Abstract
LK-157 is a novel tricyclic carbapenem with potent activity against class A and class C β-lactamases. When tested against the purified TEM-1 and SHV-1 enzymes, LK-157 exhibited 50% inhibitory concentrations (IC50s) in the ranges of the clavulanic acid and tazobactam IC50s (55 nM and 151 nM, respectively). Moreover, LK-157 significantly inhibited AmpC β-lactamase (IC50, 62 nM), as LK-157 was >2,000-fold more potent than clavulanic acid and approximately 28-fold more active than tazobactam. The in vitro activities of LK-157 in combination with amoxicillin, piperacillin, ceftazidime, cefotaxime, ceftriaxone, cefepime, cefpirome, and aztreonam against an array of Ambler class A (TEM-, SHV-, CTX-M-, KPC-, PER-, BRO-, and PC-type)- and class C-producing bacterial strains derived from clinical settings were evaluated in synergism experiments and compared with those of clavulanic acid, tazobactam, and sulbactam. In vitro MICs against ESBL-producing strains (except CTX-M-containing strains) were reduced 2- to >256-fold, and those against AmpC-producing strains were reduced even up to >32-fold. The lowest MICs (≤0.025 to 1.6 μg/ml) were observed for the combination of cefepime and cefpirome with a constant LK-157 concentration of 4 μg/ml, thus raising an interest for further development. LK-157 proved to be a potent β-lactamase inhibitor, combining activity against class A and class C β-lactamases, which is an absolute necessity for use in the clinical setting due to the worldwide increasing prevalence of bacterial strains resistant to β-lactam antibiotics.
Antibiotics have drastically reduced illness and death due to infectious diseases. However, bacteria have exhibited a remarkable capacity to quickly become resistant to one or several classes of antibiotics, which is widely considered to be one of the major problems in human medicine today (1, 19). The dramatic increase in antibacterial resistance is now a global threat, both for nosocomial and for community-acquired infections.
Although bacteria have developed several strategies for escaping the lethal action of β-lactam antibiotics, the most common and clinically important mechanism is the synthesis of β-lactamases, leading to hydrolysis of the antibiotic. Currently, the β-lactamase superfamily has more than 700 members, many of which differ only by a single amino acid (K. Bush and G. A. Jacoby, personal communication).
Class A enzymes TEM-1 and SHV-1 (functional group 2b) are the most widely disseminated. The mutated β-lactamases either have broader substrate specificities, including extended-spectrum cephalosporins (extended-spectrum β-lactamases [ESBLs]; functional group 2be), or exhibit decreased sensitivities to β-lactamase inhibitors (inhibitor-resistant TEMs; functional group 2br) (4).
Among the cephalosporin-resistant organisms causing concern were mutants of Enterobacter spp., Citrobacter freundii, Serratia spp., Morganella morganii, Providencia spp., and Pseudomonas aeruginosa, where high- or low-level constitutive expression of class C β-lactamases may be induced by exposure to certain β-lactams. Chromosomal or plasmid-encoded AmpC cephalosporinases (functional group 1) may be hyperproduced through reversible induction or stable derepression and typically confer resistance to most cephalosporins and monobactams.
Commercially available inhibitors, such as potassium clavulanate, sulbactam, and tazobactam, have been successfully used against bacteria producing the ubiquitous and prevalent class A and some class D β-lactamases, but activity against class C types is poor in general (13). In recent years, several quite potent inhibitors, such as alkylidene penems, 2β-substituted penam sulfones, oxapenems, cephalosporin-derived compounds, and cyclic acyl phosphonates, which exhibit antibacterial synergistic activity against both class A and class C β-lactamases, have been developed, but none has been approved by the FDA yet (3, 5, 9, 10, 12, 14, 21). In addition, bacterial susceptibility to such combinations has recently been challenged by the spontaneous appearance of new β-lactamases of the TEM family, which are resistant to the mechanism-based inhibitors on the market (inhibitor-resistant TEM β-lactamases [www.lahey.org/Studies]). Given the current situation, it is vital to develop new, effective agents and to sustain the clinical utility of these and existing therapies.
Recently, following a rational structure-based drug design approach, novel tricyclic carbapenem compounds (trinems) with potent inhibitory activity against serine β-lactamases have been synthesized by Lek Pharmaceuticals, d.d. (Fig. 1). Lead LK-157 was identified as a promising β-lactamase inhibitor to be coadministered with a selected cephalosporin antibiotic in bacterial infections caused by β-lactam-resistant bacteria (7, 8, 11, 15-17, 20). LK-157 is a close structural analogue of a broad-spectrum antibiotic, sanfetrinem, whose development was stopped in phase II clinical trials (2, 18).
FIG. 1.
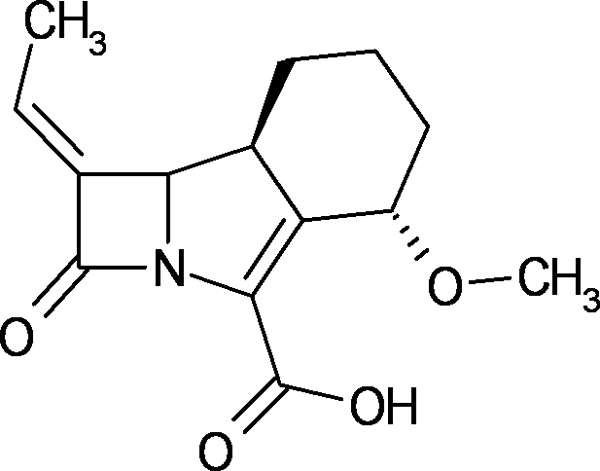
Structural formula of tricyclic carbapenem LK-157.
The main objective of the present study was to evaluate the activity of LK-157 by in vitro synergism experiments with various β-lactam antibiotics against a wide range of clinically relevant bacteria expressing class A and class C β-lactamases (4). We also present the 50% inhibitory concentrations (IC50s) of LK-157 against purified TEM-1, SHV-1, and AmpC enzymes.
MATERIALS AND METHODS
Antibacterial agents.
