Abstract
Aspergillus fumigatus must be able to properly form hyphae and maintain cell wall integrity in order to establish invasive disease. Ras proteins and calcineurin each have been implicated as having roles in these processes. Here, we further delineate the roles of calcineurin and Ras activity in cell wall biosynthesis and hyphal morphology using genetic and pharmacologic tools. Strains deleted for three genes encoding proteins of these pathways, rasA (the Ras protein), cnaA (calcineurin), or crzA (the zinc finger transcription factor downstream of calcineurin), all displayed decreased cell wall 1,3-β-d-glucan content. Echinocandin treatment further decreased the levels of 1,3-β-d-glucan for all strains tested yet also partially corrected the hyphal growth defect of the ΔrasA strain. The inhibition of glucan synthesis caused an increase in chitin content for wild-type, dominant-active rasA, and ΔrasA strains. However, this important compensatory response was diminished in the calcineurin pathway mutants (ΔcnaA and ΔcrzA). Taken together, our data suggest that the Ras and calcineurin pathways act in parallel to regulate cell wall formation and hyphal growth. Additionally, the calcineurin pathway elements cnaA and crzA play a major role in proper chitin and glucan incorporation into the A. fumigatus cell wall.
The composition, architecture, and biosynthetic mechanisms of the fungal cell wall are critically important to understanding invasive aspergillosis pathogenesis (14). Because of its essential role in cell protection and hyphal growth, the inhibition of the cell wall is an attractive antifungal target against Aspergillus fumigatus infections (16). Caspofungin, micafungin, and anidulafungin are echinocandin antifungal agents that reduce total 1,3-β-d-glucan content by the inhibition of glucan synthase activity, resulting in the formation of blunted hyphae in A. fumigatus (2). Though not employed clinically, nikkomycin Z is a chitin synthase inhibitor shown to have in vitro activity against many fungi, including A. fumigatus (7). The treatment of Aspergillus hyphae with nikkomycin Z yields swollen, balloon-like segments interspersed along normal hyphal strands, a phenotype reminiscent of chitin synthase deletion (5, 25).
Interestingly, the inhibition of the synthesis of one type of cell wall polysaccharide can lead to an increase in another, a presumably protective mechanism. For example, in Candida albicans, the inhibition of 1,3-β-d-glucan synthesis by caspofungin treatment leads to a compensatory increase in chitin, allowing the cell to escape lethality (27, 29). In addition, this compensatory increase in chitin is at least partially regulated by the C. albicans calcineurin pathway (30). Interestingly, the dual inhibition of glucan synthesis (via caspofungin treatment) and calcineurin activity (via FK506 or cyclosporine A treatment) leads to severely attenuated growth in A. fumigatus (12, 26). Although these data suggest that similar mechanisms exist, the compensatory relationship of chitin and glucan synthesis has not been characterized in A. fumigatus.
Ras (RasA) and calcineurin (CnaA) are highly conserved signaling proteins that are necessary for proper hyphal growth and response to cell wall stress in A. fumigatus (5, 6, 24, 25). The deletion of cnaA causes the formation of blunted hyphae, with reduced radial growth and decreased cell wall 1,3-β-d-glucan content (24, 25). The deletion of the transcription factor crzA leads to germination, cell wall content levels, and asexual development that are similar to defects seen in the ΔcnaA strain (4). The ΔrasA mutant similarly displays a reduced radial growth rate, with wide, blunted hyphae that continually change growth axes, thereby exhibiting a defect in polarized growth (5). Another mutant expressing a constitutively active form of rasA, dominant-active rasA (designated DArasA), displays delayed conidiophore development and the increased development of aerial hyphae (6). Both RasA and CnaA signaling proteins play roles in the proper germination and asexual development of this important human pathogen (5, 6, 24). By the targeted pharmacologic inhibition of cell wall synthesis in the ΔcnaA, ΔcrzA, ΔrasA, and DArasA mutants, we identify the RasA protein as being important for echinocandin sensitivity and show that the calcineurin pathway regulates the compensatory chitin response to glucan inhibition in A. fumigatus. Through these studies, we begin to discern the shared and unique roles of these two important signaling pathways in A. fumigatus.
MATERIALS AND METHODS
A. fumigatus strains and growth conditions.
Strains used for this study are listed in Table 1. H237 (the parental strain for ΔrasA and DArasA) and Af293 (the parental strain for ΔcnaA and ΔcrzA) were employed as the wild-type strains. All strains were maintained on glucose minimal medium (GMM) as previously described (24). Nikkomycin Z, caspofungin, and FK506 were dissolved in sterile water and stored at −20°C until use. Micafungin was dissolved in sterile water and stored at 4°C. Anidulafungin was dissolved in 20% (wt/vol) dehydrated alcohol in water, with further dilutions made using sterile water, and was stored at 4°C. All liquid medium assays were performed in RPMI 1640 prepared according to CLSI standards (19).
