Abstract
In vitro selection of mutants with decreased susceptibility to ertapenem was performed using Escherichia coli and Klebsiella pneumoniae clinical strains producing either the blaCTX-M-2, blaCTX-M-3, blaCTX-M-9, or blaCTX-M-15 gene. Frequencies of mutants with decreased susceptibilities to ertapenem were similar for all β-lactamases expressed.
Extended-spectrum β-lactamases (ESBLs) of the CTX-M type are emerging worldwide mostly in Enterobacteriaceae as a source of community-acquired and nosocomial infections (11).
Most CTX-M-type β-lactamases hydrolyze cefotaxime at a higher level than that of ceftazidime. However, several amino acid substitutions result in an increased hydrolytic activity against ceftazidime, as seen for the CTX-M enzyme that has spread worldwide, CTX-M-15, a variant of CTX-M-3 (14). Specific substitutions at Ambler positions 167 (P167S/T/Q) and 240 (D240G) in CTX-M-2 (21) and in CTX-M-3 (6, 14) conferring resistance to ceftazidime have been selected in vitro after a ceftazidime selection. Although rare ESBLs such as several GES-like enzymes may hydrolyze carbapenems (12), no CTX-M enzyme has been reported to possess carbapenemase activity. Among β-lactam molecules, carbapenems (imipenem, ertapenem, and meropenem) are the drugs of choice for treating infections by ESBL-producing Enterobacteriaceae (16, 17, 18).
The objective of this study was to determine if CTX-M β-lactamases with carbapenemase activity may be selected in vitro.
Therefore, the frequency of in vitro selection of mutant strains with reduced susceptibility to ertapenem was evaluated with Escherichia coli and Klebsiella pneumoniae strains expressing different CTX-M β-lactamases. The risk of selection of mutated blaCTX-M genes and the level of porin expression were investigated also.
The blaCTX-M genes were expressed under the same promoter and in the same genetic background. The blaCTX-M genes (blaCTX-M-2, blaCTX-M-3, blaCTX-M-9, and blaCTX-M-15) were amplified from clinical isolates without their promoter sequence (primer sequence in Table 1), cloned into the low-copy-number pACYC184 plasmid (New England Biolabs, Ozyme, Saint-Quentin-en-Yvelines, France), and expressed in clinical isolates of E. coli Wi and K. pneumoniae M (Hôpital Bicêtre strain collection). Transformants were selected overnight at 37°C on Trypticase soy agar (bioMérieux, Craponne, France) containing chloramphenicol (Euromedex, Souffelweyersheim, France) at 30 μg/ml.
TABLE 1.
Nucleotide sequences of primers used for amplification and sequence analysis
| Primer | Sequence (5′→3′) | Reference or GenBank accession no. |
|---|---|---|
| CTX-M-2Fa | AATGTATATTGAAGGCCGAGGG | This study |
| CTX-M-2R | ATACCTCGCTCCATTTATTGC | This study |
| CTX-M-3F | TCGTCTCTTCCAGAATAAG | This study |
| CTX-M-3R | TACCTATTACAAACCGTCGGTG | This study |
| CTX-M-9F | CTGATGTAACACGGATTGAC | This study |
| CTX-M-9R | AGCGCCCCATTATTGAGAG | This study |
| CTX-M-15Fb | TCGTATCTTCCAGAATAAGG | This study |
| EcOmpC-F | GTTAAAGTACTGTCCCTCCTG | 15 |
| EcOmpC-R | TAACCGGTCAGCTGGTCAGTAA | This study |
| EcOmpF-F | TCGTATCTTCCAGAATAAGG | AM040706 |
| EcOmpF-R | CAGGTACTGCAAACGCTGC | 15 |
| GapA-F | TATGACTGGTCCGTCTAAAGACAA | 19 |
| GapA-R | GGTTTTCTGAGTAGCGGTAGTAGC | 19 |
| OmpK35-F | TGATCCCTGCCCTGCTGGT | 8 |
| OmpK35-R | TCCATGTTGTATTCCCACTGG | This study |
| OmpK36-F | TTAGACCTGTACGGCAAAATCG | Z33506 |
| OmpK36-R | AATGCCAGACGAGTCCATGC | Z33506 |
| Kp16S rRNA-F | GGACGGGTGAGTAATGTC | EU048272 |
| Kp16S rRNA-R | TCTCAGACCAGCTAGGGATCG | EU048272 |
Expected sizes of PCR products with combinations of forward (F) and reverse (R) primers: 943 bp for the blaCTX-M-2 gene, 916 bp for the blaCTX-M-3 gene, 932 bp for the blaCTX-M-9 gene, 904 bp for the blaCTX-M-15 gene, 215 bp for the ompC gene, 204 bp for the ompF gene, and 201 bp for the gapA gene of E. coli and 202 bp for the ompK36 gene, 222 bp for the ompK35 gene, and 193 bp for the 16S rRNA gene of K. pneumoniae.
