Abstract
In concert with the development of novel β-lactams and broad-spectrum cephalosporins, bacterially encoded β-lactamases have evolved to accommodate the new agents. This study was designed to identify, at the sequence level, the genes responsible for the extended-spectrum-β-lactamase (ESBL) phenotypes of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates collected during the global tigecycline phase 3 clinical trials. PCR assays were developed to identify and clone the blaTEM, blaSHV, blaOXA, and blaCTX genes from clinical strains. Isolates were also screened for AmpC genes of the blaCMY, blaACT, blaFOX, and blaDHA families as well as the blaKPC genes encoding class A carbapenemases. E. coli, K. pneumoniae, and P. mirabilis isolates with ceftazidime MICs of ≥2 μg/ml were designated possible ESBL-producing pathogens and were then subjected to a confirmatory test for ESBLs by use of Etest. Of 272 unique patient isolates, 239 were confirmed by PCR and sequencing to carry the genes for at least one ESBL, with 44% of the positive isolates harboring the genes for multiple ESBLs. In agreement with current trends for ESBL distribution, blaCTX-M-type β-lactamase genes were found in 83% and 71% of the ESBL-positive E. coli and K. pneumoniae isolates, respectively, whereas blaSHV genes were found in 41% and 28% of the ESBL-positive K. pneumoniae and E. coli isolates, respectively. Ninety-seven percent of the E. coli and K. pneumoniae isolates were tigecycline susceptible (MIC90 = 2 μg/ml), warranting further studies to define the therapeutic utility of tigecycline against strains producing ESBLs in a clinical setting.
Throughout the 60-year clinical experience with β-lactams and broad-spectrum cephalosporins, bacterial pathogens have kept pace with the introduction of novel agents through the evolution of new β-lactamases that efficiently conform to the new antibacterials (5, 10, 20, 33). One of the most clinically important groups of β-lactamases is the extended-spectrum β-lactamases (ESBLs). Numerous studies have revealed increased mortality and, in general, poorer clinical outcomes for patients infected with bacteria encoding ESBLs (35, 45). According to Bush et al. (11), ESBLs are defined as oxyimino-cephalosporin-hydrolyzing β-lactamases that are inhibited by clavulanic acid.
The current rising threat in the clinic is the CTX-M family of ESBLs; these enzymes have appeared in both community and nosocomial settings (3, 12, 38). CTX-M, a class A enzyme, was originally defined by the preferential cleavage of cefotaxime (CTX) versus ceftazidime (CAZ), although several recent derivatives that cleave both agents equivalently have been described (4, 39). In most European countries, Latin America, and East Asia, CTX-M variants have displaced TEM and SHV enzymes as the predominant β-lactamases produced by gram-negative pathogens, especially Klebsiella pneumoniae and Escherichia coli (3, 12, 26). The Lahey website (http://www.lahey.org/Studies/) reports that 82 CTX-M derivatives have been described.
TEM-1, the first plasmid-mediated β-lactamase identified in gram-negative organisms, initially described in E. coli in the 1960s (16), is now present in a number of members of the family Enterobacteriaceae, including Klebsiella pneumoniae and Proteus mirabilis, and has spread into additional gram-negative pathogens (5). At last count (http://www.lahey.org/Studies/), more than 160 TEM derivatives have been reported, although not all have an expanded spectrum. In a similar fashion, SHV-type ESBLs have evolved from the SHV-1 enzyme common to K. pneumoniae. They are found on both the chromosome and plasmids and have migrated into Citrobacter spp., E. coli, Pseudomonas aeruginosa, and other genera. Approximately 112 SHV derivatives have been reported; however, not all of the SHV derivatives described are ESBLs (http://www.lahey.org/Studies/). Lastly, OXA, a diverse family of enzymes belonging to the class D family, was initially characterized by rates of hydrolysis of oxacillin that were faster than the rates of hydrolysis of benzylpenicillin and relatively less sensitivity to inhibition by clavulanic acid (3, 5, 11). Over 130 OXA derivatives have been reported; however, not all of the OXA derivatives described have an extended spectrum (http://www.lahey.org/Studies/).
The current study was designed to identify, at the sequence level, the genetic determinants responsible for the ESBL phenotype of E. coli, K. pneumoniae, and P. mirabilis isolates collected during the global tigecycline phase 3 clinical trials. In addition, isolates were screened for several AmpC determinants and the carbapenemase-encoding blaKPC genes.
MATERIALS AND METHODS
Bacterial isolates.
Clinical isolates of E. coli, K. pneumoniae, and P. mirabilis were isolated from patients enrolled in phase 3 clinical trials of tigecycline conducted from 2002 to 2006 for the following indications: complicated skin and skin structure infections (cSSSI), complicated intra-abdominal infections (cIAI), community-acquired pneumonia (CAP), and hospital-acquired pneumonia (HAP) (1, 17, 18, 27). All of the studies were global in nature, and enrollment at all sites was ongoing until the completion of the study. Therefore, the number of patients enrolled and the subsequent bacterial isolates used in this study were not equal among various regions. Individual investigators sent all bacterial pathogens to a central laboratory for identification and susceptibility tests, which were performed in real time for the duration of the clinical trials. Primary cultures representing all isolates, regardless of treatment with tigecycline or the comparator agent, were provided to Wyeth Research in frozen vials. In instances in which multiple isolates were received from a single patient, riboprinting (Qualicon, Wilmington, DE) was used, according to the manufacturer's instructions, to determine if the isolates were serial isolates of the same strain or represented unique strains. If the isolates were identical, only the first isolate, according to the site collection date, was included in the analysis. Control strains encoding various β-lactamase genes were used to establish and qualify the PCR assays and are listed in Table 1.
TABLE 1.
Control strains used in study
| Strain designation | Organism | β-Lactamase | Reference or source |
|---|---|---|---|
| R6K | E. coli | TEM-1 | Wyeth Strain Collection |
| GC 1481 | E. coli | SHV-1 | Wyeth Strain Collection |
| J53.2 | E. coli | CTX-M-1 | Wyeth Strain Collection |
| GC 5429 | E. coli | CTX-M-5 | 9 |
| R6N238 | E. coli | OXA-1 | Wyeth Strain Collection |
| GC 1777 | E. coli | OXA-2 | Wyeth Strain Collection |
| GC 1806 | E. coli | OXA-5 | Wyeth Strain Collection |
| GC 7870 | P. mirabilis | PER-1 | Gift of M.-J. Ferraro |
| GC 2809 | E. coli | ACT-1 | 8 |
| GAR 1703 | K. pneumoniae | MIR-1 | Wyeth Strain Collection |
| GC 6202 | E. coli | CMY | Wyeth Strain Collection |
| G52328 | K. pneumoniae | FOX-5 | Gift of G. Jacoby |
| UCLA 14 | K. pneumoniae | DHA-1 | Gift of G. Jacoby |
| GC 3805 | Hafnia alvei | ACC-1 | Wyeth Strain Collection |
| KP1569 | E. coli | KPC-2 | 6 |
| DH5α | E. coli | None | New England BioLabs |
Antibiotic susceptibility testing.
