Abstract
Familial dysautonomia (FD), a devastating hereditary sensory and autonomic neuropathy, results from an intronic mutation in the IKBKAP gene that disrupts normal mRNA splicing and leads to tissue-specific reduction of IKBKAP protein (IKAP) in the nervous system. To better understand the roles of IKAP in vivo, an Ikbkap knockout mouse model was created. Results from our study show that ablating Ikbkap leads to embryonic lethality, with no homozygous Ikbkap knockout (Ikbkap−/−) embryos surviving beyond 12.5 days postcoitum. Morphological analyses of the Ikbkap−/− conceptus at different stages revealed abnormalities in both the visceral yolk sac and the embryo, including stunted extraembryonic blood vessel formation, delayed entry into midgastrulation, disoriented dorsal primitive neural alignment, and failure to establish the embryonic vascular system. Further, we demonstrate downregulation of several genes that are important for neurulation and vascular development in the Ikbkap−/− embryos and show that this correlates with a defect in transcriptional elongation-coupled histone acetylation. Finally, we show that the embryonic lethality resulting from Ikbkap ablation can be rescued by a human IKBKAP transgene. For the first time, we demonstrate that IKAP is crucial for both vascular and neural development during embryogenesis and that protein function is conserved between mouse and human.
IKBKAP (encoding IκB kinase-associated protein, also called Elongator protein 1) is the gene mutated in hereditary sensory and autonomic neuropathy type III, or familial dysautonomia (FD). All FD patients carry at least one copy of a splicing mutation in IKBKAP, which causes aberrant exon skipping and subsequent tissue-specific reduction of protein expression in FD patients (1, 41, 42). The IKBKAP gene is highly conserved across species, with the human and mouse proteins (IKAP and Ikap, respectively) sharing more than 80% amino acid homology (12). The IKBKAP protein, IKAP, was first reported to act as a scaffolding protein for the IκB kinase complex (11). Recent studies, however, have shown that IKAP does not play a role in NF-κB (nuclear factor κB) signaling, but rather, it is a subunit of the human Elongator complex, which is important for efficient transcriptional elongation (19, 28, 36).
FD (or Riley-Day syndrome) is one of the best known recessive hereditary neuropathies, with an extremely high carrier frequency in the Ashkenazi Jewish population, which ranges from 1 in 17 to 1 in 28 depending on the country of origin (29, 33, 42). Clinical characteristics of FD include diminished tear secretion, dysphagia, esophageal and gastric dysmotility, gastroesophageal reflux, spinal curvature, postural hypotension, blotching, excessive sweating, and decreased deep-tendon reflexes (2). Fatality in FD patients is high, and only half survive to 40 years of age. Clinical reports have shown that the failure of autonomic function is one of the major causes of death (21). To date, three FD-causing mutations have been identified in the IKBKAP gene: an intronic noncoding point mutation, IVS20+6T>C, and two missense mutations, R696P and P914L. All FD patients carry at least one copy of the noncoding point mutation in the IKBKAP gene, with over 99.5% homozygous for this mutation. The IVS20+6T>C mutation in intron 20 disrupts the splicing of IKBKAP and results in variable skipping of exon 20 in the IKBKAP transcript (1, 30, 41). Our studies have demonstrated that homozygous mutant cells derived from FD patients express both wild-type and mutant IKBKAP mRNA and are capable of synthesizing full-length functional IKAP protein (41). Thus, the IKBKAP mutation weakens but does not completely inactivate the 5′ splice site of exon 20. Indeed, this finding was further supported by the presence of both wild-type and mutant IKBKAP mRNAs in tissues from FD patients (17, 41). Interestingly, the relative amounts of wild-type and mutant IKBKAP transcripts vary between tissues. In particular, the central and peripheral nervous systems contain the lowest levels of wild-type IKBKAP mRNA and protein (13, 41), corresponding to the observed developmental absence and ongoing degeneration of unmyelinated sensory and autonomic neurons seen in FD (2, 38). Heterozygous carriers also show reduced IKAP expression; however, no phenotype is evident, suggesting that there is a tissue-specific minimum threshold of IKAP expression required for normal development and maintenance of the nervous system.
Although the correlation between the IKBKAP mutation and FD is well documented, precisely how a tissue-specific reduction of IKAP leads to the development of FD remains to be elucidated. Recently, by using RNA interference technology in HeLa cells, it was shown that an 80% reduction of IKBKAP transcript leads to a loss of integrity of the Elongator complex, which subsequently diminishes the expression of several genes that are known to be essential for cell motility. A subset of these genes was also shown to be downregulated in fibroblast lines derived from FD patients. Further, Elongator complex reduction was demonstrated to directly interfere with histone H3 acetylation of a specific subset of genes (10). Since the development of the nervous system requires extensive migration of differentiated neuronal progenitors to their target destinations, these findings suggest that defective cellular motility could be one underlying cause of the developmental neuropathology of FD. IKAP was also implicated in myelination based on minor gene expression differences detected by microarray analyses of RNAs isolated from the frontal cortexes of two normal and two FD individuals (8); however, this putative role for IKAP has not yet been functionally confirmed. In addition to regulating the transcriptional machinery, IKAP has been proposed to play a role in exocytosis, activation of JNK signaling, and tRNA modification (24, 25, 39). As pointed out in a recent review of the many putative functions of IKAP/Elongator, considerable work remains to sort out the precise function of IKAP/Elongator (46).
To better understand the role of IKAP in vivo, we created a mouse with a targeted disruption of the Ikbkap gene (Ikbkap−/−) (GeneID, 230233). We demonstrate that homozygous disruption of the mouse Ikbkap gene leads to embryonic lethality prior to midgestation, since no Ikbkap−/− embryos could be recovered after 12.5 days postcoitum (dpc). Further analyses of the Ikbkap−/− conceptus from 7.5 to 10.5 dpc revealed several abnormal configurations compared with Ikbkap+/+ controls, including a dramatic reduction in overall size, disruption of the extraembryonic vascular networks, failure of germ layer inversion, and interruption of cephalic neural-tube closure. Further, the expression of several genes that have been shown to be essential for embryogenesis is downregulated in the Ikbkap−/− embryos due to defective elongation of the transcript, suggesting a crucial role for Ikbkap during development. The murine Ikap protein is 80% identical to human IKAP, and we show that the embryonic lethality of Ikbkap ablation can be rescued by a human IKBKAP transgene. By crossing the Ikbkap knockout mouse line with human transgenic lines, we confirmed IKAP functional conservation between human and mouse. Taken together, our studies show for the first time that Ikap is required for embryogenesis in mammals. Elucidating how IKAP functions during development is a crucial first step toward discovering how reduction of IKAP expression leads to neuronal loss in FD.
MATERIALS AND METHODS
Animals.
The Ikbkap knockout mouse line was generated in collaboration with BayGenomics (currently known as the International Gene Trap Consortium [IGTC]) (35). In brief, chimeras with the Ikbkap knockout allele were derived from embryonic cell lines that contain a gene trap vector at intron 9 of the Ikbkap gene. The gene trap vectors used by the IGTC were inserted randomly into introns and contained a splice-acceptor sequence followed by a β-galactosidase and neomycin phosphotransferase II (β-geo) cassette. The resulting insertion created a fusion transcript containing the exons upstream of the insertion joined to the β-geo cassette. The identity of the trapped/disrupted gene could easily be determined by 5′ rapid amplification of cDNA ends, followed by sequencing. Cell line BGB184 produced a transcript containing Ikbkap exons 1 to 9 and the β-geo cassette, and we later confirmed that the vector was inserted into intron 9. Mice with the C57BL/6J background used in this study were generated by serial backcrossing. The Ikbkap+/+ and Ikbkap−/− embryos for this study were produced from heterozygous breeding pairs. The day of vaginal-plug discovery was designated 0.5 dpc. The transgenic mouse lines that carry human IKBKAP transgenes were generated as previously reported (23). The mice used for this study were housed in the animal facility of Massachusetts General Hospital (Boston, MA), provided with constant access to a standard diet of food and water, and maintained on a 12-hour light/dark cycle, and all experimental protocols were approved by the Subcommittee on Research Animal Care at the Massachusetts General Hospital.
