Abstract
The CTCF protein is a highly conserved zinc finger protein that is implicated in many aspects of gene regulation and nuclear organization. Its functions include the ability to act as a repressor of genes, including the c-myc oncogene. In this paper, we show that the CTCF protein can be posttranslationally modified by the small ubiquitin-like protein SUMO. CTCF is SUMOylated both in vivo and in vitro, and we identify two major sites of SUMOylation in the protein. The posttranslational modification of CTCF by the SUMO proteins does not affect its ability to bind to DNA in vitro. SUMOylation of CTCF contributes to the repressive function of CTCF on the c-myc P2 promoter. We also found that CTCF and the repressive Polycomb protein, Pc2, are colocalized to nuclear Polycomb bodies. The Pc2 protein may act as a SUMO E3 ligase for CTCF, strongly enhancing its modification by SUMO 2 and 3. These studies expand the repertoire of posttranslational modifications of CTCF and suggest roles for such modifications in its regulation of epigenetic states.
CTCF is a highly conserved zinc finger protein that is involved in many cellular functions, including gene repression (3, 8, 49), gene activation (9), chromatin insulator function (5, 6, 9, 36), and the maintenance of genomic imprinting (21, 25, 55). CTCF prevents initiation and/or spreading of DNA methylation of imprinted genes (24, 56, 62, 66). It is also implicated in carcinogenesis, and missense mutations in its zinc finger DNA binding domain have been described in breast, prostate, and kidney tumors (27). The CTCF protein plays a role in another epigenetic process, X chromosome inactivation (11, 58).
Recently, CTCF has been implicated in genomic organization, mediating intrachromosomal interactions at the β-globin HS4 insulator (64), the H19/IGF2 imprinting control region (44, 74), and the major histocompatibility complex class II genes (50), as well as interchromosomal interactions between the IGF2 locus on human chromosome 11 and the Wsb1 locus on chromosome 7 (47). Interestingly, CTCF is required for site-specific binding of cohesin proteins throughout the genome, and cohesin has been implicated in insulator activity (57, 65, 69). Several bioinformatic and chromatin immunoprecipitation studies have recently estimated that there are around 15,000 CTCF binding sites in the human genome. These CTCF binding sites have been found to delineate the boundaries of different chromatin regions, and many of these binding sites possess insulator activity (4, 41, 53, 71, 72). Despite the important role that CTCF plays in gene expression, the molecular mechanisms underlying its many functions remain enigmatic.
CTCF is posttranslationally modified by both phosphorylation and poly(ADP) ribosylation (43, 75). Phosphorylation of CTCF relieves its repressive activity at the c-myc P2 promoter (22, 43), and its poly(ADP) ribosylation is needed for it to act as an insulator protein (42, 75). We have now added another level of complexity to CTCF's multiple functions by finding that it is posttranslationally modified by the SUMO (for small ubiquitin-like modifier) proteins.
The SUMO proteins are members of the family of ubiquitin-like proteins that covalently modify their target proteins (40). SUMO proteins are roughly 100 amino acids long, about 20 amino acids longer than ubiquitin. Their three-dimensional structure is similar to that of ubiquitin, although they are only 18% identical in sequence (28, 29, 60, 63). SUMO 1 differs in sequence by about 50% from SUMO 2 and 3, which are about 97% identical. The biochemical pathway for the modification of proteins by SUMO proteins is mechanistically similar to, but distinct from, that of ubiquitin (28, 29).
Unlike polyubiquitin, SUMO modification of a protein does not target it for degradation. The list of SUMO-modified proteins is expanding rapidly. Many of them play key roles in transcriptional regulation, chromatin structure, and DNA repair. Since SUMOylation is often associated with the repression of gene expression, and genomic imprinting and insulator function involve the silencing of genes, we asked whether the CTCF insulator protein was SUMOylated.
We report that the CTCF protein can be modified by SUMO 1, 2, and 3. We have identified two major sites of SUMOylation, one in the COOH-terminal domain and one in the NH2-terminal domain. These sites are conserved from fish to mammals. We show that the SUMO modification is functionally important for the repressive action of CTCF on the c-myc P2 promoter. Furthermore, we found that the Polycomb group protein Polycomb 2 (Pc2) likely acts as a SUMO E3 ligase in the SUMOylation of CTCF. Finally, we report that CTCF is colocalized with the Pc2 protein to nuclear particles called Polycomb bodies. Our findings suggest that the SUMOylation of CTCF is involved in the organization of repressive chromatin domains involving CTCF-responsive genes.
MATERIALS AND METHODS
Cell culture and transfection.
HEK 293 and HeLa cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. HEK 293 cells were transfected using calcium phosphate as previously described (19, 20). HeLa cells were transfected using Fugene 6 transfection reagent (Roche Diagnostic Products), according to the manufacturer's instructions.
Plasmids and cloning.
HEK 293 cDNA was prepared from total RNA isolated using Tri Reagent (Sigma) and was reverse transcribed with Superscript II (Invitrogen) using random hexamers. The coding sequence of Ufm1 (for ubiquitin fold-like modifier 1) (NM_016617) and the coding sequences for the processed forms of SUMO 1 (NM_001005781.1; nucleotides [nt] 149 to 439), SUMO 2 (NM_006937.3; nt 170 to 448), and SUMO 3 (NM_006936.2; nt 162 to 437) were PCR amplified from HEK 293 cDNA using the primers listed in Table 1. The PCR products were cloned into the plasmid pCMVmyc, which contains the cytomegalovirus promoter and an N-terminal myc tag (2). CTCF mouse cDNA (36) (a gift from S. Tilghman and C. Schoenherr) was PCR amplified and cloned into the plasmid pMZS3F (78) (a generous gift of J. Greenblatt) containing a C-terminal calmodulin binding peptide, a tobacco etch virus protease cleavage site, and a three-FLAG tag. Mutagenesis reactions were carried out using Stratagene's QuikChange Multi site directed mutagenesis kit or QuikChange XL site directed mutagenesis kit. The sentrin-specific protease 1 (SENP1) and SENP5 expression vectors (a generous gift from E. T. Yeh) were previously described (12, 31). The T7-Pc2 expression vector (a generous gift from D. Wotton) was previously described (39). The DNA encoding the NH2-terminal and COOH-terminal domains of CTCF (amino acids 1 to 266 and 570 to 736, respectively) was PCR amplified from a cDNA clone and cloned into the vector pGEX4T-1 (Amersham) using the primers listed in Table 1 to create NH2-terminal glutathione S-transferase (GST) fusions of the two domains.
TABLE 1.
Primers used for cloning
| Primer | Orientationa | Sequenceb | Description |
|---|---|---|---|
| pCMVmyc-SUMO1 | F | ATCCCC[AAGCTT]ATGTCTGACCAGGAGGCA | HindIII |
| R | ATTCGC[GGATCC][CTA]ACCCCCCGTTTGTTCCTG | BamHI; stop codon | |
| pCMVmyc-SUMO2 | F | AATCCG[GAATTC]ATGGCCGACGAAAAGCCCAA | EcoRI |
| R | TAACGC[GGATCC][TCA]ACCTCCCGTCTGCTGTTGG | BamHI; stop codon | |
| pCMVmyc-SUMO3 | F | AATCCG[GAATTC]ATGTCCGAGGAGAAGCCC | EcoRI |
| R | AATCGC[GGATCC][CTA]ACCTCCCGTCTGCTGCTG | BamHI | |
| pCMV myc-Ufm1 | F | CACCCG[GAATTC]ATGTCGAAGGTTTCCTTTAAG | EcoRI |
| R | ATTCGC[GGATCC]TTAACAACTTCCAACACGATC | BamHI | |
| pGEX4T-1 N-CTCF (amino acids 1-266) | F | TACCG[GAATTC]ATGGAAGGTGAGGCGGTTG | EcoRI |
| R | TATCCG[CTCGAG]CTGGAATGTTTTCTTTACACC | XhoI | |
| pGEX4T-1 C-CTCF (amino acids 570-736) | F | TTACGC[GGATCC]ATGGCAAGACATGCAGATAACTG | BamHI |
| R | TATCCG[CTCGAG]TCACCGGTCCATCATGCTGAG | XhoI |
F, forward; R, reverse.
Brackets indicate restriction enzyme sites and stop codons engineered into the primers.
Purification of NH2- and COOH-terminal domains and in vitro SUMOylation.
Overnight cultures of Escherichia coli BL21 cells grown in LB containing 100 μg/ml ampicillin at 37°C and 300 rpm were diluted 1:10 in fresh LB plus ampicillin and grown at 30°C and 250 rpm until an optical density at 600 nm between 0.8 and 1.0 was reached. The GST fusion domains were expressed by induction with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 20°C. Protein was purified using glutathione resin (Amersham) and eluted using reduced glutathione. Purified protein was dialyzed against 20 mM HEPES, pH 8.0, 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 1 mM dithiothreitol and quantified using the Bio-Rad protein assay.