TABLE 1.
Strains used for this study
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed according to CLSI standards and as previously reported (25, 26). Morphological effects and growth inhibition were recorded after treatment with caspofungin (0.012 to 32 μg/ml), micafungin (0.001 to 4 μg/ml), anidulafungin (0.001 to 4 μg/ml), nikkomycin Z (0.25 to 32 μg/ml), or FK506 (0.07 to 160 ng/ml), and results are reported as the minimum effective concentration (MEC) of drug treatment (25). The MEC was defined as the lowest concentration of drug that produced significantly abnormal hyphal growth (13). For cotreatment, caspofungin (1 μg/ml) was administered at a constant concentration, while nikkomycin Z was administered in ascending doses (0.15 to 16 μg/ml). The synergistic relationship between the two cell wall inhibitors during cotreatment was calculated using the fractional inhibitory concentration index (FICI), with synergy defined as an FICI of ≤0.5 (21). All strains were incubated in the presence or absence of drug for 48 h at 37°C, and all experiments were performed in triplicate.
Quantification of 1,3-β-d-glucan.
1,3-β-d-Glucan content was examined using the aniline blue assay, as previously described (4, 10, 25). Briefly, 106 conidia/ml from each strain was inoculated into RPMI 1640 medium and incubated in the presence or absence of drug at the indicated concentrations for 48 h at 37°C and 250 rpm. Hyphae were harvested by centrifugation, washed in 0.1 M NaOH, and lyophilized for 16 h. Five milligrams of lyophilized hyphal mat from each strain was resuspended in 250 μl of 1 M NaOH and sonicated for 30 s, followed by incubation at 52°C for 30 min. After incubation, three 50-μl volumes from each sample were aliquoted into the wells of a masked, 96-well fluorescence plate. A volume of 185 μl of aniline blue mix (0.067% aniline blue, 0.35N HCl, 0.98 M glycine-NaOH, pH 9.5) was added to each well, and the plate was incubated for an additional 30 min at 52°C. The 96-well plate then was allowed to cool at room temperature for 30 min, and fluorescence readings were acquired on a SPECTRAmax M2 fluorimeter (Molecular Devices, Sunnyvale, CA) at 405-nm excitation and 460-nm emission. To normalize values, a standard curve was created using curdlan, a β-1,3-glucan analog (Sigma, St. Louis, MO). Values are expressed as the percent change in relative fluorescence units per milligram of mycelial tissue, using the nontreated wild type as a control. Final results represent the averages from five independent experiments. Statistical analyses were performed in Excel using the two-sample Student's t test assuming equal variances.
Quantification of cell wall chitin.
The growth, drug treatment, and tissue harvesting of strains used for chitin assays were performed as described for 1,3-β-d-glucan measurement. Chitin assays were performed based on a slightly modified protocol of Lehmann et al. (15). Briefly, 5 mg of lyophilized hyphal mat was resuspended in 3 ml of saturated KOH and incubated at 130°C for 1 h. After being cooled to room temperature, 8 ml of ice-cold 75% ethanol was added. The resulting suspension was vortexed until the KOH and ethanol formed a single phase and then was incubated in an ice bath for 15 min. A volume of 300 μl of a 13.3% (wt/vol) Celtite 545 (C212; Fisher Chemicals) suspension was added, and the tubes were centrifuged at 1,500 × g for 5 min at 2°C. Pellets were washed once with 10 ml of ice-cold 40% ethanol, followed by two washes in 10 ml ice-cold water, with centrifuging as before. Standards consisting of a water-only sample and a known-glucosamine (10 μg/ml) sample were used in all subsequent steps. The final pellet from each sample was resuspended in 0.5 ml of water and 0.5 ml of 5% (wt/vol) NaNO2, and 0.5 ml of 5% (wt/vol) KHSO4 was added to each tube. For controls, 0.2 ml of both NaNO2 and KHSO4 were added. All tubes were gently mixed three times for a 15-min period, followed by centrifugation at 1,500 × g for 2 min at 2°C. Aliquots (150 μl each) were removed from each supernatant and added to 450 μl of water in a new tube. A volume of 0.2 ml of 12.5% (wt/vol) NH4 sulfamate was added to each tube and mixed vigorously each minute for 5 min, followed by the addition of 0.2 ml of 3-methylbenzthiazolinone-2-hydrazone (5 mg/ml) and incubation at 130°C for 3 min. Tubes were allowed to cool, and 0.2 ml of 0.83% (wt/vol) ferric chloride was added, followed by incubation at room temperature for 25 min. Absorbance was read at 650 nm on a Nanodrop ND-1000 spectrophotometer. Concentrations of glucosamine levels in the samples were calculated as follows: [(A650 unknown − A650 water) × 10 μg/ml]/(A650 standard − A650 water). Chitin levels were reported as glucosamine equivalents (in micrograms per milliliter). Final results represent the averages from three independent experiments. Statistical analyses were performed in Excel using the two-sample Student's t test assuming equal variances.