LK-157 was synthesized by Lek Pharmaceuticals, d.d. (Ljubljana, Slovenia). Ceftazidime, cefotaxime, ceftriaxone, aztreonam, clavulanic acid, sulbactam, and tazobactam were supplied by Sandoz, GmbH (Kundl, Austria). Amoxicillin and piperacillin were purchased from Sigma-Aldrich, and cefepime was derived from cefepime hydrochloride (Maxipime), purchased from a pharmacy, by physicochemical means at Nabvriva Therapeutics AG (Vienna, Austria).
Stock solutions of the test compounds and control antibiotics were prepared in distilled water according to the Clinical and Laboratory Standards Institute (CLSI) (6) guidelines and further diluted with water or Mueller-Hinton bouillon. All drug weights were corrected for salt forms and referred to the pure drug substance.
Bacterial strains.
All tested strains and clinical isolates were either purchased from the American Type Culture Collection (ATCC); kindly provided by A. Georgopoulos (Vienna General Hospital, Vienna, Austria), I. Chopra (Leeds, United Kingdom), Novartis (Vienna, Austria), Naeja (Edmonton, Canada), or D. Livermore (Health Protection Agency, London, United Kingdom); or collected from the SENTRY study (F. J. Schmitz, Minden, Germany).
All strain identities were confirmed by BBL Crystal Identification Systems (Becton Dickinson, Cockeysville, MD). Stock cultures were prepared from broth cultures grown at 35°C for 18 to 22 h without agitation, with subsequent addition of 5% dimethyl sulfoxide (DMSO) (vol/vol, final concentration) as a cryoprotectant, and stored frozen in liquid nitrogen.
Cell-free β-lactamase inhibition study.
The IC50s (μM) of the β-lactamase inhibitors against purified TEM-1, SHV-1, and AmpC ß-lactamases were assessed by determining the concentration of inhibitor at which 50% of the nitrocefin hydrolysis by the particular enzyme was inhibited. Nitrocefin was provided by Sandoz (Kundl, Austria). Assays were performed with TEM-1 derived from pB322, SHV-1 derived from a clinical Klebsiella pneumoniae isolate, and P99 AmpC derived from the clinical isolate E. cloacae B311. ß-Lactamases were expressed in the pET system (pET-28 and pET-30; Novagen, San Diego, CA) without signal peptides. They contained an N-terminal six-histidine tag that was used for purification on Ni-nitrilotriacetic acid (Qiagen, Hilden, Germany). As the IC50 values of the reference compounds largely concurred with the values reported in the literature (3, 10), the histidine tag was not split off for IC50 determination although the tag could have altered the overall activity of the enzymes. Compounds were prepared as 50 mM stocks in DMSO and diluted into buffer P1 (50 mM phosphate, pH 7) to give a final concentration of 10% DMSO. All further dilutions were done with P2 (P1 with 10% DMSO). Enzyme (final concentration, 0.25 μg/ml) and compound dilutions were preincubated for 10 min at 37°C, and the reaction was started with the addition of prewarmed (37°C) nitrocefin to give a final concentration of 50 mM. The change in absorption at 490 nm was followed at 37°C for 10 min by reading the absorption every 30 s using a Spectramax 384 Plus microplate reader (Molecular Devices, Sunnyvale, CA), using 96-well plates. The initial rate of nitrocefin degradation was determined with an excess of nitrocefin in order to maintain linearity during the time of measurement. IC50s were calculated using nonlinear regression and sigmoidal dose response analysis with PRISM 4.0 software (Graphpad Software, Inc., San Diego, CA). IC50 data were expressed in μM with 95% confidence intervals and were calculated from at least two independent experiments.
In vitro susceptibility tests.
Bacterial susceptibilities, expressed as MICs, were determined by the broth microdilution technique and the agar dilution technique as recommended by the approved standard reference recommendations of the CLSI (6). The selected antibiotics were diluted by serial twofold dilutions ranging either from 256 to 0.125 μg/ml or from 25.6 to 0.0125 μg/ml. In combination with ß-lactam antibiotics, the inhibitor was added at a constant concentration of 4 μg/ml. The inoculum (final size, 5 × 105 CFU/ml) was prepared by the direct colony suspension method as described by the CLSI (6).
RESULTS AND DISCUSSION
The increasing number of β-lactamase-producing strains is becoming a serious threat to the clinical use of β-lactam antibiotics. Considering that the prevalence of ESBLs and AmpC β-lactamases is underestimated, the clinical situation appears to be severe, with, for example, ESBL rates of up to 44% in Klebsiella spp. and up to 25.4% in E. coli (19) and AmpC rates up to 11% in K. pneumoniae (1). The clinically available β-lactamase inhibitors clavulanic acid, tazobactam, and sulbactam have only limited activity against ESBLs and lack activity against class C enzymes (13). Therefore, development of extended-spectrum β-lactamase inhibitors capable of combating emerging resistances in both class A and class C β-lactamases would be highly appreciated (13).
IC50s of LK-157 against isolated β-lactamases TEM-1, SHV-1, and AmpC.
The inhibitory activities of LK-157 against isolated class A and class C enzymes were compared to those of clavulanic acid, tazobactam, and sulbactam (Table 1). LK-157, with an IC50 of 55 nM, was as active as clavulanic acid against TEM-1, half as active as tazobactam, and approximately 20-fold more active than sulbactam. According to the literature, the IC50s of clavulanic acid and tazobactam against TEM-1 are in the range of earlier reported IC50s (3, 10), except that the IC50s of clavulanic acid against AmpC differ from earlier reported values, as Jamieson et al. (10) reported a 10-fold-lower IC50 and Bonnefoy et al. (3) a 10-fold-higher IC50. Both of these previous studies used a nitrocefin-based assay but β-lactamases were purified from different species and therefore probably exhibited different activities.
TABLE 1.
IC50s of LK-157, clavulanic acid, tazobactam, and sulbactam against purified TEM-1, SHV-1, and AmpC β-lactamasesa
| Enzyme | β-Lactamase type | IC50 (μM) (95% CI)
|
|||
|---|---|---|---|---|---|
| LK-157 | TZB | SUL | CLA | ||
| TEM-1 | A | 0.055 (0.030-0.100) | 0.014 (0.011-0.018) | 1.060 (0.829-1.350) | 0.030 (0.024-0.037) |
| SHV-1 | A | 0.151 (0.111-0.205) | 0.206 (0.107-0.397) | 3.960 (2.950-5.310) | 0.028 (0.025-0.032) |
| AmpC | C | 0.062 (0.047-0.084) | 1.760 (1.450-2.120) | 11.90 (8.280-17.10) | 136.2 (120.9-153.4) |
IC50s were determined by a nitrocefin-based assay. CLA, clavulanic acid; TZB, tazobactam; SUL, sulbactam.
