Abstract
HIV and drugs of abuse affect common neural systems underlying procedural memory, including the striatum. We compared performance of 48 HIV seropositive (HIV+) and 48 HIV seronegative (HIV−) participants with history of cocaine and/or heroin dependence across multiple Trial Blocks of three procedural learning (PL) tasks: Rotary Pursuit (RPT), Mirror Star Tracing (MST), and Weather Prediction (WPT). Groups were well matched on demographic, psychiatric, and substance use parameters, and all participants were verified abstinent from drugs. Mixed model ANOVAs revealed that the HIV+ group performed more poorly across all tasks, with a significant main effect of HIV serostatus observed on the MST and a trend toward significance obtained for the RPT. No significant differences were observed on the WPT. Both groups demonstrated significant improvements in performance across all three PL tasks. Importantly, no significant Serostatus X Trial Block interactions were observed on any task. Thus, the HIV+ group tended to perform worse than the HIV− group across all trial blocks of PL tasks with motor demands, but showed no differences in their rate of improvement across all tasks. These findings are consistent with HIV-associated deficits in complex motor skills, but not in procedural learning.
Keywords: HIV, substance use disorders, basal ganglia, procedural memory, nondeclarative memory, neuropsychology
Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence
Current taxonomy of memory systems identifies multiple functionally distinct components with differing neuroanatomical substrates, making a primary distinction between declarative (explicit) and non-declarative (implicit) memory (Squire, 1992; Squire & Zola, 1996). Briefly, declarative memory systems are responsible for memory of facts and events that are consciously recalled and dependent on integrity of medial temporal lobe structures. Such abilities are usually assessed through tasks requiring memorization of word-lists, stories, and pictures that subjects are later asked to recall. On the other hand, non-declarative memory includes such functions as procedural learning (i.e., motor skills and habits), priming, and simple classical conditioning, which do not require conscious recollection and rely primarily on brain structures outside of the mid-temporal lobes. Integrity of basal ganglia structures has been deemed critical for procedural learning, which refers to gradual, incremental learning of skills and habits that can be demonstrated through improvements in task performance, but do not require conscious memorization or recollection (e.g., riding a bike, tennis swing, driving). In this study, we examined procedural learning among HIV-seropositive (HIV+) and HIV-seronegative (HIV−) individuals with history of substance use disorders using three common neurocognitive probes of procedural learning.
Basal ganglia structures (especially caudate and putamen) have been consistently reported as vital for procedural learning (for reviews see Packard & Knowlton, 2002; Salmon & Butters, 1995; Squire et al., 1996; Yin & Knowlton, 2006). Patients with basal ganglia abnormalities, particularly those with degenerative dementias affecting subcortical brain structures, show impairments on non-declarative memory tasks but often demonstrate normal performance on declarative memory measures compared to both healthy controls and persons with damage involving mid-temporal structures or other brain regions outside basal ganglia (e.g., Knowlton, Mangels, & Squire, 1996). Conversely, densely amnestic individuals with mid-temporal lobe damage demonstrate adequate procedural learning despite no conscious recall of the testing situation (e.g., Knowlton, Squire, & Gluck, 1994). Other investigations with human subjects support dissociable neural systems for declarative and non-declarative memory, with structures of the basal ganglia supporting procedural learning (e.g., Bayley, Frascino, & Squire, 2005; Poldrack & Packard, 2003).
Several tasks have been used as successful probes of procedural learning and basal ganglia function, including the Rotary Pursuit, Star Mirror Tracing, and the Weather Prediction task. The Rotary Pursuit task and the Star Mirror Tracing task are measures of motor skills learning that are performed abnormally by patients with disease that primarily affects the basal ganglia, such as Parkinson’s (e.g., Heindel, Salmon, Shults, Walicke, & Butters, 1989; Sarazin et al., 2002) and Huntington’s disease (e.g., Heindel, Butters, & Salmon, 1988; Heindel et al., 1989) compared to persons with brain disorders with relative sparing of the basal ganglia (for review see Salmon et al., 1995). Similar findings have been reported using the Weather Prediction task, a measure of procedural learning that requires participants to make probabilistic classifications. Shohamy, Myers, Onlaor, & Gluck (2004) found deficits in Weather Prediction task performances as indicated by use of less effective strategies among patients with Parkinson's disease when compared to healthy controls. Poldrack, Seger, and Gabrieli (1999) reported increased basal ganglia activity in a sample of healthy subjects performing the Weather Prediction task. Further, activity in mid-temporal lobe structures and striatum differ depending on whether the task demands are declarative or non-declarative in nature, and activation in these regions is inversely correlated (Poldrack et al., 2001).
Damage to structures of the basal ganglia attributable to HIV is commonly reported in the scientific literature. The HIV virus has been reported at highest concentration in basal ganglia of brains examined at autopsy (Navia, Cho, Petito, & Price, 1986), with further neuropathological studies supporting preferential damage to basal ganglia from HIV (Masliah, Ge, Achim, DeTeresa, & Wiley, 1996; Nath et al., 2000; Wiley et al., 1998). Structural magnetic resonance imaging (MRI) studies report white matter abnormalities and decreased volume of subcortical nuclei, particularly in the basal ganglia (Aylward et al., 1993; Jernigan et al., 1993; Stout et al., 1998) and caudate volume is often cited as a significant predictor of neuropsychological performance in HIV+ individuals (e.g., Kieburtz et al., 1996; Paul, Cohen, Navia, & Tashima, 2002). Biochemical abnormalities in subcortical brain structures, including basal ganglia, have been noted in both early and later stage HIV disease using magnetic resonance spectroscopy (MRS) (e.g., Chang et al., 2004; Ernst, Itti, Itti, & Chang, 2000; Meyerhoff et al., 1999; Paul et al., 2007). Similarly, functional abnormalities in basal ganglia have been observed with PET (Rottenberg et al., 1996; van Gorp et al., 1992; von Giesen et al., 2000; cf. Ernst et al., 2000) and functional MRI (e.g., Chang et al., 2001).
HIV-related impairments on neuropsychological tests reflect dysfunction of basal ganglia and prefrontal-striatal brain circuits (e.g., Heaton et al., 1995; Reger, Welsh, Razani, Martin, & Boone, 2002; Sadek et al., 2004). Neuropsychological impairments among those infected with HIV are generally seen in attention/working memory, information processing speed, executive functions, learning, and motor skills compared to healthy or risk-matched controls (e.g., Bornstein et al., 1992; Hardy & Hinkin, 2002; Heaton et al., 1995; Miller et al., 1990; Peavy et al., 1994; Reger et al., 2002).