Fluorescence staining and microscopy.
To assess the morphological effects of drug treatment, the conidia of each strain were grown as stated for antifungal susceptibility testing in the presence or absence of caspofungin for 48 h at 37°C. Bright-field photomicrographs were acquired on a Nikon inverted microscope equipped with a Nikon D50 digital camera.
Calcofluor white staining for the visualization of chitin was performed by first inoculating 103 conidia/ml from each strain onto coverslips immersed in RPMI 1640 medium. Cultures were incubated in the presence or absence of the indicated amount of drug for 16 h at 37°C. Coverslips were removed, rinsed briefly in sterile water, and inverted onto a 200-μl drop of calcofluor white staining solution (18909; Sigma) for 5 min at room temperature. Coverslips then were rinsed twice for 10 min in sterile water and mounted in Cytoseal 60 mounting medium (catalog no. 8310-4; Richard-Allan Scientific). To ensure that the calcofluor white staining of different samples was appropriately analyzed, the ultraviolet light exposure time for each sample was set for the wild-type untreated control at 25 ms, and all subsequent strains were exposed for the same length of time.
RESULTS
RasA mutants display altered sensitivity to echinocandins.
We previously showed that calcineurin pathway mutants display increased sensitivity to caspofungin treatment (4, 25). However, a possible role for Ras in sensitivity to echinocandins has not been explored. To further delineate a role for the calcineurin and Ras pathways in glucan synthesis and sensitivity to echinocandin antifungals, we first utilized caspofungin and two other enchinocadins, micafungin and anidulafungin, in antifungal susceptibility testing. The ΔcnaA and ΔcrzA strains displayed increased sensitivity to echinocandins compared to that of the wild-type strain (Table 2). In contrast, the ΔrasA mutant displayed greatly reduced sensitivity to echinocandin treatment (Table 2). When incubated with caspofungin (1 μg/ml), micafungin (1 μg/ml), or anidulafungin (1 μg/ml), wild-type hyphae were blunted and ΔcnaA hyphae terminated at the germling stage (Fig. 1 and data not shown). In contrast, the ΔrasA mutant grown in the presence of echinocandins displayed longer hyphae with fewer branches (Fig. 1 and data not shown). Similarly to the case for the ΔcnaA mutant, the echinocandin MECs for the DArasA strain were decreased compared to those of the wild type (Table 2).
TABLE 2.
Antifungal susceptibility testing
| Strain | MECa (μg/ml)
|
FICI of NZ and CA | ||||
|---|---|---|---|---|---|---|
| MA | AN | CA | FK506 | NZ | ||
| Af293 | 0.125 | 0.0625 | 1 | 0.02 | 2 | 0.09 |
| ΔcnaA | 0.0039 | 0.0039 | 0.25 | >80 | 0.125 | 0.19 |
| ΔcnaA + cnaA | 0.125 | 0.0625 | 1 | 0.02 | 2 | 0.09 |
| ΔcrzA | 0.0312 | 0.0156 | 0.5 | 0.01 | 0.5 | 0.13 |
| ΔcrzA + crzA | 0.0625 | 0.125 | 1 | 0.02 | 4 | 0.16 |
| H237 | 0.125 | 0.0625 | 1 | 0.02 | 4 | 0.16 |
| ΔrasA | >4 | 2 | 8 | 0.001 | 0.5 | 0.03 |
| ΔrasA + rasA | 0.125 | 0.125 | 1 | 0.02 | 2 | 0.09 |
| DArasA | 0.0156 | 0.0156 | 0.25 | 0.01 | 4 | 0.5 |
MA, micafungin; AN, anidulafungin; CA, caspofungin; and NZ, nikkomycin Z.
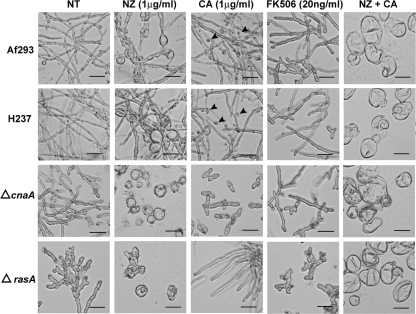
FIG. 1.
Cell wall inhibition, coupled with the loss of Ras or calcineurin signaling, causes morphological aberrancies. Shown is the no treatment (NT) and the nikkomycin Z (NZ), caspofungin (CA), FK506, and nikkomycin Z plus caspofungin (NZ + CA) treatment of the wild-type (Af293 and H237), ΔcnaA, and ΔrasA strains in RPMI 1640 medium at 37°C. Conidia from each strain were incubated in the presence or absence of drug for 48 h, followed by microscopic examination. Arrowheads denote hyphal tips that have swollen or lysed due to caspofungin treatment. Representative fields are demonstrated. Scale bar, 40 μm.
