Abstract
Semaphorins are a large family of secreted, transmembrane and GPI-linked proteins initially characterized in the development of the nervous system and axonal guidance. Semaphorins are expressed in many tissues where they regulate normal development, organ morphogenesis, immunity and angiogenesis. They affect the cytoskeleton, actin filament organization, microtubules and cell adhesion. Semaphorin signaling is transduced by plexins, which in the case of most class-3 semaphorins requires high affinity neuropilin receptors. The neuropilins also function as receptors for VEGF and other growth factors, and their expression is often abnormal in tumors. In cancer, semaphorins have both tumor suppressor and tumor promoting functions. We review here the current status of semaphorins and their receptors in tumor development with a focus on lung cancer.
Keywords: semaphorin, neuropilin, plexin, VEGF, angiogenesis, lung cancer
1. Introduction
Shortly after semaphorins were identified as axon guidance molecules, their role in cancer development began to be understood. Knowledge about their receptors, signal transduction and functional roles has significantly evolved. Today, it is clear that several semaphorins and their receptors (neuropilins and plexins) participate in vascular development, angiogenesis, and cancer. Neuropilins, which are high-affinity receptors for class-3 semaphorins, also function as co-receptors for VEGF and other growth factors, and their expression is often abnormal in cancer. We review here the status of semaphorins and their receptors in signaling and tumor development with a focus on lung cancer. Attention will be placed on semaphorin-VEGF antagonism and semaphorin signaling as these molecular events likely account for the ability of specific semaphorins to affect tumorigenesis. Lastly, we discuss the therapeutic potential of this pathway in cancer.
2. Lung cancer overview
Lung cancer ranks among the most common malignant diseases and currently is the leading cause of cancer-related death worldwide. In the United States, lung cancer is the most common cause of cancer-related deaths with an incidence approximating 70 per 100,000 individuals. In 2003, an estimated 171,900 Americans were diagnosed with lung cancer and approximately 152,200 succumbed to this disease. In the European Union, lung cancer accounts for one-third of all cancer-related deaths. In addition, lung cancer is rapidly emerging as a major cause of mortality in Asia and death rates are expected to substantially increase over the next decades. Unfortunately, the overall 5-year survival of patients is less than 15 % [1].
The major risk factor for lung cancer is tobacco exposure (90% of cases diagnosed). Exposure to other environmental respiratory carcinogens, such as asbestos, benzene, coal tar, and other industrial chemicals may interact with tobacco smoke to increase risk. The effect of low-energy-transfer radiation appears variable. Epidemiologic data suggest that lung cancer risk is associated with urbanization, and vehicle density is an excellent predictor of cancer mortality. It is assumed that 1% to 2% of lung cancers are directly attributable to air pollution. Although considerable attention has been given to household radon gas exposure, the risk remains controversial. In addition, several genetic and non-genetic defects are associated with this disease and have identified familial clusters and populations at increased risk for lung cancer.
Lung cancers are divided into 2 main classes depending on their histologic appearance and presumed cellular origin. Small Cell Lung Cancers (SCLCs) are of neuroendocrine origin, while Non-Small Cell Lung Cancers (NSCLC) are predominantly epithelial. NSCLCs include adenocarcinoma (now the dominant cell type), bronchioalveolar (BAC), squamous and large-cell carcinoma (which has neuroendocrine features).
The distinction between SCLC and NSCLC has a major therapeutic implication. While surgical resection remains the standard of care for localized NSCLC, with ongoing international attempts to improve early diagnosis, SCLC is not routinely treated with surgery even when the disease appears localized to the chest. This is because nearly all patients subsequently relapse with metastatic disease. For patients with metastatic disease and either NSCLC or SCLC, combination chemotherapy is the mainstay of treatment, with radiation used for local problematic areas such as a brain lesion or bone metastasis that is painful or prone to fracture. For SCLC, a platinum-based combination (doublet) such as cis-platinum plus etoposide would be considered standard of care - see National Comprehensive Cancer Network (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp). For advanced NSCLC, a platinum-based doublet is standard of care with bevacizumab suggested for patients without squamous histology, brain metastasis or those on anti-coagulants, all of which are associated with an unacceptable risk of fatal hemorrhage. Following disease progression, patients generally receive either a taxane (e.g., Docetaxol, which acts by stabilizing microtubules), Pemetrexed (an antifolate that specifically inhibits thymidylate synthase), or an EGFR inhibitor (Erlotinib/Tarceva). Of potential interest, class-3 semaphorins impair microtubule assembly. Whether there might be antagonism between the semaphorin and taxane effects has not been investigated. The use of EGFR inhibitors is particularly relevant in adenocarcinomas and bronchioloalveolar carcinomas, which have an increased frequency of EGFR activating mutations or increased copy number [2, 3].
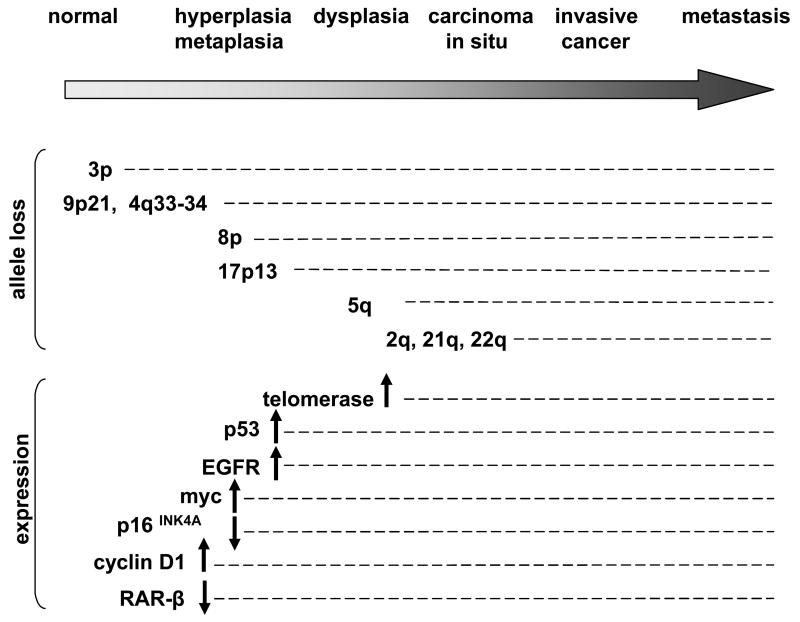
A number of genetic and epigenetic molecular lesions are necessary to transform normal bronchial epithelial cells to overt lung cancer. SCLCs and NSCLCs present distinct cytogenetic or expression profiles, as depicted in Table 1, adapted from [4]. These include mutations in oncogenes and tumor suppressor genes (TSG), aberrant DNA methylation, overexpression of growth factors and growth factors receptors, association with metabolic phenotypes affecting P450 family genes, Glutathione S-transferase genes (GSTM1), Aryl hydrocarbon hydroxylase (AHH), NAD(P)H:Quinone Oxidoreductase (NQO1), genetic variation in metabolism of carcinogens, DNA damage or repair and genomic instability. Sequential events are depicted in Figure 1 and 3p allele loss is currently the earliest known change, suggesting that either a 3p TSG may act as a “gatekeeper” for lung cancer or that 3p alterations are a particularly sensitive marker of genomic instability. Interestingly, several genes involved as guidance cues are localized in 3p (Table 2), i.e. semaphorins (SEMA3B [5], SEMA3F [6, 7], SEMA3G), plexin B1 (a semaphorin receptor), and Robo1 (a SLIT receptor) [8]. Because these guidance molecules act in general to regulate cell migration, adhesion (and invasion of cancer cells), we suspect they are involved in mechanisms of disease progression in lung cancer development.
Table 1.
Major genetic characteristics of small (SCLC) and non-small cell (NSCLC) lung cancers.
| SCLC | NSCLC | |
|---|---|---|
| frequency in lung cancer patients | 20% | 80% |
| neuroendocrine properties | 100% | rare |
| FHIT deletion/mutations | 80% | 40% |
| p53 deletion/mutations | 85% | 50% |
| p16 deletion/mutations | rare | 60% |
| Rb deletion/mutations | 90% | 20% |
| LKB1 mutations | rare | 30% |
| PTEN mutations | 15–20% | infrequent |
| E-cadherin loss | ? | 10% |
| K-Ras activating mutations | rare | 20% |
| c-myc overexpression | 20% | rare |
| EGFR1 overexpression | rare | 60% |
| HER2/Neu overexpression | rare | 20% |
| Bcl-2 overexpression | 85% | 20% |
| autocrine loop | GRP-GRP receptor, SCF-Kit | HGF-Met, TGF-α |
| PI3K activation | + | + |
| microsatellite instability | 35% | 20% |
| telomerase activity | 100% | 80% |
| frequent allelic loss | 4p, 4q, 5q, 8p, 10q, 13q, 17p, 22q | 6q, 8p, 9p, 13p, 17p, 19q |
| 3p deletion | 100% | 80% |
| 3q amplification | + | + |
Fig. 1. Sequential events in lung cancer studied in squamous cell lung carcinoma.