Susceptibility tests were performed by broth microdilution with Mueller-Hinton II broth, as recommended by the Clinical and Laboratory Standards Institute (14, 15). Confirmatory testing for ESBL production was performed by using Etest ESBL strips containing either CAZ or CTX in combination with clavulanic acid (CLA), according to the manufacturer's instructions (AB Biodisk, Solna, Sweden). A positive test result (a reduction of ≥3 log2 dilutions in the MIC in the presence of CLA) for either antibiotic was considered to be a confirmation of the presence of an ESBL. According to the manufacturer's instructions, the result of the test for ESBLs was considered nondeterminable if both MICs were above the test range (CAZ MIC > 32 μg/ml, CTX MIC > 16 μg/ml; CAZ MIC plus CLA MIC > 4 μg/ml; CTX MIC plus CLA MIC > 1 μg/ml). A nondeterminable interpretation was also given if one test strip was ESBL negative and the other test strip was nondeterminable.
PCR amplification.
Clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis from patients enrolled in tigecycline phase 3 clinical trials reporting both a CAZ MIC of ≥2 μg/ml and testing either positive or nondeterminable by the ESBL Etest were examined by PCR with diagnostic primer sets to determine each β-lactamase present (Table 2). Because of the reported low prevalence, tests with the blaPER and blaACC primer sets were performed only if isolates were negative with all the other primer sets. PCR amplifications were performed as described previously (22).
TABLE 2.
Oligonucleotide primers used in study
| Target(s) | Sequence (5′-3′)a | Start point (bp)b | Amplicon size (bp)b | GenBank accession no.c |
|---|---|---|---|---|
| TEM | F: 5′-GTGCGCGGAACCCCTATT | 107 | 968 | AB194682 |
| R: 5′-TTACCAATGCTTAATCAGTGAGGC | 1075 | |||
| SHV | F: 5′-CTTTACTCGCCTTTATCGGC | 174 | 982 | AF124984 |
| R: 5′-TTACCGACCGGCATCTTTCC | 1156 | |||
| CTX-M-2 and -5 | F: 5′-ATGTGCAGYACCAGTAAGG | 292 | 564 | X92506 |
| R: 5′-TAAGTGACCAGAATMAGCGG | 856 | |||
| CTX-M | F: 5′-ATGTGCAGYACCAGTAAAG | 292 | 562 | X92506 |
| R: 5′-GGTCACCAGAAGGAGC | 854 | |||
| OXA-1 | F: 5′-CAGATTCAACTTTCAAGATCG | 1564 | 612 | J02967 |
| R: 5′-GTGTTTAGAATGGTGATCG | 2176 | |||
| OXA-2 and -20 | F: 5′-GCRTCSACATTCAAGATWCC | 332 | 524 | X03037 |
| R: 5′-TCWTCCATYCTGTTTGGCG | 856 | |||
| OXA-5 and -10 | F: 5′-AGCATCAACATTYAARATYCC | 1855 | 597 | AF347074 |
| R: 5′-ATGATGCCYTCACTTKCC | 2452 | |||
| PER-1 and -2 | F: 5′-GGCCTGACGATCTGGAACC | 1841 | 855 | AY866517 |
| R: 5′-TAACTGCATAACCTACTCC | 2696 | |||
| ACT-1 | F: 5′-ACAGGCAAGCAGTGGCAGG | 379 | 619 | U58495 |
| R: 5′-GGATTCACTTCTCTCGCAGGC | 998 | |||
| MIR-1d,e | F: 5′-TCGGTAAAGCCGATGTTGCG | 1115 | 301 | M37839 |
| R: 5′-CTTCCACTGCGGCTGCC | 1416 | |||
| CMY and LAT | F: 5′-ATTCCGGGTATGGCCGT | 133 | 835 | EF406116 |
| R: 5′-GGGTTTACCTCAACGGC | 968 | |||
| FOX-1 to -5 | F: 5′-GACGGCATTATCCAGCCG | 800 | 856 | X77455 |
| R: 5′-GTAACCGGATTGGCCTGGAAGC | 1656 | |||
| DHA-1 and -2 | F: 5′-ATCTGCAACACTGATTTCCG | 1001 | 1,115 | Y16410 |
| R: 5′-GCACTCAAAATAGCCTGTGC | 2116 | |||
| ACC-1 and -2e | F: 5′-AACAGCCTCAGCAGCCGG | 253 | 342 | EF554600 |
| R: 5′-GCCGCAATCATCCCTAGC | 595 | |||
| KPC-1 to -3 | F: 5′-ATGTCACTGTATCGCCGTCT | 131 | 892 | AF297554 |
| R: 5′-TTTTCAGAGCCTTACTGCCC | 1023 | |||
| 16S rRNA | F: 5′-GCCAGCAGCCGCGGTAATACG | 467 | 289 | M87484 |
| R: 5′-GGACTACCAGGGTATCTAATCC | 756 |
IUB codes (underlined) are as follows: K, G+T; M, A+C; R, A+G; S, G+C; W, A+T; Y, C+T. F, forward; R, reverse.
The numbering indicates the 5′ nucleotide of each primer and is according to the sequence referenced.
This primer set also amplifies ACT-1.
Primers modified from those described previously (36).
Isoelectric focusing.
Analysis of bacterial extracts and determination of the isoelectric points (pIs) of the active enzymes were employed as described previously (7, 28) to characterize the β-lactamases present in each isolate and correlate the results with those of the PCR assays.
Sequence determination.
The nucleotide sequences of the blaTEM, blaSHV, blaOXA, and blaCTX genes were determined for all PCR-positive isolates. In some cases, the PCR amplicons were cloned into pCR-XL-TOPO (Invitrogen, Carlsbad, CA) for sequence analysis, whereas in other cases the PCR products were sequenced directly. As the PCR products did not always contain the entire coding sequence, the available sequence data (from an internal fragment) were used to determine the general family of ESBL encoded (e.g., the CTX-M-1 family or the CTX-M-2 family). Similarly, for the AmpC β-lactamase genes, the sequencing of an internal portion of the gene was sufficient to determine the AmpC-type family. The ABI Prism BigDye Terminator cycle sequencing ready reaction kit mix (version 3.1; Applied Biosystems, Foster City, CA) was used for automated sequence analysis.
Pyrosequencing.
Pyrosequencing was carried our according to the manufacturer's instructions (Biotage, Uppsala, Sweden) to determine if the blaSHV genes contained nucleotide substitutions consistent with known ESBLs through determination of the sequences of two codons encoding glycine 238 and glutamic acid 240, which are critical to the ESBL phenotype. The nucleotide triplet encoding glutamine 35 was also monitored, as this is a substitution common to many SHV derivatives. For those isolates that had the SHV-1 sequence at positions 238 and 240, complete nucleotide sequencing was carried out to ascertain if additional nucleotide substitutions that correlated with the ESBL phenotype were present.
RESULTS
Resistance determinant identification.
