To the Editor: Only 2 cases of Rickettsia aeschlimannii infection have been reported. We report 2 additional cases documented in Algeria by immunofluorescence (IF) assays and confirmed by Western blot (WB) assays and cross-adsorption studies.
Tick-borne rickettsioses are now recognized as emerging or reemerging human infections worldwide. These zoonoses, caused by intracellular bacteria within spotted fever group (SFG) Rickettsia spp., share characteristic clinical features including fever, rash, and sometimes inoculation eschar at the bite site (1). In North Africa, cases of rickettsioses are rarely documented (2). In Algeria, only Mediterranean spotted fever caused by R. conorii has been described (3).
From 2000 through 2006 in Algeria, all patients with suspected rickettsioses seen at the infectious diseases units of Constantine and Batna hospitals were included in a prospective study; clinical and epidemiologic data and acute-and convalescent-phase serum samples obtained 2–4 weeks later were collected. Serum samples were sent to Marseille, France, where they were analyzed by an IF assay, using 9 SFG rickettsial antigens (R. conorii conorii, R. conorii israelensis, R. africae, R. sibirica mongolitimonae, R. aeschlimannii, R. massiliae, R. helvetica, R. slovaca, and R. felis) and a typhus group antigen (R. typhi) (3). The IF assay result was considered positive 1) if immunoglobulin (Ig) G titers were >128 and/or IgM titers were >64 for R. conorii and 2) if IgG titers were >64 and/or IgM titers were >32 for other rickettsial antigens (3). When cross-reactions between several antigens were noted, rickettsial antigen was considered to represent the infectious agent if titers of IgG and/or IgM antibody against this antigen were at least 2-fold higher than titers of IgG and/or IgM antibody against other rickettsial antigens (3,4). When the difference in titers among several antigens was lower than 2-fold, WB assays and cross-adsorption studies were performed (4,5). A total of 135 patients were included in the study. We describe 2 cases of R. aeschlimannii infection. Cases caused by other SFG rickettsiae will be reported elsewhere.
An 80-year-old man who reported contact with dogs parasitized by ticks had a 7-day history of high fever, headache, myalgia, and vomiting. On physical examination, a generalized maculopapular rash, 2 eschars (right shoulder and knee), and bilateral hemorrhagic signs on the retina were noticed. Elevated levels of liver enzymes (aspartate aminotransferase 187 U/L, alanine aminotransferase 108 U/L), hyponatremia (sodium 120 mmol/L), and hypokalemia (potassium 2.9 mmol/L) were found. IF assay showed raised levels of IgG/IgM against R. aeschlimannii (512/64) and R. conorii (128/0).
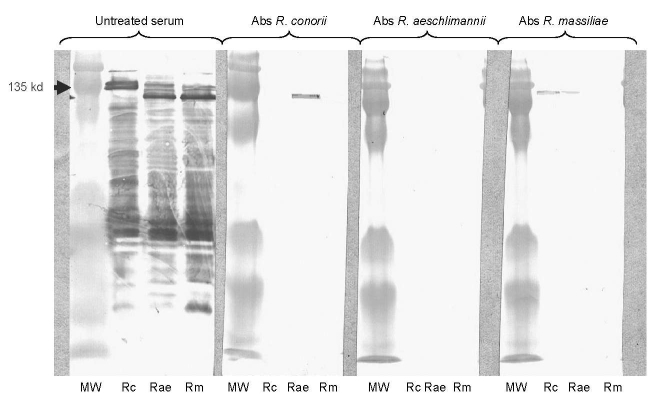
The second patient, a 36-year-old man, reported a 15-day history of fever with headache and failure of amoxicillin and cotrimoxazole treatments. Oral aphtous, a maculopapular rash, and purpuric lesions on the arms were noticed. IF assay showed raised levels of IgG/IgM at the same titer (2,048/32) against R. conorii, R. aeschlimannii, and R. massiliae. WB assays and cross-adsorption studies confirmed that antibodies were directed against R. aeschlimannii (Figure). Both patients recovered after doxycycline treatment (1).
Figure.
Western blot assay (WB) and cross-adsorption studies in serum of a patient with rickettsiosis in Algeria. Immunofluorescent assay showed raised levels of immunoglobulin (Ig) G/M at the same titer (2,048/32) against Rickettsia conorii, R. aeschlimannii, and R. massiliae. Lanes Rc, Rae, and Rm: WB assay using R. conorii, R. aeschlimannii, and R. massiliae antigens, respectively. MW, molecular weights are indicated on the left. Untreated serum, late serum samples tested by WB. When adsorption is performed with R. aeschlimannii antigens, homologous and heterologous antibodies disappear, but when it is performed with R. conorii antigens and R. massiliae, homologous antibodies disappear but heterologous antibodies persist. This result indicates that antibodies are specifically directed against R. aeschlimannii. Abs, absorbed.