Not every change is necessary and the timing may be subjected to variation. Adapted from [4].
Table 2.
Chromosomal localization of human semaphorin, neuropilin, and plexin genes.
| semaphorin |
|||||
|---|---|---|---|---|---|
| class 3 | class 4 | class 5 | class 6 | class 7 | |
| A | 7p12.1 | 1q22 | 5p15.2 | 5q23.1 | 15q22.3-q23 |
| B | 3p21.3 | 15q25 | 3q21.1 | 19p13.3 | |
| C | 7q21-q31 | 2q11.2 | 1q21.2 | ||
| D | 7q21.11 | 9q22-q31 | 15q21.1 | ||
| E | 7q21.11 | ? | |||
| F | 3p21.3 | 2p13.1 | |||
| G | 3p21.1 | 10q24.31 | |||
|
plexin |
neuropilin |
||||
| class A | class B | class C | class D | ||
|
|
|||||
| 1 | 3q21.2 | 3p21.31 | 12q23.3 | 3q21.3 | 10p12 |
| 2 | 1q32.2 | 22q13.33 | 2q33.3 | ||
| 3 | Xq28 | Xq28 | |||
| 4 | 7q32.3 | ||||
3. The semaphorin family
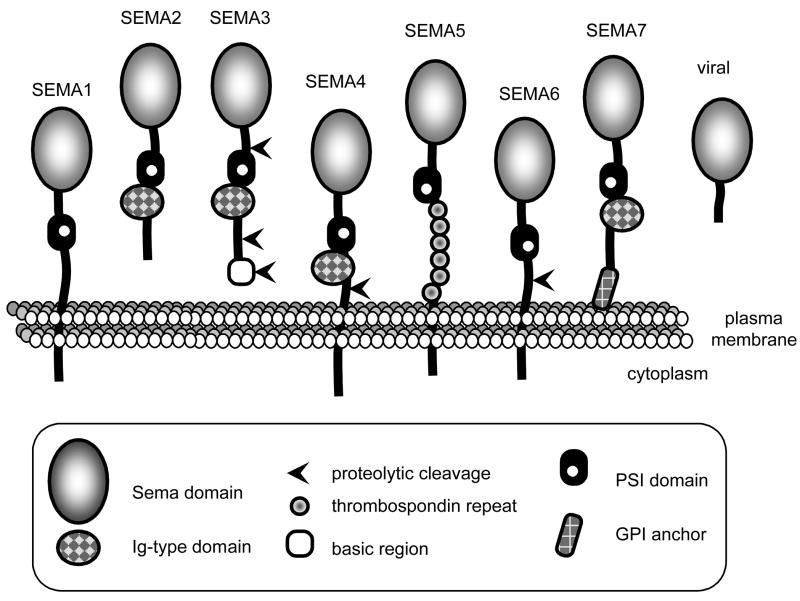
Semaphorins, initially named collapsins, belong to a large family of about 30 proteins found in multi-cellular organisms ranging from worms to flies, fish and mammals. They are not present in unicellular eukaryotes or procaryotes, although do occur in a few viruses. Semaphorins have been divided into eight classes based on structural features, with classes 3 to 7 representing the vertebrate proteins (Fig. 2; for recent reviews see [9, 10]). The hallmark of semaphorins is the sema domain, an approximately 500 amino acid, highly disulfide-linked, segment. This domain is shared with receptors of the plexin and Met/Ron families [11]. Other recurrent domains are the PSI domain (“plexin-semaphorin-integrin”) and an immunoglobulin-type domain. In contrast, semaphorins diverge in their C termini, and can be either transmembranous, anchored to the plasma membrane or exclusively secreted. Proteolytic processing converts membrane-associated semaphorins to diffusible forms [12, 13], and their localization to the extracellular matrix might be additionally controlled by proteoglycan binding [14, 15]. Class-3 semaphorins (SEMA3s) can be further processed by furin-like cleavage with large effects on biological activity. Sema3A cleavage is essential but not sufficient for the acquisition of high repulsive activity for neuritis [16] and furin-dependent cleavage of Sema3E promotes induction of invasive growth and lung metastasis in vivo, and stimulates growth and motility in vitro [17]. This complexity has often been ignored in most studies.
Fig. 2. The semaphorin family structure.
Semaphorins are either secreted, membranous or associated to the membrane. GPI: glycosyl phosphatidyl inositol. Ig: immunoglobulin-like domain. PSI: plexin-semaphorin-integrin.
4. Semaphorin receptors and signaling
4.1 A multiple receptor-ligand system
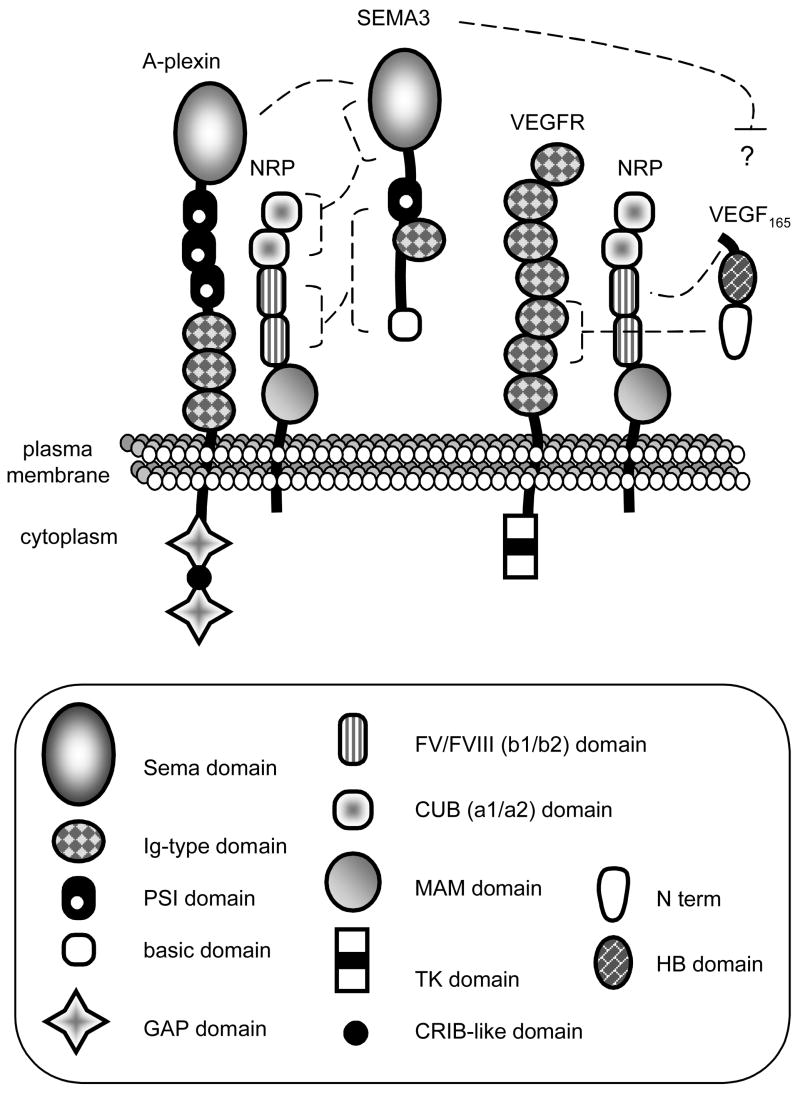
Several receptors function to transduce semaphorin stimuli (Table 3). For brevity, atypical receptors of the immune system are not presented (for reviews see [18–20]). Most semaphorins directly bind to plexins which elicit intracellular signals [21]. However, secreted SEMA3s are recruited to plexins by neuropilin (NRP) receptors [22, 23] (Fig. 3). Two recent reviews describe NRP structure and function [24, 25]. Due to their short cytoplasmic segment, neuropilins generally serve as binding subunits. However, their three C-terminal amino acids (SEA) are essential for endothelial cell migration [26]. NRP1 preferentially binds SEMA3A, SEMA3B and SEMA3E, whereas NRP2 has higher affinity for SEMA3F and SEMA3G [9]. The sema domain of SEMA3s binds the NRP a1a2 domain, while the PSI/Ig/basic domains bind the NRP b1b2 region [27]. Alternatively, some semaphorins and plexins may interact directly through their sema domains [28] and SEMA7A has been reported to directly bind to beta-1 integrins [29].
Table 3.