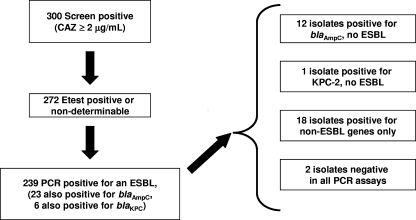
A total of 1,680 E. coli isolates, 749 K. pneumoniae isolates, and 253 P. mirabilis isolates were collected during the phase 3 clinical trials of tigecycline for the treatment of cSSSI, cIAI, CAP, and HAP (1, 17, 18, 27). A total of 178 E. coli isolates, 287 K. pneumoniae isolates, and 34 P. mirabilis isolates showed reduced susceptibility to CAZ (MIC ≥ 2 μg/ml), suggestive of the possible expression of an ESBL. By ribotyping, 98 E. coli isolates, 153 K. pneumoniae isolates, and 21 P. mirabilis isolates were determined to be unique patient isolates (Fig. 1) and were confirmed to express an ESBL by the use of ESBL Etest test strips (AB Biodisk) (15). These 272 isolates were further examined for identification of the encoded β-lactamase(s) at the sequence level.
FIG. 1.
Screening flow for ESBL analysis.
Geographically, the majority of ESBL-producing isolates (46%) came from Eastern Europe, with the majority of isolates originating in Ukraine and Romania (Table 3). Twenty percent of the ESBL-producing isolates came from India and 15% came from Latin America, the majority of which were from Brazil and Chile. The Asia Pacific region was the source for 8% of the ESBL-producing isolates, the majority of which were from China, with the remaining 10% of the isolates expressing ESBLs collected in Africa (South Africa and Morocco), North America, and Western Europe. In terms of clinical presentation, half of the isolates were from patients with pneumonia (46% from patients with HAP, 4% from patients with CAP) and 21% and 15% of the isolates were from patients with cIAI and cSSSI, respectively; 10% of the isolates came from blood.
TABLE 3.
Distribution of β-lactamases in strain collection
| Organism (total no. of isolates) | Regiona | Countryb | No. of isolates | β-Lactamase derivative(s) identifiedc
|
AmpC, C'pased | |||
|---|---|---|---|---|---|---|---|---|
| CTX-M | SHVe | TEM | OXA | |||||
| E. coli (98) | AP | CHN | 10 | 2, 3, 5, 14, 15, 22, 28 | 1, 2a, 5, 11, 12 | 1 | 1, 5, 10 | DHA-1 |
| AP | TWN | 3 | 2, 5 | 1, 5 | 1 | CMY-2 | ||
| AP | AUS | 2 | ACT-1 | |||||
| EE | BGR | 4 | 2, 15 | 5, 11, 12 | 1 | 1, 5 | ACT-1 | |
| EE | HRV | 1 | 5 | 1 | 1, 5 | |||
| EE | HUN | 1 | 1 | ACT-1 | ||||
| EE | LTU | 2 | 2, 3, 15 | 12 | ||||
| EE | POL | 1 | 2, 5 | 1 | 1 | |||
| EE | ROM | 3 | 2, 5, 15 | 1 | 1 | |||
| EE | RUS | 4 | 2, 3, 5, 15, 22, 28 | 1 | 1 | ACT-1 | ||
| EE | SVK | 1 | 2 | 5 | 1 | |||
| EE | UKR | 22 | 2, 3, 5, 14, 15, 22, 28 | 1, 5, 12 | 1 | 1, 2, 10 | ACT-1, DHA-1 | |
| IND | IND | 25 | 2, 5, 15, 28 | 1, 2a, 5, 11, 12 | 1, 2 | 1 | ACT-1, MIR-1, KPCf | |
| LA | CHL | 4 | 2, 5, 15 | 1 | 1, 5 | |||
| LA | GTL | 3 | 15, 28 | 2a, 11 | 1 | 1 | ||
| LA | MEX | 5 | 2, 5, 15 | 5, 12 | 1 | 1 | ACT-1, CMY-2, DHA | |
| NA | USA | 2 | 5 | 12 | CMY | |||
| WE | BEL | 1 | 5 | 1 | ||||
| WE | ESP | 4 | 2, 5, 15, 28 | 5, 12 | 1 | 1 | ||
| K. pneumoniae (153) | AP | CHN | 7 | 2, 5, 12, 14, 15 | 1, 5, 11, 12 | 1 | ||
| AP | KOR | 5 | 14 | 1, 5, 11, 12 | 1 | 1 | DHA-1 | |
| AP | TWN | 4 | 2, 5 | 1, 5, 11, 12 | 1 | 10 | DHA-1 | |
| EE | BGR | 3 | 5, 15 | 1, 5, 12, 28 | 1 | |||
| EE | CZE | 1 | 28 | 29 | ||||
| EE | HRV | 2 | 5 | 11 | 1 | 1 | ||
| EE | LTU | 3 | 2 | 2, 2a, 5, 11, 12 | 1 | 2 | ||
| EE | LVA | 1 | 1, 2a | 1 | ||||
| EE | ROM | 11 | 2, 5, 15 | 1, 5, 11, 12, 45 | 1 | 1 | DHA | |
| EE | RUS | 6 | 2, 5, 14, 15 | 1, 11, 12, 33 | 1 | 10 | ||
| EE | SVK | 5 | 2, 28 | 11 | 1, 110, 116 | 1 | ||
| EE | UKR | 33 | 2, 3, 5, 14, 15, 28 | 1, 2, 2a, 5, 11, 12, 28, 63 | 1 | 1, 2, 5 | KPC-3, DHA | |
| IND | IND | 33 | 2, 5, 15, 28 | 1, 2a, 5, 11, 12, 28 | 1 | 1, 2, 5, 10 | KPC | |
| LA | ARG | 8 | 2 | 1, 5, 11 | 1 | 1, 2, 10 | ||
| LA | BRA | 12 | 2, 15 | 1, 5, 11, 12 | 1, 12 | 1, 2, 5, 10 | KPC, KPC-3 | |
| LA | CHL | 5 | 2 | 1, 5, 27 | 1 | 5, 10 | ||
| LA | MEX | 2 | 15 | 1, 2a | 1 | |||
| LA | PER | 1 | 2a | |||||
| NA | USA | 5 | 2, 5 | 5, 11, 12 | 1 | KPC-2, DHA | ||
| AFR | MOR | 2 | 2, 5 | 5 | 1, 29 | |||
| AFR | ZAF | 3 | 2, 15 | 1, 5, 11 | 1 | ACT-1 | ||
| WE | GRC | 1 | 5 | |||||
| P. mirabilis (21) | EE | LVA | 1 | 1 | ACT-1/MIR-1 | |||
| EE | POL | 1 | 5 | 1 | ||||
| EE | ROM | 7 | 15, 28 | 1, 2, 5, 12 | 1, 2, 110 | 2 | CMY-2, DHA | |
| EE | RUS | 1 | 2 | 5 | 2 | |||
| EE | UKR | 1 | 14, 15 | 1 | ||||
| IND | IND | 5 | 2, 5, 28 | 1, 5, 11, 12 | 1 | 1 | DHA | |
| LA | BRA | 2 | 2, 15 | 1 | ACT-1/MIR-1 | |||
| LA | CHL | 2 | 2 | 1, 5, 11, 12 | 1 | 1, 2 | ||
| NA | USA | 1 | 155 | |||||
AFR, Africa; AP, Asia Pacific; EE, Eastern Europe; IND, India; LA, Latin America; NA, North America; WE, Western Europe.
