Abstract
Increasing pneumococcal resistance to extended-spectrum cephalosporins warrants the search for novel agents with activity against such resistant strains. Ceftaroline, a parenteral cephalosporin currently in phase 3 clinical development, has demonstrated potent in vitro activity against resistant gram-positive organisms, including penicillin-resistant Streptococcus pneumoniae. In this study, the activity of ceftaroline was evaluated against highly cefotaxime-resistant isolates of pneumococci from the Active Bacterial Core surveillance program of the Centers for Disease Control and Prevention and against laboratory-derived cephalosporin-resistant mutants of S. pneumoniae. The MICs of ceftaroline and comparators were determined by broth microdilution. In total, 120 U.S. isolates of cefotaxime-resistant (MIC ≥ 4 μg/ml) S. pneumoniae were tested along with 18 laboratory-derived R6 strains with known penicillin-binding protein (PBP) mutations. Clinical isolates were characterized by multilocus sequence typing, and the DNAs of selected isolates were sequenced to identify mutations affecting pbp genes. Ceftaroline (MIC90 = 0.5 μg/ml) had greater in vitro activity than penicillin, cefotaxime, or ceftriaxone (MIC90 = 8 μg/ml for all comparators) against the set of highly cephalosporin-resistant clinical isolates of S. pneumoniae. Ceftaroline was also more active against the defined R6 PBP mutant strains, which suggests that ceftaroline can overcome common mechanisms of PBP-mediated cephalosporin resistance. These data indicate that ceftaroline has significant potency against S. pneumoniae strains resistant to existing parenteral cephalosporins and support its continued development for the treatment of infections caused by resistant S. pneumoniae strains.
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia (18, 35). While the introduction of pneumococcal conjugate vaccine led to a large decrease in the incidence of disease caused by resistant strains (21), the numbers of highly resistant pneumococcal strains not covered by the vaccine are now increasing (25); and S. pneumoniae strains resistant to penicillin, cephalosporins, macrolides, tetracyclines, and sulfonamides continue to be observed in the United States (2-4, 9, 13, 19).
Although the breakpoint for penicillin resistance for nonmeningeal isolates has recently been increased to 8 μg/ml (6), highly penicillin- and cephalosporin-resistant pneumococci are still emerging (25, 30), and the effectiveness of existing β-lactams in the treatment of such infections is unlikely to be successful, as determined on the basis of the pharmacokinetics and pharmacodynamics of existing agents (1, 27, 29). Ceftaroline is a novel parenteral cephalosporin with broad-spectrum in vitro antimicrobial activity against resistant gram-positive pathogens, including penicillin-resistant S. pneumoniae strains and methicillin-resistant Staphylococcus aureus strains (14, 28), as well as common gram-negative organisms.
The aim of the study described here was to evaluate the activity of ceftaroline against a panel of cefotaxime-resistant clinical isolates and laboratory mutants with defined penicillin-binding protein (PBP) mutations. Multilocus sequence typing (MLST) analysis was used to group the isolates; and the genes encoding PBPs 1A, 2B, and 2X from selected isolates were sequenced to characterize key mutations.
(A preliminary report of these results was presented in 2007 at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy in Chicago, IL.)
MATERIALS AND METHODS
Bacterial strains.
One hundred twenty invasive S. pneumoniae strains with cefotaxime MICs of ≥4 μg/ml that were collected as part of the Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance and Emerging Infections Programs Network from 2001 through 2006 were included. The strains were isolated from children and adults (age range, <1 to 96 years) in eight states. Eighteen strain R6 laboratory-derived mutants with defined pbp and murM gene mutations known to confer β-lactam resistance were also included in the study. These mutants were previously generated by transforming well-characterized antimicrobial-susceptible laboratory strain R6 with various combinations of pbp genes and the murM gene containing mutations known to confer resistance to β-lactam antimicrobials (11, 31, 32). Donor strains of pbp and murM genes were strain 3191, a representative of a Hungarian pneumococcal clone that was isolated during the period from 1997 to 1998 and that was found to have notably high levels of penicillin resistance (MIC, 16 μg/ml) and cefotaxime resistance (MIC, 4 μg/ml) (31, 32), and strain 72521, which was isolated in France and which has an amoxicillin MIC (8 μg/ml) higher than that of penicillin (MIC, 4 μg/ml) (11).