HIV and substance use disorders are often comorbid and evidence suggests additive effects on neurocognitive functioning (for review see Gonzalez and Cherner, 2008). HIV-associated neurocognitive deficits have also been reliably detected among samples of substance dependent individuals (Durvasula et al., 2000; Marder et al., 1995; Rippeth et al., 2004) and studies focusing on neurocognitive tasks sensitive to prefrontal-striatal dysfunction reveal deficits in working memory (Bartok et al., 1997; Farinpour et al., 2000; Martin et al., 2001; Martin et al., 2003) and decision making (Martin et al., 2004) among HIV infected substance users compared to HIV−, substance-using comparison groups. Moreover, co-morbid substance use disorders appear to worsen HIV-associated brain dysfunction as assessed with neuropsychological tests (Basso & Bornstein, 2000; Rippeth et al., 2004) and neuroimaging protocols (Chang, Ernst, Speck, & Grob, 2005; Jernigan et al., 2005; Taylor, Alhassoon, Schweinsburg, Videen, & Grant, 2000). Thus, the high comorbidity of substance use disorders and HIV, as well as evidence to suggest additive detrimental effects on neurocognitive functioning underscores the importance of conducting studies on effects of HIV in a substance using population.
Dysfunction of basal ganglia structures and associated neural pathways are considered a primary underlying cause of neuropsychological impairments among individuals with HIV (with and without history of substance use disorders), yet investigations examining procedural learning in this population are virtually nonexistent. Only a few studies have examined procedural learning among persons with HIV disease or with a history of substance abuse, and (to our knowledge) none have reported results for individuals with both. For example, van Gorp and colleagues (1999) found evidence of improved Rotary Pursuit performance among abstinent cocaine users compared to healthy controls. All participants in their investigation were HIV-seronegative. Kalechstein et al. (1998) found that mood disorder symptoms were correlated with poorer performance on Rotary Pursuit, but their investigation was limited only to HIV+ individuals. An early study that has been widely cited as evidence for procedural learning deficits in HIV examined Rotary Pursuit performance among 29 relatively high functioning non-drug using HIV+ individuals compared to 15 HIV− controls (A. Martin, Heyes, Salazar, Law, & Williams, 1993). They reported that a subset of HIV+ individuals showed significantly decreased motor-skill learning compared to HIV− controls, consistent with basal ganglia pathology. Further, performance deficits among the HIV+ persons were associated with higher levels of the neurotoxin quinolinic acid in cerebrospinal fluid. Although evidence from this initial investigation was promising, there have been practically no follow up studies of procedural learning abilities among HIV+ patients, particularly among those with substance use disorders despite the high comorbidity of these disorders.
In the current investigation, we compare performance of HIV+ and HIV− individuals with a history of substance dependence on three separate measures of procedural learning. We hypothesize that the HIV+ group will perform more poorly, overall, than the HIV− group across all three procedural learning tasks consistent with general neuropsychological dysfunction. In addition, the HIV+ group is expected to demonstrate a deficit in procedural learning compared to the HIV− group by showing decreased rate of improvement across Trial Blocks of each task. Finally, we anticipate that HIV+ subjects with more advanced disease as indexed by CD4 lymphocyte counts and HIV viral load in plasma will show the poorest performance.
Method
Participants
Participants were 48 HIV-seropositive (HIV+) and 48 ELISA-verified HIV-seronegative (HIV−) adults enrolled in a larger study of HIV and neurocognition among substance abusers. Subjects were recruited from the Chicago metropolitan area through flyers placed throughout the community, at infectious disease clinics, and substance use treatment programs, as well as through word-of-mouth. All participants provided informed consent for study procedures, which were approved by the University of Illinois Chicago Institutional Review Board and the Jesse Brown VA Medical Center. Recent (past 3 months) CD4 T-lymphocyte cell counts and level of HIV RNA viral load in plasma were available for 94% of HIV+ participants. Inclusion criteria included a history of cocaine and/or heroin dependence as assessed by the Substance Abuse Module of the Structured Clinical Interview for DSM-IV (SCID-SAM; First, Spitzer, Gibbon, & Williams, 1995). Current SCID-diagnosed alcohol abuse or dependence were grounds for exclusion. Subjects were instructed to abstain from street drug use and none reported use of cocaine or heroin for at least 7 days prior to each study visit. Those testing positive for opiates or cocaine on urine toxicology testing (Visualine, SunBiomedical) or those with positive alcohol breath test (Inoxilyzer, CMI Inc.) did not undergo study protocol and were rescheduled. Additional exclusion criteria included history of head injury with loss of consciousness greater than thirty minutes or open head injury of any kind, history of schizophrenia or unmedicated bipolar disorder, and history of neurological illness (e.g., dementia, stroke, tumor, neurosyphilis). No statistically significant differences were observed between HIV+ and HIV− groups on demographic characteristics and estimated premorbid intelligence as assessed by the American National Adult Reading Test (AmNART; Grober & Sliwinski, 1991), which are described in Table 1.
Table 1.
Demographic Characteristics of Sample
| HIV − (n = 48) |
HIV + (n = 48) |
|
|---|---|---|
| Age (M, SD) | 44.6 (7.2) | 44.5 (6.7) |
| years of education (M, SD) | 12.0 (1.8) | 12.1(1.8) |
| AmNART estimated IQ (M, SD) | 102.2 (9.5) | 101.7 (8.3) |
| % men | 81% | 67% |
| Ethnicity/Race | ||
| % Caucasian | 11% | 6% |
| % Hispanic | 4% | 4% |
| % African American | 85% | 88% |
| % Other | 0 | 2% |
| % Right-handed | 88% | 96% |
| BDI-2 (Md, IQR) | 8 [3, 14] | 8 [3, 19] |
| STAI-State (M, SD) | 35.5 (12.8) | 37.7 (13.2) |
| WURS (M, SD) | 32.6 (20.7) | 30.8 (17.7) |
| % Hepatitis C seropositive | 27% | 44% |
Note: AmNART, American National Adult Reading Test; BDI-2, Beck Depression Inventory – 2nd Edition; STAI-State; State-Trait Anxiety Inventory – State portion; WURS, Wender-Utah Rating Scale.
Assessment Protocol
Trained personnel administered several structured clinical interviews, self-report questionnaires, and neurocognitive exams to participants. In order to minimize participant fatigue, the assessment was conducted across two study visits, each requiring one to two hours to complete. Data were collected on substance use history, mental health indices, and performance on several measures of procedural learning, as described below.