Against SHV-1, clavulanic acid was the most active inhibitor (IC50, 28 nM). However, LK-157, with an IC50 of 151 nM, was still very active compared to tazobactam (IC50, 206 nM) and sulbactam (IC50, 3,960 nM). Moreover, LK-157 exhibited an excellent activity against AmpC (IC50, 62 nM), 28-fold higher than that of tazobactam. Clavulanic acid and sulbactam were inactive against AmpC.
The IC50 of LK-157 against AmpC was comparable to the IC50 value of NXL-104, a non-β-lactam inhibitor (Novexel), and AM-112, an oxapenem derivative (Amura) (3, 10). LK-157 was slightly less active against TEM-1 than NXL-104 but significantly more active (>100-fold) than AM-112. Moreover, the low IC50 values of LK-157 against the purified enzymes were also confirmed in a cell-based screen, indicating good penetration through the outer bacterial membrane.
Intrinsic in vitro antibacterial activity of LK-157.
The novel β-lactamase inhibitor LK-157 alone did not exhibit any intrinsic antimicrobial activity against several gram-negative and gram-positive bacterial species, including Escherichia coli, Enterobacter cloacae, Klebsiella spp., and Enterococcus spp., with MICs of >25.6 μg/ml (Table 2). LK-157 exhibited weak antimicrobial activity against Moraxella catarrhalis (MIC90, 0.8 μg/ml; range, 0.2 to 0.8 μg/ml; n = 17) and methicillin-susceptible S. aureus (MSSA) strains, with MICs between 0.8 and 1.6 μg/ml (n = 5), but was inactive against methicillin-resistant S. aureus (MRSA) strains (MIC90, >256 μg/ml; n = 26) (Table 2).
TABLE 2.
Intrinsic antibacterial activity of LK-157
| Microorganism | No. of strains | MIC50 | MIC90 | Range |
|---|---|---|---|---|
| C. freundii | 7 | >25.6->25.6 | ||
| E. coli | 45 | >25.6 | >25.6 | >25.6->25.6 |
| E. cloacae | 12 | >25.6 | >25.6 | >25.6->25.6 |
| Enterococcus spp. | 3 | >25.6->25.6 | ||
| Klebsiella spp.a | 12 | >25.6 | >25.6 | 6.4-25.6 |
| M. catarrhalis | 7 | 0.8 | 0.8 | 0.2-0.08 |
| P. aeruginosa | 5 | >25.6->25.6 | ||
| S. aureus (MSSA) | 7 | 0.8-1.6 | ||
| S. aureus (MRSA) | 26 | >256 | >256 | 32->256 |
K. pneumoniae (n = 9), K. oxytoca (n = 2), and K. edwardsii (n = 1).
Activity of LK-157 against class A β-lactamases.
For the in vitro evaluation of LK-157 as a β-lactamase inhibitor, various combinations with amoxicillin, ceftazidime, piperacillin, cefotaxime, ceftriaxone, cefepime, cefpirome, and aztreonam were assayed against a panel of β-lactamase-producing strains (Table 2; also see Tables 3 to 5) and compared with the commercially available β-lactamase inhibitors clavulanic acid, tazobactam, and sulbactam in the respective combinations. All inhibitors were used at a constant concentration of 4 μg/ml.
TABLE 3.
MICs of LK-157 in combination with amoxicillin, ceftazidime, and piperacillin against class A and class C β-lactamase-producing bacteriaa
| Microorganism | Strain | β-Lactamase(s)b | MIC (μg/ml)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMX
|
CAZ
|
PIP
|
|||||||||||||
| Alone | CLA | SUL | TZB | LK-157 | Alone | CLA | SUL | TZB | LK-157 | Alone | TZB | LK-157 | |||
| E. coli | B 266 | SHV-3 | >25.6 | 6.4 | >25.6 | 12.8 | 12.8 | 1.6 | 0.1 | 0.1 | 0.1 | 0.1 | >25.6 | 1.6 | 1.6 |
| C. freundii | B 271 | SHV-5 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 1.6 | 3.2 | 0.8 | 0.8 | >25.6 | 3.2 | 3.2 |
| E. coli | B 264 | SHV-5 | >25.6 | 25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 | 3.2 | >25.6 | >25.6 | 3.2 |
| E. coli | B 270 | SHV-5, BIL-1 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 | 6.4 | >25.6 | >25.6 | >25.6 |
| E. cloacae | B 273 | SHV-12 | >25.6 | >25.6 | >25.6 | 25.6 | >25.6 | >25.6 | 3.2 | 3.2 | 0.4 | 0.8 | >25.6 | 3.2 | 12.8 |
| K. pneumoniae | B 313 | SHV* | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 3.2 | |||
| K. pneumoniae | B 314 | SHV* | >25.6 | 6.4 | >25.6 | >25.6 | >25.6 | 12.8 | 1.6 | 0.8 | 1.6 | 0.4 | |||
| K. pneumoniae | B 32 | SHV* | 12.8 | 0.4 | 0.2 | 0.8 | 0.1 | 0.05 | 0.05 | ≤0.025 | 0.05 | 0.025 | |||
| K. pneumoniae | B 312 | SHV-18 | >25.6 | 12.8 | >25.6 | >25.6 | >25.6 | 25.6 | 3.2 | 1.6 | 6.4 | 1.6 | >25.6 | 6.4 | 12.8 |
| E. coli | B 306 | TEM-1 | >25.6 | 12.8 | >25.6 | 25.6 | >25.6 | 0.2 | 0.4 | 0.2 | 0.2 | 0.2 | >25.6 | 0.8 | 25.6 |