Antimicrobials tested.
Ceftaroline (lot M599-R1001) was supplied by Forest Laboratories, Inc. (New York, NY). The other antimicrobials used in the study were purchased from commercial sources.
MIC testing.
The MICs of ceftaroline, penicillin, ceftriaxone, and cefotaxime for all clinical strains and the R6 laboratory mutants were determined by the reference broth microdilution method, according to the guidelines of the Clinical and Laboratory Standards Institute (5, 6). In addition, MIC data (determined by broth microdilution) for amoxicillin and meropenem were available from the CDC database for the clinical isolates, and the MICs for amoxicillin (determined by Etest; AB Biodisk; Solna, Sweden) were available for the R6 mutants.
MLST.
MLST was performed with all clinical isolates by previously described methods (12, 15). The sequence types (STs) obtained were compared with those at the MLST website (http://spneumoniae.mlst.net). New alleles and allelic profiles were submitted to the MLST database for MLST assignment.
PBP sequencing.
On the basis of genotypic characterization of the 120 strains by MLST, 15 strains were selected for sequencing of the pbp1a, pbp2b, and pbp2x genes to identify mutations. Nine isolates were selected from each of the three major STs identified, and six isolates were selected from the remaining clonal complexes. For sequencing, the PCR amplification products were purified with the ExoSAP-IT reagent (USB Corp., Cleveland, OH). The nucleotide sequences of the penicillin-binding domains of PBPs 1A, 2B, and 2X were determined by sequencing with a series of oligonucleotides that were primed at intervals of ±300 nucleotides along each gene. Cycle sequencing was performed with a BigDye Terminator (version 1.1) kit (Applied Biosystems; Foster City, CA), and electrophoresis was carried out with an ABI 3100 automated sequence analyzer. The nucleotide and deduced amino acid sequences of PBPs 1A, 2B, and 2X were aligned by using the MegAlign program (DNAStar, Madison, WI) and compared with the published sequence of penicillin-susceptible S. pneumoniae strain R6 (23).
RESULTS
Antimicrobial activity of ceftaroline.
Ceftaroline was the most active agent tested against the 120 clinical isolates of cefotaxime-resistant S. pneumoniae, with MICs ranging from 0.125 μg/ml to 2 μg/ml; the MIC90 was 0.5 μg/ml (Table 1). The single isolate of S. pneumoniae with a ceftaroline MIC of 2 μg/ml was highly resistant to cefotaxime and ceftriaxone (MICs ≥ 16 μg/ml for both agents). Overall, ceftaroline was more than 16-fold more active in vitro than cefotaxime, penicillin, ceftriaxone, amoxicillin, and meropenem.
TABLE 1.
MIC ranges, MIC50s, and MIC90s for cefotaxime, penicillin, ceftriaxone, ceftaroline, amoxicillin, and meropenem against 120 clinical isolates
| Antimicrobial | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Cefotaxime | 4-≥16 | 8 | 8 |
| Penicillin | 0.5-16 | 8 | 8 |
| Ceftriaxone | 2-≥16 | 8 | 8 |
| Ceftaroline | 0.125-2 | 0.5 | 0.5 |
| Amoxicillin | 0.25->8 | 8 | >8 |
| Meropenem | 0.12-2 | 1 | 2 |
The results in Table 2 (11, 31, 32) show that ceftaroline also exhibited potent activity against 18 laboratory strains with defined PBP and murM mutations known to confer resistance to β-lactams. The MICs of ceftaroline for these isolates ranged from 0.015 μg/ml to 0.25 μg/ml; the MIC90 was 0.03 μg/ml, whereas the MIC90s of penicillin, ceftriaxone, and amoxicillin were ≥4 μg/ml.