ARG, Argentina; AUS, Australia; BEL, Belgium, BGR, Bulgaria; BRA, Brazil; CHL, Chile; CHN, China; CZE, Czech Republic; ESP, Spain; GRC, Greece; GTL, Guatemala; HRV, Herzegovina; HUN, Hungary; IND, India; KOR, Korea, LTU, Lithuania; LVA, Latvia; MEX, Mexico; MOR, Morocco; PER, Peru; POL, Poland; ROM, Romania; RUS, Russia; SVK, Slovakia; TWN, Taiwan; UKR, Ukraine; USA, United States; ZAF, South Africa.
Each row indicates the β-lactamase species identified in each region (e.g., CTX-M-2, SHV-12, TEM-1, and OXA-5).
C'pase, class A carbapenemase.
Thirty-one percent of the blaSHV genes were analyzed by pyrosequencing; and sequence information was derived only for nucleotides encoding residues at positions 35, 238, and 240. Therefore, these genes encode critical residues for the ESBL phenotype but have not been fully sequenced for unambiguous determination of the blaSHV derivative.
β-Lactamase names in italics indicate that sequence data were not sufficient to determine the specific derivative, so only the BLA family is listed.
Diagnostic PCR assays for 11 β-lactamase genes were established by using previously characterized control strains (Table 1) and primer sets based on GenBank sequence listings (Table 2). One or more ESBL genes were detected in 239 (88%) of the isolates tested (Fig. 1; Table 3). Both an ESBL and an AmpC-type enzyme and both an ESBL and a KPC enzyme were identified in 23 (8.5%) and 6 (2.2%) isolates, respectively, and 12 isolates carried only a blaAmpC gene (including DHA, ACT, MIR, and CMY-type β-lactamases), with a single isolate carrying only blaKPC-2. Eighteen (13 K. pneumoniae, 3 P. mirabilis, and 2 E. coli isolates) isolates were positive by PCR for a number of non-ESBL enzymes of the blaTEM, blaSHV, and blaOXA families (Table 3) and negative for ESBLs. Two isolates (one isolate each of E. coli and K. pneumoniae) in the study were negative by the use of all primer sets tested.
The predominant ESBL type found among the isolates from this study was CTX-M, with 85%, 71%, and 43% of the ESBL-positive E. coli, K. pneumoniae, and P. mirabilis isolates, respectively, being found to carry a blaCTX-M-type β-lactamase gene (Table 3). The CTX-M β-lactamases representing both the CTX-M-1 and CTX-M-2 families were broadly distributed in isolates from all regions of the world included in the study. The less common derivatives in our analysis, CTX-M-3 and CTX-M-14, were restricted to Eastern Europe and the Asia Pacific regions, whereas CTX-M-22 was seen only in Eastern Europe and Latin America.
Thirty-six percent of the isolates carried a blaSHV determinant with an extended spectrum; the majority (65%) of these were K. pneumoniae isolates. Only 28% of the E. coli isolates were positive for an SHV-type ESBL. Of the 67 blaSHV ESBL genes that were fully sequenced, 28% were from isolates that harbored a single blaSHV β-lactamase gene alone, whereas the remaining 70% of the isolates carried a blaCTX determinant, in addition to the blaSHV ESBL gene (Table 3).
This study identified a novel ESBL, TEM-155, in a P. mirabilis isolate (Table 3) (23); however, TEM-type enzymes with an extended spectrum were rare in the study, with only six isolates carrying genes encoding a TEM-type ESBL. In contrast, TEM-1 was found in 64% of the isolates in the collection.
Over 40% of the isolates collected carried genes encoding an OXA-type β-lactamase; however, none of the genes identified by sequence analysis encoded enzymes with an extended spectrum. The OXA-1 (formerly OXA-30) determinant was prevalent in the isolate collection and was found in 76% of the isolates.
Various genes of the blaAmpC class as well as the KPC family of carbapenemases were identified by PCR, and the majority were sequenced for identification of the gene (Table 3). In total, 15% of the isolates were positive for an AmpC or carbapenemase, with 5% of the isolates harboring only a blaAmpC or a blaKPC family determinant.
Antibiotic susceptibilities.
As shown in Table 4, all of the isolates showed reduced susceptibility to CAZ (MIC range, 2 to >64 μg/ml; MIC90s, >64 μg/ml) as well as the other expanded-spectrum cephalosporins tested (MIC90s > 32 μg/ml). Nearly 30% of the isolates were resistant to cefepime (MICs > 16 μg/ml), with a very high percentage (87%) of these isolates encoding an enzyme of the CTX family.
TABLE 4.
Susceptibilities of ESBL-producing isolates from tigecycline clinical trials
| Organism (no. of isolates) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| All isolates (272) | Ceftazidime | 2->64 | 32 | >64 |
| Cefotaxime | ≤4->32 | >32 | >32 | |
| Ceftriaxone | ≤4->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 8 | >16 | |
| Imipenem | ≤0.12-32 | 0.25 | 1 | |
| Piperacillin-tazobactam | ≤8->64 | ≤8 | >64 | |
| Tobramycin | ≤0.25->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 8 | >16 | |
| Minocycline | ≤0.12->64 | 8 | 64 | |
| Tigecycline | 0.12-16 | 0.5 | 2 | |
| All E. coli isolates (98) | Ceftazidime | 2->64 | 32 | >64 |
| Cefotaxime | ≤4->32 | >32 | >32 | |
| Ceftriaxone | ≤4->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 16 | >16 | |
| Imipenem | ≤0.12-0.5 | ≤0.12 | 0.25 | |
| Piperacillin-tazobactam | ≤8->64 | ≤8 | 16 | |
| Tobramycin | ≤0.25->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 16 | >16 | |
| Minocycline | 1->64 | 4 | 32 | |
| Tigecycline | 0.12-2 | 0.25 | 0.5 | |
| All K. pneumoniae isolates (153) | Ceftazidime | 2->64 | 32 | >64 |
| Cefotaxime | ≤4->32 | >32 | >32 | |
| Ceftriaxone | ≤4->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 8 | >16 | |