Genotyping.
The genotypes of animals and embryos were determined by PCR analysis of genomic DNA from tail slips and from embryos and/or visceral yolk sacs, respectively. The primer sets used were as follows: for determining the wild-type Ikbkap allele, 5′-ACCCTCAGGCAGTTTGATTG-3′ and 5′-CATGGCTCCATAAAACAAACAC-3′; for determining the knockout allele, 5′ACCCTCAGGCAGTTTGATTG-3′ and 5′-GGCTACCGGCTAAAACTTGA-3′; and for determining the human wild-type IKBKAP transgenes, TgProbe1F (5′-GCCATTGTACTGTTTGCGACT-3′) and TgProbe1R (5′-TGAGTGTCACGATTCTTTCTGC-3′).
Morphological analysis of embryos.
Photographs of visceral yolk sacs and embryos at different stages were taken with a digital camera (Diagnostic Instruments) mounted on an Olympus dissection microscope. SPOT software (Diagnostic Instruments) was used for image processing.
X-Gal staining of embryos.
Embryos at different stages were dissected in cold phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde overnight. The embryos were then rinsed with PBS three times for 10 min each time and incubated with 1 mM 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) (Molecular Probes) in PBS at 37°C for 16 to 24 h. The embryos were rinsed with PBS and fixed in 4% paraformaldehyde before being imaged.
RT-PCR.
Total RNA from visceral yolk sacs and whole embryos was extracted by using TRI Reagent (Molecular Research Center) according to the manufacturer's protocol. Reverse transcription (RT) was then performed using 1 μg total RNA, oligo(dT) primer, and Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. For gene expression assessment, semiquantitative PCR was performed with the cDNA equivalent of 100 ng of starting RNA in a 30-μl reaction mixture, with the use of Taq polymerase (Roche) with target-specific primer pairs that were custom designed by OligoPerfect Designer (Invitrogen). For the primer sequences for RT-PCR analysis, see the supplemental material. Thirty-two amplification cycles (94°C for 30 s, 60°C for 35 s, and 72°C for 30 s) were then performed. The PCR products were separated on 1.5% agarose gels, stained with ethidium bromide, and visualized with UV light using the Alphaimager 2200 system (Alpha Innotech). Relative band intensities were determined by evaluating the integrated density values as determined by the Alphaimager 2000 software.
Acetyl-histone H3 ChIP assay.
The immunocomplex containing chromatin fragments/anti-acetyl-histone H3 antibody/protein A/G agarose was extracted using an acetyl-histone H3 chromatin immunoprecipitation (ChIP) assay kit (Millipore) according to the manufacturer's protocol. In brief, formaldehyde-treated embryos were washed with PBS containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, and 1 mg/ml pepstatin A) and centrifuged at 4°C. The pellets were suspended in sodium dodecyl sulfate lysis buffer and incubated for 10 min, followed by sonication at 30% amplitude with a Microson XL2000 Ultrasonic cell disruptor (Misonix). The sonicated samples were then centrifuged, and the supernatant containing chromatin fragments was diluted with ChIP dilution buffer. The chromatin-containing dilutions were precleared with salmon sperm DNA/protein A agarose 50% slurry before overnight incubation with anti-acetyl-histone H3 antibody. The next day, samples were incubated with salmon sperm DNA-protein A-agarose 50% slurry at 4°C for 1 h. Finally, the protein A-agarose/antibody/histone complex was recovered by centrifugation and sequentially washed with low-salt, high-salt, LiCl, and Tris-EDTA buffers. For controls, an aliquot of the cross-linked/sonicated chromatin fraction was treated as described above without adding antibody, and the first supernatant, after being precleared with salmon sperm DNA-protein A-agarose 50% slurry, was saved as an input control. For PCR analysis, the histone-DNA complex was first eluted from the antibody, and the DNA fragment was released from the histone-DNA complex by adding NaCl and incubated for 4 h at 65°C. The DNA was then purified with a PCR purification kit (Qiagen) and analyzed by PCR with appropriate primer pairs corresponding to the coding regions of the testing genes. For the primer sequences, see the supplemental material. The PCR products were size fractionated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized with UV light using the Alphaimager 2200 system (Alpha Innotech).
Western blots.
Protein from brains was extracted using RIPA buffer (Boston BioProducts). An equivalent amount of protein (30 μg) was run on a 10% Bis-Tris gel (Invitrogen) and then transferred onto a Hybond-N+ membrane (Amersham) using XCell SureLock Mini-Cell and XCell II Blot Module kits, respectively (Invitrogen), according to the manufacturer's protocol. Antibodies against the carboxyl terminus of human IKAP (1:3,000) (41), β-tubulin (1:3,000) (Santa Cruz Biotech), and rabbit/mouse immunoglobulin G-horseradish peroxidase conjugate secondary antibody (1:2,000) (Santa Cruz Biotech) were applied subsequently and visualized using the enhanced-chemiluminescence system (GE Healthcare UK, Ltd.) on X-ray film (Kodak).
RESULTS
Generation of an Ikbkap knockout mouse line.
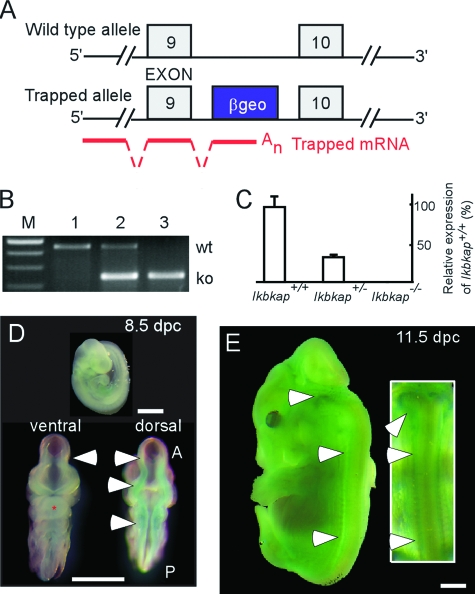
The Ikbkap knockout mouse line was generated utilizing gene trap technology in collaboration with the IGTC (35). This strategy has been widely used for functional analysis of mammalian genes (43). The gene trap vectors contain a splice-acceptor sequence, followed by the β-geo reporter gene and selection cassettes (a fusion of β-galactosidase and neomycin phosphotransferase II), which are randomly targeted to the 5′ ends of introns. The resulting inserted mutation creates a fusion transcript containing the exons upstream of the insertion joined to the β-geo marker. As a result, the identity of the trapped/disrupted gene can be determined by 5′ rapid amplification of cDNA ends followed by sequencing. Not only does successful gene trap insertion disrupt expression of the gene of interest, but the reporter cassette also provides a tag for characterizing the pattern of expression of the target gene. Chimeras that bear an Ikbkap knockout allele were derived from embryonic cell lines that contained a gene trap vector at intron 9 of the Ikbkap gene (Fig. 1A). The genotypes of specimens were determined by amplification of genomic DNA using wild-type and knockout primer sets (Fig. 1B). Mice heterozygous for the mouse Ikbkap knockout allele are fertile and phenotypically normal and show approximately 60% reduction in mouse Ikbkap expression when evaluated by quantitative RT-PCR (Fig. 1C). The mice used in this study were on a C57BL/6J background and were created by serial backcrossing for 10 generations; concurrently, heterozygote matings were used to generate offspring (Ikbkap+/+, Ikbkap+/−, and Ikbkap−/−). In addition, X-Gal staining was employed to visualize the expression of Ikbkap, as well as to confirm successful trap insertion in heterozygous embryos during development. At 8.5 and 11.5 dpc, positive signal could be observed from the entire embryo; however, intensities differed across tissues, with particularly high levels in the mid- and hindbrain walls, as well as the otic vesicle and dorsal ganglia (Fig. 1D and E). This IKAP expression pattern is similar to that reported in rats (34).