TABLE 2.
MICs of cefotaxime, penicillin, ceftriaxone, ceftaroline, and amoxicillin for 18 R6 mutants
| R6 mutanta | MIC (μg/ml)b
|
||||
|---|---|---|---|---|---|
| CTX | PEN | CRO | CPT | AMX | |
| R6 (parental strain) | 0.03 | 0.015 | 0.03 | ≤0.015 | ≤0.015 |
| 3191 (donor strain) | 4 | 16 | 8 | 0.25 | 8 |
| 72521 (donor strain) | 2 | 4 | 4 | 0.25 | 8 |
| R6 2x/3191 | 0.03 | 2 | 2 | 0.125 | 0.047 |
| R6 2b/1a/mur/3191 | 0.03 | 0.03 | 0.06 | ≤0.015 | 0.023 |
| R6 2x/72521 | 0.06 | 0.5 | 0.5 | 0.06 | ≤0.015 |
| R6 2x/1a/3191 | 0.06 | 2 | 2 | 0.125 | 0.032 |
| R6 2x/2b/72521 | 0.5 | 0.5 | 1 | 0.06 | 0.094 |
| R6 2x/2b/3191 | 0.5 | 1 | 1 | 0.125 | 0.19 |
| R6 2x/3191 + 2b/72521 | 1 | 2 | 2 | 0.125 | 0.19 |
| R6 2x/2b/1a/72521 | 2 | 0.5 | 0.5 | 0.06 | 0.5 |
| R6 2x/2b/72521 + 1a/3191 | 2 | 1 | 2 | 0.125 | 0.75 |
| R6 2x/2b/1a/gDNA/72521 | 2 | 1 | 0.5 | 0.125 | 1 |
| R6 2x/2b/1a/72521 + gDNA/3191 | 2 | 8 | 2 | 0.25 | 1 |
| R6 2x/2b/1a/72521 + mur/3191 | 2 | 1 | 1 | 0.06 | 1 |
| R6 2x/2b/3191 + 1a/72521 + mur/3191 | 2 | 8 | 4 | 0.125 | 1.5 |
| R6 2x/2b/1a/72521 + 1a/3191 + gDNA/72521 | 4 | 2 | 2 | 0.125 | 1.5 |
| R6 2x/2b/72521 + 1a/3191 + mur/72521 | 4 | 2 | 4 | 0.25 | 3 |
| R6 2x/2b/1a/3191 + mur/72521 | 16 | 8 | 8 | 0.25 | 4 |
| R6 2x/2b/1a/3191 | 16 | 4 | 4 | 0.125 | 4 |
Isogenic R6 transformants containing known pbp and murM mutations were generated from previous studies (11, 31, 32) by transforming parental strain R6 with various combinations of the pbp1a, pbp2b, pbp2x, and murM genes from donor strains 3191 and 72521; 2x, pbp2x; 2b, pbp2b; 1a, pbp1a; mur, murM; gDNA, genomic DNA.
AMX, amoxicillin; CPT, ceftaroline; CRO, ceftriaxone; CTX, cefotaxime; PEN, penicillin.
MLST.
MLST identified 31 STs associated with the 120 strains characterized. Three STs accounted for the majority of strains (57.8%): ST320 (n = 41), ST13 (n = 14), and ST37 (n = 14).
DNA sequencing of penicillin-binding domains.
Irrespective of the genetic background, as determined by the MLST type (Fig. 1) (23), the 15 clinical isolates selected for sequencing had similar constellations of mutations associated with resistance in their PBP 1A, 2B, and 2X genes. Isolate 2192 (MIC = 8 μg/ml for penicillin, cefotaxime, and ceftriaxone) had only single amino acid substitutions affecting PBP 2X and PBP 1A, and it remains possible that mutations in other genes affect β-lactam resistance in this strain.
