Abstract
The effects of chronic alcohol consumption on the bowel flora and the potential therapeutic role of probiotics in alcohol-induced liver injury have not previously been evaluated. In this study, sixty-six adult Russian males admitted to a psychiatric hospital with a diagnosis of alcoholic psychosis were enrolled in a prospective, randomized, clinical trial to study the effects of alcohol and probiotics on the bowel flora and alcohol-induced liver injury. Patients were randomized to receive 5 days of Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 vs. standard therapy alone (abstinence plus vitamins). Stool cultures and liver enzymes were performed at baseline and again after therapy. Results were compared between groups and with 24 healthy, matched controls who did not consume alcohol. Compared to healthy controls, alcoholic patients had significantly reduced numbers of bifidobacteria (6.3 vs. 7.5 log CFU/g), lactobacilli (3.15 vs. 4.59 log CFU/g), and enterococci (4.43 vs. 5.5 log CFU/g). The mean baseline AST, ALT and GGT activities were significantly elevated in the alcoholic group when compared to the healthy control group (AST: 104.1 vs. 29.15 U/L; ALT: 50.49 vs. 22.96 U/L; GGT 161.5 vs. 51.88 U/L) indicating that these patients did have mild alcohol-induced liver injury. After 5 days of probiotic therapy, alcoholic patients had significantly increased numbers of both bifidobacteria (7.9 vs. 6.81 log CFU/g) and lactobacilli (4.2 vs. 3.2 log CFU/g) when compared to the standard therapy arm. Despite similar values at study initiation, patients treated with probiotics had significantly lower AST and ALT activity at the end of treatment than those treated with standard therapy alone (AST: 54.67 vs. 76.43 U/L; ALT 36.69 vs. 51.26 U/L). In a subgroup of 26 subjects with well-characterized mild alcoholic hepatitis (defined as AST and ALT greater than 30 U/L with AST to ALT ratio greater than one), probiotic therapy was associated with a significant end of treatment reduction in ALT, AST, GGT, LDH and total bilirubin. In this subgroup, there was a significant end of treatment mean ALT reduction in the probiotic arm vs. the standard therapy arm. In conclusion, patients with alcohol-induced liver injury have altered bowel flora when compared to healthy controls. Short-term oral supplementation with Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 was associated with restoration of the bowel flora and greater improvement in alcohol-induced liver injury than standard therapy alone.
Keywords: alcohol, alcoholic liver disease, bowel flora, bifidobacteria, lactobacilli, probiotics
Introduction
Alcoholic liver disease (ALD) remains a major cause of morbidity and mortality in the United States and worldwide. Of the 14 million alcoholics in the United States, approximately 10-20% will develop cirrhosis, and of those 5-7% will decompensate per year (D'Amico et al., 2006; Wakim-Fleming and Mullen, 2005). ALD accounts for 40% of deaths from cirrhosis and more than 30% of the cases of hepatocellular carcinoma (Hassan et al., 2002; Kim et al., 2002). Alcoholic hepatitis occurs in approximately 10-35% of heavy drinkers, and carries with it a long term frequency of cirrhosis which is nine fold higher than milder forms of alcoholic liver disease (Carithers and McClain, 2006). Severe alcoholic hepatitis has high short term mortality approaching 35-45% (Carithers and McClain, 2006). Unfortunately, there is no FDA-approved therapy for ALD. The cornerstones of therapy in ALD are abstinence and the correction of associated malnutrition.
Bowel-liver interactions are well described in ALD. Importantly, alcohol abuse is associated with increased gut permeability, and the frequency of endotoxemia is high (McClain et al., 1999), (Carithers and McClain, 2006). Lipopolysacharide (LPS) derived from gram negative bowel flora stimulates pro-inflammatory cytokine production in Kupffer cells (McClain et al., 1999). Tumor necrosis factor alpha (TNF-α) has emerged as a critical cytokine in alcoholic liver disease (McClain and Cohen, 1989). Because products of bowel flora such as LPS are important mediators of ALD, it is reasonable to hypothesize that alterations in bowel flora could play a pathological role in the genesis of ALD. Accordingly, restoring normal bowel flora could be a potential therapy for ALD.