FIG. 1.
Gene targeting strategy and Ikbkap expression. (A) Schematic of the wild-type and knockout Ikbkap alleles. The blue cassette (β-geo) represents the vector containing β-galactosidase, neomycin phosphotransferase II, and stop codons that is inserted into intron 9. The insertion creates a fusion transcript containing the exons upstream of the insertion joined to the β-geo marker, as illustrated by the red lines. (B) PCR genotyping results of genomic DNA from embryonic day 9.5 samples. Lanes 1, 2, and 3 represent the wild-type (Ikbkap+/+), heterozygous (Ikbkap+/−), and homozygous (Ikbkap−/−) genotypes, respectively. wt, wild-type fragment (454 bp); ko, knockout fragment (244 bp). (C) The relative amounts of Ikbkap transcripts expressed in embryos with different genotypes at 8.5 dpc as demonstrated by quantitative RT-PCR. The error bars indicate standard deviations. (D and E) X-Gal staining of whole-mount Ikbkap+/− embryos at 8.5 and 11.5 dpc, respectively. The arrowheads in panel D point to the ventral and dorsal neural tubes; note that the primitive hindbrain region shows higher positive reactivity. The arrowheads in panel E point to the hindbrain and dorsal ganglia. A, anterior; P, posterior. Scale bars, 1 mm.
Ablation of Ikbkap results in embryonic lethality in mice.
Heterozygous matings (Ikbkap+/− × Ikbkap+/−) generated 74 Ikbkap+/− and 42 Ikbkap+/+ mice, while no Ikbkap−/− animals were born, statistically demonstrating that knocking out Ikbkap results in embryonic lethality (P < 0.005). To pinpoint the time of embryonic death, timed matings were performed using 25 dams. Pregnant mice were sacrificed at time points ranging from 6.5 to 12.5 dpc, and 230 embryos were isolated. By performing PCR of genomic DNA isolated from either the embryo or yolk sac, the genotype of each embryo was determined. At 7.5 to 12.5 dpc, 59 Ikbkap+/+, 121 Ikbkap+/−, and 50 Ikbkap−/− embryos were obtained, values that are consistent with Mendelian segregation (Table 1). No embryos were recovered inside the Ikbkap−/− yolk sacs at 12.5 dpc, suggesting that the degeneration of embryos occurs prior to this stage.
TABLE 1.
Genotype ratios of yolk sacs and neonates produced by heterozygous pairings
| Timea | No. (%) of animals
|
Total | ||
|---|---|---|---|---|
| Ikbkap+/+ | Ikbkap+/− | Ikbkap−/− | ||
| 7.5-10.5 dpc | 50 (26) | 99 (52) | 43 (22) | 192 |
| 11.2-12.5 dpc | 9 (24) | 22 (58) | 7 (18) | 38 |
| 14.5-16.5 dpc | 18 (33) | 36 (67) | 0 (0) | 54 |
| P0 | 42 (29) | 74 (71) | 0 (0) | 116 |
The time of conception is estimated to be 0.5 day prior to the observation of a vaginal plug. P0, postnatal day zero.
Vascular abnormalities in the Ikbkap−/− extraembryonic components.
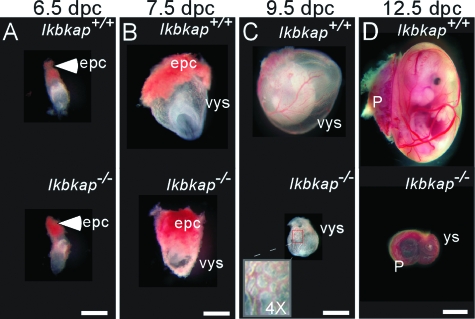
Morphologically, at 6.5 dpc, the Ikbkap+/+ and Ikbkap−/− concepti were comparable in size (Fig. 2A). However, by 7.5 and 9.5 dpc, the extraembryonic components were clearly smaller in the Ikbkap−/− conceptus (Fig. 2B and C). Further, at 7.5 dpc, while blood islands formed in the Ikbkap+/+ visceral yolk sac, corresponding structures were not observed in the Ikbkap−/− conceptus (Fig. 2B). At 9.5 dpc, the extraembryonic vessels were established in the Ikbkap+/+ visceral yolk sac; however, only the primary capillary plexus was observed in the Ikbkap−/− visceral yolk sac (Fig. 2C, inset). By 12.5 dpc, the Ikbkap−/− embryo had degenerated and was no longer visible in the yolk sac, leaving only brown aggregates of debris. Although the placenta was present in the Ikbkap−/− conceptus at this stage, it was dramatically reduced in size (Fig. 2D).
FIG. 2.
Appearance of Ikbkap+/+ and Ikbkap−/− extraembryonic components at different stages. Shown is the morphology of the Ikbkap+/+ and Ikbkap−/− conceptus at 6.5 to 12.5 dpc under a dissection microscope. (A) At 6.5 dpc, no gross abnormalities are found in the Ikbkap−/− conceptus compared to a wild-type control. epc, ectoplacental cone. (B) At 7.5 dpc, the Ikbkap−/− epc and visceral yolk sac (vys), as well as the embryo inside, are smaller than those found in Ikbkap+/+ controls. Note that at this stage the blood islands are readily observable in the Ikbkap+/+ vys; however, no corresponding architectures are found in the Ikbkap−/− vys. (C) At 9.5 dpc, the Ikbkap−/− vys, as well as the embryo inside, are smaller than those found in the Ikbkap+/+ control. At this stage, the blood vessels can easily be identified in the Ikbkap+/+ vys; however, in the Ikbkap−/− vys, only the primary capillary plexus is observed (inset). (D) At 12.5 dpc, the placenta can be found in both genotypes; in contrast, no embryo can be seen inside the Ikbkap−/− vys. P, placenta. Scale bars, 0.5 mm (A and B), 1 mm (C), and 2.5 mm (D).
Disturbed vascular development in the Ikbkap−/− visceral yolk sac suggests that vasculogenesis and angiogenesis might be compromised. Since IKAP is required for efficient transcription of a vast number of genes, many of which have yet to be identified, we examined the expression of several genes known to be vital for the development of extraembryonic blood vessels (6, 9, 14, 16, 27, 40, 45, 47). Studies of Tgfb1, Vegfa, Gata6, and Smad2 knockout mouse models have shown that ablating each of these genes results in stunted extraembryonic blood vessel formation, which subsequently leads to embryonic lethality. In addition, we evaluated Flt1, the receptor for Vegfa; Hnf4a, a target gene of Gata6; and Tie1 and Tie2 and their ligands Angpt1 and Angpt2, which have been shown to be independent from TGFb1 or VEGF signaling for the development and integrity of extraembyronic vascular networks. RNA was isolated from Ikbkap+/+ and Ikbkap−/− visceral yolk sacs, and gene expression was evaluated by RT-PCR. Interestingly, with the exception of Hnf4a, we did not see downregulation of any of these genes in the Ikbkap−/− visceral yolk sac. In fact, the expression of Tgfb1, Flt1, Tie1, and Tie2 was clearly elevated in the Ikbkap−/− yolk sac, which may be a compensatory response (Fig. 3). Given the robust expression of Ikbkap in the wild-type visceral yolk sac at this stage, these results suggest that the observed failure of yolk sac vascular development is not directly due to inefficient transcription of the genes tested.
FIG. 3.