| E. coli | B 269 | TEM-1 | >25.6 | 12.8 | >25.6 | 12.8 | >25.6 | 3.2 | 0.4 | 0.8 | 0.4 | 0.4 | |||
| E. coli | B 265 | TEM-3 | >25.6 | 12.8 | 12.8 | 12.8 | 12.8 | >25.6 | 0.4 | 0.4 | 0.4 | 0.8 | >25.6 | 1.6 | 1.6 |
| E. coli | B 267 | TEM* | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 0.8 | >25.6 | 6.4 | >25.6 | |||
| K. pneumoniae | B 401 | TEM-10, TEM-12 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 6.4 | 6.4 | 12.8 | >25.6 | >25.6 | 12.8 | >25.6 |
| M. catarrhalis | B 167 | BRO-1 | 3.2 | 0.2 | 0.1 | 0.1 | ≤0.0125 | 0.2 | 0.1 | 0.1 | 0.05 | ≤0.0125 | |||
| M. catarrhalis | B 169 | BRO-1 | 6.4 | 0.2 | 0.1 | 0.1 | ≤0.0125 | 0.1 | 0.1 | 0.1 | 0.05 | ≤0.0125 | |||
| M. catarrhalis | B 407 | BRO-1 | 0.8 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | 0.05 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | 1.6 | ≤0.0125 | ≤0.0125 |
| M. catarrhalis | B 162 | BRO-2 | 3.2 | ≤0.0125 | 0.05 | 0.025 | ≤0.0125 | 0.1 | ≤0.0125 | 0.1 | 0.025 | ≤0.0125 | |||
| M. catarrhalis | B 161 | BRO-2 | 0.4 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | 0.05 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | |||
| M. catarrhalis | B 406 | BRO-2 | 0.8 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | 0.05 | ≤0.0125 | ≤0.0125 | ≤0.0125 | ≤0.0125 | 1.6 | ≤0.0125 | ≤0.0125 |
| S. aureus | B 7 | PC* | 1.6 | 0.2 | 0.2 | 0.2 | ≤0.0125 | 12.8 | 6.4 | 3.2 | 12.8 | ≤0.0125 | |||
| S. aureus | D 111 | PC* | 25.6 | 0.2 | 0.4 | 0.2 | ≤0.0125 | 12.8 | 12.8 | 6.4 | 6.4 | ≤0.0125 | 25.6 | 0.8 | ≤0.0125 |
| S. aureus | D 557 | PC* | 12.8 | 0.4 | 1.6 | 0.8 | ≤0.0125 | 25.6 | 12.8 | 6.4 | 12.8 | ≤0.0125 | |||
| S. aureus (MRSA) | B 84 | PC* | >25.6 | 6.4 | 0.1 | 3.2 | 3.2 | >25.6 | >25.6 | 12.8 | 25.6 | 25.6 | |||
| S. aureus (MRSA) | D 1231 | PC* | >25.6 | 6.4 | 6.4 | 3.2 | 3.2 | >25.6 | >25.6 | 25.6 | 25.6 | >25.6 | |||
| C. freundii | B 272 | AmpC | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 6.4 | >25.6 | 25.6 | 6.4 |
| E. cloacae | B 274 | AmpC | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 3.2 | >25.6 | 25.6 | 6.4 |
| E. cloacae | B 275 | AmpC | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 3.2 | >25.6 | >25.6 | 6.4 |
| E. cloacae | B 311 | AmpC | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 6.4 | >25.6 | >25.6 | 25.6 |
| E. cloacae | D 1089 | AmpC | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 25.6 | 12.8 | |||
| E. coli | D 643 | ESBL | >25.6 | 12.8 | 3.2 | 3.2 | 6.4 | 1.6 | 0.8 | 0.2 | 0.4 | 0.2 | 12.8 | 0.8 | 1.6 |
| E. coli | B 268 | ESBL | >25.6 | 6.4 | >25.6 | 6.4 | 6.4 | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | |||
| E. coli | B 259 | ESBL | 12.8 | 6.4 | 3.2 | 3.2 | 3.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | |||
| E. coli | B 261 | ESBL | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 0.4 | 0.8 | 0.2 | 0.4 | 0.2 | |||
| E. coli | B 263 | ESBL | 6.4 | 6.4 | 3.2 | 3.2 | 3.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | |||
| E. coli | B 3 | ESBL | 3.2 | 6.4 | 3.2 | 3.2 | 1.6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||
| E. coli | B 308 | ESBL | >25.6 | 25.6 | >25.6 | 25.6 | >25.6 | 3.2 | 1.6 | 1.6 | 0.8 | 1.6 | |||
| E. coli | B 309 | ESBL | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 25.6 | 25.6 | 6.4 | |||
| C. freundii | B 319 | ESBL | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 25.6 | 1.6 | |||
| K. oxytoca | B 287 | ESBL | 25.6 | 0.8 | 3.2 | 1.6 | 3.2 | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | 3.2 | 1.6 | 3.2 |
| K. oxytoca | B 289 | ESBL | >25.6 | 3.2 | 6.4 | 1.6 | 25.6 | 0.4 | 0.1 | 0.05 | 0.1 | 0.1 | 12.8 | 0.8 | 1.6 |
The MICs of the β-lactam antibiotics in the presence of a constant concentration (4 μg/ml) of the β-lactamase inhibitors LK-157, clavulanic acid (CLA), sulbactam (SUL), and tazobactam (TZB) were determined by agar dilution tests. AMX, amoxicillin; CAZ, ceftazidime; PIP, piperacillin.
“ESBL” indicates an ESBL not further characterized. “*” indicates that the β-lactamase type was confirmed by PCR using specific primers but not further characterized by sequencing.
TABLE 5.