There are approximately 100 trillion microorganisms residing in the human bowel (Cani and Delzenne, 2007). This means that the human body contains more prokaryotic cells than eukaryotic cells. Alterations in normal bowel flora contribute to many disease states, most obviously acute bacterial gastroenteritis, small bowel bacterial overgrowth, and antibiotic-associated diarrhea. Although the data are limited, bowel flora alterations have also been described in both animal models and humans with liver disease. Rats with carbon tetrachloride induced cirrhosis demonstrated reduced numbers of enterococci (Chiva et al., 2002). In human patients with cirrhosis (primarily due to hepatitis B) and minimal hepatic encephalopathy, a significant fecal overgrowth of Escherichia coli and Staphylococcal species was found in comparison to healthy controls (Liu et al., 2004). In a second study of human patients with cirrhosis primarily due to viral hepatitis, a significant increase in Enterococcus, Enterobacter, and Clostridium species was found along with a significant decrease in Bifidobacterium species (Zhao et al., 2004). Preliminary work from our group demonstrated that human alcoholics have altered bowel flora with decreased bifidobacteria and lactobacilli (Kirpich I.A., 2000).
Probiotics are defined as live microorganisms which, when administered in adequate amounts, confer a health benefit on the host, apart from their simple caloric value. The therapeutic potential of probiotics has been supported by several high quality human clinical trials. For example, clinical studies have unequivocally established the value of probiotics for several luminal diseases including the prevention of recurrent Clostridium difficile colitis (McFarland et al., 1994) and the maintenance of remission of pouchitis (Gionchetti et al., 2000). Also, prebiotics, such as the fermentable, soluble fiber, inulin, modulate the bowel flora to confer a health benefit to the host. For example, fructooligosaccharides (FOS) have been shown to stimulate the growth of Bifidobacterium spp. to decrease high fat diet induced endotoxemia in mice (Cani et al., 2007b), and improve transaminases and insulin resistance in humans with nonalcoholic steatohepatitis (NASH) (Daubioul et al., 2005).
Animal (Adawi et al., 2001; Adawi et al., 1997; Daubioul et al., 2000; Ewaschuk et al., 2007; Han et al., 2005; Keshavarzian et al., 2001; Li et al., 2003; Nanji et al., 1994; Osman et al., 2008) and human data (Daubioul et al., 2005; Liu et al., 2004; Loguercio et al., 2005; Riordan et al., 2003) suggest an emerging role for prebiotic and probiotic therapy in the treatment of liver disease, including alcoholic liver disease. Because our preliminary data showed reduced numbers of bowel bifidobacteria and lactobacilli in human alcoholics, we were interested in replacing these stains in human alcoholics via oral supplementation. Importantly, several prebiotic / probiotic studies also suggest a potential role for Bifidobacterium spp. and / or Lactobacillus spp. in the treatment of human alcoholic cirrhosis (Liu et al., 2004; Loguercio et al., 2005; Riordan et al., 2003), human NASH (Daubioul et al., 2005), rodent models of alcoholic liver disease (Nanji et al., 1994), rodent models of NASH (Daubioul et al., 2000; Li et al., 2003), as well as rodent models of galactosamine (Adawi et al., 2001; Adawi et al., 1997; Ewaschuk et al., 2007; Osman et al., 2008) or carbon tetrachloride induced acute liver injury (Chiva et al., 2002; Han et al., 2005). However, no human studies have been done to date addressing the potential role of Bifidobacterium spp. or Lactobacillus spp. in the treatment of human ALD.
The purpose of the present study was (i) to confirm our previous work demonstrating that human alcoholics have reduced numbers of bowel bifidobacteria and lactobacilli, and (ii) to explore the potential for supplementation with Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 to restore the bowel flora and improve liver enzymes in patients with ALD.
Materials and methods
Study Design
First, we prospectively studied differences in bowel flora between adult alcoholics and healthy, non-drinking controls. Next, we conducted a 7-day, open-labeled, randomized, prospective clinical trial comparing the efficacy of a probiotic preparation in restoring bowel flora and improving liver enzymes in these alcoholic patients.
Patient Selection and Eligibility
This single center, open-labeled, randomized, prospective study was conducted at the Psychiatric Hospital (Arkhangelsk, Russia) during a twelve week period from January through March 2005. The study protocol was approved by the ethics committees of the Northern State Medical University and Psychiatric Hospital (Arkhangelsk, Russia) and was in accordance with the Helsinki Declaration. These Russian ethics committees are equivalent to American Institutional Review Boards. Informed consent was obtained from all study subjects, or their families when appropriate. All alcoholic adult male patients (age 18 or older) admitted with diagnosis of alcohol-related psychosis, were eligible for this clinical trial. None of the patients had stigmata of cirrhosis by physical exam. Their last drinks were within 48 hours prior to admission. Alcohol consumption history was assessed by psychiatrists who interviewed the patients and, whenever possible, their family members. The average daily volume of alcohol consumed 1-2 weeks prior to admission was approximately 750 ml of Russian vodka (40% ethanol). The diagnosis of alcoholism was established according to International Classification of Diseases, X. The control group consisted of 24 healthy, non-drinking, Russian, male volunteers matched by age and smoking status to the alcohol group.