Semaphorin receptor complexes. Semaphorins can interact with complexes that include NRPs, plexins, various membrane proteins such as growth factor receptors and cell adhesion molecules (CAMs) which themselves interact. Asterisk: Sema3E is an exception, it cand bind D1-plexin directly.
| receptor complex |
|||
|---|---|---|---|
| ligand | transducing unit | binding unit | additional unit |
| SEMA3 * | plexin A | NRP | L1/Nr-CAM, integrin |
| SEMA4 | plexin B + Met/ErbB2 | plexin B | CLCP1 |
| SEMA5 | plexin B | plexin B | |
| SEMA6 | plexin A | plexin A | VEGFR/Off-Track |
| SEMA7 | plexin C, integrin | plexin C, integrin | |
|
| |||
| VEGF | VEGFR | VEGFR, NRP | |
| HGF | Met | NRP, Met | |
| Galectin | ? | NRP | |
| TGFβ | ? | NRP | |
Fig. 3. Receptors of class-3 semaphorins and VEGF.
Left: binding of SEMA3 to NRP and A-plexin. The sema domain of SEMA3 binds the a domains (CUB) of NRP. The PSI/Ig/basic region binds the b1b2 domain. Right: binding of VEGF165 to NRP and VEGFR2. The HB domain of VEGF165 binds the b1 domain of NRP and the N terminus binds the 2d and 3rd Ig domains of VEGFR2. VEGFR2 has a tyrosine kinase activity, but not plexins. CRIB: cdc42/rac interactive binding; GAP: GTPase activating protein; HB: heparin binding; MAM: meprin/A5 antigen/tyrosine protein phosphatase μ; N term: exons 1 to 5; TK: tyrosine kinase.
Neuropilins, plexins and semaphorins form complexes with other membrane-associated proteins including receptors and cell adhesion molecules. Neuropilins are co-receptors for vascular endothelial growth factor VEGF-A, VEGF-B, VEGF-C, VEGF-D and the viral ORFV2-VEGF (Fig. 3) [30–34] as they form complexes with VEGFR1 (Flt1), VEGFR2 (KDR) and VEGFR3 [24, 35]. Multimerization of NRPs and VEGFRs is probably mediated by VEGF binding to both receptors [33, 36]. In this complex, NRPs may increase VEGF affinity toward VEGFRs [30], although this issue is still under investigation [37].
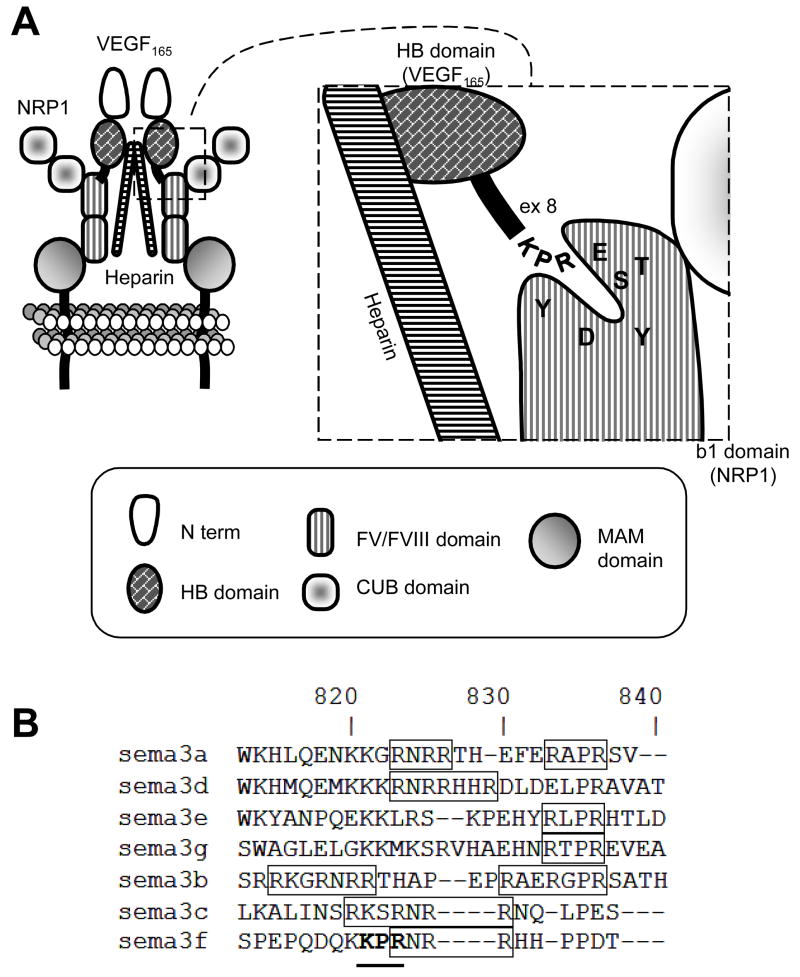
Multiple VEGF-A isoforms are generated by alternative splicing with VEGF165 being the best studied [35]. Originally, only the heparin binding forms (e.g. with exons 6–7) of VEGF-A were believed to bind NRPs [30]. However, it was recently shown that VEGF121 that does not contain exons 6–7, also binds NRPs, although it does not promote NRP-VEGFR interaction [36, 38, 39]. VEGF-A binding to NRP might be due to the exon 8 C-terminus KPR motif, which inserts into a negative cleft in the NRP b1 domain (Fig. 4A) [40, 41]. Consistent with this, a variant of VEGF lacking exon 8, VEGF165b, acts as an endogenous inhibitor of angiogenesis [42]. Because the carboxyl tip of SEMA3s is basic, it was suspected that it might also bind the NRP b1 domain [43], which would support the hypothesis that SEMA3s are competitive inhibitors of VEGF binding to NRPs [44]. Interestingly, among class-3 semaphorins, SEMA3F possesses a KPR motif that overlaps a furin cleavage site (Fig. 4B). We can only speculate that this motif might be involved in SEMA3F binding to NRP b1 groove and that furin cleavage would regulate this binding. Although a recent structural report indicated that VEGF and SEMA3s may have distinct binding sites on NRPs [45], this study did not address the proteolytically processed forms of SEMA3s.
Fig. 4. VEGF binding to neuropilins.
(A) A model of VEGF binding to NRPs with involvement of heparin. The KPR motif interacts with amino acids of the NRP b1 domain groove. (B) The amino acid composition of SEMA3s basic C-ter domain. SEMA3s have different amino acids motifs in the basic domain: only SEMA3F possesses the KPR (bold) that overlaps the RxxR furin cleavage motif (squared).
Other NRP ligands have been described including placenta growth factor 2 (PlGF-2) [46], fibroblast growth factors (FGF) [47], galectin [48], hepatocyte growth factor (HGF) [49–51] and TGF-β [52]. In addition, NRP1 interacts with c-Met [50]. There is further complexity in semaphorin receptor complexes. For instance, SEMA3E binds D1-plexin independently of NRPs [53]. More recent work indicates that NRP1 may participate in the SEMA3E-receptor complex, but when so switches its effects from repulsion to attraction [54]. Similar switches have been reported for Sema3A when the cell adhesion molecule L1-CAM is associated with NRP1 [55], and for Sema3B and Sema3F when Nr-CAM associates with NRP2 [56]. Also, CLCP1 (a NRP-related protein) is a receptor for the transmembrane SEMA4B [57]. It is thus possible that NRPs are part of larger complexes containing multiple components such as receptors and adhesion molecules. NRPs contribute to multiple relevant growth factor receptors. Whether semaphorins and other growth factors compete or have distinct binding sites is important from a potential therapeutic standpoint (see below). The interactions of semaphorins with the HGF and EGF pathways are particularly interesting with regard to lung cancer, given that both pathways are altered in this pathology.
4.2 Signaling from plexins
It is important to understand the molecular events mediated by plexins, the canonical semaphorin receptors, since these are likely responsible for the role of semaphorins in tumor development.
Sema3A and Sema3F inhibit cell attachment and cell migration (for review see [58, 59]. At least in part, this results from signaling changes that affect the activation or stabilization of surface integrins. Depending on the semaphorin, plexin, and the cellular context, distinct molecular cascades can be triggered with the recurrent participation of small GTPases.
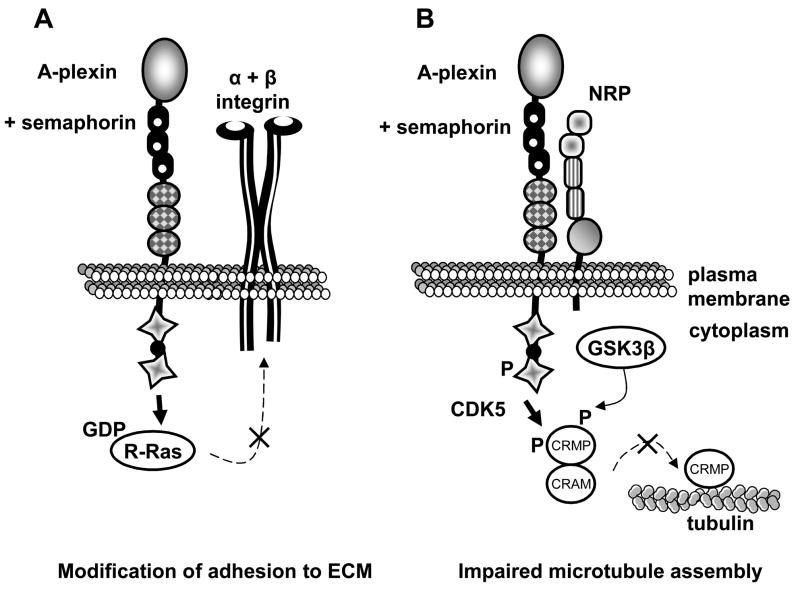
One pathway, thought common to all plexins involves FARP2, R-Ras and talin in which semaphorin binding leads to a conformational change in integrins (Fig. 5A) [60, 61]. Indeed, Sema3A and Sema3F cause downregulation of activated integrins due in part to inhibition of R-Ras. Integrin inhibition by SEMA3A and 3F could explain both endothelial cell and tumor cell migration blockade, leading to reduced tumor angiogenesis and metastasis.