MICs of LK-157 in combination with cefotaxime, ceftriaxone, cefepime, ceftazidime, cefpirome, and aztreonam against CTX-M-producing E. colia
| Microorganism | Strain | β-Lactamase
|
MIC (μg/ml)
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX
|
CRO
|
FEP
|
Cefpirome
|
CAZ
|
ATM
|
LK-157 alone | TZB-LK-157 | ||||||||||||||||
| Type | Class | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | ||||
| E. coli | B 1147 | CTX-M-1 | A | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 | 1.6 | >25.6 | 12.8 | 0.8 | >25.6 | 25.6 | 1.6 | 25.6 | >25.6 | 0.4 | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 |
| E. coli | B 1094 | CTX-M-2 | A | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 | 0.2 | 12.8 | 12.8 | 0.05 | 12.8 | 12.8 | 0.05 | 1.6 | 1.6 | 0.2 | 12.8 | 12.8 | 0.1 | >25.6 | >25.6 |
| E. coli | B 1095 | CTX-M-14 | A | >25.6 | >25.6 | 6.4 | >25.6 | >25.6 | 3.2 | 6.4 | 6.4 | 0.1 | >25.6 | 12.8 | 0.1 | 12.8 | 12.8 | 12.8 | 12.8 | 12.8 | 6.4 | >25.6 | >25.6 |
| E. coli | B 1096 | CTX-M-9 | A | 25.6 | 3.2 | 0.4 | >25.6 | 6.4 | 0.4 | 6.4 | 0.4 | 0.2 | 12.8 | 1.6 | 0.2 | 1.6 | 0.4 | 0.4 | 6.4 | 1.6 | 0.2 | >25.6 | >25.6 |
| E. coli | B 1097 | CTX-M-15 | A | >25.6 | >25.6 | 0.1 | >25.6 | >25.6 | 0.4 | 6.4 | 3.2 | 0.05 | 25.6 | 3.2 | 0.2 | 12.8 | 6.4 | 0.2 | >25.6 | 25.6 | 0.1 | 25.6 | >25.6 |
| E. coli | B 1098 | CTX-M-15 | A | >25.6 | >25.6 | 12.8 | >25.6 | >25.6 | 1.6 | 25.6 | 12.8 | 0.2 | >25.6 | 25.6 | 0.4 | 25.6 | 25.6 | 0.8 | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 |
| C. freundii | B 1099 | CTX-M-15 (strain E) | A | >25.6 | 12.8 | 0.1 | >25.6 | 25.6 | 0.2 | 3.2 | 0.8 | 0.1 | 25.6 | 1.6 | 0.2 | 6.4 | 3.2 | 0.2 | >25.6 | 6.4 | 0.1 | 12.8 | >25.6 |
| E. coli | B 1100 | CTX-M-15 (strain D) | A | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 | 12.8 | 25.6 | 12.8 | 0.4 | >25.6 | >25.6 | 0.4 | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 | 0.8 | 25.6 | >25.6 |
| E. coli | B 1101 | CTX-M-3 (strain C) | A | >25.6 | 25.6 | 0.2 | >25.6 | >25.6 | 1.6 | 6.4 | 6.4 | 0.1 | 12.8 | 6.4 | 0.05 | 1.6 | 1.6 | 0.2 | 12.8 | 12.8 | 0.2 | >25.6 | >25.6 |
| E. coli | B 1102 | CTX-M-15 (strain B) | A | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 | 1.6 | 25.6 | 12.8 | 0.2 | >25.6 | 25.6 | 0.8 | >25.6 | 25.6 | 0.4 | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 |
| E. coli | B 1103 | CTX-M-15 (strain A) | A | 25.6 | 0.8 | 0.4 | 12.8 | 0.8 | 0.1 | 0.8 | 0.05 | 0.2 | 3.2 | 0.1 | 0.1 | 1.6 | 0.8 | 0.2 | 6.4 | 1.6 | 0.2 | 25.6 | >25.6 |
| E. coli | B 1146 | CTX-M-15 | A | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 | 1.6 | >25.6 | 12.8 | 0.8 | >25.6 | >25.6 | 0.8 | >25.6 | 25.6 | 0.8 | >25.6 | >25.6 | 0.8 | >25.6 | >25.6 |
The MICs of various cephalosporins in the presence of a constant concentration (4 μg/ml) of the β-lactamase inhibitors LK-157 and tazobactam (TZB) were determined by a microdilution test. CTX, cefotaxime; CRO, ceftriaxone; FEP, cefepime; CAZ, ceftazidime; ATM, aztreonam.
LK-157 was tested against an array of clinical isolates expressing SHV-type and TEM-type β-lactamases. The comparative MICs of amoxicillin, ceftazidime, and piperacillin alone and combined with clavulanic acid, sulbactam, tazobactam, and LK-157 are reported in Table 3. Amoxicillin and piperacillin alone were inactive against the bacterial strains expressing SHV- and TEM-type β-lactamases, and 57% of the tested strains were nonsusceptible to ceftazidime.
Against strains expressing SHV-type and TEM-type ESBLs, only the use of ceftazidime combined with an inhibitor resulted in clinically relevant MICs, while the activities of piperacillin and amoxicillin were not significantly reduced after inhibitor addition. Within strains expressing TEM- and SHV-type β-lactamases, the activity of LK-157 was strongly dependent on the specific β-lactamase type and on the bacterial strain tested. For instance, the MIC of piperacillin (25.6 μg/ml) against the SHV-5-producing E. coli B264 and C. freundii B271 was significantly reduced by the addition of LK-157, while no synergy against the SHV-5-producing E. coli B270 was observed, although in the same strain, synergy was observed for LK-157 and ceftazidime. One possible assumption could be an additional resistance mechanism besides SHV-5 production, but we did not further investigate that issue. However, the susceptibility of SHV-containing strains to ceftazidime was restored by the addition of LK-157, reducing the MICs by factors of >16 to the MIC range of clavulanic acid.
Against TEM-type ESBL-expressing strains, the in vitro activities of ceftazidime-LK-157 were in the same ranges as those of ceftazidime-clavulanic acid and ceftazidime-tazobactam, according to the IC50s of the purified TEM-1 enzyme, with two exceptions: E. coli B267 (TEM) and K. pneumoniae B401 (TEM-10 and -12), against which LK-157 was inactive. Although clavulanic acid appeared to be the most active inhibitor of purified TEM-1, amoxicillin-clavulanic acid did not exhibit clinically relevant MICs (MICs ≥ 12.8 μg/ml) against the tested isolates containing TEM-type enzymes, including not only TEM-1 but also other ESBL enzymes that were obviously resistant to clavulanic acid.
