Abstract
The insulin receptor (IR) exists as two isoforms, IR-A and IR-B, which result from alternative splicing of exon 11 in the primary transcript. This alternative splicing is cell specific, and the relative proportions of exon 11 isoforms also vary during development, aging, and different disease states. We have previously demonstrated that both intron 10 and exon 11 contain regulatory sequences that affect IR splicing both positively and negatively. In this study, we sought to define the precise sequence elements within exon 11 that control exon recognition and cellular factors that recognize these elements. Using minigenes carrying linker-scanning mutations within exon 11, we detected both exonic splicing enhancer and exonic splicing silencer elements. We identified binding of SRp20 and SF2/ASF to the exonic enhancers and CUG-BP1 to the exonic silencer by RNA affinity chromatography. Overexpression and knockdown studies with hepatoma and embryonic kidney cells demonstrated that SRp20 and SF2/ASF increase exon inclusion but that CUG-BP1 causes exon skipping. We found that CUG-BP1 also binds to an additional intronic splicing silencer, located at the 3′ end of intron 10, to promote exon 11 skipping. Thus, we propose that SRp20, SF2/ASF, and CUG-BP1 act antagonistically to regulate IR alternative splicing in vivo and that the relative ratios of SRp20 and SF2/ASF to CUG-BP1 in different cells determine the degree of exon inclusion.
In mammals, alternative splicing is a common strategy for creating functional diversities of proteins that have cell and developmentally specific functions. Given the important role for splicing, it is not surprising that a recent estimate has proposed that 50 to 60% of mutations linked to disease affect splicing (21, 43). The majority of human genes undergo alternative pre-mRNA splicing through the use of competing 5′ or 3′ splice sites or through alternative inclusion/exclusion of exons in the pre-mRNA. These alternative exons often contain splice sites that diverge from the consensus site, and the presence of cis regulatory elements within the exon and/or the flanking introns determines whether these exons are recognized (18, 20, 31). These cis elements can have either a positive (enhancer) or a negative (silencer) effect on splicing. Both enhancers and silencers are thought to function through binding to specific trans-acting protein factors (1). Differences in the expression or activities of these trans-acting factors may modulate the recognition of the alternative exon and lead to developmental or tissue-specific differences in splicing. Proteins that bind to specific sequence elements to affect splice site selection include SR proteins, hnRNPs, and other related RNA binding proteins, such as the CELF family, TIA-1, and Raver-1 (11, 12, 14, 25, 32). Adding a further layer of regulation, local context, such as RNA secondary structure, may influence the way that binding motifs are recognized by their cognate factors (3, 10, 13).
The human insulin receptor (IR) is encoded by a single INSR gene that is located on chromosome 19 and composed of 22 exons. Transcription of the gene gives rise to two protein isoforms, however, that differ by a 12-amino-acid insertion in the hormone-binding domain of the receptor, due to alternative splicing of exon 11. In the embryo, the IR lacking exon 11 (IR-A) promotes growth due to its ability to bind both insulin and insulin-like growth factor II; in the adult, the IR containing exon 11 (IR-B) is expressed predominantly in the insulin-sensitive tissues comprising the liver, muscle, adipocytes, and kidney, which regulate glucose homeostasis, and binds only insulin. Inclusion of IR exon 11 is both developmentally and hormonally regulated and is altered in a number of disease states, such as type II diabetes, myotonic dystrophy, aging, and cancer (15, 17, 27-29, 33). The dysregulation of the alternative splicing of the IR may therefore have important consequences for insulin and insulin-like growth factor II sensitivity and responsiveness. This makes the IR gene an attractive model system for studying the mechanism of alternative splicing, and identification of regulatory sequences and factors that control the IR-B/IR-A ratio is of critical importance for the understanding of the role of the IR in different disease states.
We have previously shown that exon 11 of the human INSR gene conforms to the general model of alternative splicing described above. The exon is small (36 nucleotides [nt]) and is flanked by large introns (2.3 kb and 7.5 kb). The splice sites flanking exon 11 are weak and diverge from the consensus site, and strengthening either site by mutation to the consensus site renders the exon constitutive (46). We have also defined putative splicing enhancers and silencers in the precursor RNA through a combination of deletions and mutations, using a minigene transfection system (16). An intronic splicing enhancer was found at the 5′ end of intron 10 near the 5′ splice site and an intronic splicing silencer (ISS) near the 3′ splice site (16). Regulatory elements, both an exonic splicing enhancer (ESE) and an exonic splicing silencer (ESS), were also proposed to occur in the alternatively spliced exon itself. The precise locations of these elements as well as the splicing factors that recognize these elements to modulate exon definition were not known, and identification of these factors constituted the goal of this study.
Herein, we report that SRp20 and SF2/ASF mediate the activity of the ESE elements in vivo, whereas the ESS and ISS elements require the binding of CUG-BP1. Overexpression of SRp20 enhances exon inclusion, whereas overexpression of CUG-BP1 impairs exon inclusion. Our results suggest that the balance of SR proteins and CUG-BP1 is critical for regulation of exon 11 inclusion in vivo.
MATERIALS AND METHODS
Plasmid constructs.
The wild-type human INSR minigene (minigene B) and a deletion mutant (minigene N) have previously been described (16). All other plasmids were constructed using standard techniques. Linker-scanning mutants in exon 11 and the ISS were generated by PCR from minigene B, using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions.
Cell culture, transfections, and RNA extraction.
Unless otherwise stated, all tissue culture media and supplements were purchased from Invitrogen (Carlsbad, CA). Human hepatoma liver (HepG2) cells and human embryonic kidney 293 (HEK293) cells were maintained routinely in minimum essential medium plus Earle's salts with 10% fetal bovine serum and gentamicin sulfate antibiotic at 37°C under 5% CO2. The cells were plated at a density of ∼1 × 106 cells/well in six-well dishes. Medium was changed every 2 days. Transient transfections of cells with plasmid DNA were performed with Fugene-6 (Roche, Indianapolis, IN) according to the manufacturer's protocol. For cotransfection experiments, cells were transfected with the indicated amounts of minigene plasmid DNA and an expression vector for splicing factors of interest. In a given experiment, the total amount of DNA was maintained constant by adding control vector. Cells were harvested 48 h after transfection, and total cellular RNA was prepared using RNAzol B (Tel-Test, Inc., Friendswood, TX), following the manufacturer's directions, and precipitated twice.
Reverse transcription and amplification of cDNA.
To generate cDNA, total RNA (1.0 μg) was reverse transcribed using SuperScript II and an oligo(dT) primer (Applied Biosystems). PCR amplification of IR splice products derived from the minigenes was performed as published previously (16), using minigene-specific primer sets. Spliced products were visualized on 12% polyacrylamide gels, stained with ethidium bromide, and quantified using Kodak Electrophoresis Documentation and Analysis System 290. Results were confirmed by three independent experiments and expressed as percentages of IR-B. Statistical comparisons of exon inclusion were performed by analysis of variance or a t test, using Microsoft Excel (Redmond, WA).
Immobilization of RNA on agarose beads and RNA binding assays.