A SUMOylation control kit for SUMO 1 was purchased from LAE Biotech International (catalog no. K007) and used according to the manufacturer's instructions. Additional SUMO 1, 2, and 3 proteins were purchased from Boston Biochem, Inc. (catalog no. Uh-752 and Uh-762). Briefly, 5 μl of in vitro-translated CTCF or 0.5 μg of the purified GST-tagged CTCF domain was incubated with the recommended amounts of E1 and E2 enzymes and 2 mM ATP, with or without SUMO proteins, in a 20- or 10-μl reaction mixture for 1 h at 37°C. The reaction mixture was used in electrophoretic mobility shift assays or analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting and probing with the appropriate antibody (anti-CTCF C-20 or N-17; Santa Cruz Biotechnology). In the case of 35S-labeled CTCF, the gel was dried and exposed to a phosphorimager screen.
Immunoprecipitation and Western blotting.
Ten-centimeter dishes of confluent HEK 293 cells overexpressing the tagged proteins were lysed in 200 μl of buffer containing 62.5 mM Tris-Cl, pH 6.8, 2% SDS, 10% glycerol, protease inhibitor cocktail (Sigma), and 1 mM N-ethyl maleimide by boiling them for 5 min. The lysates were then sonicated three times for 5 s each time to reduce viscosity, followed by centrifugation at 15,000 rpm for 15 min to remove cellular debris. The lysates were diluted fivefold with dilution buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40) incubated with Sepharose Protein G resin (GE Healthcare) and 5 μg of anti-FLAG antibody at 4°C for 3 to 16 h. The immunoprecipitates were washed three times in wash buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.1% NP-40), boiled in 2× SDS-PAGE loading buffer, separated by SDS-PAGE, and electroblotted onto polyvinylidene difluoride (PVDF) membranes (GE Healthcare). The membranes were incubated in mouse anti-FLAG M2 (Sigma; F-3165), rabbit anti-myc (Abcam; ab9106), or goat anti-beta-actin (Abcam; ab8229) primary antibody and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) and detected using ECL Western Blotting Detection Reagents (GE Healthcare; RPN2109). In immunoprecipitation experiments for Pc2 E3 ligase activity, HeLa cells were transfected using Fugene 6. The T7-Pc2-transfected HeLa cell lysates were precleared of Pc2 using 20 μl of a 50% slurry of anti-T7 agarose (Abcam; ab1230) prior to immunoprecipitation of CTCF with anti-FLAG antibody as described above. The detection of the endogenous SUMOylation of CTCF was performed by lysing five confluent 15-cm tissue culture dishes of HEK 293 cells in 1 ml of lysis buffer by boiling them in a kettle for 10 min. The lysate was sonicated to reduce the viscosity, centrifuged as before, and diluted 10-fold with dilution buffer containing 1 mM N-ethyl maleimide. In each immunoprecipitation, 2 ml of the diluted lysate was added to Sepharose-protein G resin and 10 μg of anti-SUMO 1 (D-11) antibody (Santa Cruz) or 10 μg of mouse immunoglobulin G (IgG) (Santa Cruz) as a control and was incubated at 4°C for 16 h. The immunoprecipitates were washed three times in wash buffer (50 mM Tris-Cl, pH 8.0, 250 mM NaCl, 0.1% NP-40, and 1 mM N-ethyl maleimide), boiled in 2× SDS-PAGE loading buffer, separated by SDS-PAGE, and electroblotted onto a PVDF membrane as before. The membrane was probed with anti-CTCF (C-20) antibody (Santa Cruz) and horseradish peroxidase-conjugated secondary antibody and detected using an ECL Plus Western blotting detection system (GE Healthcare; RPN2132).
Immunofluorescence staining and confocal microscopy.
HeLa cells grown on glass coverslips were fixed in 3% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at 37°C. The cells were then washed two times in PBS at 37°C. The cells were permeabilized with 1% (wt/vol) Triton X-100 in PBS for 5 min at room temperature and blocked in 3% (wt/vol) bovine serum albumin in PBS for 30 min at 37°C. The coverslips were then incubated with anti-CTCF primary antibody (1:50 dilution) (BD Biosciences; 612148) or anti-CTCF (1:25 or 1:10 dilution) (Santa Cruz Biotechnology; N-17), anti-Pc2 (1:200 dilution) (Abcam; ab4189), anti-promyelocytic leukemia protein (PML) (1:50) (Santa Cruz Biotechnology; PG-M3), or anti-SC-35 (1:200 dilution) (Sigma) at 37°C for 1 h, followed by two washes in 0.05% Tween 20 in PBS at 37°C. The coverslips were incubated in secondary antibodies conjugated with Texas Red or fluorescein isothiocyanate (Jackson ImmunoResearch), Alexa 488, or Alexa 647 (Invitrogen) at 37°C for 1 h, washed with 0.05% Tween 20 in PBS at 37°C, and mounted with Prolong Gold antifade reagent (Invitrogen). Cell samples were examined using a Zeiss 63× numerical aperture PlanApo objective lens. Images were recorded using a Zeiss LSM 510 confocal laser microscope. The colocalization of CTCF and Pc2, SC-35, or PML was determined using the ImageJ JaCOP colocalization plug-in (7; http://rsb.info.nih.gov/ij/).
Luciferase reporter assays.
The HEK 293 cells were split and seeded into 12-well plates at a density of 2 × 104 to 2.5 × 104 cells per well 24 h prior to transfection. The cells were transfected using the calcium phosphate-mediated transfection technique. The c-myc P2 promoter-luciferase reporter construct, pGL2XNM-Luc, was the generous gift of L. Penn (23). The c-myc P2 minimal promoter contains 142 bp of DNA, which includes a CTCF binding site at its 3′ end (26). For a typical transfection, 0.5 μg of the reporter plasmid DNA was mixed with different amounts of wild-type or mutant CTCF expression vectors plus 0.25 ng of pRL-TK plasmid DNA, which contains the Renilla luciferase gene used as a control for transfection efficiency. The total amount of DNA was kept constant by the addition of pUC18 or pBR322 DNA. The assays were performed as described previously (20). To assess the transcriptional activity of the c-myc P2 promoter, the firefly luciferase activity was normalized to the Renilla luciferase activity. Each transfection was performed in triplicate, and the experiment was repeated with at least three different DNA preparations. DNA was prepared using the Qiagen Plasmid Midi kit. DNA concentrations were measured by UV spectrophotometry and confirmed by agarose gel electrophoresis.
In vitro transcription/translation of CTCF.
Full-length CTCF was prepared by in vitro transcription/translation as described previously (19). A translation reaction in the absence of CTCF mRNA was used as a negative (unprogrammed) control. To make 35S-labeled CTCF, we used a translation kit depleted of methionine (Ambion) and supplemented the reaction with l-[35S]methionine (Amersham); 80 μCi was used per 100-μl reaction mixture.
Electrophoretic mobility shift assay.
Assays of in vitro DNA binding were carried out as described previously (19). The coordinates of the 32P-labeled PCR fragments used for binding are listed in Table 2.
TABLE 2.
Primers used to amplify DNA fragments used in the electrophoretic mobility shift assays
| Primer | Accession no. | Nucleotides amplified | Orientationa | Sequence |
|---|---|---|---|---|
| H19-B1 | AF125183 | 7893-8079 | F | GAGGCTTCTCCTTCGGTCTC |
| R | AGATGACCCCCGTGAACC | |||
| c-myc 5′ insulator | D10493 | 292-468 | F | CCTCGGACGCTCCTGCTC |
| R | TCGCTATGCTGGATTTTGCTG | |||
| c-myc P2 promoter | D10493 | 2446-2610 | F | GATCGCGCTGAGTATAAAAGC |
| R | CCTATTCGCTCCGGATCTC | |||
| Chicken β-globin insulator FII | AY040835 | 20-175 | F | CCCAAAGCCCCCAGGGATGT |
| R | AAGCGATCCCGTGCCACCTT |
F, forward; R, reverse.
RESULTS
CTCF is modified by SUMO 1, 2, and 3.