LK-157 in combinations was more active than the other tested β-lactamase inhibitors against BRO-1 and BRO-2 β-lactamases of M. catarrhalis and against penicillinases (PC type) of S. aureus (MSSA). The MICs of amoxicillin-LK-157 against M. catarrhalis were >64-fold lower, and those against S. aureus were >128-fold lower, than that of amoxicillin alone. Because LK-157 was intrinsically active against M. catarrhalis and S. aureus, the synergistic effect of LK-157 combined with amoxicillin and ceftazidime was also confirmed by checkerboard titration (data not shown). The resistance of MRSA against cephalosporins is due to the expression of the penicillin binding protein (PBP2a) encoded by the mecA gene. In addition, most MRSA strains also express β-lactamases of the penicillinase (PC) type. By the addition of LK-157, the MICs of amoxicillin were reduced from >25.6 μg/ml to 3.2 μg/ml (S. aureus B84 and D1231) (Table 3). However, when piperacillin was tested against more MRSA strains (n = 26), its overall low activity (MIC90, >256 μg/ml; range, 256 to >256 μg/ml) was found to be only slightly improved by LK-157 (MIC90, 256 μg/ml; range, 16 to 256 μg/ml) or by tazobactam (MIC90, 128 μg/ml; range, 32 to 256 μg/ml) (data not shown in tables).
Against other enterobacteriaceae-expressing ESBLs that were not fully characterized, the overall activity of LK-157 was in the range of that of the other commercial inhibitors. The impact of LK-157 was most pronounced against strains E. coli B309 and C. freundii B319.
As CTX-M enzymes are becoming the most prevalent β-lactamases found in clinical isolates and as the K. pneumoniae carbapenemases (KPC) are a significant challenge to date, the activity of LK-157 was also evaluated against such isolates. However, the in vitro activity of LK-157 in combination with extended-spectrum cephalosporins against CTX-M enzymes and the K. pneumoniae carbapenemases (KPC) was limited (Tables 4 and 5).
TABLE 4.
MICs of LK-157 in combination with cefotaxime, ceftriaxone, cefepime, ceftazidime, cefpirome, and aztreonam against class A and class C β-lactamase-producing bacteriaa
| Microorganism | Strain | β-Lactamase
|
MIC (μg/ml)
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX
|
CRO
|
FEP
|
Cefpirome
|
CAZ
|
ATM
|
LK-157 alone | TZB alone | ||||||||||||||||
| Type | Class | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | Alone | LK-157 | TZB | ||||
| M. catarrhalis | B 407 | BRO-1 | A | 0.1 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | 0.2 | ≤0.025 | ≤0.025 | 0.1 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | 0.8 | ≤0.025 | ≤0.025 | 0.2 | 0.4 |
| M. catarrhalis | B 406 | BRO-2 | A | 0.1 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | 0.4 | ≤0.025 | ≤0.025 | 0.2 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | 0.8 | ≤0.025 | ≤0.025 | 0.4 | 0.8 |
| S. aureus | D 111 | PC-1 | A | 1.6 | ≤0.025 | 0.8 | 1.6 | ≤0.025 | 0.8 | 3.2 | ≤0.025 | 1.6 | 0.8 | ≤0.025 | 0.4 | 3.2 | ≤0.025 | 6.4 | >25.6 | ≤0.025 | >25.6 | 1.6 | >25.6 |
| E. coli | B 269 | TEM-1 | A | >25.6 | 0.4 | 0.4 | >25.6 | 1.6 | 0.2 | 12.8 | 0.2 | 0.2 | 25.6 | 0.2 | 0.2 | 3.2 | 0.8 | 0.8 | 12.8 | 0.2 | 0.2 | >25.6 | >25.6 |
| E. coli | D 643 | TEM-2 | A | 0.1 | 0.05 | 0.05 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | ≤0.025 | 0.05 | ≤0.025 | 1.6 | 0.2 | 0.1 | 0.1 | 0.05 | 0.05 | >25.6 | >25.6 |
| E. coli | B 265 | TEM-3 | A | >25.6 | 0.1 | 0.1 | >25.6 | 0.1 | 0.05 | 6.4 | 0.05 | 0.05 | 12.8 | 0.1 | 0.05 | >25.6 | 0.4 | 0.2 | 25.6 | 0.2 | 0.1 | >25.6 | >25.6 |
| K. pneumoniae | B 401 | TEM-10 TEM-12 | A | 6.4 | 0.4 | 0.2 | 12.8 | 0.8 | 0.1 | 25.6 | 1.6 | 0.2 | >25.6 | 0.8 | 0.2 | >25.6 | >25.6 | 12.8 | >25.6 | >25.6 | 1.6 | >25.6 | >25.6 |
| K. pneumoniae | B 312 | SHV-18 | A | 3.2 | 0.4 | 0.4 | 6.4 | 0.2 | 0.4 | 0.8 | 0.2 | 0.2 | 0.8 | 0.2 | 0.2 | 25.6 | 0.8 | 0.8 | >25.6 | 0.4 | 0.4 | >25.6 | >25.6 |
| K. pneumoniae ATCC 700603 | B 1149 | SHV-5 | A | 12.8 | 0.2 | 0.8 | 25.6 | 0.4 | 1.6 | 1.6 | 0.2 | 0.1 | 0.8 | 0.2 | 0.2 | >25.6 | 0.8 | 6.4 | >25.6 | 3.2 | 25.6 | >25.6 | >25.6 |
| E. coli | B 266 | SHV-3 | A | 3.2 | ≤0.025 | ≤0.025 | 3.2 | ≤0.025 | ≤0.025 | 0.4 | ≤0.025 | 0.05 | 0.8 | ≤0.025 | ≤0.025 | 0.8 | 0.05 | ≤0.025 | 0.4 | ≤0.025 | 0.2 | 25.6 | >25.6 |
| C. freundii | B 271 | SHV-5 | A | 25.6 | 0.05 | 0.05 | 25.6 | ≤0.025 | ≤0.025 | 0.8 | ≤0.025 | 0.05 | 3.2 | 0.1 | 0.05 | >25.6 | 0.2 | 0.4 | >25.6 | 0.2 | 0.1 | >25.6 | >25.6 |
| E. coli | B 264 | SHV-5 | A | >25.6 | 0.1 | >25.6 | >25.6 | 0.4 | >25.6 | 25.6 | 0.1 | 3.2 | >25.6 | 0.1 | 6.4 | >25.6 | 1.6 | >25.6 | >25.6 | 3.2 | >25.6 | >25.6 | >25.6 |
| K. pneumoniae | B 1150 | KPC-3 | A | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 |
| P. aeruginosa | B 1151 | PER-1 | A | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | 12.8 | >25.6 | >25.6 | 25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 | >25.6 |
| E. coli | B 1148 | AmpC | C | 3.2 | 3.2 | 25.6 | 0.1 | 0.2 | 0-2 | 0.1 | 0.1 | 0.4 | |||||||||||
| C. freundii | B 272 | AmpC | C | >25.6 | 3.2 | 12.8 | >25.6 | 3.2 | >25.6 | 0.8 | 0.05 | 0.1 | 1.6 | 0.1 | 0.2 | >25.6 | 1.6 | 12.8 | >25.6 | 6.4 | 25.6 | >25.6 | >25.6 |
| E. cloacae | B 274 | AmpC | C | >25.6 | 12.8 | >25.6 | >25.6 | 25.6 | >25.6 | 0.8 | 0.1 | 0.2 | 6.4 | 0.4 | 1.6 | >25.6 | 3.2 | 12.8 | 25.6 | 12.8 | 12.8 | >25.6 | >25.6 |
| E. cloacae | B 275 | AmpC | C | >25.6 | 3.2 | >25.6 | >25.6 | 3.2 | >25.6 | 1.6 | 0.1 | 0.2 | 3.2 | 0.1 | 3.2 | >25.6 | 3.2 | 25.6 | >25.6 | 3.2 | 12.8 | >25.6 | >25.6 |
| E. cloacae | B 311 | AmpC | C | >25.6 | 3.2 | 25.6 | >25.6 | 6.4 | 25.6 | 1.6 | 0.2 | 0.2 | 3.2 | 0.2 | 0.8 | >25.6 | 3.2 | 12.8 | 12.8 | 6.4 | 25.6 | >25.6 | >25.6 |
The MICs of various cephalosporins in the presence of a constant concentration (4 μg/ml) of the β-lactamase inhibitors LK-157 and tazobactam (TZB) were determined by a microdilution test. CTX, cefotaxime; CRO, ceftriaxone; FEP, cefepime; CAZ, ceftazidime; ATM, aztreonam.