Substrate RNAs for bead immobilization were chemically synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). RNA affinity chromatography was performed by modification of a published procedure (4). Briefly, 1,000 pmol of RNA was oxidized with sodium m-periodate (Sigma, St. Louis, MO) and covalently coupled to 400 μl of a 50% slurry of adipic acid dihydrazide agarose beads (Sigma). The beads were washed three times with 2 M NaCl and then equilibrated with buffer D (20 mM HEPES-KOH, pH 7.6, 10% [vol/vol] glycerol, 150 mM KCl, 0.2 mM EDTA, 0.5 mM dithiothreitol). An RNA agarose bead slurry was incubated with 75 μl of HeLa nuclear extract under splicing conditions at 30°C for 25 min in buffer D (total volume, 600 μl). The beads were washed five times with buffer D. Bound proteins were then eluted by boiling them in 60 μl of 2× protein sample buffer. The affinity-selected proteins were electrophoresed on a 4 to 12% bis-Tris gel (Bio-Rad Laboratories, Hercules, CA) and analyzed by Western blotting.
siRNA transfections and analysis.
Double-stranded, preannealed small interfering RNA (siRNA) oligonucleotides directed against SRp20, SF2/ASF, CUG-BP1, and scrambled siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and siRNA directed against SRp20 was purchased from Integrated DNA Technologies, Inc. Transfections with si-CUG-BP1 were performed with HEK293, using TransFast reagent (Promega, Madison, WI). Transfections with si-SRp20 and SF2/ASF in HepG2 cells were performed with Fugene 6 (Roche). The final siRNA concentration for transfection was 100 nM. Forty-eight hours after transfections, cells were harvested and assayed for protein or mRNA expression.
Whole-cell lysate preparation and Western blot analysis.
Whole-cell extracts were prepared by harvesting the same number of HepG2 and HEK293 cells. Lysates were prepared by sonication in harvesting buffer (10 mM Tris, 1% sodium dodecyl sulfate). Total proteins were quantified with a DC protein assay kit (Bio-Rad Laboratories), using bovine serum albumin as a standard. Equal amounts of protein were resolved in 4 to 12% bis-Tris gels and transferred to a polyvinyl difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with 3% nonfat dried milk in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween 20) and then exposed to the appropriate concentrations of primary antibodies overnight at 4°C. The following primary antibodies were used: mouse monoclonal anti-SRp20 (1:1,000; Zymed Laboratory, San Francisco, CA), mouse monoclonal anti-CUG-BP1 (1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA), goat monoclonal anti-SF2/ASF (1:3,000; Santa Cruz Biotechnology), and mouse monoclonal anti-β-tubulin (1:10,000; Santa Cruz Biotechnology). A culture supernatant from hybridoma cells expressing mAb104 (ATCC, Manassas, VA) was used as a source of mAb104 antibody. After being washed with TBS-T, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G or donkey anti-goat immunoglobulin G (Jackson Immunoresearch, West Grove, PA), and then proteins were detected by enhanced chemiluminescence plus Western blotting detection reagents (GE Healthcare, Piscataway, NJ). Results were confirmed by at least three independent experiments.
Immunodepletion.
HeLa nuclear extracts were immunodepleted with a culture supernatant from hybridoma cells expressing antibodies to SRp20 (CRL-2384; ATCC). Briefly, immunodepletion was performed using 300 μl of antisera. Immunocomplexes were precipitated using GammaBind G Sepharose (GE Healthcare), and depleted extracts were used in the RNA affinity purification assay described above. In order to check the efficiency of the immunodepletion, a fraction of the immunodepleted extract was loaded on a 4 to 12% bis-Tris gel and subjected to Western blotting using anti-SRp20.
RESULTS
Exon 11 contains both an ESE and an ESS.
We have previously defined putative splicing enhancers and silencers in the INSR gene through a combination of deletion and substitution mutations using a minigene transfection system. Notably, point mutations, small insertions, or deletions within exon 11 could both increase and decrease exon incorporation, suggesting the presence of both an enhancer (ESE) and a silencer (ESS) in the alternatively spliced exon itself. To define the splicing regulatory elements in exon 11 more precisely, we have systematically mutated IR exon 11 in the minigene B splicing construct (Fig. 1A) and assessed in vivo splicing in transfected HepG2 and HEK293 cells. The endogenous INSR gene in HepG2 cells generates mRNA for the A and B isoforms of the IR in a 20:80 ratio, whereas HEK293 cells produce mainly the A isoform (>95%). So, we used these two cell lines to assess IR splicing and the splicing enhancer and silencer.
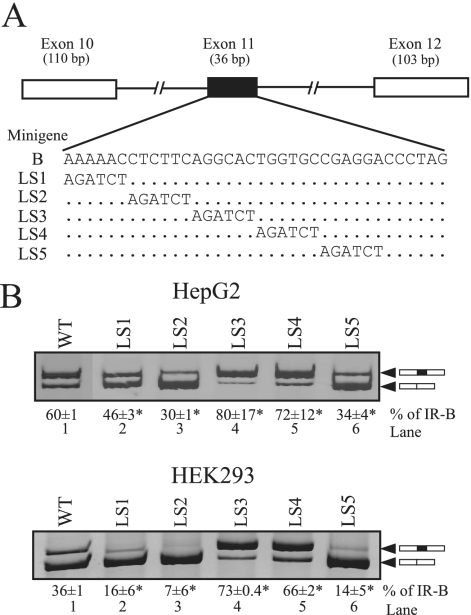
FIG. 1.
Linker-scanning mutagenesis of exon 11 identifies both ESEs and an ESS. (A) Schematic of the IR minigene carrying exons 10, 11, and 12, with the wild-type sequence of exon 11 (row B) and the linker-scanning (LS) substitutions shown below. All mutant constructs are the same length as the wild-type minigene. (B) Spliced products from the wild-type minigene (WT) and the linker-scanning mutations in transfected HepG2 and HEK293 cells. Total RNA was isolated at 48 h posttransfection and was subjected to reverse transcription-PCR (RT-PCR) analysis using primers specific to the transfected IR minigene mRNA. A representative gel is shown. The upper band contains exon 11 (black box), and the lower band lacks exon 11. The means and standard deviations (SD) for percentages of exon 11 inclusion (IR-B) are shown below the gel image. Results are derived from three independent experiments, and asterisks represent significant differences from the WT control level (P < 0.05.).
Transfection of the wild-type minigene (minigene B) into HepG2 and HEK293 cells gave RNA corresponding to both IR-A and IR-B isoform splicing patterns, comparable to what was found for the endogenous INSR gene (Fig. 1B, lane 1). We created six linker-scanning mutants that replaced blocks of 6 nt with a BglII restriction site (AGATCT). The last mutant, LS6, was uninformative, as it prevented recognition of the downstream 5′ splice site, and so was excluded from further analysis. The remaining five LS mutants were transfected into HepG2 and HEK293 cells. Analysis of exon inclusion in RNA derived from the mutant minigenes revealed that replacement of the first 12 nt of exon 11 (LS1 and LS2) caused significant decreases in exon 11 incorporation in both HepG2 and HEK293 cells (Fig. 1B, lanes 2 and 3). This is in agreement with a small deletion (nt 7 to 14) in this region that we had previously characterized (16). Replacement of nt 24 to 30 in exon 11 (LS5) also decreased exon incorporation (Fig. 1B, lane 6). These results suggest two potential ESEs at either end of exon 11. In contrast, the levels of exon 11 inclusion were greatly enhanced in LS3 and LS4 substitution mutants (Fig. 1B, lanes 4 and 5). This region coincides with a previous substitution mutation (16) that caused the exon to be spliced constitutively; thus, the improved inclusion of exon 11 in LS3 and LS4 mutants was most probably due to the loss of an ESS. Taken together, these results indicate the presence of multiple regulatory elements, both positive and negative, within exon 11.