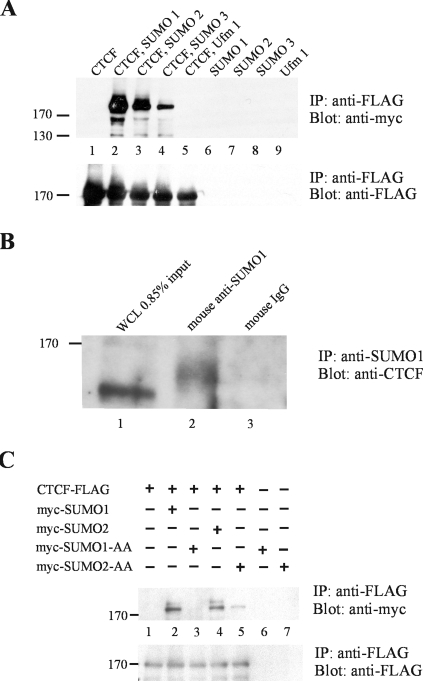
To show that CTCF is posttranslationally modified by the SUMO proteins, we transiently transfected HEK 293 cells with vectors expressing both three-FLAG-tagged mouse CTCF and one of the three myc-tagged SUMO proteins. Cells were lysed under denaturing conditions, and CTCF was immunoprecipitated using an antibody against the FLAG epitope. The immunoprecipitate was run on a denaturing polyacrylamide gel and electroblotted onto a PVDF membrane. Probing the membrane with an antibody against the myc epitope to detect SUMO-modified proteins revealed that CTCF was modified by each of the three SUMO 1, 2, and 3 proteins (Fig. 1A, lanes 2, 3, and 4). SUMOylated CTCF runs at a position corresponding to a molecular mass slightly above 170 kDa. Transfection of cells with FLAG-tagged CTCF or myc-tagged SUMO proteins alone did not result in a 170-kDa band (Fig. 1A, lanes 1, 6, 7, and 8). The reciprocal experiment, immunoprecipitation with anti-myc antibody for the SUMO proteins and Western blotting for FLAG-tagged CTCF, also showed that CTCF is SUMOylated by all three SUMO proteins (data not shown). To demonstrate that the modification of CTCF is specific to the SUMO proteins, we used a vector containing myc-tagged Ufm1, along with FLAG-CTCF. Ufm1 does not modify CTCF (Fig. 1A, lane 5). To show that the tag itself was not SUMOylated, the same three-FLAG-tag vector encoding FLAG-tagged E. coli beta-galactosidase was cotransfected with the SUMO proteins; LacZ was not modified by the SUMO proteins (data not shown). To demonstrate that endogenous CTCF is modified by SUMO 1, HEK 293 cells were harvested and lysed under denaturing conditions. The lysate was immunoprecipitated using an anti-SUMO 1 antibody and mouse IgG as a control. The immunoprecipitate was run on a denaturing polyacrylamide gel and electroblotted onto a PVDF membrane. Probing the membrane with an antibody against CTCF revealed a high-molecular-weight band of SUMOylated CTCF protein (Fig. 1B, lane 2) that was absent when mouse IgG was used (lane 3). We performed the reciprocal experiment, but we were unable to detect SUMOylated CTCF when immunoprecipitation was done with anti-CTCF antibody due to a lack of sensitivity in Western blotting with anti-SUMO 1 (data not shown).
FIG. 1.
CTCF is SUMOylated by SUMO 1, 2, and 3, and SUMOylation depends on the SUMO protein's terminal diglycine residues. (A) CTCF is SUMOylated by SUMO 1, 2, and 3. HEK 293 cells were transfected with plasmids encoding FLAG-tagged CTCF and one of the three SUMO proteins or the ubiquitin-like protein Ufm1, all tagged with the myc epitope, as indicated above the top blot. (Top) The whole-cell lysates were immunoprecipitated (IP) with an antibody directed against the FLAG epitope (for CTCF), analyzed by SDS-PAGE and Western blotting, and probed with anti-myc antibody to detect SUMO or Ufm1 modifications. CTCF (>170,000-molecular-weight band) is modified by SUMO 1, 2, and 3 (lanes 2, 3, and 4) but not by Ufm1 (lane 5). Single transfections with CTCF and SUMO 1, 2, or 3 plasmids gave no signal. (Bottom) The same blot was stripped and reprobed with anti-FLAG antibody to show that CTCF was efficiently immunoprecipitated (lanes 1 to 5), whereas no signal was detected after transfection by SUMO- or Ufm1-encoding plasmids alone (lanes 6 to 9). (B) Endogenous CTCF is SUMOylated by SUMO 1. HEK 293 cells were harvested, lysed, and immunoprecipitated with antibody against SUMO 1 or mouse IgG as a control. The immunoprecipitates were analyzed by SDS-PAGE and Western blotting. Probing with anti-CTCF antibody detected a high-molecular-weight form of CTCF modified by SUMO 1 (lane 2). Whole-cell lysate (WCL) (0.85% of the input into each immunoprecipitation) was run in lane 1 to show the unmodified form of CTCF, which migrates faster through the SDS-PAGE gel than the SUMOylated form of CTCF (lane 2). (C) SUMOylation of CTCF by SUMO 1 and 2 is dependent on the two C-terminal glycine residues. HEK 293 cells were transfected with the plasmids indicated at the top. (Top) Whole-cell lysates were immunoprecipitated with anti-FLAG antibody, and Western blots were probed with anti-myc antibody. SUMOylated CTCF was detected after transfection with wild-type SUMO plasmids (lanes 2 and 4) but was absent or greatly reduced with the GG→AA mutant constructs (lanes 3 and 5). The bands were absent after transfection with single plasmids (lanes 1, 6, and 7). (Bottom) Probing the same blot with anti-FLAG antibody (for CTCF) showed that CTCF was efficiently immunoprecipitated.
The SUMOylation reaction requires the presence of a COOH-terminal diglycine sequence on the SUMO molecule for the covalent attachment of the SUMO to the side chain of the target lysine residue. When we mutated the terminal diglycine of SUMO 1 and 2 to dialanine (SUMO 1-AA and SUMO 2-AA), there was a reduction of the SUMOylation of CTCF, thus demonstrating that the modification of CTCF by the SUMO proteins is due to the formation of a covalent bond through diglycine (Fig. 1C, lanes 2 versus 3, and 4 versus 5). Residual SUMOylation of CTCF by SUMO 2-AA may be due to the noncovalent interaction of CTCF and SUMO 2.
SUMOylation of CTCF is sensitive to the presence of SENP1 and SENP5 isopeptidases.
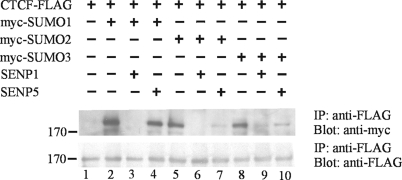
SENPs are isopeptidases that remove SUMO proteins from their target proteins by cleaving the isopeptide bond between the SUMO and the target lysine residue. These enzymes play a critical role in regulating the amount of SUMOylated target protein in the cell. To gain insight into the specificity of the SUMOylation of CTCF, we cotransfected 293 cells with plasmids that encoded either the SENP1 or the SENP5 protease (generous gifts from E. T. Yeh), in addition to those encoding FLAG-tagged CTCF and one of the three myc-tagged SUMO proteins. The overexpression of SENP1 reduces the SUMOylation of CTCF by SUMO 1 (Fig. 2, lane 3), whereas the posttranslational modification of CTCF by SUMO 1 is insensitive to the overexpression of SENP5 (Fig. 2, lane 4). The SUMOylation of CTCF by either SUMO 2 or SUMO 3 was sensitive to the presence of both the SENP1 and SENP5 isopeptidases (Fig. 2, lanes 6, 7, 9, and 10). These results are consistent with the known specificity of the SENP5 isopeptidase for SUMO 2 and 3 and its known inability to hydrolyze SUMO 1 from target proteins. The SENP1 isopeptidase is known to hydrolyze all three SUMO proteins (31).
FIG. 2.
SUMOylation of CTCF is sensitive to the SENP1 and SENP5 isopeptidases. HEK 293 cells were transfected with plasmids encoding FLAG-tagged CTCF, one of the three myc-tagged SUMO proteins (lanes 2 to 10), and SENP1 (lanes 3, 6, and 9) or SENP5 (lanes 4, 7, and 10). (Top) Whole-cell lysates were immunoprecipitated (IP) with an anti-FLAG antibody (for CTCF), and the immunoprecipitates were subjected to SDS-PAGE, followed by Western blotting with an anti-myc antibody to detect the SUMO modifications. The SUMO 1 modification of CTCF (lane 2) was sensitive to SENP1 (lane 3) but not SENP5 (lane 4), whereas the modifications of CTCF by SUMO 2 and SUMO 3 (lanes 5 and 8) were sensitive to both SENP 1 (lanes 6 and 9) and SENP5 (lanes 7 and 10). This was the expected specificity of the SENPs. (Bottom) The same blot was reprobed with anti-FLAG antibody to show that CTCF was efficiently immunoprecipitated and that the proteases were not simply degrading CTCF nonspecifically.
CTCF is SUMOylated in vitro by SUMO 1, 2, and 3.
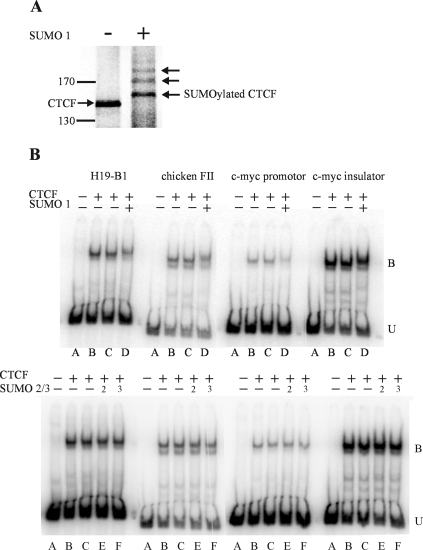
To verify the results of the transfection experiments, we asked whether CTCF could also be SUMOylated in vitro. We synthesized 35S-labeled CTCF using in vitro transcription followed by translation in a rabbit reticulocyte lysate in the presence of 35S-radiolabeled methionine. We then incubated an aliquot of the lysate in the presence of the SUMO E1-activating enzymes (SAE1/SAE2), the SUMO E2-conjugating enzyme (Ubc9), and one of the three SUMO proteins. As can be seen in Fig. 3A, SUMO 1 efficiently modified the radiolabeled CTCF protein in vitro, as demonstrated by its shift to a more slowly migrating species when run on a denaturing polyacrylamide gel. CTCF was also efficiently modified by SUMO 2 and 3 (data not shown).