Substance Use History
As previously noted, participants completed the SCID-SAM to assess history of past and current dependence or abuse for drugs and alcohol. Additionally, a modified version of the Kreek-McHugh-Schluger-Kellogg scale (KMSK; Kellogg et al., 2003) was employed to index severity of alcohol and drug use history by obtaining information on frequency, amount, and duration of alcohol and drug use during an individual’s most intense period of drug consumption (i.e., their peak use). Additional questions were added to the KMSK in order to obtain information on history of cannabis use, as well as information on drug use during the prior month for all drug classes. The possible range of scores for peak use vary depending on the substance queried (Alcohol: 0 – 13; Cocaine: 0 – 16; Heroin: 0 – 13; Cannabis: 0 – 16). Finally, additional information on substance use topography (e.g., years of drug use, days since last use) was obtained via self-report. Information on participants' substance use characteristics are presented in Table 2.
Table 2.
Substance Use Characteristics of Sample
| HIV − (n = 48) |
HIV + (n = 48) |
|
|---|---|---|
| Years of cocaine and/or heroin use (M, SD) | 22.1 (7.9) | 21.9 (9.1) |
| Days since last use of cocaine and/or heroin (Md, IQR) | 210 [90, 410] | 276 [120, 652] |
| History of past SCID-SAM Dependence | ||
| Alcohol | 81% | 85% |
| Cocaine | 94% | 96% |
| Heroin* | 69% | 46% |
| Cannabis | 54% | 35% |
| Stimulants* | 4% | 21% |
| KMSK peak (Md, IQR) | ||
| Alcohol | 10 [8, 12] | 11 [8, 12] |
| Cocaine | 13 [10, 16] | 15 [13, 15] |
| Heroin | 8 [0, 11] | 4 [0, 11] |
| Cannabis | 10 [6, 13] | 11 [4, 13] |
p < 0.05
note: SCID-SAM, Structured Clinical Interview for DSM-IV – Substance Abuse Module; KMSK, Kreek-McHugh-Schluger-Kellogg Scale
Current Mental Health
Participants completed the Beck Depression Inventory, 2nd Edition (BDI-II) to obtain information on current severity of symptoms associated with depression (Beck, Steer, Ball, & Ranieri, 1996). Current levels of anxiety were assessed with the “State” portion of the State-Trait Anxiety Inventory (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Childhood symptoms of Attention Deficit Hyperactivity Disorder (ADHD) were queried using the Wender-Utah Rating Scale (WURS; Stein et al., 1995; Ward, Wender, & Reimherr, 1993).
Procedural Learning
Three separate measures were used to assess procedural learning in our sample.
The photoelectric Rotary Pursuit task (RPT; Lafayette Instruments, Model 30014A), is a well-studied measure of motor skills learning. The task requires subjects to hold a plastic stylus over a rotating disk, keeping the stylus directly over a patch of light that appears to spin around the circumference of the disk at a set speed. Participants were instructed to “…hold on to the stylus and hold it over the light as it spins around. [They] must keep following the light until it stops spinning.” Additional instructions were provided as needed until it was evident to the examiner that the participant understood the instructions. Based on data from previous studies (e.g., see Table 1 in Weickert et al., 2002), the age of our participants, and our own pilot investigations, the speed of the turntable was set at 55 revolutions per minute (RPM) for all participants across all trials given. This approach allowed us to use a standardized setting with all participants that showed minimal "floor" and "ceiling" effects. This method also obviated the need for practice trials, and ensured that all improvements in performance were captured during testing trials, as all participants would be starting the task with no prior exposure or training. Eight trials lasting 20 seconds each were conducted. The time (in seconds) that the stylus was kept on the target during each trial was recorded. Participants were allowed to rest for approximately 20 seconds between each trial, with a 15-minute break halfway through the task between the fourth and fifth trial.
The Mirror Star Tracing task (MST; Lafayette Instruments, Model 58024A) requires participants to trace within an outline of a six-point star on a flat metallic plate using a metal stylus similar in shape to a pen. Participants are unable to see the actual star (or their hand) as they are tracing the star, but rather see only a mirror image of the two. The subjects are instructed to maintain contact between the stylus and the surface of the apparatus and to trace within the black outline of the star, trying not to stray from within the outline. They are told to trace as quickly as they can, while trying to remain within the outline of the star. Eight trials were conducted and the amount of time required for the participant to trace the full outline of the star, one time, was recorded for each trial. If 240 seconds elapsed during a trial, the participant would be asked to stop, the trial would be discontinued, and a time of 241 seconds would be recorded for that participant on that trial. Participants were allowed to rest for approximately 20 seconds between trials, except between the fourth and fifth trials where they had a five-minute break.
Participants also completed the Weather Prediction task (WPT), a 200-trial two-choice probabilistic classification task (Knowlton et al., 1994). On each trial, the participant views a display that contains one, two, or three cards (from a set of four cards, each with a distinct abstract design). As the cards are presented, participants must press one of two keys on a computer keyboard to indicate if they think the cards are associated with an outcome of “sunshine” or “rain.” Each card display (pattern) has a fixed probability of each outcome that is not told to participants. They are only informed that they may have to “guess at first” but that they should try “to get better at predicting the weather” as the task goes on. We administered the WPT with the method put forth in Experiment 2 of Gluck, Shohamy, and Myers (2002), which employs the same procedures of Knowlton et al. (1994), but uses a probability structure that is somewhat easier to learn and would thus have a difficulty level more appropriate for our sample of inner-city substance users with relatively low levels of educational attainment. As in previous studies, responses were classified as "correct" if participants chose the response that was more strongly associated with the cards presented. The percentage of correct responses made during each of four 50-trial blocks was used as the dependent variables.
There was one noteworthy difference in our method for administering the WPT. Instead of allowing participants up to 5s to make their responses, we set the task parameters to allow participants up to 10s to respond. This was done to minimize the impact of processing speed problems (that are common in HIV and sometimes observed in substance use disorders) on the participants' ability to perform the task. Our data suggest that the response time limit we employed was sufficiently lengthy to prevent possible deficits in processing speed from interfering with task performance, as HIV+ and HIV− groups did not differ significantly in their response times (p = 0.15; HIV−, M = 1.31s, SD = 0.51; HIV+, M = 1.45s, SD = 0.43). Moreover, groups did not differ in the total number of trials in which they failed to provide a response within the 10s time limit (p = 0.76; HIV−, Mdn = 0, Range [0, 2]; HIV+, Mdn = 0, Range [0, 2]). Indeed, only four HIV− and five HIV+ participants failed to provide a response within the time limit on any one trial.
