Abstract
The purpose of this study was to describe the plasma pharmacokinetics (PK) of elvucitabine at different doses when administered daily or every other day for 21 days with lopinavir-ritonavir (Kaletra) in human immunodeficiency virus (HIV)-infected subjects. Three different dosing regimens of elvucitabine were administered with lopinavir-ritonavir to 24 subjects with moderate levels of HIV. Plasma samples were collected over 35 days. Elvucitabine concentrations were analyzed using a validated liquid chromatography-tandem mass spectrometry assay. The PK of elvucitabine was determined using both noncompartmental and compartmental analyses. Models were developed and tested using ADAPT II, while a population analysis was performed using IT2S. The PK behavior of elvucitabine was best described by a two-compartment linear model using two absorption rates and an increase in the bioavailability after day 1. The augmentation in the bioavailability after day 1 was variable, with some subjects demonstrating a major increase while others had little or no increase. Elvucitabine has a long half-life of approximately 100 h. The increase in elvucitabine bioavailability may be due to ritonavir inhibiting an efflux gut transporter with activity present in various levels between subjects. The proposed PK model may be utilized and improved further by linking the PK behavior of elvucitabine to various markers of efficacy.
Lack of adherence is a major problem in the treatment of human immunodeficiency virus (HIV) infection. It is estimated that more than 50% of HIV-infected people do not adhere to their highly active antiretroviral therapy (HAART) regimen within the first year, and this can lead to the emergence of resistance (6, 24). A common reason for a lack of HAART adherence is the complicated regimens (e.g., dosing more than once daily; need for taking the medication with or without food). The administration of a complicated therapy such as HAART may be more difficult in developing countries where resources are limited. Another reason for the lack of adherence is due to adverse effects (toxicity) suffered by patients from the numerous medications taken. These adverse effects can be amplified by the numerous drug interactions present with HAART and by other medications administered to HIV patients (18, 2). There is, therefore, a clear need for new HIV drugs with more favorable pharmacokinetic (PK) profiles as well as improved safety profiles. Additionally, new medications providing innovative dosing regimens such as twice-weekly or once-a-week dosing would simplify the HAART regimen and hopefully increase compliance.
In order to have such dosing regimens, it is obvious that a new drug would have to display a long terminal half-life (t1/2) and demonstrate activity even at low concentrations so as to minimize potential drug-related toxicities. A small deviation to the timing of the intake of a long t1/2 drug becomes less important at steady state as suppressive drug levels are maintained.
Nucleoside reverse transcriptase inhibitor (NRTI) drugs typically have short half-lives and often require multiple daily doses to be efficacious. For example, zidovudine, lamivudine, and dideoxyinosine have plasma half-lives of 1.1 h, 3.5 to 5 h, and 1.75 h, respectively (1).
Elvucitabine (β-l-Fd4C), an investigational l-cytosine NRTI, showed 5- to 10-fold improved in vitro antiviral activity against wild-type HIV isolates (50% inhibitory concentration of ∼1 ng/ml in peripheral blood mononuclear cells) compared to that of lamivudine. In addition, elvucitabine also showed potentially more potent activity against a variety of nucleoside-resistant viral isolates, particularly those that are resistant to zidovudine and tenofovir. Preclinical in vitro data of elvucitabine showed that elvucitabine was not significantly bound to plasma, was metabolized intracellularly into monophosphate, diphosphate, and triphosphate analytes with elvucitabine triphosphate having a t1/2 of at least 20 h, was not metabolized by cytochrome P450 (CYP) enzymes, was not an inducer of CYP enzymes, and was not an inhibitor of CYP enzymes. Additionally, preclinical animal studies demonstrated that elvucitabine had a bioavailability of approximately 50% in dogs and had increasing exposure with increasing doses.
Preliminary phase I PK studies of elvucitabine demonstrated that elvucitabine had a long half-life, greater than 60 h, giving rise to potentially innovative dosing regimens. The sampling scheme of these previous studies did not allow the accurate characterization of the t1/2. Administration of high doses of elvucitabine (50 and 100 mg once a day [QD]) has also been associated with toxicity, evidenced by reversible leucopenia and neutropenia (17). However, PK/pharmacodynamic modeling suggested that lower daily doses would be effective and nontoxic (22).
The purpose of this research was to determine the complete plasma PK of different doses of elvucitabine when administered daily or every other day for 21 days with 400 mg lopinavir-100 mg ritonavir (Kaletra) twice daily in HIV-infected subjects with a moderately elevated viral load.
MATERIALS AND METHODS
Subjects and study design (multiple-dose study in HIV-1 subjects).
