Institute, Wayne, PA.
- 3.Craig, W. A., and D. R. Andes. 2006. In vivo pharmacodynamics of vancomycin against VISA, heteroresistant VISA (hVISA) and VSSA in the neutropenic murine thigh-infection model, abstr. A-644. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27-30 September 2006.
- 4.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 5.Howden, B. P., P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cros, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayall, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521-528. [DOI] [PubMed] [Google Scholar]
- 6.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, D., Y. Murakami, T. Stamstad, K. Marchillo, A. J., D. R. Andes, and W. A. Craig. 2007. Inoculum effect of daptomycin, linezolid, vancomycin, and ceftobiprole with Staphylococcus aureus and Streptococcus pneumoniae in neutropenic mice at 105 and 107 CFU in opposite thighs, abstr. A-37. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17-20 September 2007.
- 8.Maor, Y., G. Rahav, N. Belausov, D. Ben-David, G. Smollan, and N. Keller. 2007. Prevalence and characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a tertiary care center. J. Clin. Microbiol. 45:1511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 9a.Rose, W. E., K. L. Lau, G. W. Kaatz, and M. J. Rybak. 2007. Relationships between vancomycin (V) pharmacodynamics and the emergence of V intermediate Staphylococcus aureus (VISA) from heteroresistant VISA (hVISA) in an in vitro pharmacokinetic/pharmacodynamic model (IVPM), abstr. A-13. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17-20 September 2007.
- 10.Wootton, M., A. P. MacGowan, T. R. Walsh, and R. A. Howe. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45:329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]