The ESEs bind SRp20 and SF2/ASF.
As SR proteins recognize splicing enhancers in other genes, we analyzed binding of SR proteins to exon 11 by RNA affinity purification. RNA oligonucleotides corresponding to the wild-type or mutated LS1-LS5 exon sequence were coupled to adipic acid dihydrazide agarose beads and used to affinity purify splicing factors from HeLa cell nuclear extracts. Nuclear proteins which bound to the immobilized RNAs were analyzed by 4 to 12% bis-Tris gels and immunoblotting with mAb104, an antibody that recognizes the common phosphorylated epitope on SR proteins (39). A representative Western blot is shown (Fig. 2A, upper). A number of protein bands were detected on all templates; however, a 20-kDa band was specifically eliminated by the LS2 mutation in the ESE. The reduced binding of a 40-kDa protein in mutants LS3, LS4, and LS5 was not consistently observed in other experiments. The 20-kDa protein binds to both the wild-type exon and the LS1 mutant RNA and, surprisingly, appears to bind more strongly to the LS3, LS4, and LS5 mutant RNAs (Fig. 2A, lanes 4 to 6). No binding was found with the LS2 mutant RNA (Fig. 2A, lane 3). The differences in binding are not due to unequal protein loading, since similar levels of other SR proteins were detected in each sample. The 20-kDa molecular mass suggested that this SR protein might be SRp20. We confirmed the identity of the 20-kDa protein by using a SRp20-specific monoclonal antibody (Fig. 2A, lower). As before, the LS2 mutation eliminates binding of SRp20. Interestingly, binding of SRp20 was not increased on mutants LS3, LS4, and LS5, as had been seen with mAb104, suggesting that the phosphorylation state of SRp20 might be altered on these mutant templates. The site comprising nt 7 to 12 of exon 11 resembles the consensus high-affinity SRp20 site (CUCKUCY) identified by SELEX (Fig. 2B), suggesting that SRp20 binds to the hexamer CUCUUC in the ESE (34).
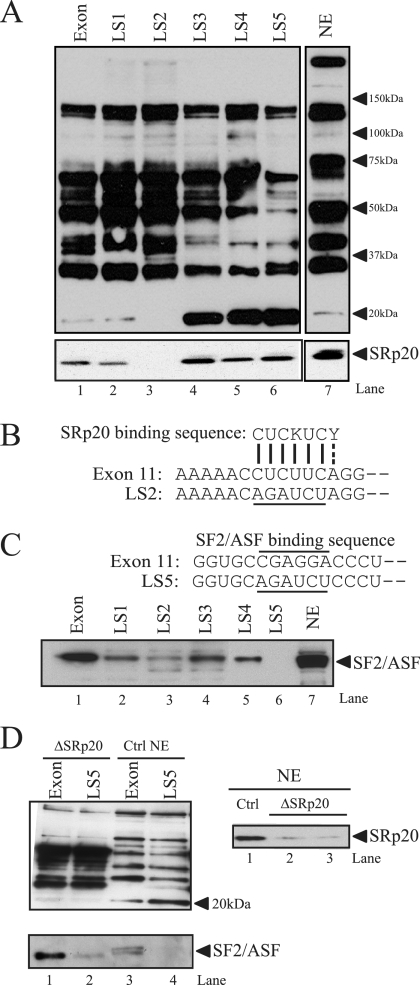
FIG. 2.
SRp20 and SF2/ASF bind to the exon 11 ESE. (A) RNA oligonucleotides corresponding to exon 11 or the LS mutants were covalently linked to adipic acid dihydrazide agarose beads. HeLa nuclear extracts (NE) were incubated with the beads and washed extensively; then, associated proteins were eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Bound proteins were separated on 4 to 12% bis-Tris gels and immunoblotted with antibody mAb104 (upper), which recognizes a common phospho-epitope on SR proteins or a monoclonal antibody to SRp20 (lower). NE indicates HeLa nuclear extract (1/25 of input). Representative blots are shown. The experiment was repeated five times, with similar results. (B) Sequence alignment of the exon 11 ESE with the consensus SRp20 binding site. The LS2 mutation (underlined) disrupts this homology. (C) The sequence of the putative SF2/ASF binding site (overlined) and the position of the LS5 linker-scanning mutant (underlined) in exon 11 are indicated (upper). A representative blot shows results for RNA affinity purification of HeLa nuclear proteins followed by immunoblotting with antibodies to SF2/ASF. (D) HeLa nuclear extracts were depleted using antibodies to SRp20, and residual SRp20 levels were determined by Western blotting (right). The same extracts were used for RNA affinity purification and Western blotting with antibody mAb104 (left). The blot was reprobed with anti-SF2/ASF antibodies to check the integrity of other SR proteins in nuclear extract after depletion.
The second ESE of exon 11 (25 to 30 nt) contains a purine rich sequence. Many SR proteins recognize purine-rich sequences; therefore, we used an ESE finder (38) to search for potential binding motifs. A high-score binding motif for SF2/ASF (CGAGGA) was found in this region (Fig. 2C, upper). To examine whether SF2/ASF binds to the ESE, we analyzed the RNA affinity-purified proteins on the exon and LS templates by using an SF2/ASF monoclonal antibody (Fig. 2C, lower). SF2/ASF binding was detected on the exon and LS1-LS4 templates (Fig. 2C, lanes 1 to 5) but was not detected on LS5 (Fig. 2C, lane 6), in which the SF2/ASF binding sequence, CGAGGA, has been replaced by AGAUCU, suggesting that SF2/ASF may participate in the enhancer function through binding to the CGAGGA motif.
To ensure that the 20-kDa band recognized by mAb104 in LS3, LS4, and LS5 mutants was phosphorylated SRp20, not another SR protein(s), the RNA affinity assay was repeated using SRp20-depleted HeLa nuclear extract with wild-type exon and LS5 templates. HeLa nuclear extracts were depleted using antibodies to SRp20, and residual SRp20 levels were determined by Western blotting and compared to levels for untreated control extract (Fig. 2D, right). No 20-kDa band was seen on LS5 or exon templates after Western blot analysis with mAb104, confirming that the 20-kDa band on LS5 was indeed SRp20. The integrity of other SR proteins in the SRp20-depleted HeLa nuclear extract was confirmed by stripping and reprobing the blot with SF2/ASF.
SRp20 increases IR exon 11 inclusion in vivo by binding to the ESE.