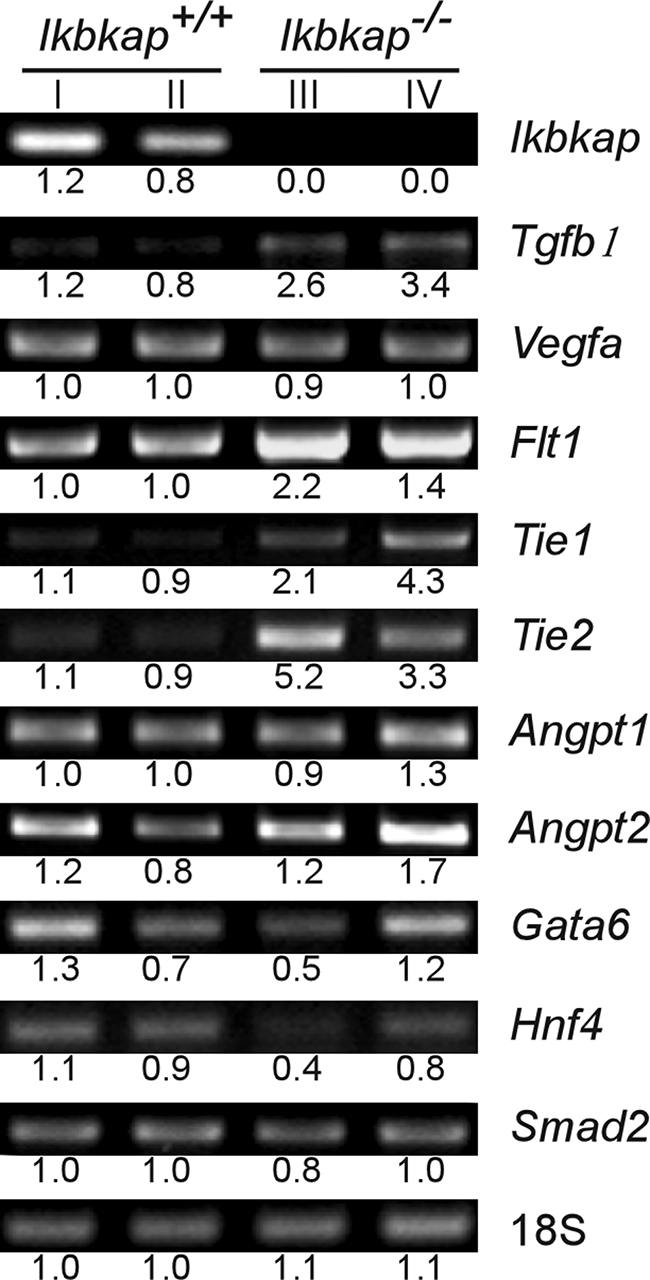
Gene expression patterns of the Ikbkap+/+ and Ikbkap−/− visceral yolk sac at 8.5 dpc. Shown is semiquantitative RT-PCR analysis of marker genes for vasculogenesis and angiogenesis at 8.5 dpc in the Ikbkap+/+ and Ikbkap−/− visceral yolk sacs. The values represent the integrated density values of the bands relative to the average of the Ikbkap+/+ expression. The names of the genes examined and the genotypes of samples are indicated. The samples in lane I and lane III, as well as lane II and lane IV, were harvested from littermates.
Abnormal architecture of Ikbkap−/− embryos during development.
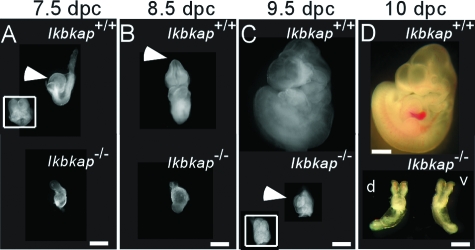
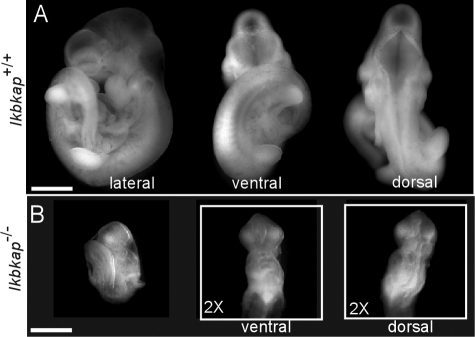
At 7.5 to 8.5 dpc, wild-type embryos underwent gastrulation, as indicated by the presence of the allantois, the definitive node, head folds, and axial rotation. However, growth of Ikbkap−/− embryos appeared to be arrested at the late egg cylinder stage, and no distinct stage-related architectures were present (Fig. 4A and B). At 9.5 and 10 dpc, several distinguishing structural characteristics, such as the primitive brain, spinal cord, cardiac contraction, branchial arch, and limb buds, were observed in the Ikbkap+/+ embryos; in contrast, the Ikbkap−/− embryos lacked these developmental features, exhibiting only a primitive heart, cephalic neural folds, and allantois, and growth appeared to be arrested at midgastrulation (Fig. 4C and D), a stage that was comparable to the Ikbkap+/+ controls at 7.5 dpc (Fig. 4A). In addition, in the Ikbkap−/− embryos, although a heart-like vascular structure was formed at 9.5 dpc, no visible primitive nucleated red blood cells were observed compared to those in the Ikbkap+/+ embryos at 10 dpc (Fig. 4C and D). At 10.5 dpc, brain structures, branchial arches, and limb buds were visible in the Ikbkap+/+ embryos but absent in the Ikbkap−/− embryos (Fig. 5A and B). At 10.5 dpc, morphological transformation had progressed in the Ikbkap−/− embryos, as the cephalic neural folds were found to be closed and there was evidence of rotation (Fig. 5B). In addition, mid- and hindbrain wall-like structures were visible but were aligned irregularly on the dorsal side of the Ikbkap−/− embryos (Fig. 5B, inset). After 10.5 dpc, the Ikbkap−/− embryos showed no further development, as the 11.5-dpc embryos isolated appeared similar in structure. As described above, all concepti isolated at 12.5 dpc had embryo degeneration.
FIG. 4.
Appearance of Ikbkap+/+ and Ikbkap−/− embryos at different embryonic stages. Shown are morphological phenotypes of the Ikbkap+/+ and Ikbkap−/− embryos from 7.5 to 10 dpc. At 7.5 dpc, the Ikbkap+/+ embryo undergoes gastrulation and formation of primitive organized structures, such as allantois and head folds (A, inset); however, the Ikbkap−/− embryos appeared to be arrested at the late primitive streak stage, and this feature persisted to 8.5 dpc in the Ikbkap−/− embryo (B). At 8.5 and 9.5 dpc, closure of the anterior neural tube (arrowheads in panels B and C) and body rotation could be observed in wild-type embryos (B and C); in contrast, the Ikbkap−/− embryos lacked these developmental features (B and C). At 9.5 and 10 dpc, the characteristics of the midgastrulation stage, such as allantois, primitive heart, and head fold (C, inset, and D), could be found in the Ikbkap−/− embryo; however, no blood vessels were observed compared with the Ikbkap+/+ control (D). Scale bars, 0.5 mm; in panel D, the scale bar adjacent to the Ikbkap+/+ embryo is 1 mm.
FIG. 5.
Appearance of Ikbkap+/+ and Ikbkap−/− embryos at 10.5 dpc. Shown is a morphological analysis of the Ikbkap+/+ and Ikbkap−/− embryos at 10.5 dpc. (A) At this stage, in the Ikbkap+/+ embryo, structures of the forebrain and hindbrain can be identified from ventral and dorsal aspects of the embryo, and the forelimb and hindlimb buds can be seen. (B) In contrast, at 10.5 dpc, incomplete turning is observed in the Ikbkap−/− embryo. No corresponding primitive brain structures or limb buds can be located; however, the anterior neural tube is closed from the ventral view, and the unsmooth zipper-like posterior neural tube can be found. Scale bars, 1 mm (A) and 0.5 mm (B).