Standard Treatment, Randomization, and Probiotic Administration
On day zero, all patients were initiated on standard alcohol detoxification therapy including scheduled vitamin B1 and B6 supplementation along with diazepam as needed for alcohol withdrawal. All patients were prescribed a regular diet. Neither fiber supplementation (prebiotics) nor antibiotics were given. The following day (day one), patients were randomized to receive standard therapy alone, or standard therapy plus probiotics. Patients were randomized based on the date of their entry into the study. Patients enrolling in the first 6 weeks of the study entered the probiotic plus standard therapy arm, while patients enrolling during the second 6 weeks of the study entered the standard therapy alone arm. Because of the simple randomization procedure, and the fact that no placebo was given, the study was open label, and neither patient nor physician were blinded to group assignment. However, scientists performing the laboratory tests including liver enzymes and stool cultures were blinded to the group assignment. Baseline blood and stool samples were obtained on day one, after randomization, but before administration of probiotics which began on day two. The probiotic therapy consisted of 0.9 × 108 Colony forming unit (CFU) Bifidobacterium bifidum and 0.9 × 109 CFU Lactobacillus plantarum 8PA3 (“Algibif” and “Algilac”, Microgen/Imbio Moscow-N.Nowgorod, Russian Federation). Probiotics were administered once daily for five consecutive days (days 2-6). The study concluded on day 7 when stool and blood samples were obtained. A single blood and stool sample was obtained from each member of the healthy control group during the 12 week study period.
Microbiological Assays of Bowel Flora
Microbiological analysis for the quantification of E. coli, bifidobacteria, lactobacilli, and enterococci, was performed on the fecal samples obtained from the study and control subjects. Samples were analyzed on day 1 and day 7 after admission and the CFU per gram stool were determined by plating different serial dilutions employing MRS agar plates for lactobacilli (de Man, Rogosa and Sharper media, Pronadiza, Spain), Bifidum-media for bifidobacteria (Obolensk, Russia), agar Endo for E. coli (Pronadiza, Spain), Enterococc-agar for enterococci (Obolensk, Russia). Lactobacilli and bifidobacteria were incubated anaerobically (37°C) for 48 hours. E. coli and enterococci were incubated aerobically (37°C) for 24 and 48 hours, respectively.
Measurement of Serum Biochemical Tests
Plasma ALT, AST, LDH, and GGT activities, and total bilirubin were measured using the Cobas Mira-S clinical biochemical analyzer (Roche, Vienna, Austria) with Cormay reagents (Warsaw, Poland).
Statistical analysis of the data
Data were expressed as M±SEM. Intergroup comparisons were performed by unpaired t-test using GraphPad Prism version 5.01 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. Intragroup comparisons were performed in two ways. First, differences in group means were compared by unpaired t-test. Next, in the subset of 26 subjects with well-characterized mild alcoholic hepatitis, a repeated measures analysis was performed before and after therapy using a paired t-test. Alcoholic hepatitis was defined as AST and ALT greater than 30 u/L with an AST to ALT ratio greater than one. A statistical probability value of ≤ 0.05 was considered significant.
Results
Bowel microflora are altered in alcoholic patients
Sixty-six adult, alcoholic, male patients and twenty-four healthy, adult, male controls were enrolled in the study. All patients completed the study and the data obtained from all the study participants were included in the analysis. In alcoholic patients, all cultures were performed within 72 hours of their last drink of alcohol. Demographic information and stool culture results from patients upon enrollment into the study (day 1) and controls are presented in Table 1. Compared to healthy controls, alcoholic patients had significantly reduced numbers of bifidobacteria (6.3 vs. 7.5 log CFU/g, p<0.05), lactobacilli (3.15 vs. 4.59 log CFU/g, p<0.05), and enterococci (4.43 vs. 5.5 log CFU/g, p<0.05). E. coli were increased in alcoholic patients (7.36 vs. 6.70 log CFU/g), but this trend did not reach statistical significance. These data document alterations in bowel flora in alcoholic patients.