To assess the role of SRp20 and SF2/ASF in alternative splicing of the IR, we evaluated the effects of overexpressing a panel of SR proteins on the alternative splicing of exon 11 in vivo. Plasmids expressing cDNAs for SRp20, SRp40, 9G8, SRp30C, SC35, SRp75, and SF2/ASF were cotransfected into HepG2 cells with the wild-type IR minigene. Overexpression of SRp20 alone enhanced exon 11 inclusion (Fig. 3A, lane 2). Surprisingly, overexpression of SF2/ASF did not alter exon inclusion. To test whether SRp20 requires the hexamer CUCUUC, the SRp20 vector was cotransfected with linker-scanning mutants LS1 to LS5. As expected, overexpression of SRp20 increased exon 11 inclusion significantly in HepG2 cells for both the wild-type exon and mutant LS5 (Fig. 3B, upper, lanes 2 and 12). In LS3 and LS4, exon 11 inclusion was already very high, so the stimulatory effect of SRp20 was weaker in these mutants (Fig. 3B, upper, lanes 8 and 10). The LS2 mutation, and to some extent the LS1 mutation, eliminated the ability of SRp20 to increase exon 11 inclusion in HepG2 cells (Fig. 3B, upper, lanes 4 and 6). In HEK293 cells, SRp20 also increases exon 11 inclusion on the wild-type exon and mutants LS3 and LS4 but not on mutants LS1, LS2, and LS5 (Fig. 3B, lower), which show almost exclusive skipping of exon 11 in these cells. To confirm the role of endogenous SRp20 in alternative splicing of exon 11, we used RNA interference to knock down SRp20. For these experiments, we used the HepG2 cells line, as it shows the greatest exon inclusion. SRp20 siRNA was cotransfected into HepG2 cells with the wild-type IR minigene. Knockdown of SRp20 led to a moderate decrease (∼50%) in SRp20 protein level (Fig. 3C, left) and also decreased exon 11 inclusion (25% versus 55%) in the wild-type minigene (Fig. 3C, right, lanes 1 and 2). No change in splicing was observed upon SRp20 knockdown in the LS2 mutant, where the SRp20 binding site had been disrupted (Fig. 3C, right, lanes 3 and 4). Identical results were obtained with siRNAs from two different sources (IDT and Santa Cruz Biotechnology), confirming the specificity of the effect.
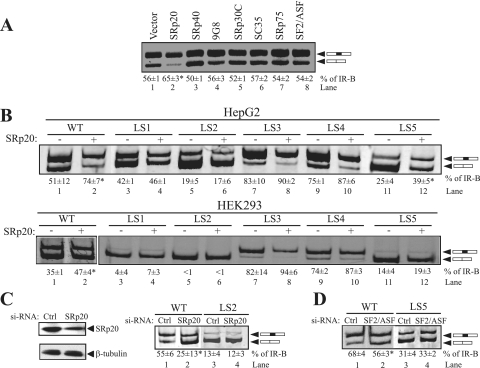
FIG. 3.
SRp20 increases IR exon 11 inclusion in vivo by binding to the ESE. (A) The IR minigene (500 ng) was cotransfected with 1 μg of seven individual SR protein expression vectors in HepG2 cells, and spliced products were analyzed by RT-PCR. A representative gel is shown. The mean percentages of exon 11 inclusion (% IR-B) ± SD derived from three independent experiments are given below the gel. (B) Linker-scanning mutants LS1 to LS5 were cotransfected with 1 μg of SRp20 protein expression vector in HepG2 (upper) and HEK293 (lower) cells, and spliced products were analyzed by RT-PCR. Representative gels are shown. The mean percentages of exon 11 inclusion (% IR-B) ± SD are shown below the gel (n = 3). WT, wild type. (C) The wild-type minigene or the LS2 mutant (500 ng) was cotransfected into HepG2 cells with 100 nM siRNAs directed against SRp20 or scrambled control siRNA. SRp20 protein content was measured by immunoblotting with anti-SRp20 monoclonal antibodies (left). The blot was stripped and reprobed for beta-tubulin protein expression as an internal control. Representative results are shown for RT-PCR analysis of the spliced products amplified from RNA isolated from HepG2 cells treated with si-SRp20 (right). (D) Representative RT-PCR analysis of the spliced products amplified from RNA isolated from HepG2 cells treated with SF2/ASF siRNA. Asterisks indicate statistical significance (P < 0.05) relative to levels for the vector control (A and B) or the control scrambled siRNA (C and D).
Knockdown of SF2/ASF decreases exon 11 inclusion.
Although SF2/ASF binds to the putative ESE, we did not see any stimulatory effect on exon 11 inclusion upon SF2/ASF overexpression in HepG2 cells (Fig. 3A, lane 8). However, HepG2 cells express high levels of endogenous SF2/ASF, which may obscure effects due to overexpression. To address whether endogenous SF2/ASF protein affects the alternative splicing of IR exon 11 in vivo, we used siRNA knockdown of SF2/ASF in HepG2 cells. In contrast to what was found in the overexpression experiments, siRNA-mediated removal of SF2/ASF decreased exon 11 inclusion significantly in HepG2 cells (Fig. 3D, lanes 1 and 2). Knockdown of SF2/ASF did not have any effect on exon 11 inclusion in the LS5 mutant, where the SF2/ASF binding site had been mutated (Fig. 3D, lanes 3 and 4).
The ESS binds CUG-BP1 in vitro.
In contrast to the ESE, the ESS did not appear to specifically bind any SR protein, as no decrease in binding was observed with LS3 or LS4 templates. A closer examination of the ESS sequence in exon 11 revealed the sequence CUGGUGCCG, which is a 7/9 match to a CUG repeat. Such CUG repeats are known to be the binding site for CUG-BP1 (22, 42). The LS3, LS4, and LS5 mutations all disrupt the CUGGUGCCG motif (Fig. 4A). Therefore, we tested whether CUG-BP1 binds to exon 11 by immunoblotting using a specific CUG-BP1 antibody following RNA affinity purification of HeLa nuclear extract on wild-type and LS mutant exon RNAs (Fig. 4B). CUG-BP1 bound strongly to the wild-type exon, LS1, and LS2 templates but weakly to the LS3, LS4, and LS5 templates (Fig. 4B). Thus, mutation of the CUG motif reduces CUG-BP1 binding in vitro.
FIG. 4.
CUG-BP1 binds to the exon 11 ESS in vitro. (A) Sequence of the ESS element and the positions of LS3, LS4, and LS5 linker-scanning mutants (underlined) in exon 11. The putative CUG-BP1 binding site is indicated by the overline. (B) RNA affinity purification of HeLa nuclear proteins followed by immunoblotting with antibodies to CUG-BP1. “NE” indicates HeLa nuclear extracts (1/25 of input). WT, wild type. (C) The WT minigene (500 ng) and linker-scanning mutants LS3, LS4, and LS5 were cotransfected with 1 μg of CUG-BP1 protein expression vector in HepG2 (upper) and HEK293 (lower) cells, and spliced products were analyzed by RT-PCR. The mean percentages of exon 11 inclusion (% IR-B) and SD derived from three independent experiments are given below the gel. Asterisks indicate statistical significance (P < 0.05) relative to levels for the vector control.
CUG-BP1 represses IR exon 11 incorporation in vivo.
To determine the functional role of CUG-BP1 in IR splicing in HepG2 and HEK293 cells, CUG-BP1 was overexpressed with the wild-type IR minigene. In agreement with the previous report of the repressive effect of CUG-BP1 on exon 11 inclusion in muscle (33), we found that CUG-BP1 expression decreased exon 11 incorporation significantly in these cell lines (Fig. 4C). The CUG-BP1 effect was greater in HepG2 cells than in HEK293 cells, as the basal exon inclusion is much higher. To establish that CUG-BP1 represses IR splicing via the sequence CUGGUGCCG in exon 11, CUG-BP1 was transfected with LS3, LS4, and LS5 linker-scanning mutants. Surprisingly, mutants LS3, LS4, and LS5 had no effect on the inhibition by CUG-BP1 in either HepG2 or HEK293 cells. This might be explained by CUG-BP1 binding being not completely abolished with these mutations and this residual binding being sufficient for CUG-BP1 inhibitory action or by CUG-BP1 binding to other sequences in the INSR gene to repress exon inclusion.