Fig. 5. Intracellular signaling by A-plexin upon semaphorin stimulus.
(A) The FARP2/R-Ras/talin pathway. When plexins are inactive, the Rac GEF FARP2 is bound to a KRK motif on their cytoplasmic region. After semaphorin binding, FARP2 is released from plexins and transiently activates Rac1. In turn, Rac1 activates plexins allowing the interaction with Rnd1. Rnd1 recruits R-Ras which is inhibited by the GAP activity of plexins. Also, free FARP2 binds and sequesters PIPKI (PIP Kinase Isoform) which is required in the final step of integrin activation by talin binding. These mechanisms result in a lack of integrin activation and stabilization in focal points. Note that this mechanism is thought to be common to all types of plexins. Thus neuropilin participation might be variable (not represented). (B) The Fes/CRMP2 pathway. When stimulated by a class-3 semaphorin, A-plexin interacting kinases Fes and Fyn phosphorylate CDK5, which subsequently phosphorylates CRMP2. CRMP2 priming phosphorylation enables an additional phosphorylation of CRMP2 by GSK3β. This results in the inability of CRMP2 to interact with tubulin and to promote microtubules assembly, suggesting a role in cell migration.
The capacity of semaphorins to affect cell migration appears to extend beyond integrins. In the Fer/CRMP2 (collapsin response mediator protein) pathway, activation of type A-plexins by SEMA3s induces phosphorylation of CRMP2 [62] which hinders its tubulin binding abilities and interferes with cell migration (Fig. 5B). To our knowledge, this pathway has not been studied in tumor cells.
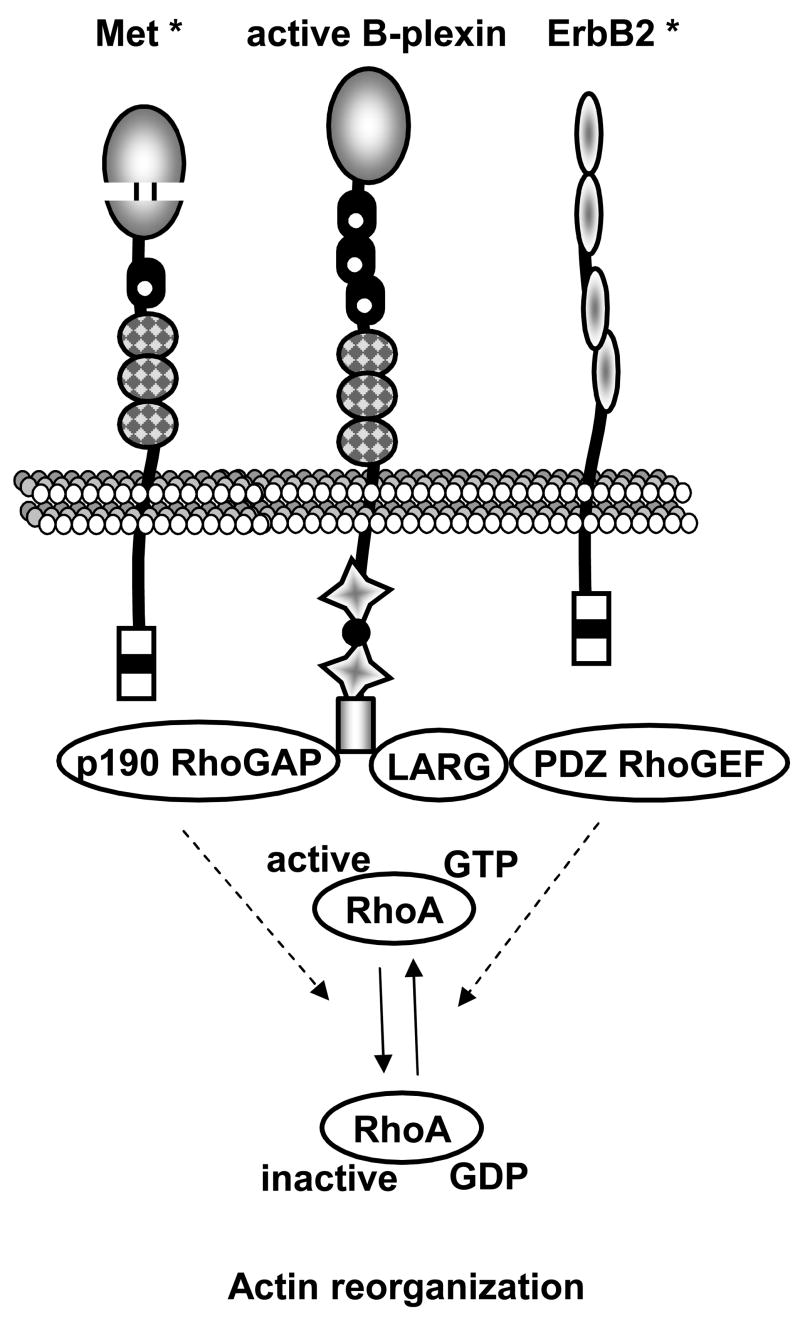
Type B-plexins also regulate the cytoskeleton organization in a tumor context (Fig. 6). B-Plexin stimulation by SEMA4D can inhibit cell migration in the presence of MET by deactivating RhoA, while the presence of ErbB2 results in opposite effect [63]. Of note, p190RhoGAP can also bind A-plexins [64]. Thus, semaphorins exhibit diverse roles in controlling cell migration through the converging processes of regulating integrin activation and reshaping the cytoskeleton. Therefore, the regulation of migration of endothelial cells and cancer cells by semaphorins might be essential in tumor progression.
Fig. 6. Regulation of actin dynamics by B-plexins.
B-plexins have an additional domain at their cytoplasmic tip that is responsible for interaction with PDZ domain-containing proteins. Stimulation by SEMA4D induces the Rnd1-dependent interaction of Rho regulators such as the GEF LARG and PDZ-RhoGEF, or the GAP p190-RhoGAP, with the PDZ domain of plexins. The association of the plexin with Met would inhibit RhoA by using p190-RhoGAP, and the association of plexin with ErbB2 would promote RhoA activity by using LARG and PDZ-RhoGEF. Asterisks: the domains of these proteins are not detailed here. Note the presence of a sema-like domain in Met. PDZ: PSD-95, DLG, ZO-1.
5. Semaphorins and Neuropilins at the heart of vascular development and angiogenesis
As VEGF receptors, NRPs are essential elements in cardiovascular development and tumor angiogenesis (for review see [24]). Both NRP1 and NRP2 are detected in human umbilical vein endothelial cells (HUVECs). In mice, NRP2 is restricted to veins and lymphatic vessels, while NRP1 is found in arteries and capillaries [65]. NRP1 overexpression in mice results in disproportionate blood vessels and heart defects [66]. In contrast, deficiency of NRP1 induces severe disorganization of vascular networks and agenesis [67], and absence of NRP2 leads to reduction of lymphatic vessels [68]. Targeting both NRPs results in greater vascular defects and death [69].
It was initially suspected that SEMA3s may control angiogenesis by competing for binding to NRP with VEGF. During vascular development, SEMA3s repulse vessels between somites in which they are expressed. In zebrafish, Sema3a1 and 2 function with D1-plexin [70, 71]. However, increasing data indicate that the effects of semaphorins and VEGF may be independent. For example, the use of a NRP1 engineered to bind solely VEGF, and not SEMA3s, has revealed that SEMA3s have little effect on VEGF/NRP1-driven vascular development [72]. Moreover, Sema3A and Sema3F-induced ERK1/2 inhibition are unrelated to the ability of VEGF to induce VEGFR2 phosphorylation [73].Thus, a major consequence of SEMA3s in angiogenesis may derive from their ability to inhibit integrin activation [74]. In contrast, Sema3C enhances adhesion of endothelial cells (ECs) in vitro [75]. Thus, as often occurs with semaphorins, regulation appears to be context-dependent.