Twenty-four subjects were enrolled in an open-label, dose-escalating PK trial of elvucitabine. Subjects between 23 and 62 years of age with moderate levels of HIV (3.6 to 5.6 log10 HIV RNA copies/ml) received elvucitabine doses of 5 or 10 mg QD or 20 mg once every 48 h (Q48h) for 21 days with concomitant treatment with 400 mg lopinavir-100 mg ritonavir (once every 12 h) at two different sites (Berlin, Germany, and Utrecht, The Netherlands). Only two females, both in the highest-dose cohorts, were dosed. Elvucitabine (enteric-coated tablets) and lopinavir-ritonavir (400 mg lopinavir and 100 mg ritonavir) were administered as follows: for elvucitabine, subjects in cohort 1 (n = 8) received 5 mg QD for 21 days, those in cohort 2 (n = 8) received 10 mg QD for 21 days, and those in cohort 3 (n = 8) received 20 mg Q48h for 21 days; for lopinavir-ritonavir, subjects in cohort 1 were dosed twice a day (BID) for 21 days, those in cohort 2 were dosed BID for 35 days, and those in cohort 3 were dosed BID for 35 days.
Due to the observed long terminal t1/2 of elvucitabine in cohort 1, lopinavir-ritonavir dosing was extended to 35 days (14 days after elvucitabine discontinuation) in the two subsequent cohorts in order to decrease the probability of the development of resistance to elvucitabine. This was done to avoid the presence of low concentrations of elvucitabine without concomitant protease inhibitor exposure. Exclusion criteria included subjects with hepatitis B virus or hepatitis C virus coinfection, previous history of HIV virologic failure, and underlying liver disease. All subjects provided written consent prior to participation in the study, which was approved by an ethics committee. When doses of elvucitabine and lopinavir-ritonavir had to coincide, elvucitabine was administered first under fasting conditions while lopinavir-ritonavir was administered with food, 2 h after elvucitabine dosing.
Plasma samples were collected over 35 days for elvucitabine PK determination. Samples were collected on days 1 and 21 predose and at 0.5, 1, 1.5, 2, 4, 7, 11, 12, and 24 h postdose as well as prior to dosing on days 3, 7, 10, and 14 and on days 25, 28, and 35.
Drug analysis.
Plasma samples were analyzed for elvucitabine concentrations by a sensitive and specific validated liquid chromatography-tandem mass spectrometry assay (21) The plasma analytical range was 0.500 ng/ml to 100 ng/ml. The precision (percent coefficient of variation [%CV]) was ≤5.2%, and accuracy ranged from 0.3 to 3.3% for concentrations at 1.5, 15, and 75 ng/ml.
Noncompartmental PK analysis.
Standard noncompartmental analyses were performed using data from elvucitabine concentration versus time. The maximum observed concentration of drug in plasma (Cmax), minimum observed concentration taken at 24 h after dosing (C24), and linear trapezoidal area under the concentration-time curve from 0 to 24 h (AUC0-24) were calculated after day 1 and day 21. Additional parameters, such as the elimination rate constant and t1/2, were also calculated after dosing on day 21. Noncompartmental analyses were performed using Kinetica version 4.3 (InnaPhase Corporation).
Population compartmental PK analysis.
Compartmental PK analyses were performed using elvucitabine data from all subjects. Individual analyses were first performed using maximum likelihood analysis in ADAPT II Release IV (5). The model discrimination process was based on the following criteria: minimization of the values of the Akaike information criterion (AIC) test, of the minimum value of the objective function, and of the residual variability. An additional criterion considered in the discrimination process was the maximization of the average coefficient of determination. A population PK analysis was then performed on the final model using an iterative two-stage methodology (IT2S) (3), using priors obtained from the ADAPT II analysis in order to obtain the most accurate population PK parameters, variance, residual variability, and individual results. All systemic concentrations of elvucitabine were modeled using the following weighting (W) procedure: Wj = 1/Sj2, where the variance Sj2 was calculated for each observation (Y) using the equation Sj2 = (a + b × Y)2. The parameters a and b are the intercept and slope of the variance model. The slope is the residual variability proportional to each concentration, and the intercept is the additive component of the error. Variance parameter estimates from the individual PK analysis (ADAPT II) were used as beginning priors and were updated iteratively during the population PK analysis until stable values were found.
Statistical analysis.
In order to compare results from different dosing regimens, appropriate statistical tests were performed. Elvucitabine PK parameters calculated from the three cohorts were compared using an analysis of variance with the GLM procedure. This model included dose as an independent variable. The PK parameters AUC0-24, Cmax, and C24 were compared using natural logarithm-transformed dose-normalized data. The time to maximum observed concentration of drug in serum (Tmax) was compared using Kruskal-Wallis nonparametric analysis. Statistical analyses were performed using Systat version 8.0 (SPSS Inc.). Statistical significance was set a priori at a P value of <0.05.
RESULTS
Noncompartmental PK analysis.