CUG-BP1 also binds to the ISS element.
We had previously (16) identified another silencer element in the intron upstream of exon 11. As the effect of CUG-BP1 is inhibitory, we tested whether CUG-BP1 binds to the ISS element. The ISS had been localized to a 42-nt sequence in intron 10. Before attempting to purify RNA binding proteins by using this motif, we created another series of linker-scanning mutants to more closely define the ISS element. Seven linker-scanning mutants (INH1 to INH7) were transfected into HepG2 cells (Fig. 5A). Two mutants, INH4 and INH5, caused statistically significant increases in exon incorporation, equivalent to deletion of the entire region (Fig. 5A, comparison to minigene N), suggesting a single cis-acting element. The ISS is thus localized to CUCCAAGUGUC, which again has similarity to an UG/GU repeat. It has been reported that CUG-BP1 exhibits a strong preference for UG/GU repeats and UGUU/UGU sequences (7, 24, 26, 40). To test whether CUG-BP1 binds to this 12-nt-long silencer sequence, we performed an RNA affinity purification assay using the 12-nt ISS, INH4, or INH5 RNA template. CUG-BP1 specifically binds the ISS, and mutation of the terminal UGUC nucleotides to AUCU (as in INH5) reduced binding of CUG-BP1 in vitro (Fig. 5B, lane 3). Mutation in the first 6 nt (as in INH4) had no effect on CUG-BP1 binding in vitro.
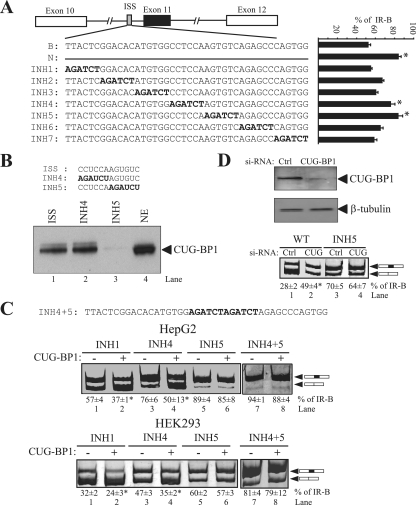
FIG. 5.
CUG-BP1 binds to the ISS element. (A) Schematic of the IR minigenes showing a deletion (N) and linker-scanning substitutions (INH1 to -7) in the ISS at the 3′ end of intron 10. The bar graph shows the percentages of exon 11 inclusion (% IR-B) for INH mutants transfected into HepG2 cells (means ± SD). Asterisks represent statistical significance (P < 0.05) relative to levels for the wild-type minigene. (B) RNA affinity purification followed by immunoblotting with antibodies to CUG-BP1. Sequences of the template RNAs are given. HeLa nuclear extract (1/25 of input) was used as a control. (C) Linker-scanning INH mutants 1, 4, 5, and 4+5 were cotransfected with the CUG-BP1 protein expression vector in HepG2 (upper) and HEK293 (lower) cells. Spliced products were analyzed by RT-PCR. The percentages of exon 11 inclusion (% IR-B) ± SD are shown (n = 3). Asterisks indicate statistical significance (P < 0.05) relative to levels for the vector control. (D) The wild-type (WT) minigene or the INH5 mutant (500 ng) was cotransfected into HEK293 cells with 100 nM CUG-BP1 siRNAs. Knockdown of CUG-BP1 protein was measured by immunoblotting with anti-CUG-BP1 monoclonal antibodies (upper). The blot was stripped and reprobed for beta-tubulin protein expression as an internal control. Representative results are shown for RT-PCR analysis of the spliced products amplified from RNA isolated from HEK293 cells treated with CUG-BP1 siRNA (lower). Control cells are treated with scrambled siRNA. Asterisks indicate statistical significance (P < 0.05) relative to levels for control scrambled siRNA.
To test whether the INH5 mutation disrupts the effect of CUG-BP1 on IR splicing, we cotransfected linker-scanning mutants INH1, INH4, and INH5 along with an expression vector for CUG-BP1 in HepG2 and HEK293 cells. CUG-BP1 decreased inclusion of exon 11 in INH1 and INH4 in both HepG2 and HEK293 cells (Fig. 5C); however, the INH5 mutant eliminated the inhibitory effect (Fig. 5C, lanes 5 and 6), suggesting that the ISS is a primary target site for CUG-BP1 in INSR. Further, mutation of the entire ISS element (INH4+5) greatly enhanced exon 11 inclusion in HEK293 cells, compared to the level for the INH4 or INH5 mutant alone (Fig. 5C, lower, lane 7). As expected, this mutant also eliminated the inhibitory effect of CUG-BP1. In HepG2 cells, exon 11 inclusion is already ∼90% in INH5, so no additive effect due to the double INH4+5 mutant was observed, and the double INH4+5 mutant eliminated the inhibitory effect of CUG-BP1, similar to INH5 alone.
To confirm the role for the endogenous protein, CUG-BP1 was knocked down using siRNA in HEK293 cells, which predominantly express the exon 11-skipped isoform. If CUG-BP1 acts as silencer, exon 11 inclusion should increase upon its depletion. HEK293 cells were treated with CUG-BP1 siRNA, and protein levels were quantified 48 h after transfection. CUG-BP1 protein was reduced by 76% (Fig. 5D, upper). To determine whether knockdown of CUG-BP1 affects IR splicing, we cotransfected the wild-type IR minigene (minigene B) into HEK293 cells (Fig. 5D, lower, lanes 1 and 2). Knockdown of CUG-BP1 resulted in a 1.8-fold increase in exon incorporation in cells treated with si-CUG-BP1, compared with the level in cells treated with control siRNA (49% versus 28%). The positive effect of si-CUG-BP1 was not observed in mutant INH5, where the primary CUG-BP1 binding site had been mutated (Fig. 5D, lower, lanes 3 and 4). These data showed that endogenous CUG-BP1 represses IR exon 11 incorporation in vivo.
SRp20 antagonizes CUG-BP1-mediated repression of IR exon 11 inclusion in vivo.
We have identified SRp20 as a splicing enhancer protein that recognizes a CUCUUC motif at the 5′ end of exon 11 and CUG-BP1 as a splicing repressor protein that recognizes two CUG-like repeats, one in exon 11 and the other in the upstream intron. To examine the interplay between SRp20 and CUG-BP1 in vivo, we cotransfected plasmids expressing these proteins together in HepG2 cells with the wild-type IR minigene. Upon transfection of increasing levels of SRp20 with a constant level of CUG-BP1, a gradual increase in exon 11 inclusion was noted (Fig. 6A, lanes 2 to 5). The opposite effect was observed when increasing levels of CUG-BP1 were transfected with a constant amount of SRp20 (Fig. 6A, lanes 3, 6, and 7). To further establish the antagonistic effect of CUG-BP1 and SRp20, a comparable experiment was performed to alter the levels of CUG-BP1 and SRp20 simultaneously. Simultaneous overexpression of CUG-BP1 and knockdown of SRp20 showed an additive effect on cotransfected minigene in HepG2 cells (Fig. 6B, upper, lane 4). In the converse experiment, simultaneous overexpression of SRp20 and knockdown of CUG-BP1 increased exon 11 inclusion in HEK293 cells; however, no additive effect was observed (Fig. 6B, lower, lane 4). Together, these experiments suggest that SRp20 antagonizes CUG-BP1-mediated repression of exon 11 inclusion in vivo and that the relative ratio of SRp20 and CUG-BP1 dictates the degree of exon 11 inclusion.
