Abstract
The combined systemic and topical administration of voriconazole has successfully been used to treat keratomycosis. Because no voriconazole eye drop product is commercially available, we prepared a sterile eye drop solution (10 mg/ml). Voriconazole remains stable over 30 days, providing an eye drop solution suitable for use for the topical treatment of fungal keratitis.
Voriconazole is a recent triazole antifungal agent that is highly effective against Aspergillus, Fusarium, and Candida species. Furthermore, voriconazole is one of the few drugs active against Scedosporium apiospermum (10). These fungi may cause severe keratomycosis, a rare but sight-threatening infection of the cornea (4).
The conventional management of keratomycosis includes the local application, with or without systemic administration, of antifungal drugs. The use of voriconazole for the treatment of visually devastating disease due to Scedosporium apiospermum has been proposed (7, 14). The treatment combines the systemic and the topical administration of voriconazole (6). More recently, voriconazole has successfully been used for the treatment of keratitis caused by a variety of fungi, most commonly, Aspergillus species, Fusarium species, and Candida species (2, 8, 9, 11).
As is the case for many antimicrobial agents, no voriconazole eye drop solution is commercially available. Although several authors have reported on the potential of voriconazole for use for topical ophthalmic administration, none of them focused on the stability or the storage of voriconazole. This paper describes how we prepared a voriconazole ophthalmic solution. In addition, we performed a study in order to assess the stability of this solution over 1 month. Finally, the sterility of the preparation was evaluated.
On the basis of information available in the literature (8, 9, 11), a 1% (10-mg/ml) voriconazole eye drop solution was prepared by using the branded product: voriconazole powder for solution for intravenous infusion (Vfend; Pfizer). Voriconazole powder (200 mg) was reconstituted with 19 ml of water for injections in order to obtain 20 ml of a 10-mg/ml voriconazole solution. Sterile filtration through a 0.20-μm-pore-size filter was then performed. Finally, the voriconazole solution was packaged in a specific sterile vial for use as an eye drop solution. In order to keep the solution sterile, reconstitution was performed according to recommendations for aseptic preparation.
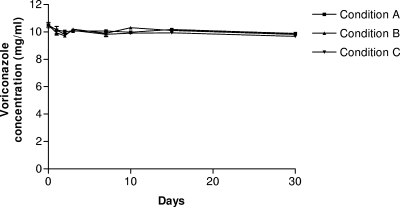
In order to cure keratomycosis, antifungal eye drops must be administered over a long period (at least 6 weeks). Although the eye drop solution needs to be kept for several days after preparation for its use by a patient, its stability was unknown. Therefore, in order to assess the stability of the voriconazole eye drop solution, three batches of the formulation were stored for up to 30 days under different projected conditions suitable for its routine use. Under condition A, the solutions (n = 6) were kept at room temperature (24 ± 3°C) and were not protected from light (colorless type I glass vial). Under condition B, the solutions (n = 6) were kept at room temperature (24 ± 3°C) and were protected from light (colored type I glass vial). Under condition C, the solutions (n = 6) were kept refrigerated (4 ± 2°C) and were protected from light (colored type I glass vial).
After storage under the different appropriate conditions, the solutions were inspected visually. An osmolarity assay was performed, and the pH of the solutions was determined. Finally, the voriconazole contents of the solutions were determined by high-performance liquid chromatography (HPLC) on days 0, 1, 2, 3, 7, 10, 15, and 30. A previously described HPLC assay (5) was used, with minor modifications. Briefly, separation was performed with a Kromasil C18 column (5 μm, 150 by 3 mm). The mobile phase consisted of 0.04 M aqueous phosphate buffer (pH 6.0) containing 50% (vol/vol) methanol, and the flow rate was 1 ml ·min−1. The retention time of voriconazole was equal to 7.5 min. The wavelength of the UV absorbance detector was set at 225 nm. The method was linear over a concentration range of 0 of 20 μg ·ml−1 (r2 ≥ 0.9997), and the limit of quantitation of voriconazole was equal to 1.25 μg ·ml−1. The intraday and interday coefficients of variation calculated with two concentrations were equal to or less than 5.7%. To ensure that the method can suitably indicate the stability of the solution, we checked that the decomposition products obtained from the voriconazole solution subjected to severe stress (100°C, pH 1) did not coelute with the intact drug (12).
Sterility tests were performed by the use of a closed-membrane filtration method (Steritest; Millipore). Samples were incubated for 14 days, and the results were read daily. Bacterial growth was determined by visual examination. A test for sterility was performed on days 0 and 30, and the requirements for sterility were met only when no growth was observed.
The mean recovery of the voriconazole content in the eye drop preparation was more than 99%, demonstrating that almost no drug was lost during preparation. The solution was clear, and no color was detected just after preparation or during storage. In addition, the sterility of the sealed preparation was preserved throughout the storage period.
Whatever storage condition was used, no significant degradation of voriconazole occurred (Fig. 1). Indeed, during the period of testing, the proportion of the initial voriconazole concentration remaining was >90%, the limit set by the U.S. Pharmacopeia (13). Voriconazole was stable under regular storage temperatures (+4°C and room temperature). Furthermore, exposure to light did not cause the degradation of voriconazole.
