To the Editor: The immunosuppressive agent alemtuzumab is a DNA-derived, humanized monoclonal antibody directed against the panlymphocyte, cell-surface antigen CD52 (1). The drug is approved for the treatment of refractory B-cell chronic lymphocytic leukemia (2) and also has been used after stem cell (3) and organ transplantations (4). Alemtuzumab causes profound and prolonged lymphocyte depletion, which results in a variety of complications involving infections (5). However, mycobacteria have rarely been reported to cause infection after alemtuzumab treatment. We describe infections with Mycobacterium haemophilum, a fastidious nontuberculous mycobacterium, in 2 patients who experienced cutaneous lesions while they received alemtuzumab.
Patient 1
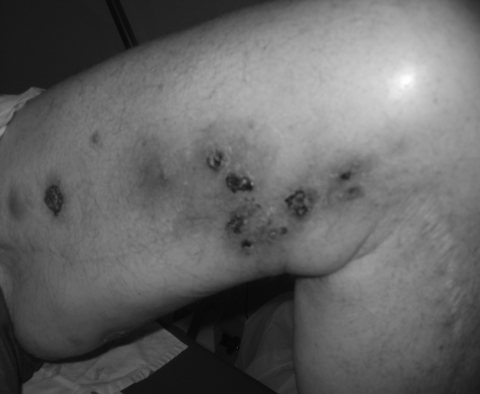
A 65-year-old man with refractory chronic lymphocytic leukemia had been receiving treatment with alemtuzumab for 3 months. During a 5-week period beginning 15 weeks after the alemtuzumab therapy started, 20–30 tender nodular-ulcerative lesions developed on the patient’s extremities. Most of the lesions were distributed along a saphenous vein site (Figure). Immediately before receiving alemtuzumab, he had been given rituximab for 3 months. A punch biopsy of the cutaneous lesion showed lymphogranulomatous inflammation in the dermis. Acid-fast stains of the skin punch biopsy specimen, as well as aspirated material from the lesions, demonstrated acid-fast bacilli. Cultures on Middlebrook 7H11 agar (Becton Dickinson and Company, Sparks, MD, USA) containing X-factor strips incubated at 30°C showed growth of the acid-fast bacilli after 13 days. The isolate was subsequently identified as M. haemophilum by using conventional biochemical profiles and assessment of morphologic features, including an optimal growth temperature of 30°C and a hemin requirement. The patient was treated with 4 drugs (rifampin, doxycycline, clarithromycin, ciprofloxacin), and he rapidly improved. Susceptibility testing, using broth MIC determinations described in Clinical and Laboratory Standards Institute publication M-24A (www.clsi.org/source/orders/free/m24-aa.pdf), indicated that the isolate was sensitive to clarithromycin, ciprofloxacin, clofazimine, and linezolid; intermediately sensitive to rifampin; but resistant to rifabutin, doxycycline, ethambutol, streptomycin, and amikacin. The antimicrobial drugs the patient was receiving were changed to only rifampin, clarithromycin, and ciprofloxacin. He completed a 6-month course of treatment course without recurrence of the lesions.
Figure.
Nodular-ulcerative skin lesions on the left thigh caused by Mycobacterium haemophilum infection in a patient with chronic lymphocytic leukemia (patient 1) whose condition had been treated with alemtuzumab.
Patient 2
A 17-year-old woman with severe systemic lupus erythematosus and secondary myelodysplastic syndrome received an unrelated T-cell depleted bone marrow transplant. Her conditioning regimen included melphalan, thiotepa, fludarabine, and 2 doses of alemtuzumab. She initially did well posttransplant and was discharged from the hospital Approximately 3 months later, 40–50 tender erythematous papular lesions developed on her extremities. A skin biopsy specimen showed mycobacterial panniculitis. Cultures from skin, blood, and bone marrow grew M. haemophilum after 18–19 days’ incubation. She was successfully treated with rifampin, clarithromycin, and gatifloxacin; however, she died several months later from unrelated complications.