FIG. 3.
SUMOylation of CTCF does not affect its DNA binding ability. (A) In-vitro translated 35S-labeled CTCF was incubated in a SUMOylation reaction without (−) and with (+) SUMO 1 protein and run on an SDS-PAGE gel. In the presence of SUMO 1, all of the CTCF was SUMOylated and shifted into more slowly migrating species (the arrows show the modified and unmodified forms of CTCF). Similar experiments also showed efficient SUMOylation by SUMO 2 and 3 (not shown). (B) CTCF was made by in vitro transcription/translation (IVT) and used in electrophoretic mobility shift assays with or without subsequent incubation in an in vitro SUMOylation reaction. The PCR fragments used in each experiment are shown and are described in Materials and Methods and Table 2. Lanes A, IVT mixture without CTCF mRNA; lanes B, IVT using CTCF mRNA; lanes C, IVT CTCF incubated in SUMOylation reaction mixture but without SUMO protein; lanes D, E, and F, IVT CTCF incubated in SUMOylation reaction mixture with SUMO 1, 2, or 3, respectively. U, unbound DNA; B, bound DNA. Careful inspection of lanes containing the SUMO proteins revealed a slight slowing of the shifted band, presumably due to SUMOylation of CTCF. Competition experiments showed that SUMOylation might affect more weakly binding sites (data not shown).
SUMOylation of CTCF does not affect its DNA binding ability.
CTCF acts as an insulator protein by binding to multiple copies of a nonmethylated consensus sequence upstream of the H19 gene. Methylation of this sequence on the paternal chromosome interferes with its DNA binding (5, 6, 9, 36). Therefore, it was of interest to know if the posttranslational modification of CTCF by the SUMO proteins would alter its ability to bind to DNA. We incubated 32P-radiolabeled DNA fragments containing the H19 CTCF binding sequence or similar CTCF binding sequences from the chicken HS4 β-globin FII insulator, the c-myc P2 promoter, and the myc N insulator with in vitro-SUMOylated CTCF and ran the products on native polyacrylamide gels. The electrophoretic mobility shifts of the fragments were not altered by the SUMOylation of the CTCF protein (Fig. 3B). Competition experiments with an unlabeled DNA fragment supported these conclusions (data not shown). Therefore, we conclude that the posttranslational modification of CTCF by SUMO does not interfere with its ability to bind DNA. It should be pointed out that the modification of CTCF by poly(ADP) ribose, while required for its insulator activity, also does not interfere with its ability to bind DNA (42, 75).
Location of the sites of SUMOylation of CTCF.
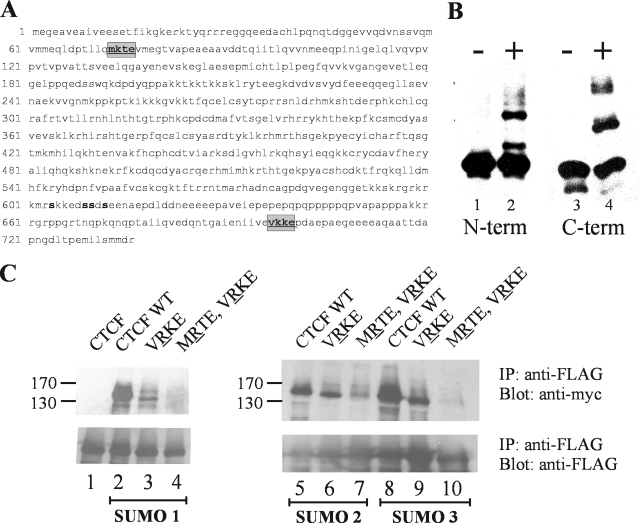
The CTCF protein has three domains. The central zinc finger DNA binding domain is flanked by an N-terminal domain and a C-terminal domain. Both the C-terminal and N-terminal domains of CTCF contain putative SUMOylation sites (Fig. 4A). To localize the sites of SUMOylation, we expressed and purified GST-tagged NH2-terminal and COOH-terminal domains of CTCF in E. coli. The purified domains were incubated with the E1 and E2 SUMOylation enzymes and SUMO 1. The enzymatic products were resolved by SDS-PAGE and were immunoblotted using an antibody to either the NH2-terminal or the COOH-terminal domain of CTCF. We found that the two domains of CTCF were efficiently SUMO modified in vitro (Fig. 4B). This suggested that there were multiple target sites for SUMOylation in the CTCF protein. This is perhaps not surprising, given the multiple functions attributed to the protein.
FIG. 4.
Identification of SUMOylation sites in CTCF. (A) The amino acid sequence of mouse CTCF protein is shown with the SUMOylation sites highlighted in the N terminus and the C terminus of the protein. The boldface serine residues were previously described by others as being posttranslationally modified by phosphorylation. (B) The C-terminal and N-terminal domains of CTCF are SUMOylated in vitro. GST-tagged N- and C-terminal domains of CTCF were expressed in E. coli, purified, and incubated in a SUMOylation reaction with (+) and without (−) SUMO 1 protein. The reactions were run on an SDS-PAGE gel, subjected to Western blotting, and probed with antibodies to the N terminus (term) or C terminus of CTCF. Purified SUMO 1 protein (lanes 2 and 4) effectively SUMOylated both domains. (C) CTCF is SUMOylated at the VKKE and MKTE motifs. HEK 293 cells were transfected with wild-type (WT) FLAG-CTCF or single-mutant FLAG-CTCF VRKE or double-mutant VRKE MRTE plasmids (as indicated above the gel), in addition to plasmids encoding myc-tagged SUMO 1, 2, or 3 (as indicated below the gel). After immunoprecipitation (IP) with anti-FLAG antibodies, SDS-PAGE, and Western blotting, the membranes were probed with anti-myc antibodies for SUMO (top) or with anti-FLAG antibodies for CTCF (bottom). The VRKE 698 mutation markedly diminished SUMOylation by SUMO 1, 2, and 3 (lanes 3, 6, and 9). The double mutations MRTE 74 and VRKE 698 diminished the residual signal (lanes 4, 7, and 10).
It was therefore of interest to identify the target lysine residues SUMOylated in the protein. The SUMO proteins modify the side chain of a lysine residue in the target protein via the formation of an isopeptide bond through the COOH-terminal glycine residues. The modified lysine (K) is often located in a consensus sequence, ψKXE/D, where ψ is a bulky hydrophobic amino acid and X is any amino acid. The fourth amino acid is commonly glutamic acid but can also be aspartic acid. We searched for likely consensus sequences and found several possibilities located among the three domains of the protein. We used site-directed mutagenesis to change the lysine residues in these putative target sites to arginine residues. We repeated our immunoprecipitation assay for the detection of SUMOylated CTCF using FLAG-tagged CTCF lysine-to-arginine mutants. When we cotransfected HEK 293 cells with the vectors encoding FLAG-tagged wild-type and mutant CTCF, along with the myc-tagged SUMO proteins, we found that the mutation of lysine 698 located in the consensus sequence VKKE caused an appreciable decrease in the levels of SUMOylated CTCF. This observed decrease in SUMOylation was seen using all three SUMO proteins (Fig. 4C, lanes 3, 6, and 9). Although the mutation of the K residue at this site caused an appreciable decrease in the levels of SUMOylated CTCF, there was still detectable SUMOylation in the presence of the lysine 698 mutation. Therefore, we combined the mutation of the VKKE sequence with mutations in other lysines that were in predicted SUMO consensus sites. We found that the mutation of lysine 74 in the MKTE site, combined with the VKKE mutation at lysine 698, further decreased the SUMOylation of CTCF (Fig. 4C, lanes 4, 7, and 10). Therefore, the CTCF protein contains at least two strong sites for SUMOylation, one in the NH2-terminal domain and one in the COOH-terminal domain. These results are in keeping with the in vitro SUMOylation studies that showed that both the NH2- and COOH-terminal domains are SUMOylated. The mutation of lysine residues in several other possible target sites had no effect on the SUMOylation of CTCF (data not shown).
CTCF and Pc2 colocalize to the Polycomb bodies.