Transcriptional deficits in Ikbkap−/− embryos.
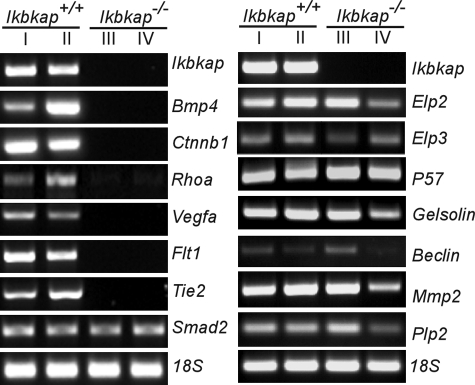
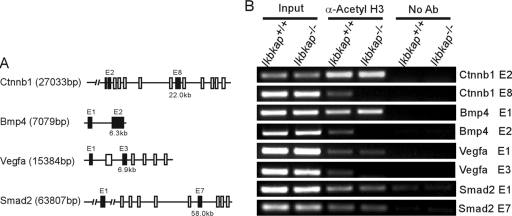
As in the visceral yolk sac, no advanced vascular network was observed in the Ikbkap−/− embryos. Thus, the expression levels of a subset of the genes that are important for vascular development were analyzed in the embryos. Since expression was evaluated in individual embryos, the amount of RNA was very limited, and therefore, we chose Vegfa, Flt1, Tie2, and Smad2 for analysis. Interestingly, RT-PCR showed dramatic downregulation of Vegfa, Flt1, and Tie 2 in the Ikbkap−/− embryos, while the expression of Smad2 was similar to that seen in normal embryos (Fig. 6). Given that the Ikbkap−/− embryos die during gastrulation, we also examined expression of Bmp4, Rhoa, and Ctnnb1, genes that show defects in neurulation in knockout mouse models (5, 18, 48, 49). These genes also show dramatic downregulation compared to expression in wild-type embryos (Fig. 6). To determine the extent of the transcriptional downregulation in Ikbkap−/− embryos, we then tested the expression of genes, such as Gelsolin, Beclin, P57, and Mmp2, that were previously shown to be downregulated in response to IKAP reduction via small interfering RNA in various in vitro models (10). Interestingly, we did not see downregulation of these genes in Ikbkap−/− embryos at 8.5 dpc. Likewise, expression levels of Elp2 (StIP1 and Statip1) and Elp3 (KAT9), which together with IKAP form the Elongator complex, were similar in Ikbkap+/+ and Ikbkap−/− embryos. Elongator has histone acetyltransferase activity via its Elp3 subunit, and histone acetylation plays a major role in transcriptional regulation by altering the chromatin structure and therefore the accessibility of polymerases to the DNA (19, 20, 44). Indeed, in a previous study, transcriptional impairment in FD patient fibroblasts was shown to be correlated with histone H3 hypoacetylation in the transcribed regions of genes (10). To examine whether a deficiency in transcriptional elongation and histone acetylation might contribute to the observed downregulation of gene expression in Ikbkap−/− embryos, the acetylation of histone H3 in various transcribed regions of the genes was assessed using ChIP. As illustrated by PCR, the acetylation patterns near the transcriptional start sites of Ctnnb1 (exon 2), Bmp4 (exon 1), Vegfa (exon 1), and Smad2 (exon 1) were compatible between Ikbkap+/+ and Ikbkap−/− samples, indicating that histone H3 acetylation at promoters and during early transcript elongation was not perturbed. However, acetylation in the downstream coding regions of Ctnnb1, Bmp4, and Vegfa (exons 8, 2, and 3, respectively) was significantly reduced in Ikbkap−/− embryos (Fig. 7). These data suggest that, at least for this subset of genes, developmental loss of Ikap leads to defective histone acetylation during transcript elongation.
FIG. 6.
Gene expression patterns of the Ikbkap+/+ and Ikbkap−/− embryos at 8.5 dpc. Shown is RT-PCR analysis of genes using RNA isolated from Ikbkap+/+ and Ikbkap−/− embryos. The names of the genes examined and genotypes of samples are indicated. Note that the samples in lane I and lane III, as well as lane II and lane IV, were harvested from littermates.
FIG. 7.
Transcriptional analysis of Ctnnb1, Bmp4, Vegfa, and Smad2 in Ikbkap+/+ and Ikbkap−/− embryos. (A) Schematic representation of the genes investigated by ChIP assay. Exons are depicted by boxes; the closed boxes indicate the localization of the amplicons. The numbers (kb) indicate the positions of these amplicons relative to the 5′ sites of genes. (B) The acetylation status of histone H3 in the transcribed regions of Ctnnb1, Bmp4, Vegfa, and Smad2 was estimated using an acetyl-histone H3 ChIP assay. Note that Ctnnb1 exon 8, Bmp4 exon 2, and Vegfa exon 3 were not pulled down with anti-acetyl-histone H3 antibody in Ikbkap−/− embryos. The genes tested, the genotypes, and the locations (E, exon) of amplicons are indicated. α-Acetyl H3, anti-acetyl-histone H3 antibody; No Ab, no antibody control.
Rescue of Ikbkap−/− embryonic lethality by human IKAP.
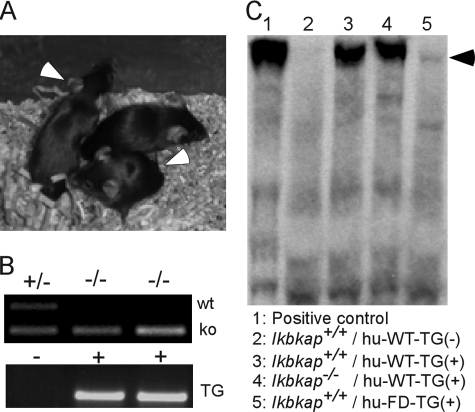
The embryonic lethality of the Ikbkap−/− mice stands in sharp contrast to the clinically observed survival of FD patients beyond the adolescent years. However, this result was anticipated, since the expression of IKAP is completely abolished in the mouse model while variable levels of normal IKAP are produced in FD patients, with marked reduction in the central and peripheral nervous system (13, 41). We postulate that there is a tissue-specific minimum IKAP level that is required for normal development. This hypothesis is supported by the fact that all FD patients carry at least one copy of the IVS20+6T>C splice mutation, which leads to the observed tissue-specific splicing differences. Further, no extremely deleterious mutations have been identified in the IKBKAP gene, as the two other known mutations lead to missense changes in the protein. Recently, we described the creation of transgenic mouse lines that carry the normal human IKBKAP gene and the FD IVS20+6T>C mutation. All of these lines are phenotypically normal, and the IVS20+6T>C transgenics missplice IKBKAP in a tissue-specific manner that mimics what is seen in FD patients (23). To test whether embryonic lethality caused by ablation of mouse Ikbkap could be rescued by human IKAP, we crossed two transgenic lines, one carrying the normal IKBKAP gene and one with the IVS20+6T>C mutation, with Ikbkap+/− mice in order to introduce the human transgenes into the Ikbkap knockout mouse line. The Ikbkap+/− mice that carried the human IKBKAP transgenes were viable, and fertility was normal with no obvious abnormalities (data not shown). Subsequent pairing of these lines with heterozygous Ikbkap knockout mice yielded animals that carried the human IKBKAP transgenes on a homozygous Ikbkap knockout background. The normal human IKBKAP transgene completely rescued embryonic lethality (Fig. 8A and B). In addition, by using an antibody that recognized only human IKAP, we demonstrated exclusive expression of human wild-type IKAP in the Ikbkap−/− knockout mice (Fig. 8C). These mice are phenotypically normal without any obvious abnormalities. The FD transgene did not rescue embryonic lethality in the Ikbkap−/− mice, likely due to the very low level of normal IKAP expression (Fig. 8C). This result indicates that the lethality of Ikbkap ablation in mice can be overcome by the presence of the human IKBKAP gene. More importantly, it confirms the functional conservation of IKAP between human and mouse during development.