FIG. 6.
SRp20 antagonizes CUG-BP1-mediated repression of exon 11 inclusion in vivo. (A) The wild-type IR minigene (500 ng) was cotransfected into HepG2 cells with CUG-BP1 expression vector (1 μg) with increasing amounts of SRp20 (1, 2, or 4 μg) or vice versa. Splicing patterns were analyzed by RT-PCR as before. The percentages of IR-B ± SD are shown (n = 3). (B) The wild-type IR minigene (500 ng) was cotransfected into HepG2 (upper) and HEK293 (lower) cells with 100 nM concentrations of siRNAs directed against CUG-BP1 or SRp20, either alone or in combination with SRp20 or CUG-BP1 expression vector, respectively. Spliced products were analyzed by RT-PCR. The mean percentages of exon 11 inclusion (% IR-B) and SD derived from three independent experiments are given below the gel. Asterisks indicate statistical significance (P < 0.05) relative to control levels. (C) Representative RT-PCR products of the endogenous INSR transcripts in HepG2 and HEK293 cells (upper) and Western blots of cell extracts prepared from HepG2 and HEK293 cells probed with antibodies for CUG-BP1, SRp20, and SF2/ASF (lower). Beta-tubulin is a control for protein loading.
A prediction from the findings presented above is that cells with a high SRp20/CUG-BP1 ratio should include exon 11 and those with a low SRp20/CUG-BP1 ratio should skip the exon. Therefore, we surveyed SRp20, SF2/ASF, and CUG-BP1 expression (Fig. 6C, lower) and endogenous INSR splicing (Fig. 6C, upper) in the two cell lines, HepG2 and HEK293. The expression levels of SRp20 and SF2/ASF were similar in both HepG2 and HEK293 cells, but the CUG-BP1 expression levels were much higher in HEK293 cells than in HepG2 cells. Thus, the ratio of SR proteins to CUG-BP1 correlates with the degree of exon inclusion in the two cell lines.
DISCUSSION
Regulation of IR alternative splicing and maintenance of the IR-B/IR-A ratio are thought to be critical for insulin sensitivity. Several studies have identified exonic and intronic sequences as the cis-acting factors that stimulate or repress the usage of suboptimal splice sites and hence play a critical role in alternative splicing (1, 37). Using a combination of RNA affinity purification and in vivo splicing, we have demonstrated that SRp20 and SF2/ASF recognize exon splicing enhancers in exon 11 and promote exon inclusion. Overexpression of SRp20 results in exon 11 inclusion, and knockdown of SRp20 leads to exon 11 skipping. A number of different ESEs have now been characterized for several genes, and in many cases, SR proteins are involved in ESE-mediated splicing enhancement (6, 9, 41, 47). However, little is known about the specific nucleotide contacts made by SRp20. Despite extensive characterization of different SR proteins, only a relatively small number of cellular and viral genes regulated by SRp20 have been identified (5, 8, 37, 45, 48), and only a small number of high-affinity binding sites have been obtained by SELEX (34). Our data add an important new member, IR exon 11, to this list. Mutation of the CUCUUC residues eliminates SRp20 binding, reduces exon 11 inclusion, and renders the minigene unresponsive to SRp20 overexpression. In contrast, although SF2/ASF also binds to the putative ESE of IR exon 11, overexpression of SF2/ASF did not enhance exon 11 inclusion. However, knockdown of SF2/ASF in HepG2 cells verified its functional importance. These results suggest that HepG2 cells express enough SF2/ASF to negate effects due to overexpression, but alterations in splicing can be observed by reducing expression.
Numerous studies have contributed to the view that SR proteins play a role in splice site selection in a concentration-dependent manner (23, 49). One consequence of this is that cells may regulate the expression or activity of individual SR proteins to control the expression of target genes in a tissue-specific and/or developmentally regulated fashion. For example, Screaton et al. (35) reported that following T-cell activation, alternative splicing of CD44 and CD45 is accompanied by changes in SF2 level and activity. While we do not find much variation in SR protein levels, we have found that SRp20-driven exon inclusion could be blocked by overexpression of a CELF family protein, CUG-BP1, which appears to bind both exonic and intronic silencer elements. Savkur et al. have shown preliminary evidence for binding of CUG-BP1 to intron 10 of the IR (33), but they did not define the binding site at the nucleotide level. In this study, we found that CUG-BP1 binds to the ISS element in intron 10 and to the ESS in exon 11 itself. We were surprised that mutation of the ESS in exon 11 did not eliminate the effect of CUG-BP1. The binding of CUG-BP1 to exon 11 is strong, and CUG-BP1 does not elute from the exon template with 1 M salt (data not shown). Using recombinant-CUG-BP1, we observed binding to the ESS and ISS directly (data not shown). Mutation of the ESS site in mutants LS3, LS4, and LS5 does not eliminate the binding of CUG-BP1 completely. Additionally, CUG-BP1 can still bind to the ISS, and this may explain why these mutants still respond to CUG-BP1. It is possible that cooperative binding of multiple CUG-BP1 monomers to the ESS and ISS is required for efficient repression in vivo. In support of this hypothesis, the functional configuration of EDEN-BP, the Xenopus homolog of CUG-BP1, is a dimer (2).
We are proposing that the ratio of SR proteins to CUG-BP1 determines the extent of exon 11 inclusion, as titration experiments show a functional antagonism. siRNA-mediated downregulation of SRp20 and overexpression of CUG-BP1 had additive effects on exon exclusion in HepG2 cells. Moreover, the correlation of differential expression levels of SR proteins and CUG-BP1 in HepG2 and HEK293 cells with exon 11 inclusion further supports this hypothesis. It is currently unclear how SRp20 and SF2/ASF facilitate IR exon 11 inclusion. The proximities of the SRp20 binding site to the 3′ splice site and of SF2/ASF to the 5′ splice site lead to the possibility that these two SR proteins are acting to facilitate the recruitment of U1 and U2 snRNPs. Another possibility is that SRp20 and SF2/ASF may favor exon 11 inclusion by preventing the sequestration of the exon by CUG-BP1. Alternatively, the activity of one factor may be regulated by the presence of the other. This is particularly intriguing given our observation that the phosphorylation state of SRp20 seems to be influenced by the presence of CUG-BP1 on the same RNA template, raising the possibility that protein binding to the ESS inhibits phosphorylation of SRp20, perhaps by recruiting a protein phosphatase. SRp20 and SF2/ASF appear to have a synergistic effect in exon 11 inclusion. Maximal exon 11 inclusion is seen in the LS3 and LS4 mutants, where both SRp20 and SF2/ASF bind, and the LS5 mutant, in which only SRp20 (rather than SF2/ASF) binds, shows greatly reduced exon inclusion. Such interactions have been reported before, as Li et al. (19) showed that splicing of bGH intron D is controlled by two SR proteins, ASF/SF2 and 9G8, acting synergistically.