Table 1. Demographic Information, Bowel Flora Results, and Liver Enzyme Activity of Control and Alcoholic Patients at Day One (M ± SEM).
| Healthy Control, n=24 | Alcoholic Patients, n=66 | |
|---|---|---|
| Age | 42.7 ± 1.2 | 42.3 ± 1.1 |
| Duration of Alcoholism, years | - | 16.4 ± 3.2 |
| Percent Smokers | 68% | 77% |
| E. coli, log CFU/g | 6.70 ± 0.34 | 7.36± 0.28 |
| Bifidobacteria, log CFU/g | 7.5 ± 0.32 | 6.30 ± 0.17a |
| Lactobacilli, log CFU/g | 4.59 ± 0.34 | 3.15 ± 0.19a |
| Enterococci, log CFU/g | 5.5 ± 0.30 | 4.43 ± 0.20a |
| ALT, U/L | 22.96 ± 2.4 | 50.49 ± 5.9a |
| AST, U/L | 29.15 ± 2.03 | 104.1 ± 9.5a |
| GGT, U/L | 51.88 ± 6.24 | 161.5 ± 13.51a |
| LDH, U/L | 326.31 ± 10.6 | 574.2 ± 22.04a |
| Total Bilirubin, μmol/l | 16.78 ± 0.79 | 22.56 ± 1.17a |
p < 0.05 vs. healthy control group
Probiotic supplementation corrects the alterations in bowel flora observed in alcoholic patients
Of the 66 enrolled alcoholic patients, 34 were randomized to the standard therapy arm and 32 were randomized to the probiotic plus standard therapy arm based on enrollment date. Demographic information and stool culture results comparing these arms are presented in Table 2. Most importantly, in the probiotic arm, bifidobacteria (7.9 vs. 6.5 log CFU/g, p<0.05), lactobacilli (4.2 vs. 3.3 log CFU/g, p<0.05), and enterococci (5.3 vs. 4.65 log CFU/g, p<0.05) significantly increased from day 1 to day 7. After probiotic supplementation, the previously depressed numbers of bifidobacteria, lactobacilli, and enterococci had returned to numbers seen in healthy controls. In contrast, no statistically different changes were observed in the bowel flora of the standard therapy group from day 1 to day 7. At day 7, the probiotic group, when compared to the standard therapy group, had significantly more fecal bifidobacteria (7.9 vs. 6.81 log CFU/g, p<0.05) and lactobacilli (4.2 vs. 3.2 log CFU/g, p<0.05). These data document that at 1 week, probiotic therapy, but not standard therapy alone, restores bowel flora in abstinent alcoholic patients.
Table 2. Demographic Information and Bowel Flora Results From the Standard Therapy and Probiotic Therapy Groups at Baseline (Day 1) and Following Treatment (Day 7) Compared to Healthy Controls, (M ± SEM).
| Control, n=24 | Standard Therapy, n=34 | Probiotic Therapy, n=32 | |||
|---|---|---|---|---|---|
| Age | 42.7 ± 1.2 | 42.06 ± 2.18 | 42.16 ± 1.89 | ||
| Duration of Alcoholism, years | - | 17.2 ± 2.8 | 15.9 ± 1.4 | ||
| Percent Smokers | 68% | 75 % | 78 % | ||
| Day 1 | Day 7 | Day 1 | Day 7 | ||
| E. coli, log CFU/g | 6.70 ± 0.34 | 7.25± 0.47 | 6.4 ± 0.37 | 7.49 ± 0.30 | 7.44 ± 0.24 |
| Bifidobacteria, log CFU/g | 7.5 ± 0.32 | 6.13 ± 0.21a | 6.81 ± 0.27 | 6.5 ± 0.28a | 7.9±0.39b,c |
| Lactobacilli, log CFU/g | 4.59 ± 0.34 | 3.0 ± 0.26a | 3.2 ± 0.27a | 3.3 ± 0.26a | 4.2 ± 0.27b,c |
| Enterococci, log CFU/g | 5.5 ± 0.30 | 4.12 ± 0.29a | 4.3 ±0.30a | 4.65 ± 0.27a | 5.3 ± 0.31c |
p < 0.05 vs. healthy control group
p < 0.05 Day 1 vs. Day 7
p < 0.05 Standard Therapy vs. Probiotic Therapy
Probiotic supplementation is associated with a greater liver enzyme reduction in alcohol-induced liver injury
The mean baseline AST, ALT and GGT activities were significantly elevated in the alcoholic group when compared to the healthy control group (AST: 104.1 vs. 29.15 U/L, ALT: 50.49 vs. 22.96 U/L, GGT: 161.5 vs. 51.88 U/L) indicating that these patients did have alcohol-induced liver injury (liver biopsies were not performed) (Table 1). Baseline (day 1) liver enzymes were not statistically different between the probiotic and standard therapy alone groups (Table 3). After five days of probiotic therapy (day 7), all liver enzymes were reduced from baseline in the probiotic group, but only AST reached statistical significance (46% reduction, 101.06 vs. 54.67 U/L). The standard therapy group also had a significant reduction in AST from baseline to the end of therapy (28% reduction, 106.8 vs. 76.43 U/L). Despite similar values at the start of treatment, patients treated with probiotics had significantly lower AST and ALT activity at the end of treatment than those treated with standard therapy alone (AST: 54.67 vs. 76.43 U/L, ALT: 36.69 vs. 51.26 U/L) (Table 3).