| Imipenem | ≤0.12-32 | 0.25 | 1 | |
| Piperacillin-tazobactam | ≤4->64 | 16 | >64 | |
| Tobramycin | ≤0.25->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 2 | >16 | |
| Minocycline | ≤0.12->64 | 4 | 64 | |
| Tigecycline | 0.25-8 | 0.5 | 2 | |
| All P. mirabilis isolates (21) | Ceftazidime | 2->64 | 64 | >64 |
| Cefotaxime | ≤0.25->32 | 32 | >32 | |
| Ceftriaxone | ≤1-64 | 8 | 32 | |
| Cefepime | ≤1->16 | 2 | >16 | |
| Imipenem | 0.5-4 | 2 | 4 | |
| Piperacillin-tazobactam | ≤4->64 | 8 | >64 | |
| Tobramycin | 0.5->32 | 8 | >32 | |
| Levofloxacin | ≤0.12->16 | 8 | >16 | |
| Minocycline | 16->64 | 64 | >64 | |
| Tigecycline | 0.5-16 | 2 | 16 | |
| E. coli blaSHVa (7) | Ceftazidime | 16->64 | NAb | NA |
| Cefotaxime | ≤4-32 | NA | NA | |
| Ceftriaxone | ≤4-32 | NA | NA | |
| Cefepime | ≤2-4 | NA | NA | |
| Imipenem | ≤0.12-0.5 | NA | NA | |
| Piperacillin-tazobactam | ≤4->64 | NA | NA | |
| Tobramycin | ≤0.25->32 | NA | NA | |
| Levofloxacin | ≤0.12->16 | NA | NA | |
| Minocycline | 2->64 | NA | NA | |
| Tigecycline | 0.12-2 | NA | NA | |
| E. coli blaCTX, single determinant (33) | Ceftazidime | 2->64 | 16 | 64 |
| Cefotaxime | ≤4->32 | >32 | >32 | |
| Ceftriaxone | ≤4->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 16 | >16 | |
| Imipenem | ≤0.12-0.5 | ≤0.12 | 0.25 | |
| Piperacillin-tazobactam | ≤8->64 | ≤8 | 32 | |
| Tobramycin | 0.5->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 16 | >16 | |
| Minocycline | 1->64 | 4 | 32 | |
| Tigecycline | 0.12-2 | 0.25 | 0.5 | |
| E. coli blaCTX, multiple determinants (18) | Ceftazidime | 2->64 | 32 | 64 |
| Cefotaxime | 32->32 | >32 | >32 | |
| Ceftriaxone | >32 | >32 | >32 | |
| Cefepime | 4->16 | 16 | >16 | |
| Imipenem | ≤0.12-0.25 | ≤0.12 | 0.25 | |
| Piperacillin-tazobactam | ≤8-16 | ≤8 | 16 | |
| Tobramycin | 0.5->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | >16 | >16 | |
| Minocycline | 2-64 | 4 | 32 | |
| Tigecycline | 0.12-1 | 0.5 | 0.5 | |
| E. coli blaCTX + blaSHVa (16) | Ceftazidime | 2->64 | 16 | 64 |
| Cefotaxime | 32->32 | >32 | >32 | |
| Ceftriaxone | >32 | >32 | >32 | |
| Cefepime | ≤2->16 | 16 | >16 | |
| Imipenem | ≤0.12-0.25 | ≤0.12 | 0.25 | |
| Piperacillin-tazobactam | ≤4-16 | <4 | 8 | |
| Tobramycin | 0.5->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 16 | >16 | |
| Minocycline | 1-32 | 4 | 16 | |
| Tigecycline | 0.25-0.5 | 0.25 | 0.5 | |
| E. coli blaAmpC (5) | Ceftazidime | 4-64 | NA | NA |
| Cefotaxime | 4-32 | NA | NA | |
| Ceftriaxone | 4-32 | NA | NA | |
| Cefepime | ≤2 | NA | NA | |
| Imipenem | 0.12-0.25 | NA | NA | |
| Piperacillin-tazobactam | ≤4-8 | NA | NA | |
| Tobramycin | 1-8 | NA | NA | |
| Levofloxacin | ≤0.12->16 | NA | NA | |
| Minocycline | 1->64 | NA | NA | |
| Tigecycline | 0.25-0.5 | NA | NA | |
| E. coli blaAmpC + ESBL (15) | Ceftazidime | 16->32 | >32 | >32 |
| Cefotaxime | 16->32 | >32 | >32 | |
| Ceftriaxone | 16->32 | >32 | >32 | |
| Cefepime | 4->16 | 16 | >16 | |
| Imipenem | ≤0.12-0.5 | 0.25 | 0.5 | |
| Piperacillin-tazobactam | ≤8-32 | ≤8 | 16 | |
| Tobramycin | 1->32 | 16 | >32 | |
| Levofloxacin | ≤0.12->16 | 16 | >16 | |
| Minocycline | 1->64 | 4 | >64 | |
| Tigecycline | 0.12-1 | 0.25 | 0.5 | |
| K. pneumoniae blaSHV,a single determinant (14) | Ceftazidime | 2->64 | 8 | >64 |
| Cefotaxime | 2->32 | 16 | >32 | |
| Ceftriaxone | ≤1->32 | 8 | >32 | |
| Cefepime | ≤2->16 | ≤2 | >16 | |
| Imipenem | 0.12-16 | 0.25 | 1 | |
| Piperacillin-tazobactam | ≤4->64 | 32 | >64 | |
| Tobramycin | 0.5->32 | 16 | >32 | |
| Levofloxacin | ≤0.12->16 | 0.5 | 16 | |
| Minocycline | 0.5-64 | 4 | 64 | |
| Tigecycline | 0.25-2 | 0.5 | 1 | |
| K. pneumoniae blaSHV,a multiple determinants (3) | Ceftazidime | 16->64 | NA | NA |
| Cefotaxime | 2-32 | NA | NA | |
| Ceftriaxone | 2-32 | NA | NA | |
| Cefepime | ≤2-16 | NA | NA | |
| Imipenem | ≤0.12-0.25 | NA | NA | |
| Piperacillin-tazobactam | ≤4->64 | NA | NA | |
| Tobramycin | 0.5-16 | NA | NA | |
| Levofloxacin | ≤0.12-0.5 | NA | NA | |
| Minocycline | 2->64 | NA | NA | |
| Tigecycline | 0.5-8 | NA | NA | |
| K. pneumoniae blaCTX, single determinant (44) | Ceftazidime | 4->64 | 32 | >64 |
| Cefotaxime | 16->32 | >32 | >32 | |
| Ceftriaxone | 16->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 16 | >16 | |
| Imipenem | ≤0.12-1 | 0.25 | 0.5 | |
| Piperacillin-tazobactam | ≤4->64 | 16 | >64 | |
| Tobramycin | ≤0.25->32 | 16 | >32 | |
| Levofloxacin | ≤0.12->16 | 4 | >16 | |
| Minocycline | ≤0.12->64 | 4 | 32 | |
| Tigecycline | 0.25-4 | 1 | 2 | |
| K. pneumoniae blaCTX, multiple determinants (20) | Ceftazidime | 2->64 | 32 | >64 |
| Cefotaxime | 1->32 | >32 | >32 | |
| Ceftriaxone | ≤1->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 16 | >16 | |
| Imipenem | ≤0.12-1 | 0.25 | 0.5 | |
| Piperacillin-tazobactam | ≤4->64 | 16 | >64 | |
| Tobramycin | ≤0.25->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 4 | 16 | |
| Minocycline | 2-32 | 4 | 16 | |
| Tigecycline | 0.25-2 | 0.5 | 1 | |
| K. pneumoniae blaSHVa + blaCTX (39) | Ceftazidime | 4->64 | 64 | >64 |
| Cefotaxime | ≤0.25->32 | >32 | >32 | |
| Ceftriaxone | 8->32 | >32 | >32 | |
| Cefepime | ≤2->16 | 8 | >16 | |
| Imipenem | ≤0.12-4 | 0.25 | 0.5 | |
| Piperacillin-tazobactam | ≤4->64 | 8 | >64 | |
| Tobramycin | 8->32 | 32 | >32 | |
| Levofloxacin | ≤0.12->16 | 0.5 | >16 | |
| Minocycline | 1->64 | 8 | 64 | |
| Tigecycline | 0.25-2 | 0.5 | 2 | |
| K. pneumoniae ESBL + blaAmpC (7) | Ceftazidime | 4->64 | NA | NA |
| Cefotaxime | 1->32 | NA | NA | |