A great deal of data showing that IR splicing is altered in myotonic dystrophy have accumulated. The Mbnl proteins are functionally inactivated by binding to the CUG and CCUG repeats, and the CUG-BP1 and hnRNP-H proteins are increased (30). These changes culminate in reductions in exon 11 inclusion in the INSR gene. Most of these studies have been performed with muscle cells, as this is the site of DMPK and Znf9 gene expression, but Mbnl and CUG-BP1 proteins are expressed in many other tissues. Like Mbnl, CUG-BP1 binds both ssRNA and dsRNA. Indeed, CUG-BP1 binds repeats that can form double-stranded structures, but with specificities different from that of Mbnl. We have identified a CUG-BP1 binding site in the middle of exon 11. Interestingly, exon 11 is predicted to form an extended stem-loop structure with the upstream polypyrimidine tract and branch point sequence (Fig. 7A). Formation of this structure would be predicted to inhibit U2AF, SF1, and U2 snRNP binding and block 3′ splice site usage. There is a precedent for a regulatory role for such structures, as a stem-loop stabilized by Mbnl has been shown to block the 3′ splice site in the cTNT gene (44). The inhibitory role of the RNA secondary structure was also demonstrated for splice site recognition of SMN2 exon 7, where the formation of an RNA hairpin close to the 5′ splice site of SMN2 exon 7 interfered with its interaction with U1 snRNP, resulting in reduced exon inclusion levels (36). The overlapping binding sites for CUG-BP1 and SF2/ASF in IR exon 11 lie in the stem-loop structure, but the SRp20 binding site is in the unstructured loop that precedes the double-stranded stem. We have some evidence for this structure from previous mutagenesis studies. Point mutations that disrupt base pairing all increase exon inclusion, whereas mutations that increase base pairing inhibit exon inclusion (Fig. 7B). It is possible that CUG-BP1 binding to this sequence and the upstream ISS stabilizes the double-stranded stem and sequesters the exon, causing skipping. Binding of SRp20 and SF2/ASF, in contrast, prevents secondary structure formation by blocking nucleation of the double-stranded stem (Fig. 7C). While this is speculation, experiments for testing this model are currently under way in our laboratory.
FIG. 7.
Proposed model for IR alternative splicing regulation by SRp20 and CUG-BP1. (A) Predicted secondary structure of exon 11 (uppercase letters) and intron 10 (lowercase letters) RNA is shown. A 180-nt region of the pre-mRNA including the 3′ end of intron 10, exon 11, and the 5′ splice site of intron 11 was folded using the M-fold server (50). The CUG-BP1 binding site is indicated in red, SRp20 in green, SF2/ASF in yellow, and the pY tract and 3′splice site in purple. (B) Mutations in the RNA secondary structure. The exon sequence is shown in uppercase letters. Stabilizing mutations are shown above the sequence and destabilizing mutations below the sequence. Exon 11 inclusion is given on the right. (C) Proposed model for stabilization of RNA secondary structure by CUG-BP1 and its antagonism by SRp20 and SF2/ASF, allowing splice site recognition by the U1 and U2 snRNPs. Exon 11 is shown in sky blue.
In summary, we have discovered novel splicing-enhancer and silencer elements, which are located in the alternatively spliced IR exon 11 sequence itself and intron 10 of the human INSR gene. The enhancers bind SRp20 and SF2/ASF, whereas the silencers bind CUG-BP1. Our study establishes novel antagonistic effects of CUG-BP1 and SRp20 that may be attributable to stabilization of an RNA secondary structure that regulates IR alternative splicing. Further studies are planned to test these models.
Acknowledgments
We are grateful to Jane Wu for providing the SRp30C, SC35, SRp75, and SF2/ASF expression plasmids. We also thank Tom Cooper for the CUG-BP1 expression plasmid.
This work was supported by a Merit Review award from the Department of Veterans Affairs.
None of the authors have any potential conflict to declare.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Black, D. L. 2003. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72291-336. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet-Corven, S., Y. Audic, F. Omilli, and H. B. Osborne. 2002. An analysis of the sequence requirements of EDEN-BP for specific RNA binding. Nucleic Acids Res. 304667-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buratti, E., M. Baralle, and F. E. Baralle. 2006. Defective splicing, disease and therapy: searching for master checkpoints in exon definition. Nucleic Acids Res. 343494-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputi, M., A. Mayeda, A. R. Krainer, and A. M. Zahler. 1999. hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. EMBO J. 184060-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Mata, M., and A. R. Kornblihtt. 2006. RNA polymerase II C-terminal domain mediates regulation of alternative splicing by SRp20. Nat. Struct. Mol. Biol. 13973-980. [DOI] [PubMed] [Google Scholar]
- 6.Dirksen, W. P., X. Li, A. Mayeda, A. R. Krainer, and F. M. Rottman. 2000. Mapping the SF2/ASF binding sites in the bovine growth hormone exonic splicing enhancer. J. Biol. Chem. 27529170-29177. [DOI] [PubMed] [Google Scholar]
- 7.Faustino, N. A., and T. A. Cooper. 2005. Identification of putative new splicing targets for ETR-3 using sequences identified by systematic evolution of ligands by exponential enrichment. Mol. Cell. Biol. 25879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiana-Arnoux, D., F. Lejeune, M. C. Gesnel, J. Stevenin, R. Breathnach, and F. Del Gatto-Konczak. 2003. The CD44 alternative v9 exon contains a splicing enhancer responsive to the SR proteins 9G8, ASF/SF2, and SRp20. J. Biol. Chem. 27832943-32953. [DOI] [PubMed] [Google Scholar]
- 9.Gontarek, R. R., and D. Derse. 1996. Interactions among SR proteins, an exonic splicing enhancer, and a lentivirus Rev protein regulate alternative splicing. Mol. Cell. Biol. 162325-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graveley, B. R. 2005. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell 12365-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gromak, N., A. J. Matlin, T. A. Cooper, and C. W. Smith. 2003. Antagonistic regulation of alpha-actinin alternative splicing by CELF proteins and polypyrimidine tract binding protein. RNA 9443-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gromak, N., A. Rideau, J. Southby, A. D. Scadden, C. Gooding, S. Huttelmaier, R. H. Singer, and C. W. Smith. 2003. The PTB interacting protein raver1 regulates alpha-tropomyosin alternative splicing. EMBO J. 226356-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller, M., Z. Zhang, R. Backofen, and S. Stamm. 2007. Pre-mRNA secondary structures influence exon recognition. PLoS Genet. 3e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo, J. M., N. Majos, S. Bonnal, C. Martinez, R. Castelo, R. Guigo, D. Bilbao, and J. Valcarcel. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 19475-484. [DOI] [PubMed] [Google Scholar]
- 15.Kellerer, M., G. Sesti, E. Seffer, B. Obermaier-Kusser, D. E. Pongratz, L. Mosthaf, and H. U. Haring. 1993. Altered pattern of insulin receptor isotypes in skeletal muscle membranes of type 2 (non-insulin-dependent) diabetic subjects. Diabetologia 36628-632. [DOI] [PubMed] [Google Scholar]