FIG. 8.
Appearance of Ikbkap−/− mice with a human wild-type IKBKAP transgene. (A) The arrowheads point to Ikbkap null mice with the human wild-type IKBKAP transgene. No significant phenotypic differences were observed compared to heterozygous littermates. (B) PCR genotyping results of genomic DNA from tail snips with dedicated primers. Lane 1 (from left), Ikbkap+/−genotype; lanes 2 and 3, Ikbkap1−/− genotype. The mice represented by lanes 2 and 3 were positive for carrying the human transgene. wt, wild-type fragment; ko, knockout fragment; TG, human IKBKAP transgene. (C) Western blot result using the human-specific IKAP antibody to confirm the presence of the human IKBKAP protein, IKAP, in mouse brains from different genotypes. Lane 1, positive control of nuclear extracts from HeLa cells, which are positive for IKAP expression; lane 2, control mouse (no human IKBKAP [WT] transgene); lane 3, Ikbkap+/+ mouse with human WT transgene; lane 4, Ikbkap−/− mouse with human WT transgene; lane 5, Ikbkap−/− mouse with human FD transgene. Arrowhead 150-kDa position.
DISCUSSION
The identification of mutations in the IKBKAP gene in FD patients was a critical first step in the endeavor to find a treatment for FD (4, 7, 41). As a result of this discovery, it became clear that cellular levels of IKAP are diminished in a tissue-specific manner due to the IVS20+6T>C mutation, with the lowest levels observed in the nervous system. Even though the defective gene is now known, it is still unclear how reduction of IKAP during development leads to the observed neuropathy in FD. In order to begin to understand the role of IKAP in mammals, we created an Ikbkap knockout mouse model.
We demonstrated that Ikbkap expression is essential for embryonic development, as evidenced by embryonic lethality in the Ikbkap−/− embryos. Examination of embryos suggested that IKAP plays a crucial role in neuronal development. Expression of mouse Ikap can be detected in embryos at the early embryonic stage (8.5 dpc), with high expression in the primitive brain regions, notably the hindbrain, at the commencement of neurogenesis. At later embryonic stages (11.5 dpc), Ikap is expressed predominately in the dorsal root ganglia beside the spinal cord and in the roof plate area, where the progenitors of the cerebellum are born. Similar dorsal root ganglion expression has been reported previously in rat embryo studies using in situ hybridization (34). These unique expression patterns in the developing central and peripheral nervous system are consistent with the disoriented neural tube and absence of a primitive brain in the Ikbkap−/− embryos.
In this study, we demonstrated for the first time a role for Ikbkap in the proper development of the extraembryonic components of the conceptus, including the placenta and yolk sac. The visceral yolk sac functions as the metabolic center for the embryo during early development (3, 15). The yolk sac not only acts as a protective barrier for the embryo against the external environment, it is also involved in the transmission of nutrition from the uterus, as well as the disposal of toxins from the embryo prior to the development of the placenta and fetal liver. In addition, several molecules that are important for tissue remodeling, such as tissue plasminogen activator, as well as proteinases and inhibitors, are secreted by the visceral yolk sac (26). Over the past decade, with the advance of gene-targeting technology, several genes, such as Vegfa (6), Flt1 (16), Tgfb1 (14), Angpt1 (45), Tie1/Tie2 (37, 40), Gata6 (27), and Hnf4a (9), have been shown to be crucial for visceral yolk sac development. The abnormalities observed in the Ikbkap−/− visceral yolk sac during gastrulation, such as the delayed onset of blood island formation and failure of blood vessel development, in part have similarities to the knockout phenotypes of these genes, suggesting that vasculogenesis and angiogenesis are impaired in the absence of Ikbkap. In the Ikbkap−/− visceral yolk sac, the formation of the primitive capillary plexus at 9.5 dpc, in conjunction with the absence of subsequent events, such as the establishment and increased thickness of the lumen and neovascularization, indicates that vasculogenesis was initiated but the subsequent angiogenesis was disrupted. Despite our demonstration of gene expression changes, further studies will be required to determine the precise role of Ikbkap in this vital developmental process.
Although the abnormalities of the Ikbkap−/− yolk sac exhibit similarities to some other gene-targeting studies that led to embryonic lethality, the developmental deficits observed in the Ikbkap−/− embryos are unique. In the Tgfb1 knockout mouse model, the authors demonstrated that 50% of the TGFb1−/− and 25% of the TGFb1+/− embryos were prenatal lethal around 10.5 dpc as a result of the failure of the vascular and hematopoietic system functions and not due to direct effects on the nervous system in null embryos (14). In contrast, the Ikbkap−/− embryo exhibited a more dramatic phenotype; no Ikbkap−/− embryos were recovered beyond 12.5 dpc and severe neurodevelopmental defects were observed at earlier stages. Interestingly, at 9.5 dpc, the Ikbkap−/− embryo exhibits the classic features of gastrulation, such as open head fold, allantois, and primitive vascular organization, which normally occur in the wild-type embryos at 7.5 dpc, suggesting that Ikbkap might be involved in the signaling cascade that controls the transition from the primitive streak stage to gastrulation, such as proliferation and/or differentiation of progenitor cells. The fact that the anterior-posterior polarity of the Ikbkap−/− embryos was established eventually, as evidenced by the head-tail appearance at 9.5 dpc, suggests that Ikbkap might be involved in modulating this event via transcriptional regulation.
Examination of the embryos suggested that death occurred at 10.5 dpc. In fact the Ikbkap−/− embryos at 10 dpc appeared to be larger than those at 9.5 dpc, suggesting that the embryos lacking Ikbkap continue to develop during this period. Further, at 10.5 dpc, the anterior neural tube was found to be closed, although the structure was distinct from that observed in the Ikbkap+/+ embryos. In addition, the demonstration of disoriented alignment of the posterior neural tube in the Ikbkap−/− embryos reinforces the argument that neural-plate morphogenesis is still ongoing between 9.5 and 10.5 dpc. The irregularity of the neural-tube architecture, as well as the reduced expression of Bmp4 and Rhoa, both of which are important for roof plate patterning in vertebrates, suggests that neurulation is disrupted in the absence of Ikbkap (31, 32, 48). Interestingly, ablating Bmp4 not only led to embryonic lethality during gastrulation, but some of the Bmp4 null embryos showed open head folds and an unturned body (49), similar to what was observed in the Ikbkap−/− embryos at the corresponding stage. However, the Bmp4 null embryos did not advance to the postgastrulation stage as seen in the Ikbkap−/− embryos, and the size of the Bmp4 null embryos was similar to that of controls while the overall size of the Ikbkap−/− embryo was dramatically reduced. In the future, it would be interesting to investigate the interaction between Ikbkap and BMP signaling, given the observed reduction of Bmp4 expression in Ikbkap−/− embryos. In the Ikbkap−/− embryos, the level of Ctnnb1, a gene that is particularly important for brain formation and craniofacial development (5), was reduced. The function of Ctnnb1 during gastrulation has been demonstrated by the embryonic lethality resulting from the failure in the formation of ectodermal cell layers in the Ctnnb1 null embryo (18). The fact that the Ctnnb1−/− embryo cannot develop beyond gastrulation, unlike the Ikbkap−/− embryos, raises the possibility that in the absence of Ikbkap, another unknown signaling pathway might be compensating for the defect of Ctnnb1 signaling, or more likely, expression of this gene might not be totally abolished in the Ikbkap−/− embryos. Taken together, our findings suggest that Ikbkap−/− embryos undergo early development and advance to the late gastrulation stage in an environment without Ikbkap. However, the disturbances in both vascular and neural development lead to lethality immediately following gastrulation.