M. haemophilum was first described in 1978 when it was isolated from cutaneous lesions of a woman from Israel with Hodgkin disease (6). M. haemophilum most often causes joint, cutaneous, and pulmonary infections in immunocompromised patients (7) and lymphadenitis in immunocompetent children (8). M. haemophilum is a fastidious organism that requires media supplemented with ferric ions in the form of hemin, hemoglobin, or ferric ammonium citrate, and incubation at 30°C–32°C for several weeks. On the basis of our experience at Memorial Sloan-Kettering Cancer Center (23 cases of M. haemophilum infection observed from 1990 through 2000) (9), the following specimens are routinely set up for culture: blood smear specimens that are positive for acid-fast bacilli, synovial or joint fluids, skin biopsy specimens, cutaneous lesions, ulcers, abscesses, lymph nodes, and lung biopsy specimens. Culture media include Middlebrook 7H11 agar plates with a hemin-containing paper strip (X-factor) placed on the agar surface that are then incubated at 30°C for 6 weeks. Growth of the organism is usually detected within 2 to 3 weeks, and the isolates are usually susceptible in vitro to the quinolones, macrolides, and rifamycins and resistant to several drugs for tuberculosis, including ethambutol, isoniazid, and pyrazinamide (9).
Alemtuzumab has been associated with the development of infections caused by a variety of microorganisms. However, mycobacteria have infrequently been the reported cause. In a review of 547 organ transplant recipients who received alemtuzumab treatment, miliary tuberculosis developed in 1 recipient of a kidney transplant, and pulmonary infection with M. kansasii developed in 2 recipients of lung transplants (5). There is also a case report of systemic M. bovis infection developing in a patient with relapsing B chronic lymphocytic leukemia after administration of alemtuzumab (10).
Although we believe that alemtuzumab is responsible for the severe immunosuppression that predisposed these patients to M. haemophilum infection, other explanations are plausible. For example, patient 1 had received rituximab and cyclophosamide for 6 months. These drugs, in addition to his underlying disease of chromic lymphocytic leukemia, may have predisposed him to M. haemophilum infection. However, his lesions did not appear until he received alemtuzumab. In patient 2, the immunosuppression associated with his transplant may have predisposed the patient to M. haemophilum infection.
This report identifies M. haemophilum as an opportunistic pathogen in patients who have received alemtuzumab. We recommend that all patients who have received at least 1 dose of alemtuzumab, and who have undiagnosed tender skin lesions located over the extremities, be evaluated by using appropriate techniques to isolate M. haemophilum. Communication with microbiology laboratory staff concerning appropriate methods for detection of the organism is crucial.
Footnotes
Suggested citation for this article: Kamboj M, Louie E, Kiehn T, Papanicolaou G, Glickman M, Sepkowitz K. Mycobacterium haemophilum infection after alemtuzumab treatment. Emerg Infect Dis [serial on the Internet]. 2008 Nov [date cited]. Available from http://www.cdc.gov/EID/content/14/11/1821.htm
References
- 1.Ferrajoli A, O’Brien S, Keating MJ. Alemtuzumab: a novel monoclonal antibody. Expert Opin Biol Ther. 2001;1:1059–65. 10.1517/14712598.1.6.1059 [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. 10.1182/blood.V99.10.3554 [DOI] [PubMed] [Google Scholar]
- 3.Ho AY, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004;104:1616–23. 10.1182/blood-2003-12-4207 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro R, Ellis D, Tan HP, Moritz ML, Basu A, Vats AN, et al. Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation. J Pediatr. 2006;148:813–8. 10.1016/j.jpeds.2006.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44:204–12. 10.1086/510388 [DOI] [PubMed] [Google Scholar]
- 6.Sompolinsky D, Lagziel A, Naveh D, Yankilevitz T. Mycobacterium haemophilum sp. nov., a new pathogen of humans. Int J Syst Bacteriol. 1978;28:67–75. [Google Scholar]
- 7.Saubolle MA, Kiehn TE, White MH, Rudinsky MF, Armstrong D. Mycobacterium haemophilum: microbiology and expanding clinical and geographic spectra of disease in humans. Clin Microbiol Rev. 1996;9:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruijnesteijn van Coppenraet LE, Kuijper EJ, Lindeboom JA, Prins JM, Class EC. Mycobacterium haemophilum and lymphadenitis in children. Emerg Infect Dis. 2005;11:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah MK, Sebti A, Kiehn TE, Massarella SA, Sepkowitz KA. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330–7. 10.1086/321894 [DOI] [PubMed] [Google Scholar]
- 10.Abad S, Gyan E, Moachon L, Bouscary D, Sicard D, Dreyfus F, et al. Tuberculosis due to Mycobacterium bovis after alemtuzumab administration. Clin Infect Dis. 2003;37:e27–8. 10.1086/375690 [DOI] [PubMed] [Google Scholar]