Results
General Statistical Procedures
Distributions of data for each variable and all statistical analyses were examined for outliers and violations of statistical assumptions (e.g., non-normal distribution, heterogeneity of variance). Non-normal data underwent transformation when appropriate. Student’s t-tests were used for between-group (HIV+ and HIV−) comparisons with one continuous dependent variable, whereas chi-square tests were employed when the single dependent variable was categorical. Analyses were deemed statistically significant when p-values were less than or equal to 0.05. In order to reduce the number of dependent variables in analyses and to reduce variability, data reduction techniques were used for data from the Rotary Pursuit task (RPT) and the Mirror Star Tracing task (MST). Specifically, performance on the eight trials of the task were reduced to four trial blocks for each of these two measures, such that each trial block represented the average performance across two successive trials (i.e., Trial Block 1 = average of Trial 1 and Trial 2; Trial Block 2 = average of Trial 3 and Trial 4; and so forth).
Group Characteristics
Groups (HIV+ and HIV−) were well matched on demographic factors, with no statistically significant differences observed in age, years of education, sex, handedness, race/ethnicity, estimated IQ, and hepatitis C serostatus (Table 1). HIV+ and HIV− groups were also matched on self-reported symptoms of depression (BDI-II), anxiety (STAI-State), and Attention-Deficit Hyperactivity Disorder (WURS), see Table 1. Similarly, no statistically significant differences were observed on various measures of substance use (Table 2), with the exception of higher prevalence of past heroin dependence among HIV− participants and a higher prevalence of past stimulant dependence in the HIV+ group. More of the participants in the HIV− group were on methadone treatment (HIV− = 21%, HIV+ = 4%; Fisher’s exact test, n = 96, p-value = 0.004) and reported using heroin more recently (HIV−, n = 35, Md = 229 days ago; HIV+, n = 28, Md = 425 days ago; Kruskal-Wallis Test, p = 0.02). Despite this, groups did not differ significantly in severity of peak substance use for all substances assessed by the KMSK, including for heroin (Table 2).
Among HIV+ participants, only 18% had an immunological AIDS diagnosis (CD4 count: Md = 359 cells/mL, IQR [249, 555]) and 44% of the HIV+ sample had undetectable HIV RNA viral load in plasma (Md = 352 copies/mL, IQR [75, 4206]). Exactly half of the HIV+ sample reported being prescribed highly active antiretroviral treatment, with 86% of HIV+ participants reporting use of any ARV.
Performance on Measures of Procedural Learning by HIV serostatus
Study hypotheses were examined using 2 (Group: HIV+; HIV−)×4 (Trial Block: Block 1, Block 2, Block 3, Block 4) mixed-model ANOVAs, with Group as the between-subjects factor and Trial-Block as the within-subjects factor. The dependent variable for each ANOVA model tested was performance for each of the four Trial Blocks associated with each procedural learning task: RPT = Rotary Pursuit seconds on target, MST = Mirror Star Tracing seconds to complete, WPT = Weather Prediction percentage of correct selections. For each model tested, we employed a Greenhouse-Geiser correction to control for violation of the assumption of sphericity.
Because HIV+ and HIV− groups differed significantly on prevalence of heroin dependence, stimulant dependence, and methadone treatment status, we examined (individually) if any of these factors was associated with performance on any of the procedural learning tasks to determine if they should be included as covariates in analyses. Briefly, each factor was included as the sole independent variable in a mixed-model ANOVA using the same procedures just described. No statistically significant effects were observed (all p-values > 0.05) for each of these variables; thus, they were not included as covariates in further analyses.
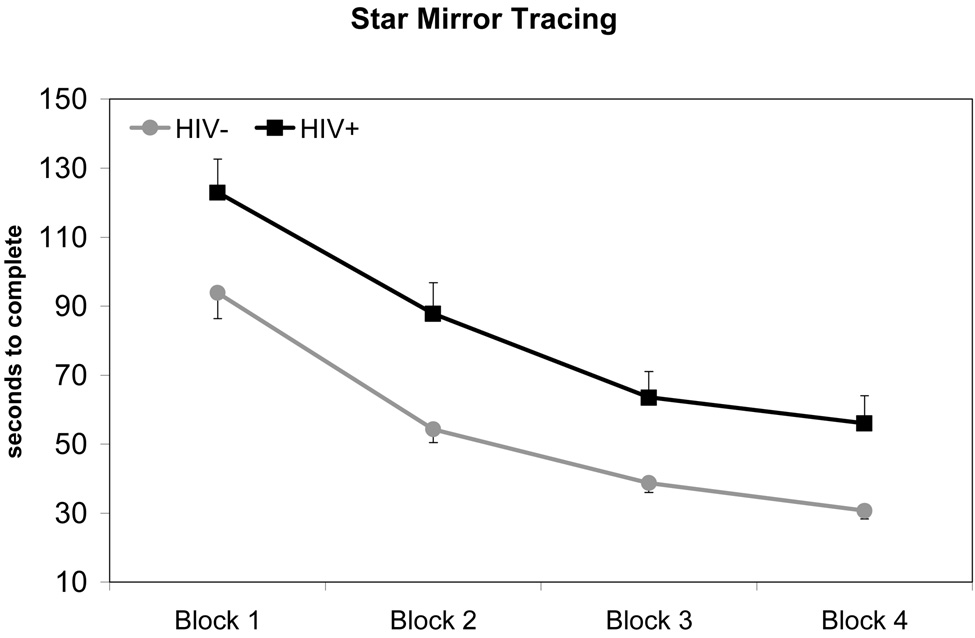