Activity of LK-157 combinations against class C β-lactamases.
LK-157 was the most active inhibitor of AmpC (class C) enzymes of C. freundii and E. cloacae (Table 3), with an IC50 of 0.062 μM, significantly lower than that of tazobactam (IC50, 1.760 μM) or clavulanic acid (IC50, 136.2 μM) (Table 1). By the addition of LK-157, the MICs of ceftazidime and piperacillin were reduced from >25.6 μg/ml to 3.2 to 12.8 μg/ml, whereas tazobactam, sulbactam, and clavulanic acid were completely inactive.
Synergy of LK-157 with various cephalosporins.
No extensive experience in therapy has been published yet with combinations of cephalosporins and β-lactamase inhibitors. Commercial combinations such as amoxicillin-clavulanic acid and piperacillin-tazobactam do not exert reasonable activity against ESBLs and class C enzymes. Therefore, LK-157 was also evaluated in synergism experiments (microbroth dilution technique) as a potential partner for various extended-spectrum cephalosporins, including cefotaxime, ceftriaxone, cefepime, cefpirome, ceftazidime, and aztreonam, which were combined with LK-157 or tazobactam at a constant inhibitor concentration of 4 μg/ml. The results presented in Table 4 demonstrate that the activities of LK-157 against isolated SHV-, TEM-, and AmpC-type β-lactamases are maintained in whole-cell assays.
Against the panel of tested strains, LK-157 and tazobactam evidently protected the cephalosporins from the SHV- and TEM-type ESBL-producing strains. LK-157 was clearly superior to commercial inhibitors in reducing the MICs of amoxicillin and piperacillin when tested against the ESBL-producing Enterobacteriaceae, MSSA, and BRO-producing M. catarrhalis. LK-157 also restored the diminished activity of aztreonam and various cephalosporins, including cefotaxime, ceftriaxone, ceftazidime, cefepime, and cefpirome, reducing their MICs by a factor of >8. LK-157 was more potent than tazobactam, especially against AmpC-producing E. cloacae and C. freundii strains. The MIC reduction due to the addition of a β-lactamase inhibitor was most pronounced in the cases of cefotaxime and ceftriaxone, with improved susceptibility compared to that of the respective β-lactam antibiotic alone. However, the lowest MICs against class A and class C β-lactamase producers were obtained by combination of cefepime or cefpirome with either LK-157 or tazobactam (MICs, ≤0.025 to 6.4 μg/ml). Cefepime is not considered to be a first-line agent for infections caused by ESBL-producing organisms, because it has been shown to exhibit a clear inoculum effect (19). Therefore, a combination of FEP and LK-157 could be an opportunity to extend the antimicrobial spectrum and restore activity of the partner antibiotic against nosocomial pathogens expressing class A (TEM-, SHV-, and BRO-type) and class C β-lactamases. Combination of both inhibitors with ceftazidime or aztreonam also confirmed that LK-157 was >4-fold more potent than tazobactam, as was seen with the other cephalosporins, piperacillin and amoxicillin. Although very potent against TEM- and SHV-type ESBLs, LK-157 exhibited significantly weaker activity against CTX-M-producing isolates than tazobactam (Table 5) and was inactive against KPC-3-producing K. pneumoniae and PER-1-producing P. aeruginosa (Table 4).
No antagonism of β-lactam-LK-157 was observed in MIC studies for any tested strain, indicating indirectly the absence of induction of β-lactamase expression. To confirm this, further investigations should be undertaken.
Conclusion.
LK-157, a novel ethylidene derivative of tricyclic carbapenem, met the in vitro criteria for an extended-spectrum β-lactamase inhibitor with potent activity against class A ESBLs and class C β-lactamases. LK-157 proved to be a potent β-lactamase inhibitor, being superior to commercial inhibitors in restoring the diminished activity of various β-lactam antibiotics against an array of bacterial isolates producing several class A ESBLs (excluding CTX-M and KPC) as well as class C β-lactamases. The most promising combinations with the lowest MIC values achieved were FEP-LK-157 and cefpirome-LK-157. The combination of LK-157 with a well-matched cephalosporin would offer substantial advantages over the current commercial inhibitors of β-lactamases in terms of both potency and spectrum. Moreover, its broad spectrum makes LK-157 a promising candidate for empirical therapy for a variety of serious hospital infections and warrants further evaluation and development.