Recently, several genes that require IKAP/Elongator have been identified by using either FD fibroblasts or HeLa cells in which RNA interference was used to reduce IKBKAP expression (8, 10). Further, RNA microarray studies using postmortem FD tissue suggest that a subset of genes involved in myelination might require IKAP for efficient transcription (8). In the current study, we tested the expression pattern of a subset of these genes in Ikbkap−/− embryos and did not find any reduction in expression. However, we did find reduced or absent expression of several genes required for embryogenesis. Our ChIP analysis of these genes demonstrated that in the absence of IKAP, histone H3 acetylation is significantly reduced in the 3′ ends of genes, although similar levels are observed close to the promoter. This is similar to the patterns observed in the FD fibroblasts and supports the previously described model for Elongator function as a histone acetyltransferase involved in transcriptional elongation (10). It is interesting that different Elongator dependences for the expression of individual genes are observed in the in vitro knockdown and in vivo knockout systems. This might be attributed to different gene expression patterns, or perhaps to changes in local chromatin structure, during development. These results may also suggest that specific genes have either developmental or tissue-specific thresholds for their reliance on IKAP/Elongator. Finally, the observed variability in Elongator dependence illustrates the complicated cascade of transcriptional regulation and the difficulty in assessing direct targets in tissues based on effects observed in cells in culture.
In conclusion, we have shown for the first time that IKAP is required for embryogenesis in mammals. Although the precise cause of embryonic lethality remains to be determined, our findings indicate that IKAP is required for entry from the egg cylinder stage into the gastrulation stage, as well as for proper neurulation and primitive organ development. Further, the function of IKAP is not restricted to the embryo, since striking abnormalities were also seen in the visceral yolk sac. Our work further demonstrating rescue of embryonic lethality by a human IKBKAP transgene is exciting, as it suggests functional conservation between the mouse and human proteins. Despite the fact that low levels of normal IKAP are expressed from the human transgene carrying the FD splicing mutation, the transgene does not rescue lethality. It does, however, suggest that increasing IKAP expression from the FD transgene using the small molecule kinetin (22) may enable us to achieve our ultimate goal of creating a phenotypically accurate mouse model of FD.
Supplementary Material
Acknowledgments
We thank Felicia Axelrod and Gabrielle Gold von Simson of the Dysautonomia Treatment and Evaluation Center at New York University Medical School for their longstanding collaboration and helpful discussions. We thank James Gusella and Marcy MacDonald for their helpful discussions when we initiated this project and Jesper Svejstrup and Pierre Close for their advice on the ChIP studies.
This work was supported by grants from the Dysautonomia Foundation, Inc., and the National Institute for Neurological Disorders and Stroke.
Footnotes
Published ahead of print on 17 November 2008.
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Anderson, S. L., R. Coli, I. W. Daly, E. A. Kichula, M. J. Rork, S. A. Volpi, J. Ekstein, and B. Y. Rubin. 2001. Familial dysautonomia is caused by mutations of the IKAP gene. Am. J. Hum. Genet. 68753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelrod, F. B. 1995. Familial dysautonomia, p. 217-231. In D. Robertson and I. Biaggioni (ed.), Disorders of the autonomic nervous system. Harwood Academic Publishers, Luxembourg.
- 3.Baron, M. H. 2003. Embryonic origins of mammalian hematopoiesis. Exp. Hematol. 311160-1169. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld, A., S. A. Slaugenhaupt, C. B. Liebert, V. Temper, C. Maayan, S. Gill, D. E. Lucente, M. Idelson, K. MacCormack, M. A. Monahan, J. Mull, M. Leyne, M. Mendillo, T. Schiripo, E. Mishori, X. Breakefield, F. B. Axelrod, and J. F. Gusella. 1999. Precise genetic mapping and haplotype analysis of the familial dysautonomia gene on human chromosome 9q31. Am. J. Hum. Genet. 641110-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brault, V., R. Moore, S. Kutsch, M. Ishibashi, D. H. Rowitch, A. P. McMahon, L. Sommer, O. Boussadia, and R. Kemler. 2001. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 1281253-1264. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, C. Declercq, J. Pawling, L. Moons, D. Collen, W. Risau, and A. Nagy. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380435-439. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick, B. P., S. Gill, M. Leyne, J. Mull, C. B. Liebert, C. M. Robbins, H. W. Pinkett, I. Makalowska, C. Maayan, A. Blumenfeld, F. B. Axelrod, M. Brownstein, and S. A. Slaugenhaupt. 1999. Cloning, genomic organization and expression of a putative human transmembrane protein related to the Caenorhabditis elegans M01F1.4 gene. Gene 24067-73. [DOI] [PubMed] [Google Scholar]
- 8.Cheishvili, D., C. Maayan, Y. Smith, G. Ast, and A. Razin. 2007. IKAP/hELP1 deficiency in the cerebrum of familial dysautonomia patients results in down regulation of genes involved in oligodendrocyte differentiation and in myelination. Hum. Mol. Genet. 162097-2104. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W. S., K. Manova, D. C. Weinstein, S. A. Duncan, A. S. Plump, V. R. Prezioso, R. F. Bachvarova, and J. E. Darnell, Jr. 1994. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 82466-2477. [DOI] [PubMed] [Google Scholar]
- 10.Close, P., N. Hawkes, I. Cornez, C. Creppe, C. A. Lambert, B. Rogister, U. Siebenlist, M. P. Merville, S. A. Slaugenhaupt, V. Bours, J. Q. Svejstrup, and A. Chariot. 2006. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol. Cell 22521-531. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, L., W. J. Henzel, and P. A. Baeuerle. 1998. IKAP is a scaffold protein of the IκB kinase complex. Nature 395292-296. [DOI] [PubMed] [Google Scholar]
- 12.Cuajungco, M. P., M. Leyne, J. Mull, S. P. Gill, J. F. Gusella, and S. A. Slaugenhaupt. 2001. Cloning, characterization, and genomic structure of the mouse Ikbkap gene. DNA Cell Biol. 20579-586. [DOI] [PubMed] [Google Scholar]
- 13.Cuajungco, M. P., M. Leyne, J. Mull, S. P. Gill, W. Lu, D. Zagzag, F. B. Axelrod, C. Maayan, J. F. Gusella, and S. A. Slaugenhaupt. 2003. Tissue-specific reduction in splicing efficiency of IKBKAP due to the major mutation associated with familial dysautonomia. Am. J. Hum. Genet. 72749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson, M. C., J. S. Martin, F. M. Cousins, A. B. Kulkarni, S. Karlsson, and R. J. Akhurst. 1995. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 1211845-1854. [DOI] [PubMed] [Google Scholar]
- 15.Dzierzak, E., and A. Medvinsky. 1995. Mouse embryonic hematopoiesis. Trends Genet. 11359-366. [DOI] [PubMed] [Google Scholar]
- 16.Fong, G. H., J. Rossant, M. Gertsenstein, and M. L. Breitman. 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 37666-70. [DOI] [PubMed] [Google Scholar]