R. aeschlimannii was first characterized as a new SFG rickettsia after its isolation from Hyalomma marginatum marginatum ticks in Morocco in 1997 (6). Thereafter, R. aeschlimannii has been detected in this tick species in southern Europe and North Africa (7), as well as in H. m. rufipes in sub-Saharan Africa (1). Preliminary data have suggested that these Hyalomma organisms may be not only vectors but also reservoirs of R. aeschlimannii and as a consequence, the geographic distribution of R. aeschlimannii would be at least that of these ticks throughout southern Europe and Africa (8).
Although WB assays and cross-adsorption studies are time-consuming and only available in specialized reference laboratories, new data can be obtained for a better understanding of rickettsioses. We have added the description of 2 more cases of infection with R. aeschlimannii. Only 2 cases of human infection caused by this rickettsia had been previously reported, including infection in a patient returning to France from Morocco, and another in a patient in South Africa (9,10).
Clinicians should be aware that several tick-borne rickettsial pathogens are present in Algeria. Specific clinical features may be directly influenced by the Rickettsia sp. involved, the rickettsial infection rate of the vector, and tick behavior. H. marginatum ticks readily bite humans, and persons may receive multiple simultaneous tick bites. Furthermore, the high infectious rate of these ticks by R. aeschlimannii has been reported (1). Therefore, the probability of being bitten by several infected H. marginatum ticks is high and can lead to several eschars in patients, a characteristic of few tick-borne rickettsioses. Finally, although doxycycline is the reference treatment for rickettsioses, rifampin has been used (1). However, although R. conorii is susceptible in vitro to this drug, R. aeschlimannii is resistant. Because patients suspected of having rickettsiosis must receive prompt presumptive treatment, the presence of R. aeschlimannii in Morocco reinforces the need to use doxycycline as a first-line drug.
Footnotes
Suggested citation for this article: Mokrani N, Parola P, Tabbal S, Dalichaouche M, Aouati A, Raoult D. Rickettsia aeschlimannii Infection, Algeria [letter]. Emerg Infect Dis [serial on the Internet]. 2008 Nov [date cited]. Available from http://www.cdc.gov/EID/content/14/11/1814.htm
References
- 1.Parola P, Paddock C, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–56. 10.1128/CMR.18.4.719-756.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letaïef A. Epidemiology of rickettsioses in North Africa. Ann N Y Acad Sci. 2006;1078:34–41. 10.1196/annals.1374.004 [DOI] [PubMed] [Google Scholar]
- 3.Mouffok N, Benabdellah A, Richet H, Rolain JM, Razik F, Belamadani D, et al. Reemergence of rickettsiosis in Oran, Algeria. Ann N Y Acad Sci. 2006;1078:180–4. 10.1196/annals.1374.033 [DOI] [PubMed] [Google Scholar]
- 4.Parola P, Miller RS, McDaniel P, Telford SR III, Wongsrichanalai C, Raoult D. Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis. 2003;9:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensenius M, Fournier PE, Vene S, Ringertz SH, Myrvang B, Raoult D. Comparison of immunofluorescence, western blotting, and cross-adsorption assays for diagnosis of African tick bite fever. Clin Diagn Lab Immunol. 2004;11:786–8. 10.1128/CDLI.11.4.786-788.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beati L, Meskini M, Thiers B, Raoult D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int J Syst Bacteriol. 1997;47:548–54. [DOI] [PubMed] [Google Scholar]
- 7.Bitam I, Parola P, Matsumoto K, Rolain JM, Baziz B, Boubidi SC, et al. Molecular detection of spotted fever group Rickettsiae in ticks from Algeria. Ann N Y Acad Sci. 2006;1078:368–72. 10.1196/annals.1374.073 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Parola P, Brouqui P, Raoult D. Rickettsia aeschlimannii in Hyalomma ticks from Corsica. Eur J Clin Microbiol Infect Dis. 2004;23:732–4. 10.1007/s10096-004-1190-9 [DOI] [PubMed] [Google Scholar]
- 9.Raoult D, Fournier PE, Abboud P, Caron F. First documented human Rickettsia aeschlimannii infection. Emerg Infect Dis. 2002;8:748–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretorius AM, Birtles RJ. Rickettsia aeschlimannii: a new pathogenic spotted fever group rickettsia, South Africa. Emerg Infect Dis. 2002;8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]