On day 1 and day 21, the parameters calculated were AUC0-24, Cmax, Tmax, and C24. The elimination rate constant and t1/2 were calculated using day 21 administration data. Results are presented in Tables 1 and 2. Throughout the range of doses administered in this study, the PK parameters appeared to be constant, suggesting dose-dependent PK linearity. A t1/2 of approximately 100 h was calculated based on 336 h of sample collection in the terminal elimination phase. The estimated t1/2 was longer than what was determined in the preliminary phase I studies (8). This is not surprising, as the collection interval in previous studies was not long enough to allow for proper characterization of the t1/2. Based on the AUC values and minimum observed concentration of drug in plasma, there was an approximately three- to fivefold accumulation between day 1 and day 21.
TABLE 1.
Noncompartmental PK parameters of elvucitabine in plasma on day 1 (eight subjects per cohort)a
| Parameter | Valuesa for dose of:
|
P valueb | ||
|---|---|---|---|---|
| 5 mg | 10 mg | 20 mg | ||
| AUC0-24 (ng·h/ml) | 59.9 (39.3),* 49.9 (26.1-88.1) | 155 (41.0),* 130 (85.6-236) | 262 (40.4), 229 (161-492) | NS*** |
| Cmax (ng/ml) | 7.25 (57.6), 6.69 (1.37-13.2) | 25.3 (50.7), 22.0 (2.45-40.0) | 39.1 (38.5), 37.2 (21.7-67.7) | NS*** |
| Tmax (h) | 6.63 (54.7), 4.00 (4.00-11.0) | 3.31 (55.3), 3.00 (1.50-7.00) | 3.75 (18.9), 4.00 (2.00-4.00) | <0.05** |
| C24 (ng/ml) | 0.832 (34.2),* 0.809 (0.521-1.42) | 1.87 (48.9),* 1.36 (1.05-3.30) | 3.41 (35.9), 3.42 (1.81-5.70) | NS*** |
Values for the dosing regimen are as follows: arithmetic mean (%CV), median (range). *, one subject from the 5-mg dose cohort and one from the 10-mg dose cohort were excluded since concentration was below the limit of quantitation by 24 h postdose (total of seven subjects per cohort).
**, 10-mg and 20-mg dose cohorts are not statistically significantly different from each other; ***, analysis of variance performed using dose-normalized parameters; NS, not statistically significant (P > 0.05).
TABLE 2.
Noncompartmental PK parameters of elvucitabine in plasma on day 21 (eight subjects per cohort)
| Parameter | Valuesa for dose of:
|
P valueb | ||
|---|---|---|---|---|
| 5 mg QD | 10 mg QD | 20 mg Q48h | ||
| AUC0-24 (ng·h/ml) | 214 (35.8), 220 (85.9-326) | 435 (45.4), 431 (114-740) | 749 (25.4),* 816 (425-999) | NS*** |
| Cmax (ng/ml) | 23.1 (49.2), 21.7 (5.58-41.3) | 49.9 (63.4), 51.0 (6.16-113) | 108 (37.3,) 110 (52.6-161) | NS*** |
| Tmax (h) | 7.00 (108.0), 4.00 (2.00-24.0) | 3.75 (46.7), 4.00 (1.00-6.98) | 4.00 (0), 4.00 (4.00-4.00) | NS |
| C24 (ng/ml) | 4.38 (22.4),** 4.86 (2.94-5.46) | 9.70 (41.7), 9.73 (4.00-16.8) | 12.0 (26.9), 10.5 (9.58-17.7) | <0.05*** |
| t1/2 (h) | 92.5 (30.9), 79.7 (67.0-132) | 112 (14.4), 112 (85.3-144) | 105 (15.1), 101 (89.5-131) | NS |
Values for the dosing regimen are as follows: arithmetic mean (%CV), median (range). *, this AUC is not the AUC during the dosing interval; **, descriptive statistics presented with one subject removed (concentration of 32.8 ng/ml).
***, analysis of variance performed using dose-normalized parameters; NS, not statistically significant (P > 0.05).
Population compartmental analysis.