The ΔcnaA, ΔcrzA, and ΔrasA strains also displayed increased sensitivity to nikkomycin Z (Table 2) compared to that of the wild-type strains. As previously reported (5, 25), the ΔrasA and ΔcnaA strains formed large, balloon-like cells with greatly decreased hyphal formation following nikkomycin Z treatment (1 μg/ml) (Fig. 1). The combination of nikkomycin Z (0 to 8 μg/ml) and caspofungin (1 μg/ml) resulted in a synergistic effect on growth in all strains, with the lowest FICI identified in the ΔrasA (0.03) strain (Table 2).
Pharmacologic inhibition of calcineurin signaling through treatment with a clinically relevant dose of FK506 (20 ng/ml) reduced growth in all strains except the ΔcnaA mutant, suggesting the presence of calcineurin activity in all other strains. Although all strains except the ΔcnaA strain were morphologically affected by pharmacologic calcineurin inhibition, the ΔrasA strain showed the greatest sensitivity to calcineurin inhibition (MEC, 0.001 μg/ml) (Table 2). Under microscopic evaluation, FK506-treated ΔrasA conidia displayed stunted germling development even after 48 h of growth (Fig. 1).
The ΔrasA mutant displays decreased cell wall 1,3-β-d-glucan in RPMI.
We next examined the content of two major cell wall polysaccharides, 1,3-β-d-glucan and chitin. Using the aniline blue assay to quantify 1,3-β-d-glucan cell wall content, we found approximately 40 to 50% decreased baseline levels of 1,3-β-d-glucan cell wall content in the ΔrasA strain compared to that of the parental strain (Fig. 2B). These results were similar to those seen for the ΔcnaA and ΔcrzA mutants (Fig. 2A) (10, 25). The treatment of the two wild-type strains and the DArasA strain with caspofungin (1 μg/ml) for 48 h decreased 1,3-β-d-glucan content to approximately the baseline level of these mutants. The caspofungin treatment of the ΔrasA, ΔcnaA, and ΔcrzA mutants further decreased the already-low 1,3-β-d-glucan levels (Fig. 2A and B).
FIG. 2.

Loss of RasA, CnaA, or CrzA signaling causes decreased baseline levels of 1,3-β-d-glucan. Analysis of 1,3-β-d-glucan levels of the ΔcnaA and ΔcrzA (A) and DArasA and ΔrasA (B) strains and those of their respective parental wild-type strains. All analyses were performed in biological triplicate, and results are normalized to values for the control strain (untreated wild type). Results are presented as the means ± standard deviations. NT, no treatment.
Nikkomycin Z treatment decreased the 1,3-β-d-glucan levels of the two wild-type strains by approximately 10 to 15% (Fig. 2A and B). In contrast, treatment with nikkomycin Z (1 μg/ml) caused a slight but statistically significant increase in the measurable 1,3-β-d-glucan levels of all three deletion mutants. No change in basal 1,3-β-d-glucan levels or in the biosynthetic response of 1,3-β-d-glucan to chitin inhibition was noted in the DArasA strain compared to that of the wild type (Fig. 2B).
The calcineurin pathway regulates compensatory chitin increase following caspofungin treatment in A. fumigatus.
In previous studies with C. albicans, caspofungin treatment resulted in a marked increase in chitin synthesis (29). When treated with caspofungin (1 μg/ml), the wild-type, ΔrasA, and DArasA strains all increased their chitin content (Fig. 3B). Surprisingly, the addition of the chitin synthase inhibitor nikkomycin Z (1 μg/ml) did not remove the caspofungin-induced chitin increase (Fig. 3A and B). The caspofungin treatment of the ΔcnaA and ΔcrzA strains did not result in large increases in chitin content (Fig. 3A). To verify that this result was not specific to caspofungin, we employed both micafungin and anidulafungin in similar experiments with the ΔcnaA mutant. When these echinocandins were used at the same physiologically relevant concentration of 1 μg/ml, the ΔcnaA mutant still was unable to upregulate chitin content to levels comparable to that of the wild type (Fig. 3C). The C. albicans strain SC5314 was used as a control in these experiments; similarly to prior studies, the incubation of this strain with caspofungin (0.125 μg/ml) resulted in a twofold increase in chitin (data not shown).
FIG. 3.