Table 3. Liver Enzyme Activity of the Standard Therapy and Probiotic Therapy Groups at Baseline (Day 1) and Following Treatment (Day 7) Compared to Healthy Controls, (M ± SEM).
| Control, n=24 | Standard Therapy, n=34 | Probiotic Therapy, n=32 | |||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | ||
| ALT, U/L | 22.96 ± 2.4 | 49.74 ± 7.17a | 51.26 ± 5.25a | 49.84 ± 6.94a | 36.69 ± 4.67a,c |
| AST, U/L | 29.15 ± 2.03 | 106.80 ± 12.78a | 76.43 ± 5.06a,b | 101.06 ± 4.33a | 54.67 ± 7.71a,b,c |
| GGT, U/L | 51.88 ± 6.24 | 152.51 ± 20.16a | 146.89 ± 18.6a | 171.48 ± 26.05a | 142.89 ±18.74a |
| LDH, U/L | 326.31 ± 10.6 | 588.57 ± 46.22a | 560.5 ± 56.29a | 560.56 ± 56.29a | 474.17 ± 41.55a |
| Total Bilirubin, μmol/l | 16.78 ± 0.79 | 24.15 ± 1.98a | 12.48 ± 1.4a,b | 20.75 ± 1.06a | 10.51 ± 0.6a,b |
p < 0.05 vs. healthy control group
p < 0.05 Day 1 vs. Day 7
p < 0.05 Standard Therapy vs. Probiotic Therapy
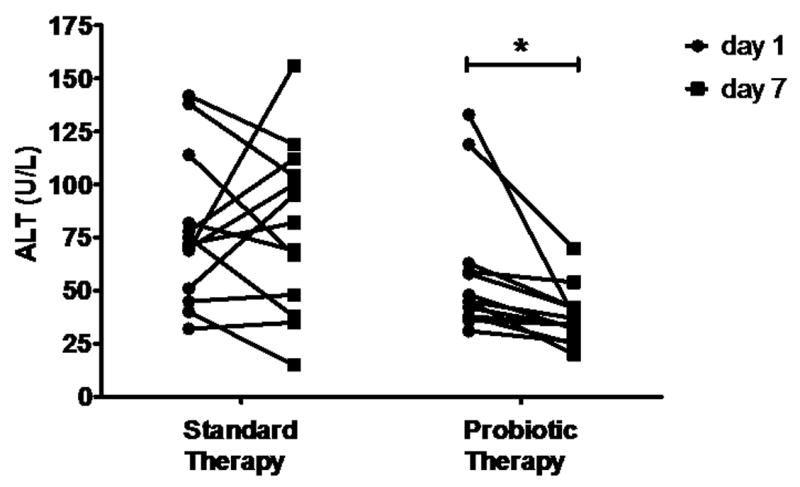
It must be noted that the overall group of alcoholic patients was enrolled with an admission diagnosis of alcoholic psychosis. Although alcoholic hepatitis was not an enrollment criterion, these subjects undoubtedly had some degree of mild ethanol-induced liver injury as all subjects had at least one abnormal liver chemistry test. However, a subgroup analysis was performed to evaluate in patients with biochemically well-characterized alcoholic hepatitis. Alcoholic subjects were selected with AST and ALT greater than 30 and AST to ALT ratio greater than one. These selection criteria identified 26 subjects, 13 from each of the two treatment groups. There were no statistically significant differences in liver enzymes between treatment groups at day one. A paired t-test was performed before (day 1) and after (day 7) therapy (Table 4). Probiotic therapy was associated with a significant decrease in ALT (58.08 vs. 37.46 U/L), AST (137.5 vs. 56.38 U/L), GGT (156.9 vs. 135.3 U/L), LDH (500.4 vs. 362.6 U/L), and total bilirubin (20.10 vs. 12.74 μmol/L). While standard therapy was associated with a significant decrease in AST (183.60 vs. 88.54 U/L) and total bilirubin (24.54 vs. 10.89 μmol/L); ALT, GGT, and LDH were unchanged. Intergroup comparison revealed a significant mean ALT reduction in the probiotic therapy group (30.03±5.64 % decrease) vs. the standard therapy group (9.15±15.08 % increase, p=0.013). Figure 1 depicts the paired analysis of these ALT data. These data demonstrate that probiotic therapy is associated with a greater improvement in liver enzymes than standard therapy alone in patients with mild ethanol induced liver injury / alcoholic hepatitis.