The CTX-M-3R primer was also used for the amplification of the blaCTX-M-15 gene.
Mutant strains with decreased susceptibilities to ertapenem were selected as described previously (6), on Trypticase soy agar containing ertapenem (Merck Sharp & Dohme-Chibret, Paris, France) at a concentration fourfold higher than the MICs (6). After overnight incubation at 37°C for 18 h, mutation frequencies were calculated, taking plate counts of viable bacteria on drug-free agar (6). Comparison of the means was performed by Student's t test on three independent experiments. In E. coli as well as in K. pneumoniae isolates, mean frequencies of selection of ertapenem-reduced susceptibility were not related to the blaCTX-M content (P > 0.1) (Table 2).
TABLE 2.
Mean frequencies of mutation to ertapenem decrease susceptibility for Escherichia coli and Klebsiella pneumoniae strains producing no CTX-M (wild type) and producing the ESBLs CTX-M-2, CTX-M-3, CTX-M-9, and CTX-M-15
| Strain | Ertapenem selection (μg/ml)b | Frequency of selection of mutants with decreased susceptibility to ertapenem (10−8)a |
|---|---|---|
| K. pneumoniae | ||
| M (wild type) | 0.032 | 4 ± 1.7 |
| M (CTX-M-2) | 0.064 | 7 ± 6 |
| M (CTX-M-3) | 0.064 | 5 ± 3 |
| M (CTX-M-9) | 0.048 | 1.8 ± 1.2 |
| M (CTX-M-15) | 0.064 | 4.2 ± 2.5 |
| E. coli | ||
| Wi (wild type) | 0.032 | 0.8 ± 0 |
| Wi (CTX-M-2) | 0.092 | 3 ± 1.4 |
| Wi (CTX-M-3) | 0.092 | 2.4 ± 1.3 |
| Wi (CTX-M-9) | 0.064 | 2.7 ± 0.6 |
| Wi (CTX-M-15) | 0.092 | 1.2 ± 0.5 |
Mutation frequencies are arithmetic means from three independent experiments, each performed in triplicate.
Ertapenem selection was performed at concentrations of four times the MICs.
Both strands of the blaCTX-M genes were sequenced from five of each blaCTX-M-harboring E. coli and K. pneumoniae mutant strain. All mutants had a wild-type blaCTX-M-2, blaCTX-M-3, blaCTX-M-9, or blaCTX-M-15 sequence, thus indicating that the reduced susceptibility to ertapenem was not due to point mutations located in the blaCTX-M genes.
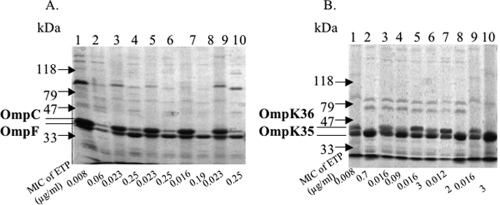
The outer membrane protein (OMP) profiles of the E. coli and K. pneumoniae isolates before and after ertapenem selection were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (15). Comparison of the OMP profiles of E. coli mutant strains selected on ertapenem-containing plates showed mainly a decrease in OmpC expression (Fig. 1). Similarly, comparison of the OMP profiles of K. pneumoniae isolates showed mainly a decrease in OmpK36 expression in ertapenem-selected isolates expressing a CTX-M β-lactamase or not (Fig. 1).
FIG. 1.
OMP profiles of E. coli Wi (A) and K. pneumoniae M (B) isolates expressing no CTX-M (lanes 1 and 2) or expressing CTX-M-2 (lanes 3 and 4), CTX-M-3 (lanes 5 and 6), CTX-M-9 (lanes 7 and 8), or CTX-M-15 (lanes 9 and 10). Lanes 1, 3, 5, 7, and 9 correspond to OMPs extracted from isolates cultured without ertapenem (ETP); lanes 2, 4, 6, 8, and 10 correspond to OMPs extracted after a single-step ertapenem selection (4× MIC for each).