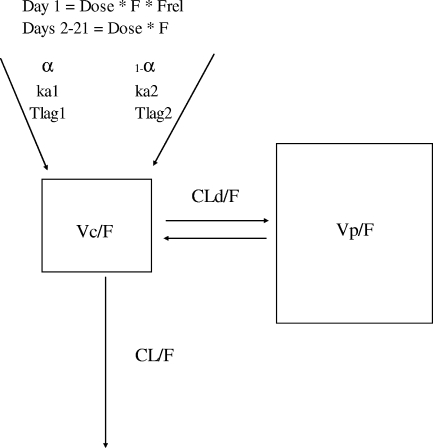
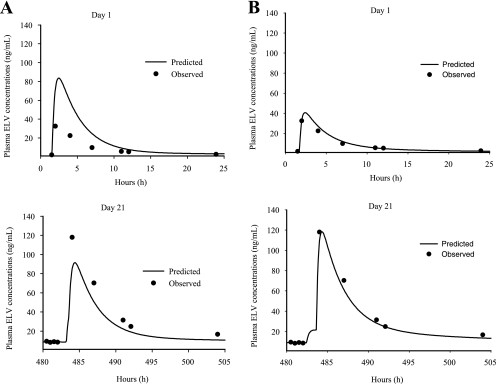
The population analysis was performed in two stages. The first consisted of determining the simplest PK model that simultaneously fitted and explained all the clinical data (collected concentrations and doses administered). Multiple linear models were tested. Although the AIC for some three-compartment models may have been slightly better than the two-compartment models, these three-compartment models were not retained since the predicted concentrations around Cmax were completely overestimated. Therefore, a two-compartment model was chosen. In addition, two separate absorption rate constants were required to properly characterize the concentrations in order to minimize the residual variability and maximize the coefficient of determination of the models. This model is presented graphically in Fig. 1. Despite this, initial analyses showed that many subjects presented predicted concentrations that were higher than the observed concentrations on day 1 and lower concentrations than those observed on day 21 (Fig. 2A), suggesting time-dependent nonlinearity. In order to determine if there were any differences in the PK of the drug between day 1 and day 21, concentrations for day 1 and day 21 were analyzed separately. These analyses suggested two important facts. The first was that the simplest model that best fitted the concentrations of elvucitabine was the same for day 1 and day 21. This was a two-compartment model, with two absorption rate constants (ka1 and ka2) and a first-order elimination process. The different discrimination criteria used to select the final model are presented in Table 3. The final and retained model contained the following PK parameters: ka1 and ka2, a different lag time (Tlag1 and Tlag2) for each absorption rate constant, apparent clearance (CL/F), volumes of distribution (apparent volume of central compartment [Vc/F] and apparent volume of peripheral compartment [Vp/F]), and apparent distributional clearance (CLD/F). The results of the PK parameters from day 1 and day 21 analyzed separately indicated that the CL/F (22.6 liters/h) and apparent volume of distribution (Vss/F) (1,494 liters) for day 21 were approximately half of the values seen on day 1 (45.6 liters/h and 2,629 liters, respectively). However, the median elimination t1/2 remained essentially the same between days 1 and 21. These results suggested that the relative bioavailability between day 1 and 21 approximately doubled.
FIG. 1.
Final PK model used in the compartmental analysis.
FIG. 2.
Day 1 and day 21 predicted versus observed elvucitabine (ELV) concentrations. (A) Predicted concentrations for a representative subject based on linear two-compartment model with two absorption rates with no change in relative bioavailability between day 1 and day 21. (B) Predicted concentrations for the same subject using the same model as that in panel A with a change in relative bioavailability between day 1 and the rest of the dosing days.
TABLE 3.
Discrimination criteria between PK modelsa
| Day | Model description | Mean ECV | Mean AIC | Mean r2 | Mean residual variability (%) | Mean CL/F (liters/h) | Mean Vss/F (liters) |
|---|---|---|---|---|---|---|---|
| 1 | 2 CPT, ka1 | −6.3 | 3.4 | 0.940 | 7.2 | ||
| 2 CPT, ka1, 2 ABS peaks | −4.6 | 10.7 | 0.913 | 6.7 | |||
| 2 CPT, ka1, ka2 | −9.5 | 3.0 | 0.993 | 2.7 | 45.6 | 2,629 | |
| 3 CPT, ka1 | −2.4 | 15.2 | 0.930 | 8.0 | |||
| 2 CPT, ka1, ka2, baseline Vss | −6 | 10.1 | 0.992 | 4.3 | |||
| 2 CPT, ka1, ka2, saturable elimination | −2.3 | 19.5 | 0.927 | 7.3 | |||
| 2 CPT, ka1, ka2, baseline Vss, saturable elimination | −2.3 | 19.4 | 0.928 | 6.9 | |||
| 21 | 2 CPT, ka1 | 16.9 | 49.8 | 0.911 | 17.1 | ||
| 2 CPT, ka1, 2 ABS peaks | 12.7 | 45.4 | 0.941 | 11.1 | |||
| 2 CPT, ka1, ka2 | 8.9 | 39.8 | 0.960 | 7.2 | 22.6 | 1,494 | |
| 3 CPT, ka1 | 12.8 | 45.7 | 0.862 | 16.8 | |||
| 2 CPT, ka1, ka2, baseline Vss | 10.9 | 43.8 | 0.949 | 10 | |||
| 2 CPT, ka1, ka2, saturable elimination | 17.2 | 58.3 | 0.952 | 17.5 | |||
| 2 CPT, ka1, ka2, baseline Vss, saturable elimination | 20.2 | 64.4 | 0.913 | 20.6 |
The selected model is in bold type. CPT, compartments; ABS, absorption; ECV, estimator criteria value.