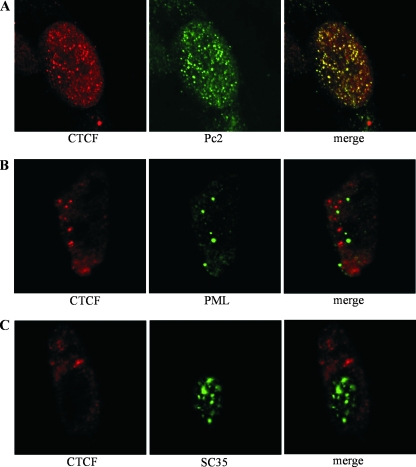
Polycomb nuclear bodies contain the Pc2 protein, a mammalian homologue of the Drosophila protein Polycomb. The Polycomb group proteins stably transmit the repressed transcriptional state of genes through mitotic cellular divisions using epigenetic marks. Recently the Pc2 protein has been shown to be a SUMO E3 ligase (38, 39, 70). Since CTCF is implicated in the silencing of genes, we asked if it could be found in Polycomb bodies. We therefore stained fixed HeLa cells with antibodies to both CTCF and Pc2 protein and examined the slides by laser scanning confocal microscopy. As shown in Fig. 5A, the two proteins clearly colocalize. As controls, we stained the HeLa cells with antibodies to other nuclear compartments. PML bodies are known to contain a large number of SUMOylated proteins. CTCF did not colocalize with the PML in the PML bodies or with the splicing factor SC-35 in splicing factor compartments (Fig. 5B and C). These nuclear bodies are distinct from each other and from the Polycomb bodies (16, 34, 79). To quantitate the extents of the colocalization of CTCF and Pc2, as well as the SC-35 and PML controls, we used the ImageJ program, with the JaCOP plug-in option (7; http://rsb.info.nih.gov/ij/). The Pearson coefficient showed a high degree of colocalization between CTCF and Pc2 (Pearson coefficient = 0.829), but not with PML (Pearson coefficient = 0.375) or SC-35 (Pearson coefficient = −0.008).
FIG. 5.
Colocalization of CTCF and Pc2 proteins in HeLa cell nuclei. HeLa cells were fixed in paraformaldehyde and stained with antibodies. (A) Cells were stained with mouse antibody to CTCF (red) and rabbit antibody to Pc2 protein (green) and counterstained with anti-mouse Texas Red-labeled antibody and anti-rabbit fluorescein isothiocyanate-labeled antibody. The merged image shows clear colocalization of CTCF and Pc2 (yellow). The Pearson coefficient for the colocalization was calculated using ImageJ and showed a high level of colocalization at 0.829. A value of 1 or −1 is considered perfect colocalization. (B) Cells were stained with goat antibody to CTCF (red) and mouse antibody to PML (green) and counterstained with anti-goat Alexa 488-labeled antibody and anti-mouse Alexa 647-labeled antibody. The merged image shows that CTCF and PML did not clearly colocalize. The Pearson coefficient for the colocalization was 0.375. These results cannot be interpreted as partial colocalization or as exclusion, since midrange coefficients between 0.5 and −0.5 are inconclusive (7). (C) Staining with goat antibody to CTCF (red) and mouse antibody to SC-35 (green) with counterstaining as in panel B. The merged image shows that CTCF and SC-35 do not colocalize and are found in different subnuclear compartments. The Pearson coefficient for the colocalization was −0.008, which is close to zero, an indication of exclusion. The images were generated using confocal laser scanning microscopy. Our protocol used nonionic detergent to permeabilize the fixed cells, and it is possible that the detergent treatment of the cells might have removed a soluble fraction of CTCF from the nucleus.
The SUMO E3 ligase Pc2 increases the SUMOylation of CTCF.
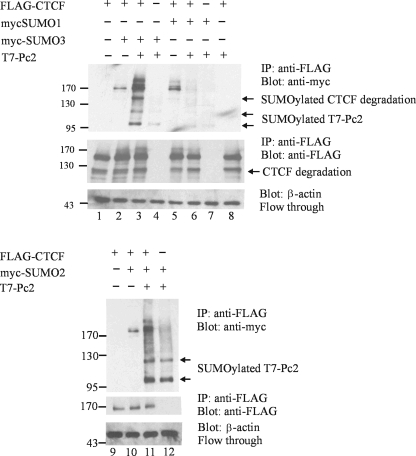
A possible reason for the colocalization of the CTCF and Pc2 proteins is that the latter acts as a SUMO E3 ligase for CTCF. To test this idea, we cotransfected HeLa cells with plasmids encoding FLAG-CTCF and myc-SUMO 1, 2, or 3 in the presence or absence of a plasmid that encoded T7 epitope-tagged Pc2 protein (a generous gift from David Wotton). Since the Pc2 protein itself undergoes SUMOylation and this might create a background, we precleared the lysate of SUMOylated Pc2 by immunoprecipitation with anti-T7-agarose resin. We subsequently immunoprecipitated this cleared lysate for CTCF using anti-FLAG antibodies. The addition of the Pc2 plasmid caused a marked increase in the SUMOylation of CTCF by SUMO 2 and 3 (Fig. 6, lanes 2 versus 3, and 10 versus 11). This shows that the Pc2 protein is likely the SUMO E3 ligase for CTCF and that it acts on SUMO 2 and 3. Conversely, the SUMOylation of CTCF by SUMO 1 was not increased in the presence of Pc2 and, moreover, was slightly reduced in the presence of the E3 ligase (Fig. 6, lane 5 versus 6). The anti-T7 immunoprecipitation cleared most of the T7-Pc2 from the reaction mixture; however, there was residual SUMOylated T7-Pc2 that bound nonspecifically to the protein G-Sepharose and was identified in the Western blot as SUMOylated T7-Pc2.
FIG. 6.
The SUMO E3 ligase Pc2 increases the SUMOylation of CTCF. HeLa cells were transfected using Fugene 6 transfection reagent with the indicated plasmids. The whole-cell lysates were precleared with anti-T7-agarose resin prior to immunoprecipitation (IP) with anti-FLAG antibodies. After SDS-PAGE and Western blotting, the membrane was probed with anti-myc antibody to detect SUMO 1, 2, or 3 (top) or with anti-FLAG antibody to detect CTCF (middle). SUMO E3 ligase activity was indicated by the increase in the SUMOylation signal in lanes 3 and 11. Pc2 exhibited no SUMO E3 ligase activity for the SUMOylation of CTCF by SUMO 1 (lane 6). CTCF degradation products were also SUMOylated by SUMO 2 and 3 in the presence of Pc2. Flowthrough from the immunoprecipitation was blotted for β-actin to show equal loading (bottom).
SUMOylation of CTCF controls its repressive activity on the c-myc P2 promoter.
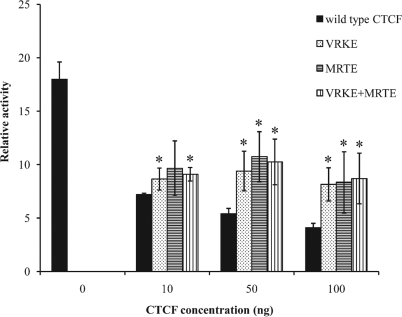
Among its many biological functions, CTCF acts to facilitate the repression of several promoters, including the c-myc P2 promoter (3, 8, 49). Therefore, we examined the importance of the SUMOylation of CTCF for the repressive activity on this promoter. We transiently transfected 293 cells with a plasmid bearing a luciferase reporter driven by a c-myc P2 promoter containing a CTCF binding sequence (a generous gift from Linda Penn). When we cotransfected cells with the reporter plasmid and a plasmid that expressed a wild-type version of CTCF, there was a statistically significant and reproducible decrease of promoter activity dependent upon the exogenous source of CTCF (Fig. 7). However, when we substituted a plasmid in which the MKTE and the VKKE SUMOylation sites were mutated singly or in combination, the repressive effect was decreased. We show that the SUMOylation of CTCF contributes to its repressive activity on the c-myc P2 promoter.
FIG. 7.
SUMOylation of CTCF contributes to its repressive effect on the c-myc P2 promoter. HEK 293 cells were transfected with 500 ng of the c-myc P2 luciferase reporter construct either with no added CTCF-encoding plasmid (far left black bar) or with 10, 50, or 100 ng of plasmid DNAs encoding either wild-type CTCF or CTCF bearing mutations in the SUMO modification sites. The luciferase activities were measured as described in Materials and Methods and were normalized to the activity of a cotransfected plasmid encoding Renilla luciferase. The repressive effect of CTCF on the promoter is shown by the concentration-dependent four- to fivefold reduction in luciferase activity (black bars). This reduction was partially decreased by either the single VRKE or MRTE mutation or the double VRKE and MRTE mutations, which decrease the SUMOylation of CTCF. The asterisks indicate that the results were statistically significant (P < 0.05) when a pairwise Student t test was used to compare the CTCF mutants to the wild-type conditions.
DISCUSSION
In this paper, we report that the multifunctional zinc finger protein CTCF is posttranslationally modified by each of the three SUMO proteins. It had been previously reported that CTCF is modified by phosphorylation and is also poly(ADP) ribosylated (22, 42, 43, 75). The phosphorylation of CTCF relieves its repressive effects at the c-myc P2 promoter, while poly(ADP) ribosylation has been linked to CTCF's insulator activity. Our findings augment the role of posttranslational modifications in the regulation of CTCF's many functions by showing that SUMOylation is involved in the repressive activity of CTCF.