The capacity of semaphorins to regulate vessel patterning is particularly relevant to cancer since tumor neoangiogenesis is a requisite for tumor growth and metastasis. In in vitro and xenograft experiments, Sema4D was shown to promote experimental angiogenesis by B1-plexin-expressing endothelial cells, both in the presence or absence of Met [76]. The regulation of angiogenesis by SEMA4D was subsequently confirmed in human head and neck cancer tissues [77]. In tumor cells, Sema4D was shown to be a novel target of MT1-MMP matrix-metalloproteinase, which processes and releases soluble Sema4D thereby inducing endothelial cell chemotaxis in vitro and tumor-angiogenesis in vivo [76]. In contrast, the SEMA6A extracellular domain inhibits VEGF165-mediated xenograft vascularization [78]. Similarly, SEMA3F impairs angiogenesis in vivo in various xenograft tumor models [79–82].
6. Semaphorins and their receptors in normal lung
Like vessels and nerves, the lung develops by successive branching, generating a complex architecture ending in bronchioles. Correct development requires cell migration and proliferation, with high coordination between epithelial and mesenchymal cells. Notably, EGF and FGF positively regulate proliferation. The lung is also highly vascularized, and semaphorins and their receptors have been detected in lung tissues. NRP1, NRP2 and A1-plexin are temporally and spatially regulated during mouse lung development, together with Sema3A, 3C and 3F [22, 23, 27, 83, 84]. In mouse lung explants grown ex vivo, Sema3A inhibits branching whereas Sema3C and Sema3F promote it [83, 84]. Localized in the mesenchyme, Sema3A may maintain a low arborescence, allowing the principal branches to form. Later in development, Sema3A expression decreases while NRP1 levels rise, suggesting a switch in NRP1 function from Sema3A receptor to VEGF receptor. In the epithelium, Sema3C and Sema3F might influence the formation of terminal buds. In adult human lungs, SEMA3F mRNA is expressed [5–7] and the protein is found at the membrane of type-II pneumocytes and in endothelial cells of large vessels [85]. In contrast, SEMA3B was barely detected by northern-blot in adult normal lung [5].
7. Semaphorins in lung cancer
7.1 SEMA3B and SEMA3F
In lung cancer, one of the earliest and common genetic change is chromosome 3p deletion. The first hypothesis implicating semaphorins in lung cancer came from the cloning of two semaphorin genes, SEMA3B and SEMA3F, from a 3p21.3 homozygous deletion region in SCLC cell lines (Table 1) [5–7]. In human lung and breast cancers, this region undergoes frequent loss of heterozygosity (LOH) and both SEMA3F and SEMA3B transcripts are underrepresented in squamous cell carcinomas [86].
It was shown that SEMA3B transfection reduces the growth of lung cancer cells in vitro and in subcutaneous mouse models [87, 88]. In vitro, SEMA3B induces apoptosis of lung and breast cancer cells, while VEGF has opposite effects [89]. In addition, SEMA3B was lost in a metastatic variant of the NSCLC cell line, NCI-H460, compared to the less metastatic parental cell line [90]. No inactivating mutation has been detected, but a single nucleotide alteration (T415I) leads to a reduced ability to suppress tumorigenesis in vitro [87]. Curiously, the T415I substitution is associated with a reduced risk of lung cancer in latino-americans in the USA [91]. Moreover, SEMA3B downregulation is sustained by gene hypermethylation in lung cancer cell lines [87, 92, 93]. Also, SEMA3B is the target of the tumor suppressor, p53 [94], suggesting it could be activated during DNA damage or other stress responses. However, Rolny et al, [95] recently found in xenograft tumor models with MDA-MB435 melanoma and A549 lung cancer cells that SEMA3B inhibited tumor growth while simultaneously and unexpectedly triggering metastasis by activating the p38 MAPK. Although p38 activated p21, a cell cycle inhibitor, it also induced IL-8 cytokine secretion from tumor cells. The consequence was macrophage infiltration which is thought to spur metastasis by producing soluble factors such as VEGF.
The second semaphorin gene mapped in 3p21.3, SEMA3F (previously SEMAIV, [6, 96] has been studied for its in vivo tumor suppressor function. The first evidence came from transfection of a mouse tumor cell line by a 80 Kb genomic clone containing SEMA3F (and additional sequences) that suppressed tumor growth [97]. In human lung cancer, while SEMA3F is expressed in normal lung, the protein is lost or delocalized in the cytoplasm of tumor cells [85]. Moreover, its loss correlates inversely with the grade and stage of lung cancer, and also with the tumor surface staining of VEGF165 [85, 98]. Similarly in ovarian cancer, an elevated VEGF/SEMA3 ratio is a poor prognostic feature [99].
SEMA3F potently inhibits tumorigenesis in several xenograft cancer models induced by ovarian cancer [100], melanoma [79], lung cancer [81, 82, 101], murine fibrosarcoma [100] and HEK293 cells [80]. One recurrent observation has been that tumors formed by SEMA3F-expressing cells display reduced vascularization [79–82]. In vitro, SEMA3F secretion by transfected tumor cells repels endothelial cells (ECs) [79]. SEMA3F also inhibits VEGF and FGF-induced ERK1/2 activation and EC proliferation [80]. Similarly, SEMA3F repels breast cancer cells [102] and has an antagonistic effect on breast cancer cells spreading induced by VEGF [103].
Consistent with the inhibition of integrin by plexin signaling, reduced beta-1 or beta-3 integrin activation was found in both melanoma cells and H157 lung cancer cells, along with reduced adhesion to fibronectin and vitronectin [79, 82, 101]. Additional signaling changes in H157 lung cancer cells included loss of activated ERK1/2, AKT and STAT3 with downstream inhibition of HIF1α translation and VEGF mRNA expression [82, 101]. Mechanistically, SEMA3F inhibited the activity of integrin-linked kinase (ILK) although this appeared to account only for the loss of phospho-ERK1/2. Thus, SEMA3F has emerged as a potent tumor suppressor and antagonist of VEGF-driven tumor neovascularization. Interestingly, reduction of VEGF transcription was also observed after treatment of myeloma cells by Sema3A, suggesting that this effect might not be restricted to SEMA3F [104].
To date, no inactivating mutation of SEMA3F has been reported, although its promoter is the target of DNA hypermethylation in lung and breast cancer cell lines. Treatment with a histone deacetylase inhibitor alone can restore SEMA3F expression [105]. SEMA3F can be turned off by direct binding of the transcriptional repressor Zeb-1 (J. Clarhaut, personal communication), a factor involved in the epithelial-mesenchymal transition in lung cancer cells and loss of E-cadherin [106]. Like SEMA3B, SEMA3F is also a transcriptional target of p53 in lung cancer cells [81].
7.2 SEMA6A
There are few data concerning SEMA6A in lung cancer, except that SEMA6A is located in 5q23.1 (Table 2), a region that can be deleted in lung cancer [107]. Interestingly, SEMA6A is moderately expressed in the lung, but not expressed in the lung adenocarcinoma cell line A549 [78, 108]. In kidney cancers, SEMA6A is more expressed in tumor tissues than in adjacent normal tissues and might contribute to tumor angiogenesis. However, treatment of ECs with the extracellular domain (ECD) of SEMA6A reduces VEGF-promoted migration in vitro, and inhibited ERK1/2, Src and focal adhesion kinase (FAK) phosphorylation [78]. This was independent of VEGFR2 phosphorylation status, and SEMA6A-ECD did not bind NRP1/2. In vivo, the SEMA6A-ECD inhibited tumor formation induced by 786-0 kidney cancer cells and VEGF-induced xenograft vascularization.
7.3 Other semaphorins
In lung adenocarcinoma, SEMA3C was reported to be upregulated in metastatic cells [109]. SEMA4B has been described as a migration and metastasis-promoting factor in the H460 NSCLC cell line [57] and can bind a homolog of NRPs, CLCP1, which was overexpressed in a metastatic variant of H460 cells [110]. SEMA4D, and its receptor B1-plexin, are highly expressed in head and neck tumors and lung carcinomas [77]. Although B1-plexin expression was detected in non-malignant bronchial epithelium of adult lungs, SEMA4D was not [111]. When released from the tumor cell membrane by a protease, SEMA4D stimulates EC chemotaxis and may therefore promote tumor angiogenesis [77]. Semaphorins implicated in other cancers are listed in Table 4.
Table 4.