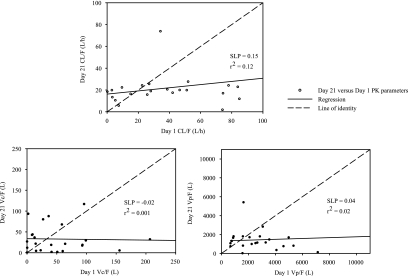
The second important fact suggested by two separate parameter analyses of day 1 and day 21 was that there was a time-dependent relationship in the results of the individual PK parameters CL/F, Vc/F, and Vp/F. Figure 3 shows that the regression slopes of the correlations between day 21 and day 1 are approximately 0 for the different PK parameters. This suggests that all subjects had similar clearances and volumes of distribution on day 21 independently of their results for day 1.
FIG. 3.
Day 21 versus day 1 correlations of PK parameters.
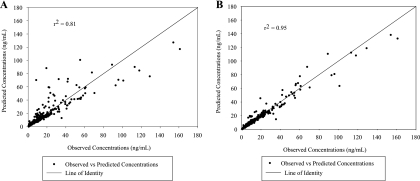
Based on these results, a linear two-compartment model with two first-order rate constants, a first-order elimination rate constant, and a change in bioavailability after day 1 (Frel) was chosen. Figure 4 represents the fitted versus observed concentrations for both the model with and without Frel. Based on visual inspection and coefficient of determination (r2) of these graphs, the model with Frel was better at explaining the observed concentrations. The reason the bioavailability was allowed to change after day 1 was that the first dose of elvucitabine was the only dose given prior to any administration of lopinavir-ritonavir, and it was hypothesized that the presence of lopinavir-ritonavir was what was affecting the bioavailability of elvucitabine on subsequent days. This was considered the final model. An additional model was tested where clearance could also change after day 1. This model, including a variable clearance after day 1, was tested since elvucitabine is eliminated primarily unchanged in urine; consequently, it was possible to consider that the same effect observed with the absorption could also happen with the elimination process. This model was not retained as it did not improve the fit; approximately half of the subjects had clearances that were lower after day 1, and the other half had higher clearances after day 1. Results of the model discrimination process for all data combined (days 1 and 21) are presented in Table 4.
FIG. 4.
Predicted versus observed concentrations for model with and without change in bioavailability after day 1. Shown are fitted versus observed concentrations using models without (A) and with (B) a change in F after day 1.
TABLE 4.
Discrimination criteria between PK models (all data simultaneously fitted)a
| Model | Median ECV | Median AIC | Median r2 | Median residual variability (%) |
|---|---|---|---|---|
| 2 CPT; ka1; Tlag1 per dose | 39.6 | 97.2 | 0.929 | 15.4 |
| 2 CPT; ka1; Tlag1, Tlag2 per dose; including peak 1% | 37.3 | 98.5 | 0.945 | 17.2 |
| 2 CPT; ka1, ka2 and Tlag1, Tlag2 per dose; including peak 1% | 36.4 | 98.9 | 0.954 | 14.9 |
| 2 CPT; ka1; Tlag1 per dose; Frel between day 1 and day 21 | 36.4 | 92.7 | 0.980 | 14.5 |
| 2 CPT; ka1, ka2; Tlag1, Tlag2 per dose; Frel for day 1 | 27.0 | 82.1 | 0.988 | 11.6 |
| 2 CPT; ka1, ka2; Tlag1, Tlag2 per dose; Frel for day 1 fixed based on the previous model, with Frel on CL/F for day 1 | 29.1 | 86.3 | 0.989 | 11.7 |
| 3 CPT; ka1; Tlag1 per dose | 36.6 | 95.2 | 0.952 | 14.1 |
| 3 CPT; ka1, ka2; peak 1% the same for all days; Tlag1, Tlag2 per dose | 33.9 | 97.7 | 0.949 | 15.3 |
| 3 CPT; ka1, ka2 and Tlag1, Tlag2 the same for all doses; including peak 1% | 47.6 | 121.3 | 0.778 | 25.6 |
| 3 CPT; ka1, ka2 the same for all doses; Tlag1, Tlag2 and 2 peak 1% per dose | 40.2 | 112.5 | 0.967 | 21.0 |
The selected model is in bold type. CPT, compartments; ECV, estimator criteria value. α, distribution phase.
Based on the model retained using ADAPT II, a population analysis was performed using a mixed-effect modeling approach (IT2S). This analysis was done to obtain better estimates of the population PK parameters, their variance (intersubject variability), the residual variability (intrasubject variability), and the subject's individual results. The PK parameters included in the model were ka1 and ka2, which were constant for all days, the percentage of the total drug absorbed associated with ka1 (peak 1%), Frel, which was the relative bioavailability of day 1 compared to the rest of the dosing days (Frel on days 2 to 21, set to 1), Tlag on day 1 and day 21 for each ka, clearance (CL/F × Frel), Vc/F, Vp/F, and CLD/F. Results of the PK parameters are presented in Table 5 with a fit of the representative subject in Fig. 2B.