We have identified two major sites of SUMOylation. The COOH-terminal VKKE sequence is the principal site, whereas modification of the NH2-terminal MKTE site is detectable only when the VKKE site has been mutated. The observation that the repressive effect on the c-myc P2 promoter occurs by mutation of a single CTCF SUMO site suggests that the SUMOylation of either site is sufficient to produce the repressive effect. This is in keeping with findings of repressive domains in both the N and C termini of the protein (13, 17, 55). Both sites are modified by all three SUMO proteins.
Unlike polyubiquitin, which targets proteins for proteolytic degradation by the 26S proteasome, SUMO modifications in vivo are usually not polymeric. Our experiments show that the modification of CTCF results in several CTCF SUMO-modified species that migrate as multiple high-molecular-weight bands during denaturing PAGE. These higher-molecular-weight species are enriched in the presence of Pc2. It is possible that the overexpression of an E3 ligase is needed to reveal minor SUMO modification sites in CTCF. It is also possible that the fastest-migrating SUMO-modified band of CTCF is a dimer of two CTCF molecules, as the protein has been previously shown to dimerize.
While CTCF is involved in many biological functions, the molecular mechanisms responsible for its repressive activity have remained enigmatic. The posttranslational modification of CTCF by SUMOylation might explain the apparent discrepancy between results that implicate CTCF as a repressor of genes such as c-myc (26) and the fact that the levels of CTCF bound to the c-myc P2 promoter (as detected by chromatin immunoprecipitation) do not change while the rate of transcription of the gene changes widely (30). It would be interesting to examine the influence of CTCF SUMOylation on the rates of transcription of other CTCF-regulated genes. While our results demonstrate that the SUMOylation of CTCF is needed for its repressive effect on the c-myc P2 promoter, we observed only a twofold effect of derepression by the CTCF mutants. It is possible that there are other SUMOylation sites in the CTCF protein that are not detected due to the limited sensitivity of our immunoprecipitation assays. It is also possible that other posttranslational modifications contribute to CTCF's repressive function at this promoter. This is especially intriguing, since others have reported that phosphorylation of CTCF relieves the repressive effects at the c-myc P2 promoter (43). Mutating the phosphorylation sites in the C terminus of CTCF to nonphosphorylatable amino acids enhances the repression of CTCF at the c-myc P2 promoter. It is interesting to speculate that the posttranslational modifications of CTCF by SUMOylation and phosphorylation compete to regulate the repressive function of CTCF at the promoter. In keeping with this idea, the SUMOylation and phosphorylation of some proteins are reciprocally regulated (1, 33, 35, 59, 67). Therefore, while searching for putative SUMO sites in CTCF, we mutagenized lysines 605 and 606 (underlined) in the sequence SKKE, which is very close to the sites of phosphorylation by CK2. However, neither of these lysine residues was found to be SUMOylated (data not shown). One may speculate that CTCF might also be modified by different combinations of the SUMO proteins at the different sites and that the change in SUMO could regulate different biological functions.
We found that CTCF and the Pc2 protein colocalized to the particulate nuclear structures called Polycomb bodies. The particles contain several Polycomb proteins, including Pc2, RING1, BMI1, Rae28, HPH1, and HPH2 (61). The Pc2 protein acts as a SUMO E3 ligase for certain proteins, notably the transcriptional corepressor protein CtBP, the protein kinase HIPK2, and the DNA methyltransferase Dnmt3a (38, 39, 46, 59, 70). We showed that Pc2 increases the SUMOylation of CTCF by SUMO 2 and 3, but not by SUMO 1, in transfection experiments. This is the first report of a SUMO E3 ligase exhibiting specificity with respect to the individual SUMO used for the modification of the substrate protein. We note that the overexpression of Pc2 decreased the modification of CTCF by SUMO 1. It is possible that Pc2 depletes the pool of SUMO 1 molecules by SUMOylating other substrate proteins with SUMO 1, thus decreasing the amount of SUMO 1 available for the modification of CTCF by another E3 ligase. In the future, it would be interesting to identify the E3 ligase for the modification of CTCF by SUMO 1. We were unable to detect the SUMO E3 ligase activity of purified Pc2 protein upon CTCF in vitro, although we did demonstrate that CTCF and Pc2 interacted directly in vitro (data not shown). It would also be interesting to determine whether the SUMOylation of CTCF is required for its localization to the Polycomb bodies and if the CTCF lysine mutants that are not SUMOylated also localize to these bodies.
The Polycomb bodies seem to act as sites of SUMOylation within the nucleus. The Pc2 protein is itself SUMOylated, although its SUMOylation is not required for its localization to the Polycomb bodies or its E3 ligase activity (38). It is noteworthy that the Drosophila myc gene is repressed by the Pc protein (32). We were unable to detect an effect of human Pc2 on the c-myc P2 promoter, since Pc2 repressed the expression of the Renilla luciferase reporter used to normalize the luciferase data. The Polycomb proteins are named after their Drosophila counterparts, which function in the establishment of repressive chromatin domains. They establish cellular memory by acting as regulators of epigenetically mediated transcriptional silencing through mitotic cell divisions. Since CTCF is involved in repression and genomic imprinting, it is attractive to postulate that it is involved in the docking of repressed genes into the Polycomb bodies, thus implicating CTCF in the maintenance of the epigenetic memory. It is also possible that CTCF tethers repressed genes to the Polycomb body in a manner similar to that suggested for its role in tethering insulators to nucleoli (76, 77). The Drosophila Ctcf homologue also acts as an insulator protein (15, 37, 51, 52, 68). A recent publication has shown that the Drosophila Ctcf homologue mediates looping between two Fab-8 insulators and one of the Abd-B promoters. This looping is thought to involve enhancers that activate transcription in the bithorax complex, overcoming the repressive effects of the Polycomb proteins (45).
Numerous publications have also highlighted CTCF's role in organizing the nuclear architecture of mammalian cells. CTCF is thought to mediate its insulator function by organizing chromatin into looped domains (47, 64) that occur even between chromosomes. A role for CTCF binding sites has been demonstrated in X chromosome inactivation (11, 54), and CTCF has been implicated in the pairing between the two X inactivation centers that precedes the choice for X inactivation (18, 73). Three recent publications have demonstrated another role for CTCF in nuclear architecture, showing that cohesins bind specifically to CTCF binding sequences (57, 65, 69). It will be important to determine the molecular mechanisms responsible for CTCF's role in genomic organization and the role that the SUMOylation of CTCF plays in these functions.
The CTCF protein has multiple functions in several eukaryotic organisms. Our observations considerably expand the repertoire of posttranslational modifications that may play roles in regulating this multiplicity of functions. It is not known whether CTCF's nuclear localization changes depending upon whether it is acting as a repressor, activator, or insulator protein. It is noteworthy that the SUMO sites we have identified in the mouse protein are conserved in vertebrates from fish to mammals. Although the actual SUMO sites in the vertebrate proteins are not conserved in the Drosophila protein, there are several high-probability putative SUMO consensus sites in both its NH2 and COOH termini. Since SUMOylation has recently been found to attenuate the function of the gypsy insulator in Drosophila (10) and since Drosophila Ctcf is involved in insulator function (45), it would be of interest to learn whether the CTCF of flies is also SUMOylated. It would also be valuable to learn the effects of SUMOylation on insulator activity in vertebrates.
In this paper, we have focused on the role of SUMOylation in CTCF function. However, it has not escaped our attention that basic residues, such as lysine and arginine, that are commonly modified in histones can also be methylated and acetylated and that such modifications can also affect the activity and stability of nonhistone proteins, for example, p53 (14, 48). Further studies of the roles of posttranslation modifications in the molecular mechanisms of CTCF functions are warranted.
Acknowledgments
This work was supported in part by funds from the Canadian Institutes for Health Research. M.J.M. was the recipient of a University of Toronto Open Fellowship.