Acknowledgments
This work was supported by Lek Pharmaceuticals, d.d., Ljubljana, Slovenia, and Nabriva Therapeutics AG, Vienna, Austria.
We thank F.J. Schmitz (Minden, Germany), D. Livermore (Health Protection Agency, London, United Kingdom), A. Georgopoulos (Vienna General Hospital, Vienna, Austria), I. Chopra (Leeds, United Kingdom), Novartis (Vienna, Austria), and Naeja (Edmonton, Canada) for supplying clinical isolates. We gratefully acknowledge the technical expertise and contributions of M. Kaučič, A. Hegyi, B. Kubik, and M. Walenta and the guidance of K. Seme. We also thank R. Novak for continuing support and encouragement.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Alvarez, M., J. H. Tran, N. Chow, and G. A. Jacoby. 2004. Epidemiology of conjugative plasmid-mediated AmpC β-lactamases in the United States. Antimicrob. Agents Chemother. 48:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babini, G. S., M. Yuan, and D. M. Livermore. 1998. Interactions of beta-lactamases with sanfetrinem (GV 104326) compared to those with imipenem and with oral beta-lactams. Antimicrob. Agents Chemother. 42:1168-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnefoy, A., C. Dupuis-Hamelin, V. Steier, C. Delachaume, C. Seys, T. Stachyra, M. Fairley, M. Guitton, and M. Lampilas. 2004. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor. J. Antimicrob. Chemother. 54:410-417. [DOI] [PubMed] [Google Scholar]
- 4.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buynak, J. D. 2004. The discovery and development of modified penicillin- and cephalosporin-derived beta-lactamase inhibitors. Curr. Med. Chem. 11:1951-1964. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed., vol. 26. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Copar, A., T. Prevec, B. Anzic, T. Mesar, L. Selic, M. Vilar, and T. Solmajer. 2002. Design, synthesis and bioactivity evaluation of tribactam beta lactamase inhibitors. Bioorg. Med. Chem. Lett. 12:971-975. [DOI] [PubMed] [Google Scholar]
- 8.Copar, A., B. Anzic, T. Kuzman, D. Kocjan, T. Mesar, and T. Solmajer. December 2002. Ethilidene derivatives of tricyclic carbapenems. U.S. patent 6,489,318.
- 9.Georgopapadakou, N. H. 2004. Beta-lactamase inhibitors: evolving compounds for evolving resistance targets. Expert Opin. Investig. Drugs 13:1307-1318. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson, C. E., P. A. Lambert, and I. N. Simpson. 2003. In vitro and in vivo activities of AM-112, a novel oxapenem. Antimicrob. Agents Chemother. 47:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kresken, M., J. Brauers, and A. Preželj. 2007. In vitro activity of LK-157, a novel tricyclic carbapenem β-lactamase inhibitor, abstr. F1-0338. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007. [DOI] [PMC free article] [PubMed]
- 12.Micetich, R. G., S. M. Salama, S. N. Maiti, A. V. N. Reddy, and R. Singh. 2002. ß-Lactamases and their inhibitors: an update. Curr. Med. Chem. Anti-Infective Agents 1:193-213. [Google Scholar]
- 13.Miller, L. A., K. Ratnam, and D. J. Payne. 2001. Beta-lactamase-inhibitor combinations in the 21st century: current agents and new developments. Curr. Opin. Pharmacol. 1:451-458. [DOI] [PubMed] [Google Scholar]
- 14.Nishida, K., C. Kunugita, T. Uji, F. Higashitani, A. Hyodo, N. Unemi, S. N. Maiti, O. A. Phillips, P. Spevak, K. P. Atchison, S. M. Salama, H. Atwal, and R. G. Micetich. 1999. In vitro and in vivo activities of Syn2190, a novel β-lactamase inhibitor. Antimicrob. Agents Chemother. 43:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plantan, I., L. Selic, T. Mesar, P. S. Anderluh, M. Oblak, A. Prezelj, L. Hesse, M. Andrejasic, M. Vilar, D. Turk, A. Kocijan, T. Prevec, G. Vilfan, D. Kocjan, A. Copar, U. Urleb, and T. Solmajer. 2007. 4-Substituted trinems as broad spectrum β-lactamase inhibitors: structure-based design, synthesis, and biological activity. J. Med. Chem. 50:4113-4121. [DOI] [PubMed] [Google Scholar]
- 16.Preželj, A., I. Plantan, G. Vilfan, P. Štefanič Anderluh, L. Selič, P. Igličar, V. Car, M. Oblak, T. Šolmajer, M. Andrejašič, D. Turk, S. Paukner, L. Hesse, A. Čopar, and U. Urleb. 2007. 10-Ethylidene trinems as broad spectrum β-lactamase inhibitors, abstr. F1-316. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007.
- 17.Preželj A., I. Plantan, G. Vilfan, P. Štefanič Anderluh, L. Selič, P. Igličar, V. Car, S. Andrenšek, I. Legen, P. Smrdel, I. Locatelli, A. Mrhar, N. Kovačič, M. Černe, and U. Urleb. 2007. Stability of 10-ethylidene trinems, PK of LK-157 and design of prodrug esters with improved solubility and GIT permeation, abstr. F1-317. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007.
- 18.Singh, K. V., T. M. Coque, and B. E. Murray. 1996. In vitro activity of the trinem sanfetrinem (GV104326) against gram-positive organisms. Antimicrob. Agents Chemother. 40:2142-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturenburg, E., and D. Mack. 2003. Extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory, therapy, and infection control. J. Infect. 47:273-295. [DOI] [PubMed] [Google Scholar]
- 20.Vilar, M., M. Galleni, T. Solmajer, B. Turk, J. M. Frere, and A. Matagne. 2001. Kinetic study of two novel enantiomeric tricyclic β-lactams which efficiently inactivate class C β-lactamases. Antimicrob. Agents Chemother. 45:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss, W. J., P. J. Petersen, T. M. Murphy, L. Tardio, Y. Yang, P. A. Bradford, A. M. Venkatesan, T. Abe, T. Isoda, A. Mihira, H. Ushirogochi, T. Takasake, S. Projan, J. O'Connell, and T. S. Mansour. 2004. In vitro and in vivo activities of novel 6-methylidene penems as β-lactamase inhibitors. Antimicrob. Agents Chemother. 48:4589-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]