| Ceftriaxone | 2->32 | NA | NA | |
| Cefepime | ≤2->16 | NA | NA | |
| Imipenem | ≤0.06-1 | NA | NA | |
| Piperacillin-tazobactam | 8->64 | NA | NA | |
| Tobramycin | ≤0.25->32 | NA | NA | |
| Levofloxacin | 2->16 | NA | NA | |
| Minocycline | 4->64 | NA | NA | |
| Tigecycline | 0.5-4 | NA | NA | |
| K. pneumoniae blaDHA (4) | Ceftazidime | 64->64 | NA | NA |
| Cefotaxime | 2->32 | NA | NA | |
| Ceftriaxone | ≤1->32 | NA | NA | |
| Cefepime | ≤2-16 | NA | NA | |
| Imipenem | 0.5-2 | NA | NA | |
| Piperacillin-tazobactam | 8->64 | NA | NA | |
| Tobramycin | 16->32 | NA | NA | |
| Levofloxacin | 1->16 | NA | NA | |
| Minocycline | 4-64 | NA | NA | |
| Tigecycline | 0.5-1 | NA | NA | |
| P. mirabilis ESBL, single determinant (6) | Ceftazidime | 2->64 | NA | NA |
| Cefotaxime | ≤0.25->64 | NA | NA | |
| Ceftriaxone | ≤1-32 | NA | NA | |
| Cefepime | ≤1->16 | NA | NA | |
| Imipenem | 0.5-4 | NA | NA | |
| Piperacillin-tazobactam | ≤4->64 | NA | NA | |
| Tobramycin | 0.5->32 | NA | NA | |
| Levofloxacin | ≤0.12-16 | NA | NA | |
| Minocycline | 16->64 | NA | NA | |
| Tigecycline | 2-16 | NA | NA | |
| P. mirabilis ESBL, multiple determinants (7) | Ceftazidime | 2->64 | NA | NA |
| Cefotaxime | 8->64 | NA | NA | |
| Ceftriaxone | 2-64 | NA | NA | |
| Cefepime | ≤1->16 | NA | NA | |
| Imipenem | 2-4 | NA | NA | |
| Piperacillin-tazobactam | ≤4->64 | NA | NA | |
| Tobramycin | 4->32 | NA | NA | |
| Levofloxacin | 2->16 | NA | NA | |
| Minocycline | 16->64 | NA | NA | |
| Tigecycline | 0.5-16 | NA | NA | |
| P. mirabilis blaAmpC (3) | Ceftazidime | 64->64 | NA | NA |
| Cefotaxime | 16->32 | NA | NA | |
| Ceftriaxone | 8-16 | NA | NA | |
| Cefepime | 2->16 | NA | NA | |
| Imipenem | 1-2 | NA | NA | |
| Piperacillin-tazobactam | 8-64 | NA | NA | |
| Tobramycin | 4->32 | NA | NA | |
| Levofloxacin | 4->16 | NA | NA | |
| Minocycline | 16-64 | NA | NA | |
| Tigecycline | 2-4 | NA | NA | |
Thirty-one percent of blaSHV genes were analyzed by pyrosequencing; and sequence information was derived only for nucleotides encoding residues at positions 35, 238, and 240. Therefore, these genes encode critical residues for the ESBL phenotype but have not been fully sequenced for unambiguous determination of the blaSHV derivative.
NA, not applicable (less than 10 isolates were analyzed).
The majority of the E. coli isolates were susceptible to piperacillin-tazobactam (MIC90 = 16 μg/ml); however, two isolates were resistant (MICs > 64 μg/ml). In contrast, 30% of the K. pneumoniae isolates were resistant to this combination (MIC90s > 64 μg/ml). Of the 21 P. mirabilis isolates in the collection, 3 were resistant to piperacillin-tazobactam (MICs > 64 μg/ml).
Tigecycline was active against the majority of the ESBL-, AmpC-, and KPC-producing E. coli and K. pneumoniae isolates, with 97% of the isolates being fully susceptible to 2 μg/ml of drug.
DISCUSSION
Multidrug-resistant gram-negative pathogens in general and E. coli and K. pneumoniae expressing ESBLs in particular are on the hit list of six microbial pathogens of concern, according to the Infectious Diseases Society of America (21, 42). The choice of inappropriate antibiotic therapy for patients with isolates harboring an ESBL is correlated with increased mortality in patients (24, 35, 45). The high level of expression of resistance to other classes of antibiotics (fluoroquinolones, aminoglycosides, trimethoprim-sulfamethoxazole, tetracyclines) by ESBL-encoding pathogens is an additional confounding factor to treatment (33, 38, 46).
Forty percent of all isolates examined in this study were isolated from patients in Eastern Europe, and the prevalence of ESBLs in that region was nearly 17%. Interestingly, 36% of the K. pneumoniae isolates collected in that region expressed ESBLs, whereas only 10% of the E. coli isolates did so. Similarly, in Latin America, the rate of ESBL carriage among the K. pneumoniae isolates (36%) was higher than that among the E. coli isolates (6%). This is in contrast to the situation in India, which showed relatively equal distributions of ESBLs among E. coli (32%) and K. pneumoniae (40%) isolates. In the remaining regions (where more than 20 isolates were collected), the prevalence rates of E. coli and K. pneumoniae isolates encoding ESBLs were 8.6% and 18%, respectively, in the Asia Pacific; 3% and 15%, respectively, in North America; and 6% and 12%, respectively, in Western Europe. The prevalence rates of ESBL producers among the P. mirabilis isolates were 7% in Eastern Europe, 10% in Latin America, and 2.2% in North America.
In this global analysis, the prevalence rates of ESBLs were 8.6%, 31.1%, and 6.3% among the E. coli, K. pneumoniae, and P. mirabilis isolates, respectively. In addition, 44% of the isolates (n = 119) harbored two or more ESBLs, with 3 of the isolates carrying the genes for five different ESBLs and 26 isolates carrying the genes for both ESBLs and AmpC or ESBLs and KPC β-lactamases in various configurations (Table 3). These percentages are comparable to those from recent global surveys for CAZ resistance; for example, ESBLs were found in 10.4% of the E. coli isolates, 14.7% of the K. pneumoniae isolates, and 2.8% of the P. mirabilis isolates in the MYSTIC program in 2006 (43). One caveat when those data are compared with the data from this study is that many of the strains in the present study were isolated in Eastern Europe (40%) and India (8.2%), whereas the centers participating in the MYSTIC program did not include sites in India and had a low representation in Eastern Europe.