We thank E. T. Yeh, D. Wotton, L. Frappier, J. Greenblatt, and L. Penn for vectors. We thank L. Frappier and A. Sakwe for their thoughtful advice, A. Cochrane for the SC-35 antibody, and Howard Lipshitz and Marc Shulman for their interest and support. We thank L. Frappier for her careful reading of the manuscript.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Anckar, J., and L. Sistonen. 2007. SUMO: getting it on. Biochem. Soc. Trans. 351409-1413. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S., D. L. Davis, H. Dahlback, H. Jornvall, and D. W. Russell. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 2648222-8229. [PubMed] [Google Scholar]
- 3.Arnold, R., M. Burcin, B. Kaiser, M. Muller, and R. Renkawitz. 1996. DNA bending by the silencer protein NeP1 is modulated by TR and RXR. Nucleic Acids Res. 242640-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barski, A., S. Cuddapah, K. Cui, T. Y. Roh, D. E. Schones, Z. Wang, G. Wei, I. Chepelev, and K. Zhao. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129823-837. [DOI] [PubMed] [Google Scholar]
- 5.Bell, A. C., and G. Felsenfeld. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405482-485. [DOI] [PubMed] [Google Scholar]
- 6.Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98387-396. [DOI] [PubMed] [Google Scholar]
- 7.Bolte, S., and F. P. Cordelieres. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224213-232. [DOI] [PubMed] [Google Scholar]
- 8.Burcin, M., R. Arnold, M. Lutz, B. Kaiser, D. Runge, F. Lottspeich, G. N. Filippova, V. V. Lobanenkov, and R. Renkawitz. 1997. Negative protein 1, which is required for function of the chicken lysozyme gene silencer in conjunction with hormone receptors, is identical to the multivalent zinc finger repressor CTCF. Mol. Cell. Biol. 171281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, T., B. Liang, A. Dibrov, and F. Amara. 2002. Transforming growth factor-beta-induced transcription of the Alzheimer beta-amyloid precursor protein gene involves interaction between the CTCF-complex and Smads. Biochem. Biophys. Res. Commun. 295713-723. [DOI] [PubMed] [Google Scholar]
- 10.Capelson, M., and V. G. Corces. 2006. SUMO conjugation attenuates the activity of the gypsy chromatin insulator. EMBO J. 251906-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao, W., K. D. Huynh, R. J. Spencer, L. S. Davidow, and J. T. Lee. 2002. CTCF, a candidate trans-acting factor for X-inactivation choice. Science 295345-347. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, J., N. D. Perkins, and E. T. Yeh. 2005. Differential regulation of c-Jun-dependent transcription by SUMO-specific proteases. J. Biol. Chem. 28014492-14498. [DOI] [PubMed] [Google Scholar]
- 13.Chernukhin, I., S. Shamsuddin, S. Y. Kang, R. Bergstrom, Y. W. Kwon, W. Yu, J. Whitehead, R. Mukhopadhyay, F. Docquier, D. Farrar, I. Morrison, M. Vigneron, S. Y. Wu, C. M. Chiang, D. Loukinov, V. Lobanenkov, R. Ohlsson, and E. Klenova. 2007. CTCF interacts with and recruits the largest subunit of RNA polymerase II to CTCF target sites genome-wide. Mol. Cell. Biol. 271631-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuikov, S., J. K. Kurash, J. R. Wilson, B. Xiao, N. Justin, G. S. Ivanov, K. McKinney, P. Tempst, C. Prives, S. J. Gamblin, N. A. Barlev, and D. Reinberg. 2004. Regulation of p53 activity through lysine methylation. Nature 432353-360. [DOI] [PubMed] [Google Scholar]
- 15.Ciavatta, D., S. Rogers, and T. Magnuson. 2007. Drosophila CTCF is required for Fab-8 enhancer blocking activity in S2 cells. J. Mol. Biol. 373233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahle, O., O. Bakke, and O. S. Gabrielsen. 2004. c-Myb associates with PML in nuclear bodies in hematopoietic cells. Exp. Cell Res. 297118-126. [DOI] [PubMed] [Google Scholar]
- 17.Defossez, P. A., and E. Gilson. 2002. The vertebrate protein CTCF functions as an insulator in Saccharomyces cerevisiae. Nucleic Acids Res. 305136-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donohoe, M. E., L. F. Zhang, N. Xu, Y. Shi, and J. T. Lee. 2007. Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol. Cell 2543-56. [DOI] [PubMed] [Google Scholar]
- 19.Du, M., L. G. Beatty, W. Zhou, J. Lew, C. Schoenherr, R. Weksberg, and P. D. Sadowski. 2003. Insulator and silencer sequences in the imprinted region of human chromosome 11p15.5. Hum. Mol. Genet. 121927-1939. [DOI] [PubMed] [Google Scholar]
- 20.Du, M., W. Zhou, L. G. Beatty, R. Weksberg, and P. D. Sadowski. 2004. The KCNQ1OT1 promoter, a key regulator of genomic imprinting in human chromosome 11p15.5. Genomics 84288-300. [DOI] [PubMed] [Google Scholar]
- 21.Dunn, K. L., and J. R. Davie. 2003. The many roles of the transcriptional regulator CTCF. Biochem. Cell Biol. 81161-167. [DOI] [PubMed] [Google Scholar]
- 22.El-Kady, A., and E. Klenova. 2005. Regulation of the transcription factor, CTCF, by phosphorylation with protein kinase CK2. FEBS Lett. 5791424-1434. [DOI] [PubMed] [Google Scholar]
- 23.Facchini, L. M., S. Chen, W. W. Marhin, J. N. Lear, and L. Z. Penn. 1997. The Myc negative autoregulation mechanism requires Myc-Max association and involves the c-Myc P2 minimal promoter. Mol. Cell. Biol. 17100-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedoriw, A. M., P. Stein, P. Svoboda, R. M. Schultz, and M. S. Bartolomei. 2004. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science 303238-240. [DOI] [PubMed] [Google Scholar]
- 25.Filippova, G. N. 2008. Genetics and epigenetics of the multifunctional protein CTCF. Curr. Top. Dev. Biol. 80337-360. [DOI] [PubMed] [Google Scholar]
- 26.Filippova, G. N., S. Fagerlie, E. M. Klenova, C. Myers, Y. Dehner, G. Goodwin, P. E. Neiman, S. J. Collins, and V. V. Lobanenkov. 1996. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-Myc oncogenes. Mol. Cell. Biol. 162802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippova, G. N., C. F. Qi, J. E. Ulmer, J. M. Moore, M. D. Ward, Y. J. Hu, D. I. Loukinov, E. M. Pugacheva, E. M. Klenova, P. E. Grundy, A. P. Feinberg, A. M. Cleton-Jansen, E. W. Moerland, C. J. Cornelisse, H. Suzuki, A. Komiya, A. Lindblom, F. Dorion-Bonnet, P. E. Neiman, H. C. Morse III, S. J. Collins, and V. V. Lobanenkov. 2002. Tumor-associated zinc finger mutations in the CTCF transcription factor selectively alter its DNA-binding specificity. Cancer Res. 6248-52. [PubMed] [Google Scholar]
- 28.Gill, G. 2005. Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 15536-541. [DOI] [PubMed] [Google Scholar]
- 29.Gill, G. 2004. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 182046-2059. [DOI] [PubMed] [Google Scholar]
- 30.Gombert, W. M., S. D. Farris, E. D. Rubio, K. M. Morey-Rosler, W. H. Schubach, and A. Krumm. 2003. The c-Myc insulator element and matrix attachment regions define the c-Myc chromosomal domain. Mol. Cell. Biol. 239338-9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong, L., and E. T. Yeh. 2006. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J. Biol. Chem. 28115869-15877. [DOI] [PubMed] [Google Scholar]
- 32.Goodliffe, J. M., E. Wieschaus, and M. D. Cole. 2005. Polycomb mediates Myc autorepression and its transcriptional control of many loci in Drosophila. Genes Dev. 192941-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregoire, S., A. M. Tremblay, L. Xiao, Q. Yang, K. Ma, J. Nie, Z. Mao, Z. Wu, V. Giguere, and X. J. Yang. 2006. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J. Biol. Chem. 2814423-4433. [DOI] [PubMed] [Google Scholar]
- 34.Guo, A., P. Salomoni, J. Luo, A. Shih, S. Zhong, W. Gu, and P. P. Pandolfi. 2000. The function of PML in p53-dependent apoptosis. Nat. Cell Biol. 2730-736. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez, G. J., and Z. Ronai. 2006. Ubiquitin and SUMO systems in the regulation of mitotic checkpoints. Trends Biochem. Sci. 31324-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hark, A. T., C. J. Schoenherr, D. J. Katz, R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405486-489. [DOI] [PubMed] [Google Scholar]
- 37.Holohan, E. E., C. Kwong, B. Adryan, M. Bartkuhn, M. Herold, R. Renkawitz, S. Russell, and R. White. 2007. CTCF genomic binding sites in Drosophila and the organisation of the bithorax complex. PLoS Genet. 3e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagey, M. H., T. A. Melhuish, S. E. Powers, and D. Wotton. 2005. Multiple activities contribute to Pc2 E3 function. EMBO J. 24108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kagey, M. H., T. A. Melhuish, and D. Wotton. 2003. The polycomb protein Pc2 is a SUMO E3. Cell 113127-137. [DOI] [PubMed] [Google Scholar]
- 40.Kerscher, O., R. Felberbaum, and M. Hochstrasser. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22159-180. [DOI] [PubMed] [Google Scholar]
- 41.Kim, T. H., Z. K. Abdullaev, A. D. Smith, K. A. Ching, D. I. Loukinov, R. D. Green, M. Q. Zhang, V. V. Lobanenkov, and B. Ren. 2007. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 1281231-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klenova, E., and R. Ohlsson. 2005. Poly(ADP-ribosyl)ation and epigenetics. Is CTCF PARt of the plot? Cell Cycle 496-101. [DOI] [PubMed] [Google Scholar]