CnaA and CrzA, but not RasA, contribute to the compensatory response of chitin to 1,3-β-d-glucan inhibition. Analysis of chitin content of the ΔcnaA and ΔcrzA (A) and DArasA and ΔrasA (B) strains and that of their respective parental wild-type strains. A total of 106 conidia/ml were inoculated into RPMI 1640 medium and grown in the presence or absence of 1 μg/ml of nikkomycin Z (NZ), caspofungin (CA), or a combination of both drugs (NZ + CA) for 48 h at 37°C and 250 rpm. (C) Analysis of the chitin content of the wild-type and ΔcnaA strains in the presence of anidulafungin (1 μg/ml) or micafungin (1 μg/ml). All analyses were performed in biological triplicate. Results and error bars indicate the means ± standard deviations. NT, no treatment.
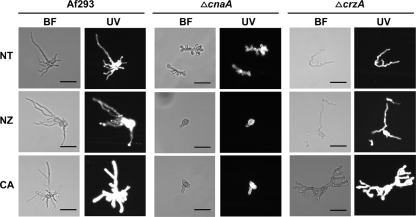
We confirmed the absence of a compensatory increase in chitin following the caspofungin treatment of calcineurin pathway mutants by calcofluor white staining. The wild-type, ΔcnaA, and ΔcrzA conidia were grown in the presence or absence of caspofungin (1 μg/ml) for 16 h and stained with calcofluor white. Under fluorescence microscopy, the staining intensity increased to a greater extent in the wild-type strain treated with caspofungin than in similarly treated ΔcnaA and ΔcrzA strains (Fig. 4).
FIG. 4.
Caspofungin treatment increases calcofluor white staining of wild-type A. fumigatus. A total of 103 conidia/ml of the Af293, ΔcnaA, or ΔcrzA strains were inoculated onto coverslips immersed in RPMI 1640 medium and grown in the presence or absence of caspofungin (1 μg/ml) or nikkomycin Z (1 μg/ml) for 16 h at 37°C. Coverslips were removed and stained with calcofluor white. BF, brightfield; UV, ultraviolet; NT, no treatment. Scale bar, 20 μm.
Nikkomycin Z treatment does not cause a measurable decrease in chitin content in A. fumigatus.
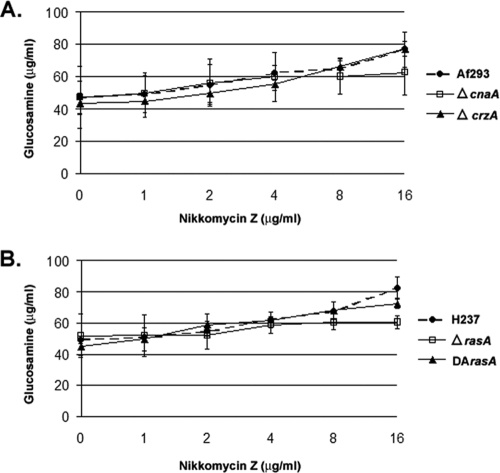
Treatment with the chitin synthase inhibitor nikkomycin Z would be expected to decrease chitin levels. In fact, the chitin content of the wild-type strain C. albicans SC5314 was significantly decreased after incubation with 4 μg/ml nikkomycin Z for 48 h (data not shown). In contrast, our initial studies indicated that the incubation of the various A. fumigatus strains in the presence of nikkomycin Z (1 μg/ml) resulted in no change in chitin content or, paradoxically, in an increase (Fig. 3A and B). To further investigate this apparent discrepancy, we incubated the A. fumigatus strains in the presence of increasing concentrations of nikkomycin Z (0 to 16 μg/ml). Even at high levels of nikkomycin Z treatment (16 μg/ml), chitin content was unchanged or trended upward for all strains (Fig. 5A and B).
FIG. 5.
Response of chitin biosynthesis to high-level nikkomycin Z treatment in A. fumigatus. Analysis of the chitin content of the ΔcnaA and ΔcrzA (A) and DArasA and ΔrasA (B) strains and that of their respective parental wild-type strains when treated with ascending doses of nikkomycin Z. A total of 106 conidia/ml were inoculated into RPMI 1640 medium and grown in the presence or absence of the indicated amount of nikkomycin Z for 48 h at 37°C and 250 rpm. All analyses were performed in biological triplicate. Results and error bars indicate the means ± standard deviations.
DISCUSSION
Although many studies have revealed important roles for calcineurin and Ras in fungi, no model currently exists for how these important signaling pathways converge on downstream targets to regulate growth. This study is the first direct comparison of Ras and calcineurin contribution to the regulation of hyphal growth and cell wall synthesis in a pathogenic, filamentous fungus, and we explored the shared and unique roles of the two important signaling pathways. The deletion of rasA or cnaA causes a similar reduction in growth and, at least under the conditions examined here, caused a similar reduction in cell wall 1,3-β-d-glucan content. Although the ΔrasA and ΔcnaA mutants responded similarly to cell wall inhibition at the glucan level, their patterns of susceptibility to echinocandins were different, with ΔrasA displaying decreased susceptibility. In addition, the testing of the ΔcnaA and ΔcrzA mutants revealed a role for the calcineurin pathway in compensatory chitin regulation following 1,3-β-d-glucan inhibition, a role that does not seem to be shared with the RasA pathway. This is the first report to characterize the compensatory chitin response to glucan inhibition in A. fumigatus and to show that the calcineurin pathway regulates this process.