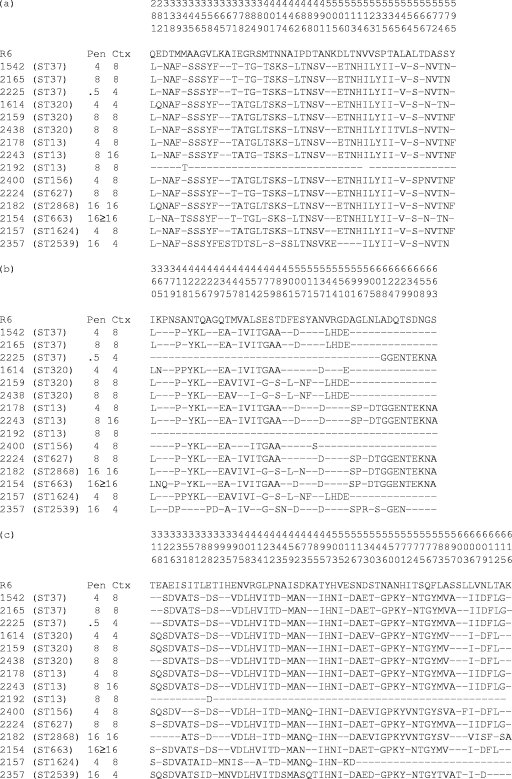
FIG. 1.
Deduced amino acid sequences encoded by the pbp2x (a), pbp2b (b), and pbp1a (c) genes. Only those amino acids that differ from the sequences of strain R6 are shown. Dashes indicate residues identical to those of R6. Amino acids are numbered according to their positions in the gene (23).
DNA sequence analysis of the PBP 1A, 2B, and 2X penicillin-binding domains from the isolates revealed extensive sequence divergence compared with the sequence of strain R6. Within the STMK motif of the PBP 2X penicillin-binding domain, isolates had a Thr338Ala substitution (Fig. 1a). Met339Phe and Met400Thr were common substitutions; there were no Thr550 or Gln552 mutations associated with cephalosporin resistance in these isolates. In the PBP 2B penicillin-binding domain, a Thr445Ala substitution (adjacent to the S442SN conserved motif) was present in most of the isolates (Fig. 1b). Numerous substitutions in close proximity to the PBP 2B active-site K614TG motif were identified. In the PBP 1A penicillin-binding domain, a substitution of Thr371 to Ser or Ala and the four consecutive substitutions at residues 574 to 577 were present in the majority of isolates (Fig. 1c). All isolates with the exception of isolate 2225 had amoxicillin MICs of ≥2 μg/ml; isolate 2225 had an MIC of 0.25 μg/ml.
DISCUSSION
Increases in S. pneumoniae resistance to cephalosporins and other β-lactam antimicrobial agents have been observed since 2005 in the United States (2-4). In view of pharmacokinetic and pharmacodynamic considerations, there may be clinical implications of very-high-level penicillin and cephalosporin resistance for patients with pneumonia. On the basis of the pharmacodynamic principle of achieving a free drug concentration greater than the MIC90 for 40% to 50% of the length of the dosing interval, the clinical failure breakpoint is 2 μg/ml for ceftriaxone given 1 g daily or cefotaxime 1 g given 3 times daily (17). The highly cephalosporin-resistant strains analyzed in this study would thus raise concern over potential clinical failure for patients with pneumonia.
In the present study, ceftaroline was more active in vitro against the set of highly cephalosporin-resistant clinical isolates of S. pneumoniae than either penicillin, ceftriaxone, or amoxicillin. Carbapenems are recognized to have activity against a broad range of gram-positive cocci, including penicillin-resistant S. pneumoniae, and meropenem was more active against the strains included in this study. Ceftaroline was also more active against laboratory R6 mutants containing pbp mutations known to confer resistance to other β-lactam agents. These observations suggest that ceftaroline could be clinically effective for the treatment of infections caused by S. pneumoniae strains resistant to the currently available antimicrobials. A clinical situation involving S. pneumoniae in which ceftaroline may be of value is meningitis. Current treatment guidelines advocate the use of combination therapy because of the risk of failure imposed by cephalosporin-resistant pneumococci (24). If cerebrospinal fluid penetration studies show adequate penetration of ceftaroline, then the in vitro activity demonstrated against cephalosporin-resistant strains warrants clinical trials on the use of ceftaroline as monotherapy for meningitis.