Table 4. Liver Enzyme Activity in Patients With Well-Defined Alcoholic Hepatitis Before (Day 1) and After (Day 7) Standard or Probiotic Therapy, (M ± SEM).
| Standard Therapy, n=13 | Probiotic Therapy, n=13 | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | P value | Day 1 | Day 7 | P value | |
| ALT, U/L | 77.54 ± 9.60 | 80.00 ± 10.9 | 0.820 | 58.08 ± 8.7 | 37.46 ± 3.61 | 0.013 |
| AST, U/L | 183.60 ±37.21 | 88.54±15.16 | 0.036 | 137.5±27.33 | 56.38 ± 9.47 | 0.0016 |
| GGT, U/L | 202.5±36.85 | 153.2±26.08 | 0.075 | 156.9±32.98 | 135.3±32.61 | 0.020 |
| LDH, U/L | 658.5±111.3 | 564.2±88.19 | 0.520 | 500.4±56.67 | 362.6±34.32 | 0.001 |
| Total Bilirubin, μmol/L | 24.54 ± 2.0 | 10.89 ± 2.0 | <0.001 | 20.10 ± 1.2 | 12.74 ± 0.7 | <0.001 |
Figure 1.
Discussion
The present study demonstrates several potentially important and novel results. First, it is the largest study to date demonstrating specific alterations in the bowel flora of alcoholics. Secondly, this is the first human pilot study demonstrating a potential therapeutic role for probiotics in the short term treatment of ALD. Probiotic therapy was associated with an increase in the number of fecal bifidobacteria and lactobacilli. This suggests that probiotic therapy played a causal role in the improvement in liver enzymes.
This study demonstrates that human alcoholics have a significant reduction in the numbers of fecal bifidobacteria, lactobacilli, and enterococci, with a trend towards increased E. coli. These results are consistent with some, but not all, of the previous animal and human studies of bowel flora composition in liver disease (Chiva et al., 2002; Liu et al., 2004; Zhao et al., 2004). It is important to note, however, that most of the previous human studies were done in patients primarily with viral hepatitis induced cirrhosis and not acute alcohol-induced liver injury. It is possible that the composition of the bowel flora could vary with the etiology and severity of the liver disease. It is also possible that additional bowel flora alterations could be present in these alcoholic patients but were missed because more sensitive detection methods such as sequencing of bacterial 16S rRNA from stool samples were not used.
Consistent with prior studies, we observed a decrease in fecal bifidobacteria (Kirpich I.A., 2000; Zhao et al., 2004). Although the potential importance of bifidobacteria in ALD has not been adequately evaluated, bifidobacteria appear to be important in obesity and NASH. Reduced fecal bifidobacteria in normal weight human infants was predictive of the subsequent development of childhood obesity at seven years of age (Kalliomaki et al., 2008). Likewise, in mice, high fat feeding was associated with a significant reduction in cecal Bifidobacterium spp. with significant increases in body weight, adipose tissue mass, liver triglycerides, endotoxemia, and hepatic tumor necrosis factor alpha (Cani et al., 2007a). The authors subsequently demonstrated that a prebiotic mixture of fructooligosaccharides (FOS) restored the quantities of bifidobacteria in high fat fed rodents to decrease endotoxemia, pro-inflammatory cytokines, insulin resistance, and fatty liver (Cani et al., 2007b; Daubioul et al., 2000). A pilot study of FOS in human NASH patients demonstrated an improvement in serum aminotransferases in 8 weeks, possibly through selective increases in bowel bifidobacteria, although this was not evaluated (Daubioul et al., 2005). Therefore, data from our study and others suggest an important emerging role for reduced bifidobacteria in the pathogenesis of steatohepatitis from both alcoholic and non-alcoholic causes.