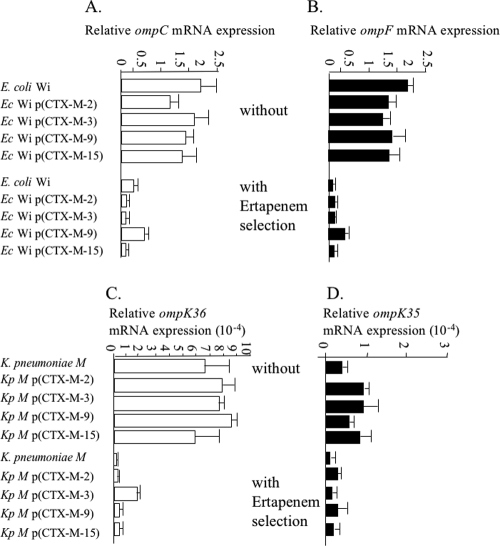
In order to quantify expression of the ompC and ompF genes in E. coli isolates and of the ompK35 and ompK36 genes in K. pneumoniae isolates, the two-step quantitative reverse transcription-PCR was used as recommended by Fey et al. (3). Expression level results were standardized relative to the transcription level of the constitutively expressed gapA (d-glyceraldehyde-3-phosphate dehydrogenase) gene in E. coli (19) and the 16S rRNA genes in K. pneumoniae (Fig. 2). Transcript quantification was performed by using the LightCycler FastStart DNA MasterPLUS kit SYBR-Green I on a LightCycler 1.0 instrument (Roche Diagnostics, Neuilly, France) at an annealing temperature of 57°C. The calibration curves were generated with serially diluted cDNA from in vitro-obtained RNA standards (10) for each gene with primers listed in Table 1. The slope of each calibration curve was used to compare the number of copies of each omp gene in E. coli and K. pneumoniae isolates before and after ertapenem selection. Real-time quantitative reverse transcription-PCR experiments showed that the E. coli isolates with decreased susceptibilities to ertapenem had similarly decreased expression of the ompC and ompF genes whatever the CTX-M expressed (Fig. 2), the converse of what was observed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Similarly, K. pneumoniae isolates with decreased susceptibilities to ertapenem had decreased expression of the ompK35 and ompK36 genes whatever the CTX-M expressed (Fig. 2). The level of ompK35 transcript was 10-fold lower than that of ompK36 in the same RNA extracts, and the decrease in transcript level upon ertapenem selection was then less significant for ompK35 (two- to sixfold) than for ompK36 (up to 30-fold) (Fig. 2).
FIG. 2.
Effects of one-step selection of ertapenem (4× MIC) on transcription of ompC (A) and ompF (B) genes in E. coli and of ompK36 (C) and ompK35 (D) genes in K. pneumoniae. Isolates expressing no CTX-M, CTX-M-2, CTX-M-3, CTX-M-9, or CTX-M-15 were used as templates. Data are expressed as the amount of target mRNA relative to the control value, i.e., the gap gene of E. coli and the 16S rRNA genes of K. pneumoniae. Upper lanes of each diagram correspond to the omp transcript level in isolates cultured without ertapenem, and lower lanes correspond to the omp transcript level after one-step selection of ertapenem.
Resistance to carbapenems in Enterobactericeae may be related to carbapenemases (13, 20) or to dual mechanisms associating the outer membrane permeability defect and β-lactamases such as AmpCs and ESBLs (2, 5, 7). In the absence of β-lactamase, no defect in outer membrane permeability is sufficient to lead to carbapenem resistance (1). Previous studies reported ertapenem resistance in CTX-M-producing K. pneumoniae (2, 9) and CTX-M-producing E. coli (7) isolates exhibiting a permeability defect. Here, we show that ertapenem selects for mutant strains with decreased susceptibility by modification of porin expression whatever the content in CTX-M β-lactamases.
Finally, this study may indicate that the frequency of selection of ertapenem resistance is not higher in isolates expressing CTX-Ms. In additional to the alteration of membrane permeability, CTX-M β-lactamases most probably contribute to the decreased ertapenem susceptibility by binding with a high affinity to this molecule. Indeed, even if poorly hydrolyzed by CTX-Ms, ertapenem has been shown to have a strong inhibitory effect (low Ki) on these β-lactamases as exemplified with CTX-M-15 (4). This finding shall be added to the current debate on usage of ertapenem for treating infections due to ESBL-producing K. pneumoniae and E. coli isolates.
Acknowledgments
We thank Hubert Chardon for fruitful discussions.