Resistance to β-lactam antimicrobial agents in S. pneumoniae is mediated by successive alterations in essential PBPs, with specific mutations being expressed in combination with mutations in other genes (16, 22, 37). The final resistance phenotype is therefore dependent on the collective actions of multiple PBPs. Irrespective of the genetic background, the 15 clinical isolates selected for sequence analysis had similar mutations in genes encoding PBP 1A, 2B, and 2X. Interestingly, isolate 2192, which exhibited high-level resistance to penicillin and cefotaxime, had almost no alterations in the sequenced portions of the genes for PBP 1A, 2B, and 2X. Resistance in this strain may be associated with changes in other genes, such as pbp2A, pbp1B, or murM (10, 34), or may be due to a non-PBP-mediated mechanism (31).
Several of the PBP mutations observed in this study are known to be important in β-lactam resistance. The Thr338Ala substitution in the STMK motif observed in the PBP 2X penicillin-binding domains of the clinical isolates used in this study has been shown to be important for β-lactam resistance (26). In addition, the amino acid mutations Met339Phe and Met400Thr, which were common substitutions in these isolates, have been shown to be important substitutions for conferring high-level cefotaxime resistance (MICs ≥ 2 μg/ml) (7).
The previously documented Thr445Ala substitution (adjacent to the S442SN conserved motif) in the PBP 2B penicillin-binding domain was present in most of the isolates in the current study; the importance of this conserved motif in interacting with penicillin has been noted (8). PBP 2B is not considered a target for extended-spectrum cephalosporins, but it appears to be important in the development of amoxicillin resistance; this is supported by the observation in the present study that all but one isolate had amoxicillin MICs of ≥2 μg/ml. Mutations in the region from residues 590 to 676 of PBP 2B, similar to the substitutions near the K614TG motif reported in this study, have been shown to be associated with strains with higher amoxicillin MICs than penicillin MICs (11, 20).
The Thr371-to-Ser or -Ala substitutions and the four consecutive substitutions at residues 574 to 577 that were present in the PBP 1A penicillin-binding domains in the majority of the isolates in this study have been documented previously, and the contribution of these residues to the development of penicillin resistance (MICs ≥ 0.25 μg/ml) has been confirmed (33). The substitution of Ala for Thr at residue 371 is homologous to the Thr338Ala substitution in PBP 2X (within the Ser337-Thr-Met-Lys conserved motif).
The activity of ceftaroline against the set of defined R6 PBP mutants in this study was superior to the activities of the comparator agents and suggests that ceftaroline may overcome common mechanisms of β-lactam resistance that are mediated by target modification. It has recently been shown that the activity of ceftaroline against methicillin-resistant S. aureus is associated with conformational changes to the active site of PBP 2a that are promoted by ceftaroline binding to an allosteric site (36); it is not clear if similar interactions occur between ceftaroline and PBPs in S. pneumoniae. The present study demonstrated that ceftaroline has significant potency against pneumococcal isolates highly resistant to existing parenteral cephalosporins. These findings support the continued development of ceftaroline for the treatment of infections caused by resistant S. pneumoniae strains.
Acknowledgments
We are indebted to all of the hospitals and laboratories participating in the CDC's Emerging Infections Programs Network and Active Bacterial Core surveillance system. We thank the CDC's Streptococcal Laboratory for antibiotic susceptibility testing and support with MLST. We acknowledge Tressa M. Chung of Scientific Therapeutics Information, Inc., Springfield, NJ, and thank her for providing editorial assistance in the development of the manuscript.
We acknowledge the use of the pneumococcal MLST database (Imperial College London), which is funded by the Wellcome Trust.