FIG. 1.
Stability of voriconazole eye drop solution under the three storage conditions, conditions A to C, as described in the text. All values are the means of six independent determinations ± standard deviations (symbols without bars correspond to values for which the standard deviation is smaller than the symbol size).
During storage, the pH of the voriconazole solution remained stable (7.0 ± 0.1), and no significant variation in the osmolarity occurred (562 ± 10 mosmol/liter). These results indicate that the voriconazole in the solution is chemically stable for at least 30 days, and the amount of intact drug remaining suggests that the microbiological activity of the solution is preserved.
The parenteral formulation of the commercial product (Vfend) contains a new excipient, sulfobutylether-β-cyclodextrin sodium (SBEβCD), that enhances the solubility of voriconazole. Cyclodextrin facilitates the formulation of the eye drop solution and improves its clinical properties (1). SBEβCD has already been used for ocular drug delivery, with success, at final concentrations ranging from 5 to 15% (1). The SBEβCD concentration in our eye drop solution was equal to 160 mg/ml, leading to a hyperosmolar ophthalmic solution compared to the physiological osmolarity of tears. Nevertheless, several anti-infectious eye drop preparations commonly used to treat infectious keratitis have similarly high osmolarities (3) but do not cause significant side effects. Therefore, the osmolarity of our preparation was considered to be safe and compatible with ocular administration. Finally, the voriconazole preparation provided a neutral pH solution close to the physiological pH of tears (about 7.4); because tears have a wide buffering capacity, no pH adjustment was made.
The recent increasing use of topical voriconazole by patients with severe keratomycosis raises the issue of eye drop stability and storage. Several authors recommend that topical voriconazole solution be kept refrigerated for no longer than 48 h, as recommended by the manufacturer (2, 9). These storage conditions are highly constraining, in particular, for outpatient treatment. Therefore, according to the findings of this work, the stability of the voriconazole eye drop solution prepared under our conditions could be extended to up to 30 days.
Acknowledgments
We thank Regis Lubat for helpful discussions.
Footnotes
Published ahead of print on 10 November 2008.
REFERENCES
- 1.Bourlais, C. L., L. Acar, H. Zia, P. A. Sado, T. Needham, and R. Leverge. 1998. Ophthalmic drug delivery systems—recent advances. Prog. Retin. Eye Res. 17:33-58. [DOI] [PubMed] [Google Scholar]
- 2.Bunya, V. Y., K. M. Hammersmith, C. J. Rapuano, B. D. Ayres, and E. J. Cohen. 2007. Topical and oral voriconazole in the treatment of fungal keratitis. Am. J. Ophthalmol. 143:151-153. [DOI] [PubMed] [Google Scholar]
- 3.Chédru-Legros, V., M. Fines-Guyon, A. Chérel, A. Perdriel, F. Albessard, D. Debruyne, and F. Mouriaux. 2007. Fortified antibiotic (vancomycin, amikacin and ceftazidime) eye drop stability assessment at −20 degrees C. J. Fr. Ophtalmol. 30:807-813. [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Valle, D., J. M. Benitez del Castillo, E. Amor, N. Toledano, M. M. Carretero, and T. Díaz-Valle. 2002. Severe keratomycosis secondary to Scedosporium apiospermum. Cornea 21:516-518. [DOI] [PubMed] [Google Scholar]
- 5.Gage, R., and D. A. Stopher. 1998. A rapid HPLC assay for voriconazole in human plasma. J. Pharm. Biomed. Anal. 17:1449-1453. [DOI] [PubMed] [Google Scholar]
- 6.Hernández Prats, C., F. Llinares Tello, A. Burgos San José, J. Selva Otaolaurruchi, and J. P. Ordovás Baines. 2004. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann. Pharmacother. 38:414-417. [DOI] [PubMed] [Google Scholar]
- 7.Lai, T. F., R. Malhotra, R. Esmail-Zaden, A. Galanopoulas, M. Chehade, and D. Selva. 2003. Use of voriconazole in Scedosporium apiospermum keratitis. Cornea 22:391-392. [DOI] [PubMed] [Google Scholar]
- 8.Lau, D., M. Fedinands, L. Leung, R. Fullinfaw, D. Kong, G. Davies, and M. Daniell. 2008. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch. Ophthalmol. 126:343-346. [DOI] [PubMed] [Google Scholar]
- 9.Mehta, H., H. B. Mehta, P. Garg, and H. Kodial. 2008. Voriconazole for the treatment of refractory Aspergillus fumigatus keratitis. Indian J. Ophthalmol. 56:238-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott, L. J., and D. Simpson. 2007. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 67:269-298. [DOI] [PubMed] [Google Scholar]
- 11.Thiel, M. A., A. S. Zinkernagel, J. Burhenne, C. Kaufmann, and W. E. Haefeli. 2007. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob. Agents Chemother. 51:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trissel, L. A. 1983. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am. J. Hosp. Pharm. 40:1159-1160. [PubMed] [Google Scholar]
- 13.U.S. Pharmacopeial Convention. 1995. Pharmacopeia of the United States of America (the national formulary). U.S. Pharmacopeial Convention, Rockville, MD.
- 14.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum: report of two cases and review of treatment. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]