TABLE 5.
Elvucitabine PK parameters estimated using IT2S population compartmental analysesa
| Parameter(s) | Mean (%CV) | Median (range) |
|---|---|---|
| Peak 1% | 52.3 (32.8) | 47.8 (21.5-93.0) |
| Day 1, Tlag1 (h) | 0.363 (28.4) | 0.354 (0.218-0.612) |
| Day 1, Tlag2 (h) | 0.445 (42.8) | 0.492 (0.077-0.870) |
| Day 21, Tlag1 (h) | 0.341 (42.1) | 0.350 (0.069-0.721) |
| Day 21, Tlag2 (h) | 1.02 (91.7) | 0.729 (0.009-4.46) |
| ka1 (h−1) | 0.065 (64.6) | 0.057 (0.012-0.166) |
| ka2 (h−1) | 0.420 (22.5) | 0.404 (0.271-0.667) |
| % F for day 1 compared to that of other days | 55.4 (38.7) | 56.4 (17.0-90.5) |
| CL/F (liters/h) | 25.4 (48.0) | 23.4 (9.2-65.9) |
| Vc/F (liters) | 6.43 (75.4) | 5.87 (0.08-16.9) |
| CLD/F (liters/h) | 15.4 (28.9) | 15.4 (2.4-23.4) |
| Vp/F (liters) | 1,618 (47.8) | 1,398 (318-4,091) |
| Vss/F (liters) | 1,624 (47.6) | 1,396 (333-4,096) |
| λZ-HL (h) | 120 (23.0) | 115 (61.3-198) |
Residual variability, 15.7%. λZ-HL, terminal half-life.
DISCUSSION
The PK behavior of elvucitabine was best described by a two-compartment linear model using two absorption rates with different lag times for each absorption rate for each sampling day (day 1 and day 21). The same preference for two absorption rates was seen when day 1 and day 21 data were modeled separately (Table 3). In addition, once it was determined that the bioavailability was different between day 1 and the other dosing days, the model with two absorption rates was still better than the model with one absorption rate (Table 4). This is often seen with modified-release formulations or with the drug undergoing multiple absorption peaks, such as those for cyclosporine (11).
The residual variability left from the population analyses was 15.7%. This was slightly higher than the residual variability calculated in a single-dose study of elvucitabine administered in healthy subjects (∼9%) (4). However, considering all of the sources of variability in a multiple-dose patient study (e.g., analytical and clinical sources and those of the modeling exercise), a residual variability of 15.7% is perfectly acceptable and suggests that an appropriate model was used. Possible reasons for a higher residual variability include a study performed for HIV-1 subjects in which the subjects had fewer samples taken per dose, especially during the absorption phase, had multiple doses with changing bioavailability, and self-administered their medications from day 3 to day 20, adding to the potential unknown variations in the timing of dosing relative to the trough concentrations collected. Therefore, a higher residual variability was expected for this multiple-dose HIV patient study compared to that of the single-dose study of healthy subjects.
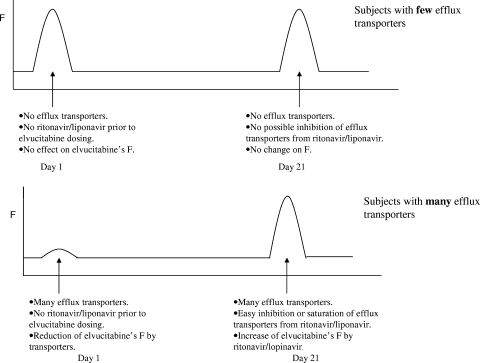
As described previously, results from the compartmental PK analyses from the multiple-dose study in HIV-1 subjects revealed that the bioavailability of elvucitabine approximately doubled between the first dose of elvucitabine and that from day 21. As demonstrated in Fig. 3, there were no correlations in the individual PK results for CL/F, Vc/F, and Vp/F between day 1 and day 21, with subjects having similar clearances and volumes of distribution on day 21 independently of their day 1 values. We are hypothesizing that the increase in elvucitabine's bioavailability could be due to the inhibition of efflux transporters (e.g., ABCB1) in the gut by ritonavir-lopinavir or by elvucitabine itself. Ritonavir has been shown to be a potent inhibitor of ABCB1 activity numerous times in the literature (7, 12, 16). In addition, polymorphisms exist in ABCB1 transporters, thus providing potential baseline differences between subjects in the activity of their gut ABCB1 transporters (9, 10, 13, 14, 19, 20, 23). We can hypothesize that subjects with little efflux transporter activity in the gut would see little change in their bioavailabilities between day 1 and day 21 and, consequently, little change in their PK parameters between day 1 and day 21. However, subjects with high levels of ABCB1 transporter activity in the gut would have significant changes in their PK parameters between day 1 and day 21 with the addition of ritonavir, and their PK parameters calculated on day 21 would resemble those of subjects with little transporter activity. The impact of this hypothesis was translated graphically in Fig. 5 and mathematically in the legend to Fig. 5.