Although two isolates in the collection were negative for all PCR targets tested, by isoelectric focusing analysis, one of the isolates expressed a β-lactamase with a pI of 9 and the other isolate had a β-lactamase with a pI of 7.6. These results suggest that our primer sets failed to cover the spectrum of bla genes carried by these isolates and that further investigation is warranted.
In agreement with recent trends (3, 12, 25, 26, 40), the most frequently identified ESBLs encoded by both E. coli and K. pneumoniae were members of the CTX-M family. CTX-M group 1 enzymes (CTX-M-3, CTX-M-15, CTX-M-22, CTX-M-28) were detected in 23% of the isolates, whereas CTX-M group 2 enzymes (CTX-M-2, CTX-M-5) were detected in 36% of the isolates. Fourteen percent of the isolates encoded both a CTX-M group 1 β-lactamase and a CTX-M group 2 β-lactamase. CTX-M-14, a CTX-M group 9 β-lactamase, was found in 1% of the isolates. It was not surprising, due to its worldwide distribution, that CTX-M-15 was the enzyme of the CTX-M family most frequently recovered (43%) in this survey (12, 31). CTX-M-15 and CTX-M-28, which accounted for 46% of all the CTX-M enzymes described in the study, are both members of the CTX-M-1 group and differ by a single amino acid substitution. These two derivatives were recovered from all geographic areas included in the study except North America. Considering that 50% of the isolates collected were from patients in Eastern Europe (42%) and India (8%), the high representation of CTX-M-15 from these areas is not unexpected (12, 13).
CTX-M-2 and CTX-M-5 were also prevalent in the study and were detected in 42% and 33% of the isolates, respectively, with 8% of the isolates encoding both of these derivatives of the CTX-M-2 group. Historically, enzymes of the CTX-M-2 group were mainly identified in South America and Japan (3, 12). In the present study, CTX-M-2 and CTX-M-5 were found in all regions sampled, including North America and Latin America; however, most were recovered from Eastern Europe and India. This is in contrast to the findings of a recent study by Ensor et al. (19), in which the only enzyme of the CTX-M class found in a survey of 130 isolates obtained from three centers in India was CTX-M-15. One explanation for this difference is that 75% of the isolates surveyed by Ensor et al. (19) were from urine samples, whereas in the present study, only 1.4% of the isolates were from patients with urinary tract infections, with the balance of the isolates being recovered from patients with nosocomial pneumonia, cSSSI, and cIAI.
Three of eight patient isolates from the United States were positive for CTX-M-type β-lactamase genes (CTX-M-2 and CTX-M-5 in K. pneumoniae and CTX-M-5 in one isolate of E. coli), and all three strains also harbored an SHV-type ESBL (SHV-5 or SHV-12) (Table 3). A report by Moland et al. in 2003 (29) described the first appearance of CTX-M-like ESBLs (CTX-M-1 group, CTX-M-9 group) in E. coli isolates in the United States, and a recent study by Lewis et al. (25) showed that CTX-M enzymes, specifically, CTX-M-15, have become the predominant ESBLs in the University Health System, San Antonio, TX. The difference in the CTX-M group may reflect the fact that the three isolates in the present study were isolated from hospitalized patients with cIAI or cSSSI, whereas 60 to 67% of the CTX-M-encoding isolates in the previous studies were recovered from urinary sources (25, 29). This is in keeping with the dramatic rise in the incidence of CTX-M ESBLs in the community as well as the nosocomial setting (3, 12, 32, 37, 38, 46).
Over 60% of isolates carried a blaSHV β-lactamase gene, more than half (59%) of which encoded ESBLs. For the 67 blaSHV ESBLs that were sequenced, 43% encoded SHV-5 and 55% encoded SHV-12, with 7.5% encoding both genes. SHV-5 and SHV-12 are the most widespread SHV class ESBLs described (34) and, accordingly, were found in all areas sampled. Thirty blaSHV ESBL genes were analyzed by pyrosequencing. This focused sequencing effort was directed at nucleotides encoding residues 238 and 240 to confirm the presence of substitutions at these residues, which are critical to the ESBL phenotype, as well as residue 35; however, since complete gene sequencing was not carried out for these isolates, precise determination of the blaSHV derivative was not possible. Non-ESBL SHV derivatives were identified in 42% of the isolates in the collection: SHV-1, SHV-11, SHV-28, SHV-33, and the recently described SHV-63 (M. Edelstein et al., GenBank accession number EU342351 [www.ncbi.nlm.nih.gov]).
ESBL-encoding isolates often coexpress resistance to fluoroquinolones, trimethoprim-sulfamethoxazole, tetracycline, and aminoglycosides and are thus often classified as multidrug-resistant pathogens (30, 33). The isolates in the current collection showed coresistance to tobramycin (76%), levofloxacin (42%), and minocycline (37.5%), with 34% of the isolates fulfilling the criteria for multidrug resistance. In the case of E. coli, 55% of the isolates were resistant to three classes of drugs.
There were four strains of K. pneumoniae that showed reduced susceptibility to imipenem, and three of those strains expressed either KPC-2 or KPC-3, as identified by sequence analysis. The fourth isolate did not appear to encode a class A carbapenemase, and further analysis of this isolate is ongoing. The remaining isolates were fully susceptible to imipenem (MIC90 = 1 μg/ml). To our knowledge, this is the first reporting of KPC β-lactamases in India and Ukraine.
The majority of the ESBL-producing E. coli and K. pneumoniae isolates were fully susceptible to tigecycline (MICs ≤ 2 μg/ml). However, eight K. pneumoniae isolates showed reduced susceptibility to tigecycline, which in earlier studies was correlated with the overexpression of the AcrAB efflux pump in this species (41). Not surprisingly, 52% of the P. mirabilis isolates in the study were resistant to tigecycline; the proposed mechanism for this resistance is upregulation of an efflux pump related to AcrAB (44).
In agreement with the findings of prior studies with smaller or less well defined strain collections, the present study shows that tigecycline activity is not compromised in ESBL-, AmpC-, or KPC-encoding E. coli and K. pneumoniae pathogens (2, 30). These results demonstrate the potential utility of tigecycline against these pathogens of great medical importance.
Acknowledgments
We thank Rosaria Camarda, Sreekala Mandiyan, and D. Tasha Weaver-Sands for technical assistance and Jan Kieleczawa and the Wyeth Core Sequencing Group for sequence analysis.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Babinchak, T., E. J. Ellis-Grosse, N. Dartois, G. M. Rose, and E. G. Loh. 2005. The efficacy and safety of tigecycline in the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin. Infect. Dis. 41:S354-S367. [DOI] [PubMed] [Google Scholar]
- 2.Biedenbach, D. J., M. L. Beach, and R. N. Jones. 2001. In vitro antimicrobial activity of GAR-936 tested against antibiotic resistant gram-positive blood stream infection isolates and strains producing extended-spectrum β-lactamases. Diagn. Microbiol. Infect. Dis. 40:173-177. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet, R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bou, G. 2005. CTX-M-β-lactamases: an update. Curr. Med. Chem. Anti-Infect. Agents 4:219-234. [Google Scholar]
- 5.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, P. A., S. Bratu, C. Urban, M. Visalli, N. Mariano, D. Landman, J. J. Rahal, S. Brooks, S. Cebular, and J. Quale. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55-60. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, P. A., C. E. Cherubin, V. Idemyor, B. A. Rasmussen, and K. Bush. 1994. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob. Agents Chemother. 38:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush, K. 2002. The impact of β-lactamases on the development of novel antimicrobial agents. Curr. Opin. Investig. Drugs 3:1284-1290. [PubMed] [Google Scholar]
- 11.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canton, R., and T. M. Coque. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 13.Canton, R., A. Novais, A. Valverde, E. Machado, L. Peixe, F. Baquero, and T. M. Coque. 2008. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14(Suppl. 1):144-153. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A7, 7th ed., vol. 26. Clinical and Laboratory Standards Institute, Wayne, PA.