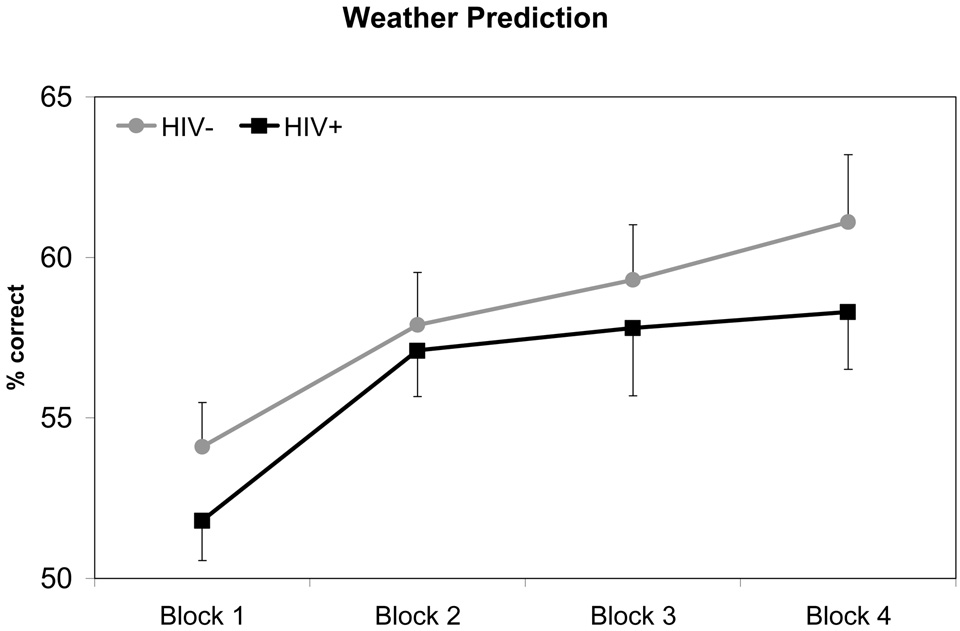
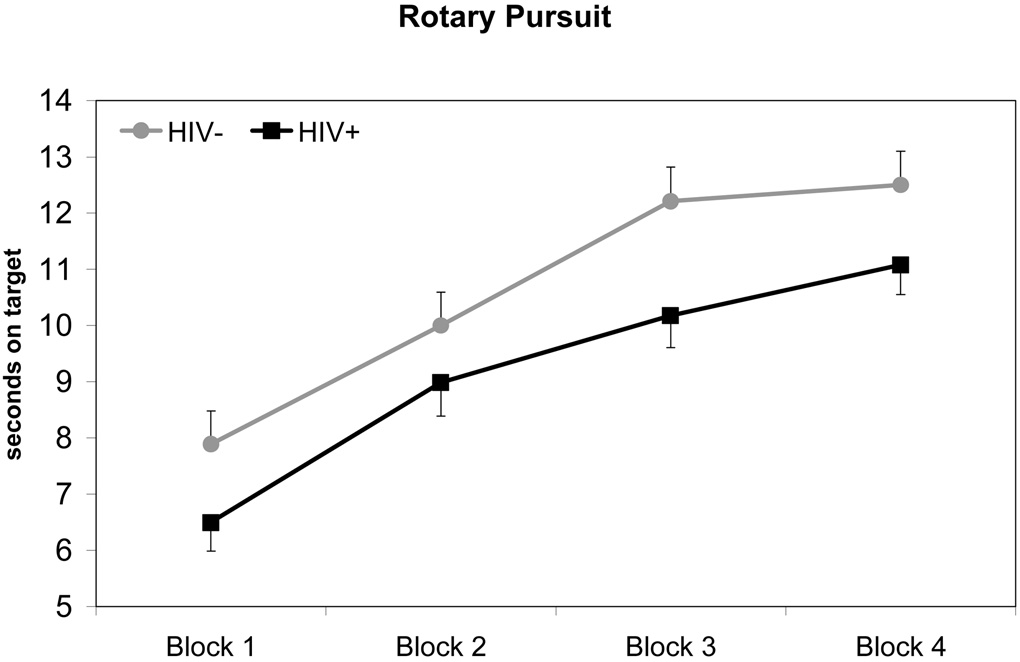
Performance of HIV+ and HIV− groups on procedural learning tasks are depicted in Figure 1 – Figure 3. For each mixed-model ANOVA, we observed a statistically significant main effect for Trial Blocks, indicating that overall participant performance improved across task trials (all p-values < 0.001). There were no significant Group x Trial Block interaction effects for any procedural learning task (SMT: F1.83, 171.59 = 0.43, p = 0.63; RPT: F2.63, 247.56 = 1.35, p = 0.26; WPT: F2.77, 255.03 = 0.34, p = 0.78), suggesting that both HIV+ and HIV− groups demonstrated similar rates of improvement in performance across Trial Blocks. However, HIV+ participants performed worse overall than HIV− participants on all tasks. A statistically significant main effect for Group was observed for the SMT (F1, 94 = 11.32, p = 0.001), such that HIV+ participants performed more poorly overall compared to HIV− participants. A similar pattern of data were observed on RPT performance, although the main effect of Group failed to reach conventional levels of significance (F1, 94 = 3.73, p = 0.057). In contrast, a statistically significant main effect of Group was not observed on the WPT (F1, 94 = 1.13, p = 0.29). Overall mean differences in performances between HIV+ and HIV− groups on the SMT and RPT evidenced effect sizes of medium to large (hedges g = 0.68) and small to medium magnitude (hedges g = 0.39), respectively. In summary, these data suggest that the HIV+ and HIV− groups evidenced no differences in their rate of procedural learning across all tasks. However, HIV+ participants performed worse, overall, on two of the three tasks.
Figure 1. Title: Star Mirror Tracing Performance by HIV Serostatus.
Note: Data points represent mean performance and standard error for each trial block.
Figure 3. Title: Weather Prediction Performance by HIV Serostatus.
Note: Data points represent mean performance and standard error for each trial block.
In order to analyze asymptotes in performance across Trial Blocks, a quadratic function was fitted separately for each PL task. Point-of-asymptote was calculated for all three tasks using data from all four Trial Blocks. As in previous analyses, a significant main effect of Trial Block was observed for all tasks (p-values ≤ .05). There were no significant Group x Trial Block interactions, indicating that point-of-asymptote did not differ between HIV+ and HIV− groups on any of the tasks. Participants were found to reach asymptote slightly before or during the fourth Trial Block (point-of-asymptote: RPT = 4.28, SMT = 3.95, WPT = 3.74). Mean performance level at the point-of-asymptote was calculated for the HIV+ and HIV− group on each of the three PL tasks. Consistent with results from our previous analyses, t-tests revealed that the HIV− group performed significantly better than the HIV+ group at asymptote on the SMT (p < 0.001), but not the WPT (p = 0.32). Difference in Group performance at point-of-asymptote for the RPT approached significance (p = 0.056).
Effects of HIV Disease Severity and Treatment Factors on Procedural Learning
Further analyses were conducted with the HIV+ sample to determine if biomarkers of disease severity (i.e., HIV RNA viral load in plasma and CD4 counts) or current treatments (on antiretrovirals or HAART) influenced performance on measures of procedural learning.
Using a median split, HIV+ participants were stratified into those with low (< 359, n = 23) and high (≥ 359, n = 22) CD4 counts. As in previous analyses, three separate mixed-model ANOVAs were conducted (one for each procedural learning task) with CD4 group as the between-subjects factor and Trial Blocks as the within-subjects factor. As with all prior analyses, a significant main effect of Trial Block was observed for each procedural learning task, indicating significant improvements in performance over time (p-values < .01). No other statistically significant main effects or interaction effects were observed (p-values > .10).