To decipher the roles played by the Ras and calcineurin pathways, we first examined the possibility of decreased CnaA activity in the ΔrasA mutant. The pharmacologic inhibition of calcineurin activity in a Ras deletion background causes a synthetic growth defect in Cryptococcus neoformans, suggesting the presence of an active calcineurin pathway (1, 28). To probe for the presence of an active calcineurin pathway in ΔrasA, FK506 was used to inhibit calcineurin activity. The ΔrasA strain treated with FK506 showed increased sensitivity by antifungal susceptibility assays and further growth inhibition by morphological analysis, unlike the ΔcnaA strain. This finding suggests that CnaA activity is intact in the ΔrasA mutant and that the impact of each signaling protein on hyphal growth occurs through distinct mechanisms.
In yeast, calcineurin signaling is known to impact 1,3-β-d-glucan synthesis through a CRZ1-mediated transcriptional induction of FKS2, a 1,3-β-d-glucan synthase (23). This mechanism of CnaA is likely intact in A. fumigatus, as the deletion of cnaA causes decreased 1,3-β-d-glucan synthesis and further inhibition by the addition of caspofungin causes the cessation of growth completely (25). Compared to the wild type, the ΔrasA mutant also displayed a reduction in baseline levels of 1,3-β-d-glucan that was similar to that of the ΔcnaA strain. In a separate study, the ΔrasA mutant had no significant decrease in 1,3-β-d-glucan levels, a finding supported by the similar expression of fksA, the 1,3-β-d-glucan synthase, in the ΔrasA mutant and wild-type strains (5). Interestingly, we found the reason for this discrepancy to be in the growth medium, with the present study using RPMI and the previous study using Aspergillus minimal medium (AMM). When we directly compared growth in AMM to that in RPMI 1640, the wild-type strain showed identical 1,3-β-d-glucan levels in each medium, whereas the ΔrasA mutant cultured in RPMI 1640 displayed a nearly 30% decrease in 1,3-β-d-glucan compared to that of growth in AMM (data not shown). This finding implies that the ΔrasA mutant responds more poorly to the environmental changes of growth in RPMI 1640 than those of AMM.
Our previous work has shown that the nikkomycin Z treatment of the ΔcnaA and ΔrasA strains resulted in large, balloon-like germlings (5, 25) and produced a compensatory increase in the 1,3-β-d-glucan content of the ΔcnaA and ΔcrzA mutants (25). We found that the treatment of the ΔrasA strain with nikkomycin Z also resulted in a similar increase of measurable 1,3-β-d-glucan. Although this increase is statistically significant for each of the deletion mutants, it is a very small change and is unlikely to be a biologically significant compensatory mechanism. However, these observations do suggest that the RasA and CnaA pathways are similarly linked to perturbations of chitin synthesis and 1,3-β-d-glucan formation in the cell wall. The use of both cell wall inhibitors displayed strong synergy, with the drug MECs for all strains being greatly decreased and the strains developing swollen, balloon-like cell morphologies. These structures mimic those seen in the ΔrasA and ΔcnaA strains treated with nikkomycin Z alone. Taken together, these data suggest that the inhibition of chitin synthesis in a background of reduced 1,3-β-d-glucan content causes severe morphological abnormalities and lend credence to the usefulness of multiple inhibitors of cell wall components and cell signaling pathways to achieve greater growth inhibition.