- 15.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, M100-S17, 17th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 16.Datta, N., and P. Kontomichalou. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208:239-241. [DOI] [PubMed] [Google Scholar]
- 17.Dukart, G., N. Dartois, C. Cooper, H. Gandjini, and E. Ellis-Grosse. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-1450.
- 18.Ellis-Grosse, E., T. Babinchak, N. Dartois, G. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin structure infections: results of two double-blind phase 3 comparison studies with vancomycin/aztreonam. Clin. Infect. Dis. 41:S341-S353. [DOI] [PubMed] [Google Scholar]
- 19.Ensor, V. M., M. Shahid, J. T. Evans, and P. M. Hawkey. 2006. Occurrence, prevalence and genetic environment of CTX-M β-lactamases in Enterobacteriaceae from Indian hospitals. J. Antimicrob. Chemother. 58:1260-1263. [DOI] [PubMed] [Google Scholar]
- 20.Helfand, M. S., and R. A. Bonomo. 2003. β-Lactamases: a survey of protein diversity. Curr. Drug Targets Infect. Disord. 3:9-23. [DOI] [PubMed] [Google Scholar]
- 21.Infectious Diseases Society of America. July 2004, posting date. Bad bugs, no drugs: as antibiotic R&D stagnates, a public health crisis brews. Infectious Diseases Society of America, Arlington, VA.
- 22.Jones, C. H., M. Tuckman, A. Y. M. Howe, M. Orlowski, S. Mullen, K. Chan, and P. A. Bradford. 2006. Diagnostic PCR analysis of the occurrence of methicillin and tetracycline resistance genes among Staphylococcus aureus isolates from phase 3 clinical trials of tigecycline for complicated skin and skin structure infections. Antimicrob. Agents Chemother. 50:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeney, D., M. Tuckman, S. Mandiyan, P. Petersen, C. H. Jones, and P. A. Bradford. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr C1-51.
- 24.Kotapati, S., J. L. Kuti, C. H. Nightingale, and D. P. Nicolau. 2005. Clinical implications of extended spectrum β-lactamase (ESBL) producing Klebsiella species and Escherichia coli on cefepime effectiveness. J. Infect. 51:211-217. [DOI] [PubMed] [Google Scholar]
- 25.Lewis, J. S., II, M. Herrera, B. Wickes, J. E. Patterson, and J. H. Jorgensen. 2007. First report of the emergence of CTX-M-type extended-spectrum β-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob. Agents Chemother. 51:4015-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 27.Maroko, R., A. Cooper, G. Dukart, N. Dartois, and H. Gandjini. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-730.
- 28.Mathew, A., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 29.Moland, E. S., J. A. Black, A. Hossain, N. D. Hanson, K. S. Thomson, and S. Pottumarthy. 2003. Discovery of CTX-M-like extended-spectrum β-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob. Agents Chemother. 47:2382-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morosini, M. I., M. Garcia-Castillo, T. M. Coque, A. Valverde, A. Novais, E. Loza, F. Baquero, and R. Canton. 2006. Antibiotic coresistance in extended-spectrum-β-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob. Agents Chemother. 50:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolas-Chanoine, M. H., J. Blanco, V. Leflon-Guibout, R. Demarty, M. P. Alonso, M. M. Canica, Y. J. Park, J. P. Lavigne, J. Pitout, and J. R. Johnson. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273-281. [DOI] [PubMed] [Google Scholar]
- 32.Oteo, J., C. Navarro, E. Cercenado, A. Delgado-Iribarren, I. Wilhelmi, B. Orden, C. Garcia, S. Miguelanez, M. Perez-Vazquez, S. Garcia-Cobos, B. Aracil, V. Bautista, and J. Campos. 2006. Spread of Escherichia coli strains with high-level cefotaxime and ceftazidime resistance between the community, long-term care facilities, and hospital institutions. J. Clin. Microbiol. 44:2359-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paterson, D. L., K. M. Hujer, A. M. Hujer, B. Yeiser, M. D. Bonomo, L. B. Rice, and R. A. Bonomo. 2003. Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob. Agents Chemother. 47:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31-37. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Perez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitout, J. D., D. B. Gregson, D. L. Church, S. Elsayed, and K. B. Laupland. 2005. Community-wide outbreaks of clonally related CTX-M-14 β-lactamase-producing Escherichia coli strains in the Calgary health region. J. Clin. Microbiol. 43:2844-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitout, J. D., P. Nordmann, K. B. Laupland, and L. Poirel. 2005. Emergence of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) in the community. J. Antimicrob. Chemother. 56:52-59. [DOI] [PubMed] [Google Scholar]
- 39.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum β-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Bano, J., and M. D. Navarro. 2008. Extended-spectrum β-lactamases in ambulatory care: a clinical perspective. Clin. Microbiol. Infect. 14(Suppl. 1):104-110. [DOI] [PubMed] [Google Scholar]
- 41.Ruzin, A., M. A. Visalli, D. Keeney, and P. A. Bradford. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 49:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 43.Turner, P. J. 2008. Meropenem activity against European isolates: report on the MYSTIC (Meropenem Yearly Susceptibility Test Information Collection) 2006 results. Diagn. Microbiol. Infect. Dis. 60:185-192. [DOI] [PubMed] [Google Scholar]
- 44.Visalli, M. A., E. Murphy, S. J. Projan, and P. A. Bradford. 2003. AcrAB multidrug efflux pump is associated with reduced levels of susceptibility to tigecycline (GAR-936) in Proteus mirabilis. Antimicrob. Agents Chemother. 47:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong-Beringer, A., J. Hindler, M. Loeloff, A. M. Queenan, N. Lee, D. A. Pegues, J. P. Quinn, and K. Bush. 2002. Molecular correlation for the treatment outcomes in bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae with reduced susceptibility to ceftazidime. Clin. Infect. Dis. 34:135-146. [DOI] [PubMed] [Google Scholar]
- 46.Woodford, N., M. E. Ward, M. E. Kaufmann, J. Turton, E. J. Fagan, D. James, A. P. Johnson, R. Pike, M. Warner, T. Cheasty, A. Pearson, S. Harry, J. B. Leach, A. Loughrey, J. A. Lowes, R. E. Warren, and D. M. Livermore. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum β-lactamases in the UK. J. Antimicrob. Chemother. 54:735-743. [DOI] [PubMed] [Google Scholar]