Using the same statistical methods previously described, three separate mixed-model ANOVAs (one for each procedural learning measure) were conducted with presence of HIV RNA viral load in plasma serving as the between-subjects factor and Trial Blocks on procedural learning tasks as the within-subjects factor. Of the 45 HIV+ participants for which plasma viral load levels were available, 25 had detectable and 20 had undetectable HIV viral load. As with our previous analyses, all models demonstrated a significant main effect for Trial Blocks across all procedural learning tasks, indicating improvements in performance over time (p-values ≤ .001). A statistically significant Group main effect for viral load was only observed on the WPT, with HIV+ participants with detectable viral load performing worse, overall, compared to those without detectable viral load (F1, 43 = 4.90, p = 0.03). Statistically significant Group main effects were not observed for performance on the SMT or RPT (p-values > .10). Similarly, no statistically significant Group x Trial Block effects were observed, regardless of procedural learning task (p-values > .10).
For the final set of analyses, the between-subjects factor was whether HIV+ participants were on antiretroviral medications (ARV+; n = 41; ARV−; n = 7). Again, three separate mixed-model ANOVAs were conducted. Consistent with prior analyses, statistically significant effects for Trial Blocks were observed across all tasks, indicating improved performance over Trial Blocks (p-values < .001). No significant Group main effect or Group x Trial Block effects were observed for any of the PL tasks (p-values > .05).
Discussion
The primary goal of this investigation was to determine if a positive HIV serostatus was associated with poorer procedural learning (PL) in a sample of individuals with a history of substance dependence. Several important conclusions emerged. First, compared to the HIV− group, HIV+ individuals showed evidence of poorer overall performance on PL tasks, particularly on the two measures with complex motor demands (i.e., Rotary Pursuit and Star Mirror Tracing). Secondly, both HIV+ and HIV− participants evidenced significant improvements in performance across trial blocks of all three tasks, suggesting successful PL. Finally, there was no evidence to substantiate a specific deficit in procedural learning among HIV+ compared to HIV− participants.
Our findings are consistent with deficits in complex motor functions and processing speed (typically indexed by slowed performance) among HIV+ participants, which have been well-documented in the scientific literature (e.g., Hardy et al., 2002; Heaton et al., 1995; Martin, Sorensen, Edelstein, & Robertson, 1992), and are often referred to as a "hallmark" neurocognitive feature resulting from known abnormalities in white matter and subcortical nuclei of HIV+ participants. Of the tasks that our participants were administered, the Rotary Pursuit Task (RPT) and Star Mirror Tracing Task (SMT) both place time demands on participant performance and depend on intact complex motor functions whereas the Weather Prediction Task (WPT) does not. Although in theory our subjects’ performance might be influenced in part by peripheral neuropathy, some evidence argues against this explanation. Peripheral neuropathies are reported in 10 – 15% of HIV+ patients, and are more common in advanced disease stages, typically affect the lower extremities first, and often do not produce objective motor deficits in the upper limbs (for reviews see Verma, 2001; Wulff, Wang, and Simpson, 2000). Given the low prevalence of immunological AIDS diagnoses in our ambulatory community sample of HIV+ participants, we do no think that peripheral neuropathies (if present) would be of sufficient severity to affect performance on the measures we employed. However, future studies may benefit from detailed evaluation of peripheral neuropathy, particularly among subjects with more advanced disease. Furthermore, inclusion of additional neuropsychological tests assessing specifically simple motor skills and processing speed would help to understand better the mechanisms for the complex motor deficits that we observed in the HIV+ sample.
It is important to note that multiple structures in addition to the caudate and putamen contribute to performance of both motor and non-motor tasks of PL, including prefrontal, occipital, parietal cortex, and cerebellum (e.g., Grafton et al., 1992; Jenkins, Brooks, Nixon, Frackowiak, & Passingham, 1994; Poldrack et al., 1999; Poldrack et al., 2001). PL tests of motor skills may rely more heavily on different structures (e.g., putamen and cerebellum) in these systems when compared to PL measure without motor demands (e.g., WPT). Furthermore, neural mechanisms that may prove compensatory for WPT may not necessarily generalize to SMT and RPT. Recent literature suggests that in some conditions individuals may recruit medial temporal lobe structures and partially rely on declarative memory to perform the WPT (e.g., Foerde 2006; Moody 2004). Further evidence indicates that individual elements of the circuitry supporting procedural learning might compensate for possible dysfunction in other structures in the network. For example, Fera and colleagues (2005) compared performance of young and older healthy adults on the WPT and found no significant group differences in task performance. However, older adults demonstrated much greater activation of parietal cortex on fMRI.
HIV-associated neurocognitive deficits were detected in our sample of individuals with history of substance dependence on two of three PL tasks, and our finding of significant improvements across Trial Blocks on all tasks indicate that the measures employed in this investigation were sensitive indicators of PL in our sample. However, despite evidence of poorer performance on the RPT and SMT, our findings did not demonstrate specific PL deficits among HIV+ substance dependent individuals compared to HIV− controls. Contrary to our hypothesis, both HIV+ and HIV− individuals demonstrated significant improvements in performance across Trial Blocks and their rate of improvement across Trial Blocks did not differ significantly across all three PL tasks. However, the absence of a non-substance using HIV+ control group and a group of healthy controls precludes coming to definitive conclusions on whether performance among our HIV+ subjects was "within normal limits" or if there are possible interactions between substance use and HIV on PL performance. Recruitment of such groups is currently underway in our laboratory to address these questions in future studies.
At first glance, our findings may appear to contradict those presented in the seminal paper by A. Martin and colleagues (1993), that is widely cited as providing evidence for procedural learning deficits associated with HIV infection. However, findings from these two studies are considerably less discrepant with careful comparison of their investigation with ours. A. Martin et al. (1993) found that a small subset of approximately 24% of the HIV+ group in their study and 7% of HIV− controls (ns= 7 and 1, respectively) could be characterized as “poor learners” on the basis of minimal improvement in performance from the first to last Rotary Pursuit trial. The level of the neurotoxin quinolinic acid in CSF among five HIV+ "poor learners" was significantly higher than in 18 HIV+ "good learners" and quinolinic acid levels were correlated with improvements in Rotary Pursuit performance. However, they also found that HIV+ subjects, as a group, did not show a deficit in rate of improvement across Trial Blocks on the Rotary Pursuit and the majority of subjects performed in the normal range. Thus, analyses of task performance in both the A. Martin study subjects and our groups showed no significant HIV serostatus by Trial Block interactions, which we deem necessary to establish a specific procedural learning deficit from HIV. In fact, despite significant differences in sample characteristics and our use of individuals with history of substance dependence, the results of both investigations are remarkably similar. Further, our conclusions are bolstered by our larger study sample and use of three separate PL tasks. Therefore, although both investigations found evidence for complex motor problems among HIV+ participants as a whole, neither investigation substantiates specific PL deficits, per se, among HIV+ persons. To our knowledge, no study has demonstrated this latter type of impairment among HIV+ participants. One can speculate that that the paucity of published investigations on HIV-associated PL deficits may stem, in part, from a "file drawer" effect, as other studies with negative outcomes may have gone unpublished (Rosenthal, 1979).