- 16.Kosaki, A., J. Nelson, and N. J. Webster. 1998. Identification of intron and exon sequences involved in alternative splicing of insulin receptor pre-mRNA. J. Biol. Chem. 27310331-10337. [DOI] [PubMed] [Google Scholar]
- 17.Kosaki, A., and N. J. Webster. 1993. Effect of dexamethasone on the alternative splicing of the insulin receptor mRNA and insulin action in HepG2 hepatoma cells. J. Biol. Chem. 26821990-21996. [PubMed] [Google Scholar]
- 18.Kramer, A. 1996. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu. Rev. Biochem. 65367-409. [DOI] [PubMed] [Google Scholar]
- 19.Li, X., M. E. Shambaugh, F. M. Rottman, and J. A. Bokar. 2000. SR proteins Asf/SF2 and 9G8 interact to activate enhancer-dependent intron D splicing of bovine growth hormone pre-mRNA in vitro. RNA 61847-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, A. J. 1998. Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu. Rev. Genet. 32279-305. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Bigas, N., B. Audit, C. Ouzounis, G. Parra, and R. Guigo. 2005. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 5791900-1903. [DOI] [PubMed] [Google Scholar]
- 22.Lu, X., N. A. Timchenko, and L. T. Timchenko. 1999. Cardiac elav-type RNA-binding protein (ETR-3) binds to RNA CUG repeats expanded in myotonic dystrophy. Hum. Mol. Genet. 853-60. [DOI] [PubMed] [Google Scholar]
- 23.Manley, J. L., and R. Tacke. 1996. SR proteins and splicing control. Genes Dev. 101569-1579. [DOI] [PubMed] [Google Scholar]
- 24.Marquis, J., L. Paillard, Y. Audic, B. Cosson, O. Danos, C. Le Bec, and H. B. Osborne. 2006. CUG-BP1/CELF1 requires UGU-rich sequences for high-affinity binding. Biochem. J. 400291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Contreras, R., P. Cloutier, L. Shkreta, J. F. Fisette, T. Revil, and B. Chabot. 2007. hnRNP proteins and splicing control. Adv. Exp. Med. Biol. 623123-147. [DOI] [PubMed] [Google Scholar]
- 26.Mori, D., N. Sasagawa, Y. Kino, and S. Ishiura. 2008. Quantitative analysis of CUG-BP1 binding to RNA repeats. J. Biochem. 143377-383. [DOI] [PubMed] [Google Scholar]
- 27.Mosthaf, L., B. Vogt, H. U. Haring, and A. Ullrich. 1991. Altered expression of insulin receptor types A and B in the skeletal muscle of non-insulin-dependent diabetes mellitus patients. Proc. Natl. Acad. Sci. USA 884728-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norgren, S., L. S. Li, and H. Luthman. 1994. Regulation of human insulin receptor RNA splicing in HepG2 cells: effects of glucocorticoid and low glucose concentration. Biochem. Biophys. Res. Commun. 199277-284. [DOI] [PubMed] [Google Scholar]
- 29.Norgren, S., J. Zierath, D. Galuska, H. Wallberg-Henriksson, and H. Luthman. 1993. Differences in the ratio of RNA encoding two isoforms of the insulin receptor between control and NIDDM patients. The RNA variant without Exon 11 predominates in both groups. Diabetes 42675-681. [DOI] [PubMed] [Google Scholar]
- 30.Paul, S., W. Dansithong, D. Kim, J. Rossi, N. J. Webster, L. Comai, and S. Reddy. 2006. Interaction of muscleblind, CUG-BP1 and hnRNP H proteins in DM1-associated aberrant IR splicing. EMBO J. 254271-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rio, D. C. 1992. RNA processing. Curr. Opin. Cell Biol. 4444-452. [DOI] [PubMed] [Google Scholar]
- 32.Sanford, J. R., J. Ellis, and J. F. Caceres. 2005. Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem. Soc. Trans. 33443-446. [DOI] [PubMed] [Google Scholar]
- 33.Savkur, R. S., A. V. Philips, and T. A. Cooper. 2001. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 2940-47. [DOI] [PubMed] [Google Scholar]
- 34.Schaal, T. D., and T. Maniatis. 1999. Selection and characterization of pre-mRNA splicing enhancers: identification of novel SR protein-specific enhancer sequences. Mol. Cell. Biol. 191705-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Screaton, G. R., J. F. Caceres, A. Mayeda, M. V. Bell, M. Plebanski, D. G. Jackson, J. I. Bell, and A. R. Krainer. 1995. Identification and characterization of three members of the human SR family of pre-mRNA splicing factors. EMBO J. 144336-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh, N. N., R. N. Singh, and E. J. Androphy. 2007. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucleic Acids Res. 35371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, C. W., and J. Valcarcel. 2000. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 25381-388. [DOI] [PubMed] [Google Scholar]
- 38.Smith, P. J., C. Zhang, J. Wang, S. L. Chew, M. Q. Zhang, and A. R. Krainer. 2006. An increased specificity score matrix for the prediction of SF2/ASF-specific exonic splicing enhancers. Hum. Mol. Genet. 152490-2508. [DOI] [PubMed] [Google Scholar]
- 39.Stark, J. M., T. A. Cooper, and M. B. Roth. 1999. The relative strengths of SR protein-mediated associations of alternative and constitutive exons can influence alternative splicing. J. Biol. Chem. 27429838-29842. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, N., N. Sasagawa, K. Suzuki, and S. Ishiura. 2000. The CUG-binding protein binds specifically to UG dinucleotide repeats in a yeast three-hybrid system. Biochem. Biophys. Res. Commun. 277518-523. [DOI] [PubMed] [Google Scholar]
- 41.Tian, M., and T. Maniatis. 1993. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell 74105-114. [DOI] [PubMed] [Google Scholar]
- 42.Timchenko, L. T., J. W. Miller, N. A. Timchenko, D. R. DeVore, K. V. Datar, L. Lin, R. Roberts, C. T. Caskey, and M. S. Swanson. 1996. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 244407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, G. S., and T. A. Cooper. 2007. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 8749-761. [DOI] [PubMed] [Google Scholar]
- 44.Warf, M. B., and J. A. Berglund. 2007. MBNL binds similar RNA structures in the CUG repeats of myotonic dystrophy and its pre-mRNA substrate cardiac troponin T. RNA 132238-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanuki, T., H. Funato, S. Uchida, T. Matsubara, A. Kobayashi, Y. Wakabayashi, K. Otsuki, A. Nishida, and Y. Watanabe. 2008. Increased expression of splicing factor SRp20 mRNA in bipolar disorder patients. J. Affect. Disord. 11062-69. [DOI] [PubMed] [Google Scholar]
- 46.Webster, N. J., L. G. Evans, M. Caples, L. Erker, and S. L. Chew. 2004. Assembly of splicing complexes on exon 11 of the human insulin receptor gene does not correlate with splicing efficiency in-vitro. BMC Mol. Biol. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeakley, J. M., J. P. Morfin, M. G. Rosenfeld, and X. D. Fu. 1996. A complex of nuclear proteins mediates SR protein binding to a purine-rich splicing enhancer. Proc. Natl. Acad. Sci. USA 937582-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuo, C. Y., H. H. Lin, Y. S. Chang, W. K. Yang, and J. G. Chang. 2008. 5-(N-ethyl-N-isopropyl)-amiloride enhances SMN2 exon 7 inclusion and protein expression in spinal muscular atrophy cells. Ann. Neurol. 6326-34. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, J., A. Mayeda, and A. R. Krainer. 2001. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell 81351-1361. [DOI] [PubMed] [Google Scholar]
- 50.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 313406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]