This study demonstrates that in ALD, probiotic therapy increased fecal bifidobacteria and lactobacilli, and was associated with lower serum transaminases after five days of therapy despite similar values at baseline. Furthermore, subgroup analysis demonstrated improved end of treatment liver enzymes in the subset of patients with biochemiclly well-characterized mild alcoholic hepatitis. This suggests that probiotics, and in particular Bifidobacterium bifidum and Lactobacillus plantarum, have a potential short term role in the treatment of ALD. Although mechanisms were not addressed by this study, potential mechanisms may be found in the literature. First, probiotic therapy may promote integrity of the intestinal mucosa to prevent gut permeability, endotoxemia, and pro-inflammatory cytokine production. Nanji et al. first demonstrated that Lactobacillus GG reduced endotoxemia and alcohol-induced liver injury in rats (Nanji et al., 1994). It was later shown in alcohol fed rats, that prebiotic oats reduced gut permeability, endotoxemia, and liver injury (Keshavarzian et al., 2001). More recently, it was demonstrated that increased basal mucosal prostaglandin E2 production was the mechanism of gastric mucosal protection by Lactobacillus GG in alcohol fed rats (Lam et al., 2007). In mice injected with LPS and D-galactosamine, pretreatment with the probiotic mixture, VSL#3™, prevented a breakdown in colonic barrier function, reduced bacterial translocation, reduced tissue TNF-α levels, and significantly attenuated liver injury (Ewaschuk et al., 2007). Synbiotic 2000™, which contains lactobacillus spp. including L. plantarum and FOS, was demonstrated to reduce primed TNF-α production by peripheral blood mononuclear cells in human cirrhotics (Riordan et al., 2003). In summary, the available evidence suggests that probiotics including Lactobacillus spp. and Bifidobacterium spp. are capable of protecting gut mucosal integrity to reduce bacterial translocation, endotoxemia, and TNF-α production in alcoholic liver disease.
In addition to these effects, probiotics exert numerous other potentially relevant actions in the treatment of alcoholic liver disease. Apart from decreasing TNF-α levels, probiotics modulate the immune system in many other ways. For example, bifidobacteria were shown to induce IL-10 production by cultured human dendritic cells (Hart et al., 2004). Likewise, L. casei ingestion by humans was associated with increased natural killer T cell activity (Takeda et al., 2006). In a recent intriguing study, liver extract protected against NASH by increasing both serum IL-10 and hepatic NKT cells (Elinav et al., 2006). Further actions of probiotics on the immune system have recently been reviewed (Boirivant and Strober, 2007). Probiotics may also reduce oxidative stress. For example, intestinal B. longum and L. acidophilus reduced in vitro plasma lipid peroxidation (Lin and Chang, 2000). Probiotics may also influence bioenergetics and metabolism to influence liver disease. VSL#3™ was shown in a mouse model of NASH to reduce the activity of Jun-N-terminal kinase (JNK) which integrates inflammatory and metabolic signals and mediates insulin resistance (Li et al., 2003). Recent papers have demonstrated that FOS fermentation, which is known to increase bifidobacteria, results in increased production of glucagon-like peptide-1 (GLP-1) by L cells in the colon with decreased GLP-1 degradation by dipeptidyl peptidase-IV (Delzenne et al., 2007). This results in an increased portal vein concentration of GLP-1 with resultant hepatic insulin sensitization (Delzenne et al., 2007). Lastly, 129S6 mice which are sensitive to both alcoholic and nonalcoholic fatty liver disease have a unique gut microbiota which disrupts intestinal choline metabolism to produce a marginally choline deficient mouse (Dumas et al., 2006). Therefore, the composition of the bowel flora and probiotics appear to modulate inflammation, oxidative stress, and metabolism in animal models relevant to alcoholic hepatitis.
Weaknesses of the present study include concerns that the severity of the alcohol-induced liver injury was mild as there were no deaths, the disease was not characterized by liver biopsy, and other forms of concomitant liver disease such as viral hepatitis were not excluded. Because all subjects were admitted with a diagnosis of alcoholic psychosis, they were most assuredly actively drinking ethanol and were at risk for alcohol induced liver injury. Furthermore, all subjects had at least one abnormal liver enzyme. In the subgroup analysis, subjects were very likely to have alcoholic hepatitis without concomitant viral hepatitis as the AST to ALT ratio was greater than one. Unfortunately, neither albumin nor prothrombin were available in these subjects. These data would have provided potentially useful information regarding the severity of the alcoholic hepatitis. Additional concerns exist over the randomization method, and the lack of a placebo or blinding. However, none of these factors would be expected to influence the composition of the subjects' bowel flora or liver enzymes. Furthermore, while the treating physicians and patients were not blinded, the scientists performing the laboratory tests were blinded to the group assignment. Although all patients were prescribed the same diet (regular), calorie counts were not performed. This could be a weakness of the study as potential differences in caloric consumption between groups could have affected both the severity of liver disease and the bowel flora composition.
In conclusion, this study demonstrates that human alcoholics have a significant reduction in the numbers of fecal bifidobacteria, lactobacilli, and enterococci. Short-term treatment of patients with alcohol-induced liver injury with a probiotic preparation containing Bifidobacterium bifidum and Lactobacillus plantarum 8RA was associated with restoration of bowel flora. Despite similar values at the start of treatment, patients treated with probiotics had significantly lower AST and ALT activity at the end of treatment than those treated with standard therapy alone. In the subgroup of patients with well-characterized alcoholic hepatitis, probiotic therapy was associated with a significant end of treatment reduction in ALT, AST, GGT, LDH and total bilirubin. In this subgroup, there was a significant end of treatment mean ALT reduction in the probiotic arm vs. the standard therapy arm.