Semaphorin involvment in diverse cancers. The very recent finding that SEMA3B inhibits tumor growth but fosters a prometastatic environment [95] adds complexity in the classification of SEMA3B as a pro- or anti-tumor semaphorin. exp., experiments
| SEMA | human tumor | in vitro exp. | in vivo exp. | effect | reference | |
|---|---|---|---|---|---|---|
| pro-tumoral | 3A | x | ≠tumor cell lines | x | inhibits the immune response | 135 |
| pancreas | x | x | correlates with shorter survival | 136 | ||
| 3B | glioblastoma | x | x | co-marker of poorer survival | 137 | |
| 3C | x | ovarian cancer cells | x | ovarian cancer drug resistance | 138 | |
| ovary | x | x | correlates with shorter survival | 139 | ||
| x | prostate cancer cells | x | invasive cellular growth | 140 | ||
| x | lung cancer cells | x | overexpression in metastatic cells | 109 | ||
| 3E | breast | breast cancer cells | breast cancer cells | pro-metastatic | 17,141 | |
| 4B | x | lung cancer cells | x | pro-migratory | 57 | |
| 4D | leukemia | x | x | pro-proliferative | 142 | |
| x | leukemia cells | x | anti-apoptotic | 143 | ||
| head and neck | h. and n. cancer cells | h. and n. cancer cells | pro-angiogenic | 13,77 | ||
| x | breast cancer cells | x | invasive growth and pro-migratory | 144 | ||
| 5A | prostate | x | x | correlates with aggressiveness | 121 | |
| 5B | kidney | x | x | increased expression in tumors | 145 | |
| 5c | x | x | drosophila model | pro-metastatic | 146 | |
| 6A | kidney | renal cancer cells | renal cancer cells | correlates with tumor vascularization | 78 | |
| 6D | stomach | x | x | increased expression in tumors | 147 | |
| anti-tumoral | 3A | x | breast cancer cells | x | anti-migratory | 148 |
| x | mesothelioma cells | x | negative retrocontrol of VEGF | 149 | ||
| myeloma | myeloma cells | x | negative retrocontrol | 104 | ||
| x | prostate cancer cells | x | anti-invasive in vitro | 140 | ||
| 3B | lung | x | x | gene deleted in tumors | 5 | |
| x | lung cancer cells | x | in vitro tumoral suppression | 87 | ||
| x | lung cancer cells | x | lower expression in metastatic cells | 90 | ||
| lung | x | x | lower expression in tumors | 86 | ||
| x | lung cancer cells | x | pro-apoptotic | 89 | ||
| x | ovarian cancer cells | ovarian cancer cells | in vivo tumoral suppression | 88 | ||
| 3F | lung | x | x | gene deleted in tumors | 6,7 | |
| lung | ≠ cell lines | x | early expression loss | 85,98 | ||
| lung | x | x | lower expression in tumors | 85,98 | ||
| x | lung cancer cells | lung cancer cells | anti-angiogenic + tumoral suppression | 81,82,101 | ||
| x | melanoma cells | melanoma cells | anti-metastatic | 79 | ||
| x | HEK293 cells | HEK293 cells | anti-angiogenic | 80 | ||
| x | x | murine fibrosarcoma cells | in vivo tumoral suppression | 100 | ||
| x | breast cancer cells | x | anti-migratory | 102,103 | ||
| 4B | B-cell lymphoma | x | x | expression loss | 150 | |
| 4D | x | breast cancer cells | x | anti-migratory | 63 |
8. Neuropilins and other semaphorin-related proteins in lung cancer
The importance of NRPs in cancer has been presented in recent excellent reviews [24, 25, 35, 112–114]. In brief, NRPs are frequently overexpressed and often associated with poor prognosis or advanced disease. In ECs, NRP1 and VEGFR2 stimulate PI3K activation [115]. Additionally, VEGF appears to be an autocrine survival factor for NRP-positive tumor cells [116, 117]. In lung cancer, high levels of NRP1 were correlated with shorter disease-free and overall survival [118]. In another study, co-expression of NRP1 and NRP2 were associated with increased tumor vascularization and poor prognosis [119]. Compared to benign bronchial hyperplasia and squamous metaplasia, progressive upregulation of NRP levels was observed in pre-malignant lesions ranging from dysplasia to microinvasive carcinoma [98].
In lung cancer, loss of the semaphorin effector, CRMP1, has been associated with a more aggressive phenotype [120]. Recent evidence indicates that plexins may participate in tumor progression as well [77, 121–123]. Another important molecule in cell migration and oncogenesis is MET which functions with B-plexins in invasive growth [124] while its ligand HGF binds NRPs [51].
9. The semaphorin pathway as a target for cancer treatment
Semaphorins and their receptors have now emerged as key components in tumor development or progression. NRPs have been viewed as VEGF co-receptors and this has led to different VEGF-inhibiting strategies, such as VEGF or NRP-blocking antibodies, NRP-blocking peptides, and NRP soluble forms [24]. A signaling inhibitor of both VEGF and SEMA3A has even been made [125].
In an animal model, injection of anti-NRP antibodies resulted in inhibition of tumor angiogenesis, which showed increased activity when combined with an anti-VEGF antibody [126, 127]. In breast cancer cells, a peptide matching the VEGF binding site of NRPs induced apoptosis [128]. Two other peptides that block VEGF-mediated NRP1 signaling have shown tumor inhibitory properties [118]. A peptide (ATWLPPR) specifically blocking the VEGF exon 8 binding to the NRP1 b1 domain has been tested with promising results [40, 41, 129, 130]. Peptides blocking NRP1 dimerization and function have also been used in the nervous system [131].
Another strategy to block NRP1 is to induce its internalization. As recently shown [132], sulfated polysaccharides like dextran sulfate and fucoidan reduce endothelial cell surface levels of NRP1, NRP2 and, to a lesser extent, VEGFR-1 and VEGFR-2, and block the binding and function of SEMA3A and VEGF165. These compounds appear to bridge the extracellular domain of NRP1 to the scavenger receptor SREC-I and promote their internalization to lysosomes.
Most therapeutic strategies have focused on NRP1 as less is known about the biological properties of NRP2. Recently, it was reported that NRP2 plays a critical role in colorectal cancer development and may represent a potential therapeutic target [133]. Indeed, NRP2 silencing rendered colon carcinoma cells less tumorigenic in nude mice. In the future, semaphorin-based therapies could be used by themselves or in combination. In vitro, the synchronized use of SEMA3A and SEMA3F demonstrated synergy in repelling and inducing EC apoptosis [73]. A strategy employing the extracellular domain of SEMA6A has been developed with success; injection of this protein fragment in mice reduced tumor and VEGF-dependent vascularization [78].
10. Conclusion
Members of class-3 semaphorins, especially SEMA3F, have emerged as important factors in the development/progression of lung cancer. While SEMA3F and SEMA3B are downregulated in many tumors, their NRP receptors are conversely upregulated, most likely due to their role as receptors for other growth factors. Recent data have suggested that SEMA3s might not compete with VEGF for NRP binding thus strengthening the argument for a combined semaphorin/anti-VEGF approach.
Relatively little is known about which semaphorin signaling pathways significantly contribute to preventing tumorigenesis, except that integrin regulation is recurrently observed. There is a need in future studies to determine which signaling pathways or components predominate in the semaphorin anti-tumor effects. This might allow small molecules to recapitulate the effects of semaphorins, which are more cumbersome and expensive to produce. Recently, antibodies targeting the VEGF-A/C binding sites on NRP1 and NRP2 have been engineered and show promising results on blocking tumor angiogenesis, lymphangiogenesis and metastasis in rodents [127, 134]. It is likely that such strategies will see their ways to clinical trials in the near future
Acknowledgments
This work was supported in part by “La Ligue Contre le Cancer” and “Association pour la Recherche sur le Cancer” (VP, JR) and the Colorado/MUSC Lung Cancer SPORE grant CA58187 (VP, HD).