The treatment of Candida albicans with low levels of caspofungin recently has been shown to cause a compensatory increase in chitin synthesis, thereby allowing for cell survival (29). This compensatory increase in chitin is partially controlled by the calcineurin pathway, as the deletion of cna1, encoding C. albicans calcineurin, causes decreased chitin synthase activity and decreased survival in response to caspofungin treatment (29). Our data show that, like C. albicans, wild-type A. fumigatus treated with caspofungin, micafungin, or anidulafungin caused a compensatory increase in chitin that is, at least partially, controlled by the calcineurin pathway. These results indicate that the compensatory increase in chitin is a class effect seen in all echinocandins, not just caspofungin. However, we found that nikkomycin Z treatment, even in the ΔcnaA and ΔcrzA mutants, did not lead to a loss of chitin content, as would be expected when using the chitin synthase inhibitor. This finding is in contrast to our C. albicans data showing that treatment with nikkomycin Z causes a decrease in total chitin content. In addition, our data with C. albicans are in line with published data showing the inhibitory effect of nikkomycin Z (11). In fact, in both C. albicans and Saccharomyces cerevisiae, nikkomycin Z has been shown to be a competitive inhibitor of chitin biosynthesis, inhibiting the action of chitin synthases 1 and 3 of S. cerevisiae and all three chitin synthases of C. albicans (3, 8, 11). The filamentous fungi, like A. fumigatus and A. nidulans, have as many as eight chitin synthase genes, divided into separate classes, that are known to play different roles in the cell (reviewed in reference 9). For example, a double deletion of chsG (a class III chitin synthase) and chsE (a class V chitin synthase), or a deletion of chsE alone, leads to a hyphal phenotype very similar to that of the wild-type organism treated with nikkomycin Z in this study (e.g., large, swollen cells spaced intermittently among normal hyphal threads) (17). However, the inactivation of A. fumigatus chsD does not lead to any obvious phenotypic defects, even though total chitin levels were decreased by almost 20% (18). As the deletion of chitin synthase genes, either individually or in combination, leads to different phenotypes, it is conceivable that nikkomycin Z treatment partially inhibits the chitin synthase activity of A. fumigatus, allowing noninhibited chitin synthase activity to compensate for this loss.
The finding that aberrancies in RasA signaling cause altered sensitivity to echinocandins is intriguing. In recent data reported by Plaine et al., a group of four C. albicans genes was described that, when deleted, lead to caspofungin resistance (22). Although the exact function of each of the identified genes is not known, the data are supportive of roles in cell wall integrity and glycosylphosphatidylinositol anchor synthesis. It is tempting to speculate the involvement of A. fumigatus RasA as well; however, nothing currently is known of a possible role for the Ras pathway in glycosylphosphatidylinositol anchor synthesis in any filamentous fungi. We do show that the deletion of rasA caused increased resistance to all echinocandins, accompanied by decreased cell wall 1,3-β-d-glucan content. In contrast, the expression of DArasA caused decreased resistance to echinocandins with no detectable change in 1,3-β-d-glucan. Taken together, these data argue that the level of active Ras plays a role in response to echinocandin treatment that may be separate from the control of glucan synthesis. Also, the paradox of decreased 1,3-β-d-glucan content and increased resistance to echinocandin treatment in the ΔrasA strain suggests that the baseline glucan levels of fungi are not directly predictive of caspofungin sensitivity. In effect, blocking 1,3-β-d-glucan synthesis appeared to remove a hyphal inhibition mechanism in the ΔrasA strain. The ΔrasA mutant also appeared to behave like the wild type with respect to the compensatory chitin response to 1,3-β-d-glucan inhibition. These findings are in direct contrast to the similarities between the ΔrasA and ΔcnaA mutants in basal glucan levels and in their responses to the inhibition of chitin synthesis. These data suggest that RasA plays a more indirect role in 1,3-β-d-glucan synthesis that is parallel to the action of the calcineurin pathway.
In Cryptococcus neoformans, Ras activity has been shown to play a role in proper actin polymerization in response to high-temperature growth (20). Likewise, the deletion of rasA in A. fumigatus may cause unstable actin polymerization at the hyphal tip and therefore lead to the defects in polarized growth observed (e.g., wide, stunted hyphae that continually change growth direction). We hypothesize that the treatment of the ΔrasA mutant with echinocandins slows general growth mechanisms enough to compensate for inefficient actin polymerization at the hyphal tip, allowing hyphal extension to appear more like the wild type in morphology. Therefore, these data could argue for an indirect relationship between the decreased 1,3-β-d-glucan synthesis caused by caspofungin treatment and improved hyphal growth in the ΔrasA strain.
In summary, the data presented here suggest that although either rasA or cnaA deletion leads to similar reductions in hyphal growth and 1,3-β-d-glucan content, these two signaling proteins yield similar phenotypes through distinct and parallel mechanisms. Further, the pharmacologic inhibition of calcineurin with FK506 revealed that calcineurin activity is still present in the rasA deletion background and that the apparent synthetic growth defect caused by a lack of Ras and calcineurin activity is particularly damaging to A. fumigatus. Although ΔrasA and ΔcnaA mutants appeared similar at the 1,3-β-d-glucan level, these strains did not share changes in the compensatory mechanism of increased hyphal chitin content in response to the inhibition of 1,3-β-d-glucan synthesis. It is possible, then, that RasA and CnaA respond similarly to cell wall stress by accessing a shared, compensatory mechanism(s) for glucan synthesis, whereas CnaA plays a separate role in the regulation of chitin biosynthesis for a protective outcome. Future studies directed toward understanding the molecular contributions of Ras and calcineurin to hyphal growth and cell wall integrity may shed light on new therapeutic possibilities for the best-focused attack on hyphal growth.