Funding for this study and for editorial assistance was provided by Forest Laboratories, Inc.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Cafini, F., L. Aguilar, L. Alou, M. J. Giménez, D. Sevillano, M. Torrico, N. González, J. J. Granizo, J. E. Martín-Herrero, and J. Prieto. 2008. Cidal activity of oral third-generation cephalosporins against Streptococcus pneumoniae in relation to cefotaxime intrinsic activity. Eur. J. Clin. Microbiol. Infect. Dis. 27:679-683. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2007. Active Bacterial Core Surveillance (ABCs) report, Emerging Infections Program Network, Streptococcus pneumoniae, 2006. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/ncidod/dbmd/abcs/survreports/spneu06.pdf.
- 3.Centers for Disease Control and Prevention. 2006. Active Bacterial Core Surveillance (ABCs) report, Emerging Infections Program Network, Streptococcus pneumoniae, 2005. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/ncidod/dbmd/abcs/survreports/spneu05.pdf.
- 4.Centers for Disease Control and Prevention. 2005. Active Bacterial Core Surveillance (ABCs) report, Emerging Infections Program Network, Streptococcus pneumoniae, 2004. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/ncidod/dbmd/abcs/survreports/spneu04.pdf.
- 5.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed.; approved standard, M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing, 18th informational supplement, M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Coffey, T. J., M. Daniels, L. L. McDougal, C. G. Dowson, F. C. Tenover, and B. G. Spratt. 1995. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 39:1306-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowson, C. G., A. Hutchison, and B. G. Spratt. 1989. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 17:7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dueger, E. L., E. J. Asturias, J. Matheu, R. Gordillo, O. Torres, and N. Halsey. 2008. Increasing penicillin and trimethoprim-sulfamethoxazole resistance in nasopharyngeal Streptococcus pneumoniae isolates from Guatemalan children, 2001-2006. Int. J. Infect. Dis. 12:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Plessis, M., A. M. Smith, and K. P. Klugman. 2000. Analysis of penicillin-binding protein lb and 2a genes from Streptococcus pneumoniae. Microb. Drug Resist. 6:127-131. [DOI] [PubMed] [Google Scholar]
- 11.du Plessis, M., E. Bingen, and K. P. Klugman. 2002. Analysis of penicillin-binding protein genes of clinical isolates of Streptococcus pneumoniae with reduced susceptibility to amoxicillin. Antimicrob. Agents Chemother. 46:2349-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 13.Felmingham, D., A. R. White, M. R. Jacobs, P. C. Appelbaum, J. Poupard, L. A. Miller, and R. N. Grüneberg. 2005. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 56(Suppl. 2):ii3-ii21. [DOI] [PubMed] [Google Scholar]
- 14.Ge, Y., D. Biek, G. H. Talbot, and D. F. Sahm. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertz, R. E., Jr., M. C. McEllistrem, D. J. Boxrud, Z. Li, V. Sakota, T. A. Thompson, R. R. Facklam, J. M. Besser, L. H. Harrison, C. G. Whitney, and B. Beall. 2003. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J. Clin. Microbiol. 41:4194-4216. (Author's correction, 43:1013, 2005.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakenbeck, R., M. Tarpay, and A. Tomasz. 1980. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 17:364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs, M. R. 2001. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589-596. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, M. R., C. E. Good, B. Beall, S. Bajaksouzian, A. R. Windau, and C. G. Whitney. 2008. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae in Cleveland: a quarter-century of experience. J. Clin. Microbiol. 46:982-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins, S. G., S. D. Brown, and D. J. Farrell. 2008. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US Years 1-4. Ann. Clin. Microbiol. Antimicrob. 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosowska, K., M. R. Jacobs, S. Bajaksouzian, L. Koeth, and P. C. Appelbaum. 2004. Alterations of penicillin-binding proteins 1A, 2X, and 2B in Streptococcus pneumoniae isolates for which amoxicillin MICs are higher than penicillin MICs. Antimicrob. Agents Chemother. 48:4020-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, C. G. Whitney, and Active Bacterial Core Surveillance of the Emerging Infections Program Network. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455-1463. (Author's correction, 355:638.) [DOI] [PubMed] [Google Scholar]