Several findings from more detailed studies of the neural systems underlying procedural learning help to clarify our results. Most initial investigations suggesting that PL is dependent on integrity of striatum were conducted with clinical populations with known basal ganglia disease (i.e., Parkinson and Huntington' disease). However, the pattern of neuropathology associated with these diseases typically extends well beyond striatum and are much more severe than in HIV. The caudate is often noted as a critical structure for PL, but widespread circuitry beyond striatum is often shown to be active in neuroimaging studies of PL (e.g., Grafton et al., 1992; Jenkins, Brooks, Nixon, Frackowiak, & Passingham, 1994; Poldrack et al., 1999; Poldrack et al., 2001). Thus, some researchers have postulated that the caudate is an important, but not essential, structure for procedural learning (e.g., Schmidtke, Manner, Kaufmann, & Schmolck, 2002). As we not above, several studies have suggested that individuals may rely on brain systems outside striatum to perform some PL tasks. It is possible that neuropathology in striatum must reach a threshold level of severity or must extend substantially to additional brain regions for frank deficits in procedural learning to emerge. Brain functioning in our HIV+ sample was compromised enough to manifest with deficits in complex motor skills, but it may be that injury to neural systems that support procedural learning was insufficient to produce detectable deficits.
We acknowledge that HIV has been shown to disrupt basal ganglia preferentially, but HIV-associated neuropathology is diffuse and affects additional brain systems, including white matter, prefrontal cortex, and hippocampus (e.g. Jernigan et al., 1993; Pomara, Crandall, Choi, Johnson, & Lim, 2001; Reyes, Mohar, Mallory, Miller, & Masliah, 1994). Furthermore, correlations between basal ganglia neuropathology and neurocognitive performance are not always detected, suggesting that there is not an invariable relationship between striatal integrity and neuropsychological performance in the HIV literature (cf. Paul et al., 2002). For example, Moore and colleagues (2006) found significant correlations between a global index of neuropsychological performance obtained shortly before death of HIV+ participants and postmortem measures of neurodegeneration in hippocampus and midfrontal cortex, but not putamen. Striatal pathology in HIV may not be of sufficient severity, in most cases, to produce deficits in procedural learning as have been reported with disorders that severely damage basal ganglia and associated circuits (e.g., Parkinson's and Huntington's Disease).
With one exception, we found no consistent evidence to suggest that markers of immune status related to performance in our sample. WPT performance was significantly worse among HIV+ participants with detectable viral load in plasma, compared to those with undetectable viral load (p = 0.03). However, performance on the WPT did not differ significantly as a consequence of CD4 count or ARV status. Furthermore, performance on the SMT and RPT, which differed based on HIV serostatus, did not differ significantly when HIV+ groups were stratified by CD4 count, plasma viral load, or ARV status. Although the observed differences in WPT performance might have resulted from Type-I error, one might speculate that HIV effects may first manifest on tests involving complex motor skills (e.g., SMT and RPT) and then progress to more "cognitive" tasks (e.g., WPT). Therefore, participants with more advanced disease would be more likely to demonstrate differences in performance on the WPT, in addition to the RPT and SMT. However, only 18% of HIV+ participants in the current study met criteria for an immunological AIDS diagnosis, 44% had undetectable HIV viral load in plasma, and 86% were on ARVs, with 50% on HAART. Future studies may benefit from including HIV+ participants with more advanced disease and from examination of additional medical markers of HIV disease severity (e.g., biomarkers of neuroinflammation, viral load in CSF, or neuroimaging data).
Our sample consisted predominantly of African-American men with a high school education, most of who were in their 4th decade of life and were recruited from the Chicago greater metropolitan area; thus, our results may not generalize to participants of differing demographics. Nonetheless, our findings are very similar to those of A. Martin et al. (1993), who tested a sample of men recruited from the U.S. military that was generally younger, more highly educated, and of higher estimated IQ than our sample. Further studies that include more diverse samples will be better poised to systematically examine possible interactions with demographic variables, including possible gender effects.
In summary, the results from the current investigation showed evidence of poorer performance on procedural learning tasks that were consistent with general difficulties in complex motor skills among HIV+ individuals with substance dependence compared to matched HIV− controls. However, there was no evidence for specific procedural learning deficits among the HIV+ group, as both groups showed significant and comparable improvements in performance across Trial Blocks on motor and non-motor tasks of procedural learning. Although our results suggest that both groups achieved asymptote within the trials we administered, future investigations may examine if HIV+ individuals may achieve levels of performance comparable to controls on motor skills tasks if they receive additional training. Overall, our findings contribute new knowledge to an initially promising but understudied research area within the literature on HIV and neurocognition. Furthermore, the current study is unique in its use of multiple measures of procedural learning (with and without motor demands), a fairly large sample size, and its focus on examining the effects of HIV among individuals with history of substance dependence. The ability to implicitly acquire skills gradually and incrementally may impact an individual's ability to learn and implement new tasks at work, efficiently complete routine tasks at home, and carry out skills taught in clinical treatment programs. Understanding procedural learning in HIV+ substance dependent individuals will help to more accurately characterize the impact of HIV and substance use on neurobehavioral performance.
Figure 2. Title: Rotary Pursuit Performance by HIV Serostatus.
Note: Data points represent mean performance and standard error for each trial block.
Table 3.
Performance of Groups on Procedural Learning Measures
| HIV− (n = 48) Mean (SD) |
HIV+ (n = 48) Mean (SD) |
|
|---|---|---|
| Star Mirror Tracing (SMT) | ||
| Trial Block 1 | 93.9 (52.1) | 122.9 (66.7) |
| Trial Block 2 | 54.4 (27.0) | 87.8 (62.1) |
| Trial Block 3 | 38.7 (19.0) | 63.6 (52.2) |
| Trial Block 4 | 30.7 (16.0) | 56.1 (55.2) |
| Overall mean performance | 54.4 (23.9) | 82.6 (55.1) |
| Rotary Pursuit (RPT) | ||
| Trial Block 1 | 7.9 (4.1) | 6.5 (3.5) |
| Trial Block 2 | 10.0 (4.1) | 9.0 (4.1) |
| Trial Block 3 | 12.2 (4.2) | 10.2 (4.0) |
| Trial Block 4 | 12.5 (4.2) | 11.1 (3.7) |
| Overall mean performance | 10.6 (3.9) | 9.2 (3.5) |
| Weather Prediction (WPT) | ||
| Trial Block 1 | 54.1 (9.4) | 51.8 (8.6) |
| Trial Block 2 | 57.9 (11.2) | 57.1 (9.9) |
| Trial Block 3 | 59.3 (11.7) | 57.8 (14.6) |
| Trial Block 4 | 61.1 (14.2) | 58.3 (12.4) |
| Overall mean performance | 58.2 (9.0) | 56.2 (8.8) |
Note: All data presented in this table is not transformed. Values for SMT are seconds to complete task (lower value is better); RPT values are seconds on target (higher value is better); WPT values are percentage of correct choices (higher value is better).