- 43.Klenova, E. M., I. V. Chernukhin, A. El-Kady, R. E. Lee, E. M. Pugacheva, D. I. Loukinov, G. H. Goodwin, D. Delgado, G. N. Filippova, J. Leon, H. C. Morse III, P. E. Neiman, and V. V. Lobanenkov. 2001. Functional phosphorylation sites in the C-terminal region of the multivalent multifunctional transcriptional factor CTCF. Mol. Cell. Biol. 212221-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurukuti, S., V. K. Tiwari, G. Tavoosidana, E. Pugacheva, A. Murrell, Z. Zhao, V. Lobanenkov, W. Reik, and R. Ohlsson. 2006. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl. Acad. Sci. USA 10310684-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyrchanova, O., S. Toshchakov, Y. Podstreshnaya, A. Parshikov, and P. Georgiev. 2008. Functional interaction between the Fab-7 and Fab-8 boundaries and the upstream promoter region in Drosophila Abd-B gene. Mol. Cell. Biol. 284188-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, B., J. Zhou, P. Liu, J. Hu, H. Jin, Y. Shimono, M. Takahashi, and G. Xu. 2007. Polycomb protein Cbx4 promotes SUMO modification of de novo DNA methyltransferase Dnmt3a. Biochem. J. 405369-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling, J. Q., T. Li, J. F. Hu, T. H. Vu, H. L. Chen, X. W. Qiu, A. M. Cherry, and A. R. Hoffman. 2006. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312269-272. [DOI] [PubMed] [Google Scholar]
- 48.Liu, L., D. M. Scolnick, R. C. Trievel, H. B. Zhang, R. Marmorstein, T. D. Halazonetis, and S. L. Berger. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 191202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobanenkov, V. V., R. H. Nicolas, V. V. Adler, H. Paterson, E. M. Klenova, A. V. Polotskaja, and G. H. Goodwin. 1990. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 51743-1753. [PubMed] [Google Scholar]
- 50.Majumder, P., J. A. Gomez, B. P. Chadwick, and J. M. Boss. 17 March 2008. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J. Exp. Med. doi 10.1084/jem.20071843. [DOI] [PMC free article] [PubMed]
- 51.Mohan, M., M. Bartkuhn, M. Herold, A. Philippen, N. Heinl, I. Bardenhagen, J. Leers, R. A. White, R. Renkawitz-Pohl, H. Saumweber, and R. Renkawitz. 2007. The Drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. EMBO J. 264203-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon, H., G. Filippova, D. Loukinov, E. Pugacheva, Q. Chen, S. T. Smith, A. Munhall, B. Grewe, M. Bartkuhn, R. Arnold, L. J. Burke, R. Renkawitz-Pohl, R. Ohlsson, J. Zhou, R. Renkawitz, and V. Lobanenkov. 2005. CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator. EMBO Rep. 6165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukhopadhyay, R., W. Yu, J. Whitehead, J. Xu, M. Lezcano, S. Pack, C. Kanduri, M. Kanduri, V. Ginjala, A. Vostrov, W. Quitschke, I. Chernukhin, E. Klenova, V. Lobanenkov, and R. Ohlsson. 2004. The binding sites for the chromatin insulator protein CTCF map to DNA methylation-free domains genome-wide. Genome Res. 141594-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro, P., D. R. Page, P. Avner, and C. Rougeulle. 2006. Tsix-mediated epigenetic switch of a CTCF-flanked region of the Xist promoter determines the Xist transcription program. Genes Dev. 202787-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohlsson, R., R. Renkawitz, and V. Lobanenkov. 2001. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 17520-527. [DOI] [PubMed] [Google Scholar]
- 56.Pant, V., S. Kurukuti, E. Pugacheva, S. Shamsuddin, P. Mariano, R. Renkawitz, E. Klenova, V. Lobanenkov, and R. Ohlsson. 2004. Mutation of a single CTCF target site within the H19 imprinting control region leads to loss of Igf2 imprinting and complex patterns of de novo methylation upon maternal inheritance. Mol. Cell. Biol. 243497-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parelho, V., S. Hadjur, M. Spivakov, M. Leleu, S. Sauer, H. C. Gregson, A. Jarmuz, C. Canzonetta, Z. Webster, T. Nesterova, B. S. Cobb, K. Yokomori, N. Dillon, L. Aragon, A. G. Fisher, and M. Merkenschlager. 2008. cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132422-433. [DOI] [PubMed] [Google Scholar]
- 58.Pugacheva, E. M., V. K. Tiwari, Z. Abdullaev, A. A. Vostrov, P. T. Flanagan, W. W. Quitschke, D. I. Loukinov, R. Ohlsson, and V. V. Lobanenkov. 2005. Familial cases of point mutations in the XIST promoter reveal a correlation between CTCF binding and pre-emptive choices of X chromosome inactivation. Hum. Mol. Genet. 14953-965. [DOI] [PubMed] [Google Scholar]
- 59.Roscic, A., A. Moller, M. A. Calzado, F. Renner, V. C. Wimmer, E. Gresko, K. S. Ludi, and M. L. Schmitz. 2006. Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol. Cell 2477-89. [DOI] [PubMed] [Google Scholar]
- 60.Saitoh, H., and J. Hinchey. 2000. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 2756252-6258. [DOI] [PubMed] [Google Scholar]
- 61.Saurin, A. J., C. Shiels, J. Williamson, D. P. Satijn, A. P. Otte, D. Sheer, and P. S. Freemont. 1998. The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol. 142887-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoenherr, C. J., J. M. Levorse, and S. M. Tilghman. 2003. CTCF maintains differential methylation at the Igf2/H19 locus. Nat. Genet. 3366-69. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz, D. C., and M. Hochstrasser. 2003. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem. Sci. 28321-328. [DOI] [PubMed] [Google Scholar]
- 64.Splinter, E., H. Heath, J. Kooren, R. J. Palstra, P. Klous, F. Grosveld, N. Galjart, and W. de Laat. 2006. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 202349-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stedman, W., H. Kang, S. Lin, J. L. Kissil, M. S. Bartolomei, and P. M. Lieberman. 2008. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 27654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szabo, P. E., S. H. Tang, F. J. Silva, W. M. Tsark, and J. R. Mann. 2004. Role of CTCF binding sites in the Igf2/H19 imprinting control region. Mol. Cell. Biol. 244791-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanhatupa, S., D. Ungureanu, M. Paakkunainen, and O. Silvennoinen. 2007. MAPK-induced Ser727 phosphorylation promotes sumoylation of STAT1. Biochem. J. 409179-185. [DOI] [PubMed] [Google Scholar]
- 68.Wallace, J. A., and G. Felsenfeld. 2007. We gather together: insulators and genome organization. Curr. Opin. Genet. Dev. 17400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wendt, K. S., K. Yoshida, T. Itoh, M. Bando, B. Koch, E. Schirghuber, S. Tsutsumi, G. Nagae, K. Ishihara, T. Mishiro, K. Yahata, F. Imamoto, H. Aburatani, M. Nakao, N. Imamoto, K. Maeshima, K. Shirahige, and J. M. Peters. 2008. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451796-801. [DOI] [PubMed] [Google Scholar]
- 70.Wotton, D., and J. C. Merrill. 2007. Pc2 and SUMOylation. Biochem. Soc. Trans. 351401-1404. [DOI] [PubMed] [Google Scholar]
- 71.Xi, H., H. P. Shulha, J. M. Lin, T. R. Vales, Y. Fu, D. M. Bodine, R. D. McKay, J. G. Chenoweth, P. J. Tesar, T. S. Furey, B. Ren, Z. Weng, and G. E. Crawford. 2007. Identification and characterization of cell type-specific and ubiquitous chromatin regulatory structures in the human genome. PLoS Genet. 3e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie, X., T. S. Mikkelsen, A. Gnirke, K. Lindblad-Toh, M. Kellis, and E. S. Lander. 2007. Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites. Proc. Natl. Acad. Sci. USA 1047145-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu, N., M. E. Donohoe, S. S. Silva, and J. T. Lee. 2007. Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nat. Genet. 391390-1396. [DOI] [PubMed] [Google Scholar]
- 74.Yoon, Y. S., S. Jeong, Q. Rong, K. Y. Park, J. H. Chung, and K. Pfeifer. 2007. Analysis of the H19ICR insulator. Mol. Cell. Biol. 273499-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu, W., V. Ginjala, V. Pant, I. Chernukhin, J. Whitehead, F. Docquier, D. Farrar, G. Tavoosidana, R. Mukhopadhyay, C. Kanduri, M. Oshimura, A. P. Feinberg, V. Lobanenkov, E. Klenova, and R. Ohlsson. 2004. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat. Genet. 361105-1110. [DOI] [PubMed] [Google Scholar]
- 76.Yusufzai, T. M., and G. Felsenfeld. 2004. The 5′-HS4 chicken beta-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc. Natl. Acad. Sci. USA 1018620-8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yusufzai, T. M., H. Tagami, Y. Nakatani, and G. Felsenfeld. 2004. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol. Cell 13291-298. [DOI] [PubMed] [Google Scholar]
- 78.Zeghouf, M., J. Li, G. Butland, A. Borkowska, V. Canadien, D. Richards, B. Beattie, A. Emili, and J. F. Greenblatt. 2004. Sequential peptide affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J. Proteome Res. 3463-468. [DOI] [PubMed] [Google Scholar]
- 79.Zhong, S., P. Salomoni, and P. P. Pandolfi. 2000. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2E85-E90. [DOI] [PubMed] [Google Scholar]