Abbreviations
- CRMP
collapsin response mediator protein
- EC
endothelial cell
- ECD
extracellular domain
- EGFR
epidermal growth factor receptor
- FHIT
fragile histidine triad
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- GRP
gastrin-releasing peptide
- HGF
hepatocyte growth factor
- LKB1
serine/threonine kinase 11
- NRP
neuropilin
- NSCLC
non-small cell lung cancer
- PTEN
phosphatase and tensin homolog
- RAR
retinoic acid receptor
- Rb
retinoblastoma protein
- SCF
stem cell factor
- SCLC
small cell lung cancer
- TGF
transforming growth factor
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schrump DS, Altorki NK, Henschke CL, Carter D, Turrisi AT, Gutierrez ME. Non-small cell lung cancer. In: De Vitta VT, Hellman S, Rosenberg SA, editors. Cancer, Principles and Practice of Oncology, Lung Cancer. Lippicott Williams and Wilkins; Philadelphia: 2005. pp. 189–246. [Google Scholar]
- 2.Helfrich BA, Raben D, Varella-Garcia M, Gustafson D, Chan DC, Bemis L, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–7125. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Varella-Garcia M, Cappuzzo F, McCoy J, Bemis L, Xavier AC, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2007;18:752–760. doi: 10.1093/annonc/mdm003. [DOI] [PubMed] [Google Scholar]
- 4.Sekido H, Fong KM, Minna JD. Molecular biology of lung cancer. In: De Vitta VT, Hellman S, Rosenberg SA, editors. Cancer, Principles and Practice of Oncology, Lung Cancer. Lippicott Williams and Wilkins; Philadelphia: 2005. pp. 181–188. [Google Scholar]
- 5.Sekido Y, Bader S, Latif F, Chen JY, Duh FM, Wei MH, et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, et al. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- 7.Xiang RH, Hensel CH, Garcia DK, Carlson HC, Kok K, Daly MC, et al. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 8.Sundaresan V, Chung G, Heppell-Parton A, Xiong J, Grundy C, Roberts I, et al. Homozygous deletions at 3p12 in breast and lung cancer. Oncogene. 1998;17:1723–1729. doi: 10.1038/sj.onc.1202103. [DOI] [PubMed] [Google Scholar]
- 9.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin Regulation of Cellular Morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 11.Comoglio PM, Tamagnone L, Boccaccio C. Plasminogen-related growth factor and semaphorin receptors: a gene superfamily controlling invasive growth. Exp Cell Res. 1999;253:88–99. doi: 10.1006/excr.1999.4684. [DOI] [PubMed] [Google Scholar]
- 12.Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 14.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. Embo J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, et al. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 18.Bismuth G, Boumsell L. Controlling the immune system through semaphorins. Sci STKE. 2002;2002:RE4. doi: 10.1126/stke.1282002re4. [DOI] [PubMed] [Google Scholar]
- 19.Potiron V, Nasarre P, Roche J, Healy C, Boumsell L. Semaphorin signaling in the immune system. Adv Exp Med Biol. 2007;600:132–144. doi: 10.1007/978-0-387-70956-7_11. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 21.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 22.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 23.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 24.Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 25.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. Faseb J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 27.Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 28.Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 29.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 30.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 31.Makinen T, Olofsson B, Karpanen T, Hellman U, Soker S, Klagsbrun M, et al. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem. 1999;274:21217–21222. doi: 10.1074/jbc.274.30.21217. [DOI] [PubMed] [Google Scholar]
- 32.Wise LM, Veikkola T, Mercer AA, Savory LJ, Fleming SB, Caesar C, et al. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc Natl Acad Sci U S A. 1999;96:3071–3076. doi: 10.1073/pnas.96.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 34.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. Faseb J. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 35.Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231:1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 36.Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, et al. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- 37.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276:25520–25531. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 38.Gluzman-Poltorak Z, Cohen T, Shibuya M, Neufeld G. Vascular endothelial growth factor receptor-1 and neuropilin-2 form complexes. J Biol Chem. 2001;276:18688–18694. doi: 10.1074/jbc.M006909200. [DOI] [PubMed] [Google Scholar]
- 39.Shraga-Heled N, Kessler O, Prahst C, Kroll J, Augustin H, Neufeld G. Neuropilin-1 and neuropilin-2 enhance VEGF121 stimulated signal transduction by the VEGFR-2 receptor. Faseb J. 2007;21:915–926. doi: 10.1096/fj.06-6277com. [DOI] [PubMed] [Google Scholar]
- 40.von Wronski MA, Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, et al. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- 41.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci U S A. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 43.Lee CC, Kreusch A, McMullan D, Ng K, Spraggon G. Crystal structure of the human neuropilin-1 b1 domain. Structure. 2003;11:99–108. doi: 10.1016/s0969-2126(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 44.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. Embo J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, et al. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 47.West DC, Rees CG, Duchesne L, Patey SJ, Terry CJ, Turnbull JE, et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem. 2005;280:13457–13464. doi: 10.1074/jbc.M410924200. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh SH, Ying NW, Wu MH, Chiang WF, Hsu CL, Wong TY, et al. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008 doi: 10.1038/sj.onc.1211029. Jan28. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, et al. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26:5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67:10309–10316. doi: 10.1158/0008-5472.CAN-07-3256. [DOI] [PubMed] [Google Scholar]
- 51.Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111:2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- 52.Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor {beta}-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008 doi: 10.1189/jlb.0208090. April24. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 54.Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. Embo J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 57.Nagai H, Sugito N, Matsubara H, Tatematsu Y, Hida T, Sekido Y, et al. CLCP1 interacts with semaphorin 4B and regulates motility of lung cancer cells. Oncogene. 2007;26:4025–4031. doi: 10.1038/sj.onc.1210183. [DOI] [PubMed] [Google Scholar]
- 58.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 59.Serini G, Napione L, Arese M, Bussolino F. Besides the Adhesion: New Perspectives of Integrin Functions in Angiogenesis. Cardiovasc Res. 2008;78:213–222. doi: 10.1093/cvr/cvn045. [DOI] [PubMed] [Google Scholar]
- 60.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 61.Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- 62.Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 63.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 64.Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, et al. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 65.Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 66.Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 67.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 68.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 69.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- 71.Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 74.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 75.Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. Faseb J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- 76.Sun Q, Nawabi-Ghasimi F, Basile JR. Semaphorins in vascular development and head and neck squamous cell carcinoma-induced angiogenesis. Oral Oncol. 2008;44:523–531. doi: 10.1016/j.oraloncology.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, MacDougall J, et al. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.4161/cbt.4.6.1733. [DOI] [PubMed] [Google Scholar]
- 79.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, et al. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, et al. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.can-03-3090. [DOI] [PubMed] [Google Scholar]
- 81.Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, et al. Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res. 2007;67:1451–1460. doi: 10.1158/0008-5472.CAN-06-2485. [DOI] [PubMed] [Google Scholar]
- 82.Potiron VA, Sharma G, Nasarre P, Clarhaut JA, Augustin HG, Gemmill RM, et al. Semaphorin SEMA3F Affects Multiple Signaling Pathways in Lung Cancer Cells. Cancer Res. 2007;67:8708–8715. doi: 10.1158/0008-5472.CAN-06-3612. [DOI] [PubMed] [Google Scholar]
- 83.Ito T, Kagoshima M, Sasaki Y, Li C, Udaka N, Kitsukawa T, et al. Repulsive axon guidance molecule Sema3A inhibits branching morphogenesis of fetal mouse lung. Mech Dev. 2000;97:35–45. doi: 10.1016/s0925-4773(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 84.Kagoshima M, Ito T. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells. 2001;6:559–571. doi: 10.1046/j.1365-2443.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 85.Brambilla E, Constantin B, Drabkin H, Roche J. Semaphorin SEMA3F localization in malignant human lung and cell lines: A suggested role in cell adhesion and cell migration. Am J Pathol. 2000;156:939–950. doi: 10.1016/S0002-9440(10)64962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujii T, Dracheva T, Player A, Chacko S, Clifford R, Strausberg RL, et al. A preliminary transcriptome map of non-small cell lung cancer. Cancer Res. 2002;62:3340–3346. [PubMed] [Google Scholar]
- 87.Tomizawa Y, Sekido Y, Kondo M, Gao B, Yokota J, Roche J, et al. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci U S A. 2001;98:13954–13959. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tse C, Xiang RH, Bracht T, Naylor SL. Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res. 2002;62:542–546. [PubMed] [Google Scholar]
- 89.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci U S A. 2004;101:11432–11437. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Lange R, Dimoudis N, Weidle UH. Identification of genes associated with enhanced metastasis of a large cell lung carcinoma cell line. Anticancer Res. 2003;23:187–194. [PubMed] [Google Scholar]
- 91.Marsit CJ, Wiencke JK, Liu M, Kelsey KT. The race associated allele of Semaphorin 3B (SEMA3B) T415I and its role in lung cancer in African-Americans and Latino-Americans. Carcinogenesis. 2005;26:1446–1449. doi: 10.1093/carcin/bgi098. [DOI] [PubMed] [Google Scholar]
- 92.Kuroki T, Trapasso F, Yendamuri S, Matsuyama A, Alder H, Williams NN, et al. Allelic loss on chromosome 3p21.3 and promoter hypermethylation of semaphorin 3B in non-small cell lung cancer. Cancer Res. 2003;63:3352–3355. [PubMed] [Google Scholar]
- 93.Ito M, Ito G, Kondo M, Uchiyama M, Fukui T, Mori S, et al. Frequent inactivation of RASSF1A, BLU, and SEMA3B on 3p21.3 by promoter hypermethylation and allele loss in non-small cell lung cancer. Cancer Lett. 2005;225:131–139. doi: 10.1016/j.canlet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 94.Ochi K, Mori T, Toyama Y, Nakamura Y, Arakawa H. Identification of semaphorin3B as a direct target of p53. Neoplasia. 2002;4:82–87. doi: 10.1038/sj.neo.7900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rolny C, Capparuccia L, Casazza A, Mazzone M, Vallario A, Cignetti A, et al. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med. 2008;205:1155–1171. doi: 10.1084/jem.20072509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potiron VA, Drabkin HA, Roche J. SEMA3F, Atlas Genet Cytogenet Oncol Haematol. 2008 http://www.atlasgeneticsoncology.org/Genes/SEMA3FID42254ch3p21.html.