Acknowledgments
The authors thank Drs. Rodney Eiger and Max Brito, as well as Mr. Gerald Nunnally at the Jesse Brown VA Medical Center for their generosity in referring participants for this investigation. This study was supported by HHS F32 DA018522 to Dr. Gonzalez and R01 DA12828 to Dr. Martin.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/neu/
References
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Bartok JA, Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM, et al. Working memory deficits in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 1997;3:451–456. [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Neurobehavioural consequences of substance abuse and HIV infection. Journal of Psychopharmacology. 2000;14:228–237. doi: 10.1177/026988110001400306. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Nasrallah HA, Para MF, Whitacre CC, Rosenberger P, Fass RJ, et al. Neuropsychological performance in asymptomatic HIV infection. Journal of Neuropsychiatry and Clinical Neuroscience. 1992;4:386–394. doi: 10.1176/jnp.4.4.386. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Durvasula RS, Myers HF, Satz P, Miller EN, Morgenstern H, Richardson MA, et al. HIV-1, cocaine, and neuropsychological performance in African American men. Journal of the International Neuropsychological Society. 2000;6:322–335. doi: 10.1017/s1355617700633076. [DOI] [PubMed] [Google Scholar]
- Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. Journal of Magnetic Resonance Imaging. 2000;12:859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, et al. Verbal working memory in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, et al. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis II Personality Disorders--Patient Edition (SCID-I/P) Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the "weather prediction" task?: individual variability in strategies for probabilistic category learning. Learning and Memory. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. Journal of Neuroscience. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time performance in adults with HIV/AIDS. Journal of Clinical and Experimental Neuropsychology. 2002;24:912–929. doi: 10.1076/jcen.24.7.912.8391. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease. Behavioral Neuroscience. 1988;102:141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. Journal of Neuroscience. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, et al. The HNRC Group. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. Archives of Neurology. 1993;50:250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Hinkin CH, van Gorp WG, Castellon SA, Satz P. Depression predicts procedural but not episodic memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 1998;20:529–535. doi: 10.1076/jcen.20.4.529.1473. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, et al. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology. 1996;53:155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Marder K, Liu X, Stern Y, Malouf R, Dooneief G, Bell K, et al. Risk of human immunodeficiency virus type 1-related neurologic disease in a cohort of intravenous drug users. Archives of Neurology. 1995;52:1174–1182. doi: 10.1001/archneur.1995.00540360052016. [DOI] [PubMed] [Google Scholar]
- Martin A, Heyes MP, Salazar AM, Law WA, Williams J. Impaired motor-skill learning, slowed reaction time, and elevated cerebrospinal fluid quinolinic acid in a subgroup of HIV-infected individuals. Neuropsychology. 1993;7:149–157. [Google Scholar]
- Martin EM, Pitrak DL, Rains N, Grbesic S, Pursell K, Nunnally G, et al. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17:283–288. doi: 10.1037/0894-4105.17.2.283. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, et al. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sorensen DJ, Edelstein HE, Robertson LC. Decision-making speed in HIV-1 infection: a preliminary report. AIDS. 1992;6:109–113. doi: 10.1097/00002030-199201000-00015. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, et al. Auditory working memory in HIV-1 infection. Journal of the International Neuropsychological Society. 2001;7:20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, DeTeresa R, Wiley CA. Patterns of neurodegeneration in HIV encephalitis. Journal of NeuroAIDS. 1996;1:161–173. doi: 10.1300/j128v01n01_08. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, et al. Neuropsychological performance in HIV-1-infected homosexual men: The Multicenter AIDS Cohort Study (MACS) Neurology. 1990;40:197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. Journal of Psychopharmacology. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Annals of Neurology. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and Biobehavioral Reviews. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Peavy G, Jacobs D, Salmon DP, Butters N, Delis DC, Taylor M, et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. Journal of Clinical and Experimental Neuropsychology. 1994;16:508–523. doi: 10.1080/01688639408402662. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso MJ, Myers C, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Research. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Reyes E, Mohar A, Mallory M, Miller A, Masliah E. Hippocampal involvement associated with human immunodeficiency virus encephalitis in Mexico. Archives of Pathology and Laboratory Medicine. 1994;118:1130–1134. [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The "file drawer problem" and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. Journal of Nuclear Medicine. 1996;37:1133–1141. [PubMed] [Google Scholar]
- Sadek JR, Johnson SA, White DA, Salmon DP, Taylor KI, Delapena JH, et al. Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer's disease, and Huntington's disease. Neuropsychology. 2004;18:692–699. doi: 10.1037/0894-4105.18.4.692. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinions in Neurobiology. 1995;5:184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Sarazin M, Deweer B, Merkl A, Von PN, Pillon B, Dubois B. Procedural learning and striatofrontal dysfunction in Parkinson's disease. Movement Disorders. 2002;17:265–273. doi: 10.1002/mds.10018. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Manner H, Kaufmann R, Schmolck H. Cognitive procedural learning in patients with fronto-striatal lesions. Learning and Memory. 2002;9:419–429. doi: 10.1101/lm.47202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Onlaor S, Gluck MA. Role of the basal ganglia in category learning: how do patients with Parkinson's disease learn? Behavioral Neuroscience. 2004;118:676–686. doi: 10.1037/0735-7044.118.4.676. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory. Palo Alto CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychology Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, et al. Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacology Bulletin. 1995;31:425–433. [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, et al. HIV Neurobehavioral Research Center Group. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Archives of Neurology. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Alhassoon OM, Schweinsburg BC, Videen JS, Grant I. MR spectroscopy in HIV and stimulant dependence HNRC Group. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 2000;6:83–85. doi: 10.1017/s1355617700611104. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, et al. Cerebral metabolic dysfunction in AIDS: findings in a sample with and without dementia. Journal of Neuropsychiatry and Clinical Neuroscience. 1992;4:280–287. doi: 10.1176/jnp.4.3.280. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, et al. Declarative and procedural memory functioning in abstinent cocaine abusers. Archives of General Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Archives of Neurology. 2000;57:1601–1607. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learning and Memory. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, et al. Distribution of brain HIV load in AIDS. Brain Pathology. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Review Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]