Acknowledgments
This work was supported through a K08 award (A1061149) to W.J.S., a Basic Science Faculty Development grant from the American Society for Transplantation, a Children's Miracle Network grant, and a Molecular Mycology and Pathogenesis Training Program grant at Duke University (5T32-AI052080) to J.R.F.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabib, E. 1991. Differential inhibition of chitin synthetases 1 and 2 from Saccharomyces cerevisiae by polyoxin D and nikkomycins. Antimicrob. Agents Chemother. 35:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer, R. A., Jr., B. Z. Perfect, N. Pinchai, S. Park, D. S. Perlin, Y. G. Asfaw, J. Heitman, J. R. Perfect, and W. J. Steinbach. 2008. The calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortwendel, J. R., K. K. Fuller, T. J. Stephens, W. C. Bacon, D. S. Askew, and J. C. Rhodes. 2008. Aspergillus fumigatus RasA regulates asexual development and cell wall integrity. Eukaryot. Cell 7:1530-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortwendel, J. R., J. C. Panepinto, A. E. Seitz, D. S. Askew, and J. C. Rhodes. 2004. Aspergillus fumigatus rasA and rasB regulate the timing and morphology of asexual development. Fung. Genet. Biol. 41:129-139. [DOI] [PubMed] [Google Scholar]
- 7.Ganesan, L. T., E. K. Manavathu, J. L. Cutright, G. J. Alangaden, and P. H. Chandrasekar. 2004. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin. Microbiol. Infect. 10:961-966. [DOI] [PubMed] [Google Scholar]
- 8.Gaughran, J. P., M. H. Lai, D. R. Kirsch, and S. J. Silverman. 1994. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J. Bacteriol. 176:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiuchi, H. 2008. Functional diversity of chitin synthases of Aspergillus nidulans in hyphal growth, conidiophore development and septum formation. Med. Mycol. 24:1-6. [DOI] [PubMed] [Google Scholar]
- 10.Kahn, J. N., M.-J. Hsu, F. Racine, R. Giacobbe, and M. Motyl. 2006. Caspofungin susceptibility in Aspergillus and non-Aspergillus molds: inhibition of glucan synthase and reduction of β-d-1,3 glucan levels in culture. Antimicrob. Agents Chemother. 50:2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, M.-K., H.-S. Park, C.-H. Kim, H.-M. Park, and W. Choi. 2002. Inhibitory effect of nikkomycin Z on chitin synthases in Candida albicans. Yeast 19:341-349. [DOI] [PubMed] [Google Scholar]
- 12.Kontoyiannis, D., R. Lewis, N. Osherov, N. Albert, and G. May. 2003. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. Antimicrob. Agents Chemother. 51:313-316. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latgé, J. P. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279-290. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 17.Mellado, E., G. Dubreucq, P. Mol, J. Sarfati, S. Paris, M. Diaquin, D. W. Holden, J. L. Rodriguez-Tudela, and J. P. Latgé. 2003. Cell wall biogenesis in a double chitin synthase mutant (chsG-/chsE-) of Aspergillus fumigatus. Fung. Genet. Biol. 38:98-109. [DOI] [PubMed] [Google Scholar]
- 18.Mellado, E., C. A. Specht, P. W. Robbins, and D. W. Holden. 1996. Cloning and characterization of chsD, a chitin synthase-like gene of Aspergillus fumigatus. FEMS Microbiol. Lett. 143:69-76. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS document M38-A. Clinical and Laboratory Standards Institute, Villanova, PA.
- 20.Nichols, C. B., Z. H. Perfect, and J. A. Alspaugh. 2007. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 63:1118-1130. [DOI] [PubMed] [Google Scholar]
- 21.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 22.Plaine, A., L. Walker, G. Da Costa, H. M. Mora-Montes, A. McKinnon, N. A. R. Gow, C. Gaillardin, C. A. Munro, and M. L. Richard. 2008. Functional analysis of Candida albicans GPI-anchored proteins: roles in cell wall integrity and caspofungin sensitivity. Fung. Genet. Biol. 45:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, C. Henn, K. Nielsen, J. Heitman, and J. R. Perfect. 2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:2979-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbach, W. J., W. A. Schell, J. R. Blankenship, C. Onyewu, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallim, M. A., L. Fernandes, and J. A. Alspaugh. 2004. The RAM1 gene encoding a protein-farnesyltransferase β-subunit homologue is essential in Cryptococcus neoformans. Microbiology 150:1925-1935. [DOI] [PubMed] [Google Scholar]
- 29.Walker, L. A., C. A. Munro, I. de Bruijn, M. D. Lenardon, A. McKinnon, and N. A. Gow. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederhold, N., D. Kontoyiannis, R. Prince, and R. Lewis. 2005. Attenuation of the Activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 49:5146-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]