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA 3539), Université Paris XI, and mostly by a grant of the European Community (6th PCRD, LSHM-CT-2005-018705) and the INSERM, France.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Cornaglia, G., K. Russell, G. Satta, and R. Fontana. 1995. Relative importances of outer membrane permeability and group 1 beta-lactamase as determinants of meropenem and imipenem activities against Enterobacter cloacae. Antimicrob. Agents Chemother. 39:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott, E., A. J. Brink, J. van Greune, Z. Els, N. Woodford, J. Turton, M. Warner, and D. M. Livermore. 2006. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiella pneumoniae with an extended-spectrum β-lactamase. Clin. Infect. Dis. 42(11):e95-e98. [DOI] [PubMed] [Google Scholar]
- 3.Fey, A., S. Eichler, S. Flavier, R. Christen, M. G. Höfle, and C. A. Guzmán. 2004. Establishment of a real-time PCR-based approach for accurate quantification of bacterial RNA targets in water, using Salmonella as a model organism. Appl. Environ. Microbiol. 70:3618-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girlich, D., L. Poirel, and P. Nordmann. 2008. Do CTX-M beta-lactamases hydrolyse ertapenem? J. Antimicrob. Chemother. 62:1155-1156. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby, G. A., D. M. Mills, and N. Chow. 2004. Role of β-lactamases and porins in resistance to ertapenem and other β-lactams in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:3203-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karisik, E., M. J. Ellington, R. Pike, D. M. Livermore, and N. Woodford. 2006. Development of high-level ceftazidime resistance via single-base substitutions of blaCTX-M-3 in hyper-mutable Escherichia coli. Clin. Microbiol. Infect. 12:803-806. [DOI] [PubMed] [Google Scholar]
- 7.Lartigue, M. F., L. Poirel, C. Poyart, H. Réglier-Poupet, and P. Nordmann. 2007. Ertapenem resistance of Escherichia coli. Emerg. Infect. Dis. 13:315-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, C. H., C. Chu, J. W. Liu, Y. S. Chen, C. J. Chiu, and L. H. Su. 2007. Collateral damage of flomoxef therapy: in vivo development of porin deficiency and acquisition of blaDHA-1 leading to ertapenem resistance in a clinical isolate of Klebsiella pneumoniae producing CTX-M-3 and SHV-5 beta-lactamases. J. Antimicrob. Chemother. 60:410-413. [DOI] [PubMed] [Google Scholar]
- 9.Livermore, D. M., K. J. Oakton, M. W. Carter, and M. Warner. 2001. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent β-lactamases. Antimicrob. Agents Chemother. 45:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillet, S., T. Naas, S. Crepin, A. M. Roque-Afonso, F. Lafay, S. Efstathiou, and M. Labetoulle. 2006. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. J. Virol. 80:9310-9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mena, A., V. Plasencia, L. García, O. Hidalgo, J. I. Ayestarán, S. Alberti, N. Borrell, J. L. Pérez, and A. Oliver. 2006. Characterization of a large outbreak by CTX-M-1-producing Klebsiella pneumoniae and mechanisms leading to in vivo carbapenem resistance development. J. Clin. Microbiol. 44:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naas, T., L. Poirel, and P. Nordmann. 2008. Minor extended-spectrum β-lactamases. Clin. Microbiol. Infect. 14:42-52. [DOI] [PubMed] [Google Scholar]
- 13.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 14.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 15.Poirel, L., C. Héritier, C. Spicq, and P. Nordmann. 2004. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J. Clin. Microbiol. 42:3831-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raveh, D., A. M. Yinnon, E. Broide, and B. Rudensky. 2007. Susceptibilities of ESBL-producing Enterobacteriaceae to ertapenem, meropenem and piperacillin-tazobactam with and without clavulanic acid. Chemotherapy 53:185-189. [DOI] [PubMed] [Google Scholar]
- 17.Tamayo, J., B. Orden, J. Cacho, J. Cuadros, J. L. Gomez-Garces, and J. I. Alos. 2007. Activity of ertapenem and other antimicrobials against ESBL-producing enterobacteria isolated from urine in patients from Madrid. Rev. Esp. Quimioter. 20:334-338. [PubMed] [Google Scholar]
- 18.Teng, C. P., H. H. Chen, J. Chan, and D. C. Lye. 2007. Ertapenem for the treatment of extended-spectrum beta-lactamase-producing Gram-negative bacterial infections. Int. J. Antimicrob. Agents 30:356-359. [DOI] [PubMed] [Google Scholar]
- 19.Toshima, H., A. Yoshimura, K. Arikawa, A. Hidaka, J. Ogasawara, A. Hase, H. Masaki, and Y. Nishikawa. 2007. Enhancement of Shiga toxin production in enterohemorrhagic Escherichia coli serotype O157:H7 by DNase colicins. Appl. Environ. Microbiol. 73:7582-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh, K. J., M. Barlow, F. C. Tenover, J. W. Biddle, J. K. Rasheed, L. A. Clark, and J. E. McGowan, Jr. 2005. Experimental prediction of the evolution of ceftazidime resistance in the CTX-M-2 extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 49:1242-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]