- 17.Gold-von Simson, G., and F. B. Axelrod. 2006. Familial dysautonomia: update and recent advances. Curr. Probl. Pediatr. Adolesc. Health Care 36218-237. [DOI] [PubMed] [Google Scholar]
- 18.Haegel, H., L. Larue, M. Ohsugi, L. Fedorov, K. Herrenknecht, and R. Kemler. 1995. Lack of beta-catenin affects mouse development at gastrulation. Development 1213529-3537. [DOI] [PubMed] [Google Scholar]
- 19.Hawkes, N. A., G. Otero, G. S. Winkler, N. Marshall, M. E. Dahmus, D. Krappmann, C. Scheidereit, C. L. Thomas, G. Schiavo, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 2002. Purification and characterization of the human elongator complex. J. Biol. Chem. 2773047-3052. [DOI] [PubMed] [Google Scholar]
- 20.Hebbes, T. R., A. W. Thorne, and C. Crane-Robinson. 1988. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 71395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilz, M. J., E. H. Kolodny, I. Neuner, B. Stemper, and F. B. Axelrod. 1998. Highly abnormal thermotests in familial dysautonomia suggest increased cardiac autonomic risk. J. Neurol. Neurosurg. Psychiatry 65338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hims, M. M., C. el Ibrahim, M. Leyne, J. Mull, L. Liu, C. Lazaro, R. S. Shetty, S. Gill, J. F. Gusella, R. Reed, and S. A. Slaugenhaupt. 2007. Therapeutic potential and mechanism of kinetin as a treatment for the human splicing disease familial dysautonomia. J. Mol. Med. 85149-161. [DOI] [PubMed] [Google Scholar]
- 23.Hims, M. M., R. S. Shetty, J. Pickel, J. Mull, M. Leyne, L. Liu, J. F. Gusella, and S. A. Slaugenhaupt. 2007. A humanized IKBKAP transgenic mouse models a tissue-specific human splicing defect. Genomics 90389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmberg, C., S. Katz, M. Lerdrup, T. Herdegen, M. Jaattela, A. Aronheim, and T. Kallunki. 2002. A novel specific role for I kappa B kinase complex-associated protein in cytosolic stress signaling. J. Biol. Chem. 27731918-31928. [DOI] [PubMed] [Google Scholar]
- 25.Jablonowski, D., S. Zink, C. Mehlgarten, G. Daum, and R. Schaffrath. 2006. tRNAGlu wobble uridine methylation by Trm9 identifies Elongator's key role for zymocin-induced cell death in yeast. Mol. Microbiol. 59677-688. [DOI] [PubMed] [Google Scholar]
- 26.Jones, C. J., and E. Jauniaux. 1995. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron 26145-173. [DOI] [PubMed] [Google Scholar]
- 27.Koutsourakis, M., A. Langeveld, R. Patient, R. Beddington, and F. Grosveld. 1999. The transcription factor GATA6 is essential for early extraembryonic development. Development 126723-732. [PubMed] [Google Scholar]
- 28.Krappmann, D., E. N. Hatada, S. Tegethoff, J. Li, A. Klippel, K. Giese, P. A. Baeuerle, and C. Scheidereit. 2000. The I kappa B kinase (IKK) complex is tripartite and contains IKK gamma but not IKAP as a regular component. J. Biol. Chem. 27529779-29787. [DOI] [PubMed] [Google Scholar]
- 29.Lehavi, O., O. Aizenstein, D. Bercovich, D. Pavzner, R. Shomrat, A. Orr-Urtreger, and Y. Yaron. 2003. Screening for familial dysautonomia in Israel: evidence for higher carrier rate among Polish Ashkenazi Jews. Genet. Test. 7139-142. [DOI] [PubMed] [Google Scholar]
- 30.Leyne, M., J. Mull, S. P. Gill, M. P. Cuajungco, C. Oddoux, A. Blumenfeld, C. Maayan, J. F. Gusella, F. B. Axelrod, and S. A. Slaugenhaupt. 2003. Identification of the first non-Jewish mutation in familial dysautonomia. Am. J. Med. Genet. A 118305-308. [DOI] [PubMed] [Google Scholar]
- 31.Liem, K. F., Jr., G. Tremml, and T. M. Jessell. 1997. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91127-138. [DOI] [PubMed] [Google Scholar]
- 32.Liu, A., and L. A. Niswander. 2005. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat. Rev. Neurosci. 6945-954. [DOI] [PubMed] [Google Scholar]
- 33.Maayan, C., E. Kaplan, S. Shachar, O. Peleg, and S. Godfrey. 1987. Incidence of familial dysautonomia in Israel 1977-1981. Clin. Genet. 32106-108. [DOI] [PubMed] [Google Scholar]
- 34.Mezey, E., A. Parmalee, I. Szalayova, S. P. Gill, M. P. Cuajungco, M. Leyne, S. A. Slaugenhaupt, and M. J. Brownstein. 2003. Of splice and men: what does the distribution of IKAP mRNA in the rat tell us about the pathogenesis of familial dysautonomia? Brain Res. 983209-214. [DOI] [PubMed] [Google Scholar]
- 35.Nord, A. S., P. J. Chang, B. R. Conklin, A. V. Cox, C. A. Harper, G. G. Hicks, C. C. Huang, S. J. Johns, M. Kawamoto, S. Liu, E. C. Meng, J. H. Morris, J. Rossant, P. Ruiz, W. C. Skarnes, P. Soriano, W. L. Stanford, D. Stryke, H. von Melchner, W. Wurst, K. Yamamura, S. G. Young, P. C. Babbitt, and T. E. Ferrin. 2006. The International Gene Trap Consortium website: a portal to all publicly available gene trap cell lines in mouse. Nucleic Acids Res. 34D642-D648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otero, G., J. Fellows, Y. Li, T. de Bizemont, A. M. Dirac, C. M. Gustafsson, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3109-118. [DOI] [PubMed] [Google Scholar]
- 37.Patan, S. 1998. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 561-21. [DOI] [PubMed] [Google Scholar]
- 38.Pearson, J. 1979. Familial dysautonomia (a brief review). J. Auton. Nerv Syst. 1119-126. [DOI] [PubMed] [Google Scholar]
- 39.Rahl, P. B., C. Z. Chen, and R. N. Collins. 2005. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell 17841-853. [DOI] [PubMed] [Google Scholar]
- 40.Sato, T. N., Y. Tozawa, U. Deutsch, K. Wolburg-Buchholz, Y. Fujiwara, M. Gendron-Maguire, T. Gridley, H. Wolburg, W. Risau, and Y. Qin. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 37670-74. [DOI] [PubMed] [Google Scholar]
- 41.Slaugenhaupt, S. A., A. Blumenfeld, S. P. Gill, M. Leyne, J. Mull, M. P. Cuajungco, C. B. Liebert, B. Chadwick, M. Idelson, L. Reznik, C. Robbins, I. Makalowska, M. Brownstein, D. Krappmann, C. Scheidereit, C. Maayan, F. B. Axelrod, and J. F. Gusella. 2001. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am. J. Hum. Genet. 68598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slaugenhaupt, S. A., and J. F. Gusella. 2002. Familial dysautonomia. Curr. Opin. Genet. Dev. 12307-311. [DOI] [PubMed] [Google Scholar]
- 43.Stanford, W. L., T. Epp, T. Reid, and J. Rossant. 2006. Gene trapping in embryonic stem cells. Methods Enzymol. 420136-162. [DOI] [PubMed] [Google Scholar]
- 44.Struhl, K. 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12599-606. [DOI] [PubMed] [Google Scholar]
- 45.Suri, C., P. F. Jones, S. Patan, S. Bartunkova, P. C. Maisonpierre, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 871171-1180. [DOI] [PubMed] [Google Scholar]
- 46.Svejstrup, J. Q. 2007. Elongator complex: how many roles does it play? Curr. Opin. Cell Biol. 19331-336. [DOI] [PubMed] [Google Scholar]
- 47.Waldrip, W. R., E. K. Bikoff, P. A. Hoodless, J. L. Wrana, and E. J. Robertson. 1998. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell 92797-808. [DOI] [PubMed] [Google Scholar]
- 48.Wallingford, J. B., and R. M. Harland. 2002. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development 1295815-5825. [DOI] [PubMed] [Google Scholar]
- 49.Winnier, G., M. Blessing, P. A. Labosky, and B. L. Hogan. 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 92105-2116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.