These data suggest that modulation of the bowel flora may play a role in the pathogenesis and treatment of ALD and indicate a need for larger and more rigorously designed clinical trials to support the use of probiotics in alcoholic liver disease. Furthermore, this study calls for future animal studies to better define the mechanism of action of Bifidobacterium bifidum and Lactobacillus plantarum 8RA in alcoholic liver disease.
Acknowledgments
This research was partially funded by the National Institutes of Health Grants AA010762 (McClain), AA010496 (McClain), AA013170 (Barve), a Sheila Sherlock Clinical and Translational Research Award in Liver Diseases from the American Association for the Study of Liver Diseases (Cave), and the Department of Veterans Affairs (McClain).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adawi D, Ahrne S, Molin G. Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol. 2001;70:213–220. doi: 10.1016/s0168-1605(01)00550-5. [DOI] [PubMed] [Google Scholar]
- Adawi D, Kasravi FB, Molin G, Jeppsson B. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997;25:642–647. doi: 10.1002/hep.510250325. [DOI] [PubMed] [Google Scholar]
- Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opinion Gastroenterol. 2007;23:679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007a;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Current Opinion Clin Nutr Metabolic Care. 2007;10:729–734. doi: 10.1097/MCO.0b013e3282efdebb. [DOI] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007b;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- Carithers RL, McClain CM. Alcoholic Liver Disease. In: Feldman M, Friedman L, Brandt L, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease Pathophysiology / Diagnosis / Management. Canada: Saunders Elsevier; 2006. pp. 1771–1792. [Google Scholar]
- Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzo J. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatology. 2002;37:456–462. doi: 10.1016/s0168-8278(02)00142-3. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatology. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Daubioul CA, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutrition. 2005;59:723–726. doi: 10.1038/sj.ejcn.1602127. [DOI] [PubMed] [Google Scholar]
- Daubioul CA, Taper HS, De Wispelaere LD, Delzenne NM. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J Nutrition. 2000;130:1314–1319. doi: 10.1093/jn/130.5.1314. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: experimental data. The J Nutrition. 2007;137:2547S–2551S. doi: 10.1093/jn/137.11.2547S. [DOI] [PubMed] [Google Scholar]
- Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Amelioration of non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice by oral immune regulation towards liver-extracted proteins is associated with elevated intrahepatic NKT lymphocytes and serum IL-10 levels. J Pathology. 2006;208:74–81. doi: 10.1002/path.1869. [DOI] [PubMed] [Google Scholar]
- Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- Han SY, Huh CS, Ahn YT, Lim KS, Baek YJ, Kim DH. Hepatoprotective effect of lactic acid bacteria, inhibitors of beta-glucuronidase production against intestinal microflora. Arch Pharmacol Res. 2005;28:325–329. doi: 10.1007/BF02977800. [DOI] [PubMed] [Google Scholar]
- Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, B TA, Simonova GN, Lebedeva OV, Sidorov PI, Solov'ev AG. Colon micro flora of patients with chronic alcoholism. News Science Technique. Alcoholic Disease. 2000;8:1–4. [Google Scholar]
- Lam EK, Tai EK, Koo MW, Wong HP, Wu WK, Yu L, So WH, Woo PC, Cho CH. Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sci. 2007;80:2128–2136. doi: 10.1016/j.lfs.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Digestive Dis and Sci. 2000;45:1617–1622. doi: 10.1023/a:1005577330695. [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Seminars in Liver Disease. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Expl Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Probiotics and Blueberry Attenuate the Severity of Dextran Sulfate Sodium (DSS)-Induced Colitis. Digestive diseases and sciences. 2008 doi: 10.1007/s10620-007-0174-x. [DOI] [PubMed] [Google Scholar]
- Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, Hamilton JA, Bengmark S, Williams R, Visvanathan K. Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–1164. doi: 10.1053/jhep.2003.50180. [DOI] [PubMed] [Google Scholar]
- Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006;146:109–115. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim-Fleming J, Mullen KD. Long-term management of alcoholic liver disease. Clinics in Liver Disease. 2005;9:135–149. doi: 10.1016/j.cld.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Zhao HY, Wang HJ, Lu Z, Xu SZ. Intestinal microflora in patients with liver cirrhosis. Chinese J Digestive Diseases. 2004;5:64–67. doi: 10.1111/j.1443-9573.2004.00157.x. [DOI] [PubMed] [Google Scholar]