FIG. 5.
Representation of the change in bioavailability based on activity of the subject's transporters. The mathematical representation of this is as follows: F = 1 − E, where E is the extraction ratio. In this case, extraction would be due to transporters and nontransporters. E = (ENT + ET), where ENT is the extraction due to nontransporters and ET is the extraction due to the transporters; F = 1 − (ENT + ET), ENT = percent × E, and ET = (1 − percent) × E. Therefore, if the transporters are inhibited after day 1, bioavailability could be represented as follows: F (day 1) = 1 − (ENT + ET) or 1 − E, and F (day 21) = 1 − ENT or 1 − (percent × E).
Preliminary urine data collected from dogs indicated that elvucitabine had a bioavailability of approximately 50% (15). In a single-dose study of healthy subjects, similar bioavailability results were obtained using urine data collected over 96 h. An average of 32% (range, 9.6 to 55%) of the elvucitabine dose was excreted unchanged in urine in 96 h. Assuming there is one elimination t1/2 (50%) by 96 h, the observed recovery is equivalent to a bioavailability of 64%. As the bioavailability is incomplete, an increase is possible. The results of the population compartmental analysis in the multiple-dose study of HIV-1 subjects estimated that the bioavailability increased by approximately 45% after day 1, ranging from 9 to 83%. Based on urine data from a single-dose study, the increase and variability in bioavailability estimated by the model are acceptable.
Comparing the noncompartmental and compartmental analyses can be useful in determining consistency between both PK methods. However, the results and study design allowed only a partial comparison between methods. The mean noncompartmental t1/2 varied between 92.5 and 112 h for the three cohorts, while the estimated mean t1/2 from the compartmental analysis was 120 h. With this study design, it was expected that noncompartmental t1/2 would be shorter than the compartmental t1/2, as the t1/2 calculated from the noncompartmental analysis was based on the last 336 h of sampling while the compartmental analysis was based on all 804 h of sampling. The fact that all 804 h of sampling were used by the compartmental analysis permitted a better characterization of elvucitabine's t1/2. The total exposure estimated by the compartmental analysis for the 5-mg dose cohort and the 10-mg dose cohort was 234 and 482 ng·h/ml, respectively. This is similar to the 214 and 435 ng·h/ml calculated by the noncompartmental analysis, demonstrating the consistency between the two methods.
The t1/2 of elvucitabine was long at approximately 100 h, and as expected, concentrations remained detectable for at least 7 days after cessation of dosing. Therefore, elvucitabine concentrations were probably not at steady state by the time the last dose was administered. Steady-state concentrations would be expected to be slightly higher. Based on minimum concentrations for elvucitabine on day 21, all cohorts had concentrations above the efficacious levels of 1 ng/ml.
A comparison of different PK parameters (e.g., AUC0-24, Cmax, and C24) for the three cohorts indicated no statistical differences for the dose-normalized parameters AUC0-24, Cmax, and C24 with the exception of C24 on day 21. It was expected that C24 on day 21 showed a statistical difference between groups, as this value for cohort 3 (20 mg Q48h) did not represent the minimum concentration of the dosing interval. Therefore, these results suggested that the PK of elvucitabine is linear at the doses tested in this study.
Further work on the modeling using triphosphate data is warranted to determine if the metabolite concentrations have a linear relationship with the plasma concentrations and if the PK/pharmacodynamic model can be described using plasma concentrations.
Conclusion.
Elvucitabine PK behavior was well described by a linear two-compartment model with two first-order absorption rates and a first-order elimination rate in two different studies. Results suggest that the bioavailability of elvucitabine increases when lopinavir-ritonavir is coadministered. This increase is prominent in subjects displaying a lower starting bioavailability. We hypothesized that this could be due to ritonavir inhibiting an efflux gut transporter with activity present in various levels between subjects. The proposed PK model may be utilized and improved further in the future by linking the now-explained PK behavior of elvucitabine, with and without ritonavir, with various markers of efficacy.
Elvucitabine has a long terminal t1/2, ensuring that concentrations can remain detectable for at least 7 days after cessation of dosing. The long plasma t1/2 of elvucitabine sets this drug apart from other NRTIs. This could possibly allow for less-complicated and less-rigid dosing regimens than those for other NRTIs. These less-rigid regimens for elvucitabine may translate to increased adherence, leading to decreased emergence of resistance and improved health for HIV patients. Therefore, continued development of elvucitabine is warranted as it responds to the need for new HIV drugs with more favorable PK profiles.