- 97.Todd MC, Xiang RH, Garcia DK, Kerbacher KE, Moore SL, Hensel CH, et al. An 80 Kb P1 clone from chromosome 3p21.3 suppresses tumor growth in vivo. Oncogene. 1996;13:2387–2396. [PubMed] [Google Scholar]
- 98.Lantuejoul S, Constantin B, Drabkin H, Brambilla C, Roche J, Brambilla E. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol. 2003;200:336–347. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 99.Osada R, Horiuchi A, Kikuchi N, Ohira S, Ota M, Katsuyama Y, et al. Expression of semaphorins, vascular endothelial growth factor, and their common receptor neuropilins and alleic loss of semaphorin locus in epithelial ovarian neoplasms: increased ratio of vascular endothelial growth factor to semaphorin is a poor prognostic factor in ovarian carcinomas. Hum Pathol. 2006;37:1414–1425. doi: 10.1016/j.humpath.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 100.Xiang R, Davalos AR, Hensel CH, Zhou XJ, Tse C, Naylor SL. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res. 2002;62:2637–2643. [PubMed] [Google Scholar]
- 101.Kusy S, Nasarre P, Chan D, Potiron V, Meyronet D, Gemmill RM, et al. Selective suppression of in vivo tumorigenicity by semaphorin SEMA3F in lung cancer cells. Neoplasia. 2005;7:457–465. doi: 10.1593/neo.04721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nasarre P, Kusy S, Constantin B, Castellani V, Drabkin HA, Bagnard D, et al. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia. 2005;7:180–189. doi: 10.1593/neo.04481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nasarre P, Constantin B, Rouhaud L, Harnois T, Raymond G, Drabkin HA, et al. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia. 2003;5:83–92. doi: 10.1016/s1476-5586(03)80020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vacca A, Scavelli C, Serini G, Di Pietro G, Cirulli T, Merchionne F, et al. Loss of inhibitory semaphorin 3A (SEMA3A) autocrine loops in bone marrow endothelial cells of patients with multiple myeloma. Blood. 2006;108:1661–1667. doi: 10.1182/blood-2006-04-014563. [DOI] [PubMed] [Google Scholar]
- 105.Kusy S, Potiron V, Zeng C, Franklin W, Brambilla E, Minna J, et al. Promoter characterization of Semaphorin SEMA3F, a tumor suppressor gene. Biochim Biophys Acta. 2005;1730:66–76. doi: 10.1016/j.bbaexp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 106.Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, et al. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003;100:10429–10434. doi: 10.1073/pnas.1734137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueno K, Kumagai T, Kijima T, Kishimoto T, Hosoe S. Cloning and tissue expression of cDNAs from chromosome 5q21–22 which is frequently deleted in advanced lung cancer. Hum Genet. 1998;102:63–68. doi: 10.1007/s004390050655. [DOI] [PubMed] [Google Scholar]
- 108.Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem. 2000;275:39647–39653. doi: 10.1074/jbc.M006316200. [DOI] [PubMed] [Google Scholar]
- 109.Martin-Satue M, Blanco J. Identification of semaphorin E gene expression in metastatic human lung adenocarcinoma cells by mRNA differential display. J Surg Oncol. 1999;72:18–23. doi: 10.1002/(sici)1096-9098(199909)72:1<18::aid-jso5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 110.Koshikawa K, Osada H, Kozaki K, Konishi H, Masuda A, Tatematsu Y, et al. Significant up-regulation of a novel gene, CLCP1, in a highly metastatic lung cancer subline as well as in lung cancers in vivo. Oncogene. 2002;21:2822–2828. doi: 10.1038/sj.onc.1205405. [DOI] [PubMed] [Google Scholar]
- 111.Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, et al. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol. 2007;7:55. doi: 10.1186/1471-213X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 113.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 114.Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 115.Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, et al. Neuropilin-1 Modulates p53/Caspases Axis to Promote Endothelial Cell Survival. PLoS ONE. 2007;2:e1161. doi: 10.1371/journal.pone.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, et al. Vascular Endothelial Growth Factor Mediates Intracrine Survival in Human Breast Carcinoma Cells through Internally Expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barr MP, Bouchier-Hayes DJ, Harmey JJ. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int J Oncol. 2008;32:41–48. [PubMed] [Google Scholar]
- 118.Hong TM, Chen YL, Wu YY, Yuan A, Chao YC, Chung YC, et al. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin Cancer Res. 2007;13:4759–4768. doi: 10.1158/1078-0432.CCR-07-0001. [DOI] [PubMed] [Google Scholar]
- 119.Kawakami T, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Abe Y, et al. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 2002;95:2196–2201. doi: 10.1002/cncr.10936. [DOI] [PubMed] [Google Scholar]
- 120.Shih JY, Lee YC, Yang SC, Hong TM, Huang CY, Yang PC. Collapsin response mediator protein-1: a novel invasion-suppressor gene. Clin Exp Metastasis. 2003;20:69–76. doi: 10.1023/a:1022598604565. [DOI] [PubMed] [Google Scholar]
- 121.Sadanandam A, Varney ML, Kinarsky L, Ali H, Mosley RL, Singh RK. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. Omics. 2007;11:41–57. doi: 10.1089/omi.2006.0004. [DOI] [PubMed] [Google Scholar]
- 122.Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, et al. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 123.Wong OG, Nitkunan T, Oinuma I, Zhou C, Blanc V, Brown RS, et al. Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci U S A. 2007;104:19040–19045. doi: 10.1073/pnas.0702544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen QD, Rodrigues S, Rodrigue CM, Rivat C, Grijelmo C, Bruyneel E, et al. Inhibition of vascular endothelial growth factor (VEGF)-165 and semaphorin 3A-mediated cellular invasion and tumor growth by the VEGF signaling inhibitor ZD4190 in human colon cancer cells and xenografts. Mol Cancer Ther. 2006;5:2070–2077. doi: 10.1158/1535-7163.MCT-06-0044. [DOI] [PubMed] [Google Scholar]
- 126.Liang WC, Dennis MS, Stawicki S, Chanthery Y, Pan Q, Chen Y, et al. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007;366:815–829. doi: 10.1016/j.jmb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 127.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 128.Barr MP, Byrne AM, Duffy AM, Condron CM, Devocelle M, Harriott P, et al. A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells. Br J Cancer. 2005;92:328–333. doi: 10.1038/sj.bjc.6602308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. Embo J. 2000;19:1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, et al. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Cremel G, Hubert P, et al. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646–654. doi: 10.1091/mbc.E07-06-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Narazaki M, Segarra M, Tosato G. Sulfated polysaccharides identified as inducers of Neuropilin-1 internalization and functional inhibition of VEGF165 and Semaphorin3A. Blood. 2008;111:4126–4136. doi: 10.1182/blood-2007-09-112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, Yang AD, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 134.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 135.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 136.Muller MW, Giese NA, Swiercz JM, Ceyhan GO, Esposito I, Hinz U, et al. Association of axon guidance factor Semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121:2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 137.Rich JN, Hans C, Jones B, Iversen ES, McLendon RE, Rasheed BK, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 2005;65:4051–4058. doi: 10.1158/0008-5472.CAN-04-3936. [DOI] [PubMed] [Google Scholar]
- 138.Yamada T, Endo R, Gotoh M, Hirohashi S. Identification of semaphorin E as a non-MDR drug resistance gene of human cancers. Proc Natl Acad Sci U S A. 1997;94:14713–14718. doi: 10.1073/pnas.94.26.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galani E, Sgouros J, Petropoulou C, Janinis J, Aravantinos G, Dionysiou-Asteriou D, et al. Correlation of MDR-1, nm23-H1 and H Sema E gene expression with histopathological findings and clinical outcome in ovarian and breast cancer patients. Anticancer Res. 2002;22:2275–2280. [PubMed] [Google Scholar]
- 140.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–1238. [PubMed] [Google Scholar]
- 141.Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58:1238–1244. [PubMed] [Google Scholar]
- 142.Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood. 2005;105:3042–3050. doi: 10.1182/blood-2004-10-3873. [DOI] [PubMed] [Google Scholar]
- 143.Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- 144.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 145.Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, et al. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer. 2001;85:1372–1382. doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF, 3rd, et al. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci U S A. 2003;100:11463–11468. doi: 10.1073/pnas.2031202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao XY, Chen L, Xu Q, Li YH. Expression of semaphorin 6D in gastric carcinoma and its significance. World J Gastroenterol. 2006;12:7388–7390. doi: 10.3748/wjg.v12.i45.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 149.Catalano A, Caprari P, Rodilossi S, Betta P, Castellucci M, Casazza A, et al. Cross-talk between vascular endothelial growth factor and semaphorin-3A pathway in the regulation of normal and malignant mesothelial cell proliferation. Faseb J. 2004;18:358–360. doi: 10.1096/fj.03-0513fje. [DOI] [PubMed] [Google Scholar]
- 150.Dorfman DM, Shahsafaei A, Nadler LM, Freeman GJ. The leukocyte semaphorin CD100 is expressed in most T-cell, but few B-cell, non-Hodgkin’s lymphomas. Am J Pathol. 1998;153:255–262. doi: 10.1016/S0002-9440(10)65566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]