- 22.Laible, G., B. G. Spratt, and R. Hakenbeck. 1991. Interspecies recombinational events during the evolution of altered PBP 2X genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993-2002. [DOI] [PubMed] [Google Scholar]
- 23.Martin, C., T. Briese, and R. Hakenbeck. 1992. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding proteins 1A and 1B. J. Bacteriol. 174:4517-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMaster, P., P. McIntyre, R. Gilmour, L. Gilbert, A. Kakakios, and C. Mellis. 2002. The emergence of resistant pneumococcal meningitis—implications for empiric therapy. Arch. Dis. Child. 87:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, M. R., R. E. Gertz, Jr., R. L. Woodbury, G. A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L. H. Harrison, J. L. Hadler, N. M. Bennett, A. R. Thomas, L. McGee, T. Pilishvili, A. B. Brueggemann, C. G. Whitney, J. H. Jorgensen, and B. Beall. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016-1027. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz, R., C. G. Dowson, M. Daniels, T. J. Coffey, C. Martin, R. Hakenbeck, and B. G. Spratt. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 6:2461-2465. [DOI] [PubMed] [Google Scholar]
- 27.Odenholt, I., I. Gustafsson, E. Löwdin, and O. Cars. 2003. Suboptimal antibiotic dosage as a risk factor for selection of penicillin-resistant Streptococcus pneumoniae: in vitro kinetic model. Antimicrob. Agents Chemother. 47:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sader, H. S., T. R. Fritsche, K. Kaniga, Y. Ge, and R. N. Jones. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sader, H. S., M. R. Jacobs, and T. R. Fritsche. 2007. Review of the spectrum and potency of orally administered cephalosporins and amoxicillin/clavulanate. Diagn. Microbiol. Infect. Dis. 57(3 Suppl.):5S-12S. [DOI] [PubMed] [Google Scholar]
- 30.Schrag, S. J., L. McGee, C. G. Whitney, B. Beall, A. S. Craig, M. E. Choate, J. H. Jorgensen, R. R. Facklam, K. P. Klugman, and Active Bacterial Core Surveillance Team. 2004. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob. Agents Chemother. 48:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, A. M., and K. P. Klugman. 2001. Alterations in MurM, a cell wall muropeptide branching enzyme, increase high-level penicillin and cephalosporin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2393-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, A. M., and K. P. Klugman. 2000. Non-penicillin-binding protein mediated high-level penicillin and cephalosporin resistance in a Hungarian clone of Streptococcus pneumoniae. Microb. Drug Resist. 6:105-110. [DOI] [PubMed] [Google Scholar]
- 33.Smith, A. M., and K. P. Klugman. 1998. Alterations in PBP 1A essential for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, A. M., C. Feldman, O. Massidda, K. McCarthy, D. Ndiweni, and K. P. Klugman. 2005. Altered PBP 2A and its role in the development of penicillin, cefotaxime, and ceftriaxone resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 49:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song, J.-H., W. S. Oh, C.-I. Kang, D. R. Chung, K. R. Peck, K. S. Ko, J. S. Yeom, C. K. Kim, S. W. Kim, H.-H. Chang, Y.-S. Kim, S.-I. Jung, Z. Tong, Q. Wang, S.-G. Huang, J.-W. Liu, M. K. Lalitha, B.-H. Tan, P. H. Van, C. C. Carlos, and T. So. 2008. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents 31:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villegas-Estrada, A., M. Lee, D. Hesek, S. B. Vakulenko, and S. Mobashery. 2008. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two novel anti-MRSA β-lactam antibiotics. J. Am. Chem. Soc. 130:9212-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zighelboim, S., and A. Tomasz. 1980. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 17:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]