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Anderson, P. O., J. E. Knoben, and W. G. Troutman. 2002. Handbook of clinical drug data, 10th ed. McGraw-Hill, New York, NY.
- 2.Barry, M., et al. 1999. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin. Pharmacokinet. 36:289-304. [DOI] [PubMed] [Google Scholar]
- 3.Collins, D., and A. Forrest. 1995. IT2S user's guide. State University of New York at Buffalo, Buffalo, NY.
- 4.Colucci, P., J. C. Pottage, H. Robison, J. Turgeon, and M. P. Ducharme. 2009. Effect of a single dose of ritonavir on the pharmacokinetic behavior of elvucitabine, a nucleoside reverse transcriptase inhibitor, administered in healthy volunteers. Antimicrob. Agents Chemother. 53:646-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Argenio, D., and A. Schumitzky. 1997. ADAPT II users manual. Biomedical Simulations Resource, University of Southern California-Los Angeles, CA.
- 6.d'Arminio Monforte, A., et al. 2000. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. AIDS 14:499-507. [DOI] [PubMed] [Google Scholar]
- 7.Drewe, J., et al. 1999. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem. Pharmacol. 57:1147-1152. [DOI] [PubMed] [Google Scholar]
- 8.Dunkle, L. M., S. C. Oshana, Y.-C. Cheng, K. Hertogs, W. G. Rice, et al. 2001. ACH-126,443: a new nucleoside analog with potent activity against wild-type and resistant HIV-1 and a promising pharmacokinetic and mitochondrial safety profile, abstr. 303. In Abstr. Eighth Conf. Retrovir. Oppor. Infect., Chicago, IL, 4 to 8 February 2001.
- 9.Evans, W. E., and H. L. McLeod. 2003. Pharmacogenomics-drug disposition, drug targets, and side effects. N. Engl. J. Med. 348:538-549. [DOI] [PubMed] [Google Scholar]
- 10.Fellay, J., et al. 2002. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 359:30-36. [DOI] [PubMed] [Google Scholar]
- 11.Fradette, C., et al. 2005. The utility of the population approach applied to bioequivalence in patients: comparison of 2 formulations of cyclosporine. Ther. Drug Monit. 27:592-600. [DOI] [PubMed] [Google Scholar]
- 12.Gutmann, H., et al. 1999. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol. Pharmacol. 56:383-389. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmeyer, S., et al. 2000. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurata, Y., et al. 2002. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin. Pharmacol. Ther. 72:209-219. [DOI] [PubMed] [Google Scholar]
- 15.Noveroske, J., J. Mao, et al. 2006. A comparative bioavailability study of β-L-FD4C and 3TC each administered by intravenous injection and orally by tablet to beagle dogs, abstr. B020-666. Oread BioSafety, Inc., Farmington, CT.
- 16.Olson, D. P., et al. 2002. The protease inhibitor ritonavir inhibits the functional activity of the multidrug resistance related-protein 1 (MRP-1). AIDS 16:1743-1747. [DOI] [PubMed] [Google Scholar]
- 17.Otto, M. J. 2004. New nucleoside reverse transcriptase inhibitors for the treatment of HIV infections. Curr. Opin. Pharmacol. 4:431-436. [DOI] [PubMed] [Google Scholar]
- 18.Piscitelli, S. C., and K. D. Gallicano. 2001. Interactions among drugs for HIV and opportunistic infections. N. Engl. J. Med. 344:984-996. [DOI] [PubMed] [Google Scholar]
- 19.Sakaeda, T., T. Nakamura, and K. Okumura. 2003. Pharmacogenetics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics 4:397-410. [DOI] [PubMed] [Google Scholar]
- 20.Schwab, M., M. Eichelbaum, and M. F. Fromm. 2003. Genetic polymorphisms of the human MDR1 drug transporter. Annu. Rev. Pharmacol. Toxicol. 43:285-307. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon, C., R. Sukovaty, and V. Andaloro. 2004. Validation of an LC-MS/MS method for the quantitation of ACH-126,443 in human EDTA plasma, abstr. 27427_1. MDS Pharma Services, Lincoln, NE.
- 22.Stypinski, D., et al. 2004. Optimization of the therapeutic index of elvucitabine through PK/PD modeling. 5th Int. Workshop Clin. Pharmacol. HIV Ther., Rome, Italy.
- 23.Woodahl, E. L., et al. 2005. MDR1 G1199A polymorphism alters permeability of HIV protease inhibitors across P-glycoprotein-expressing epithelial cells. AIDS 19:1617-1625. [DOI] [PubMed] [Google Scholar]
- 24.Wright, M. T. 2000. The old problem of adherence: research on treatment adherence and its relevance for HIV/AIDS. AIDS Care 12:703-710. [DOI] [PubMed] [Google Scholar]