Abstract
Objective
To assess participation bias in the assembly of a specimen repository for genetic studies and to examine the association of participation with outcome within the Olmsted County myocardial infarction (MI) cohort.
Participants and Methods
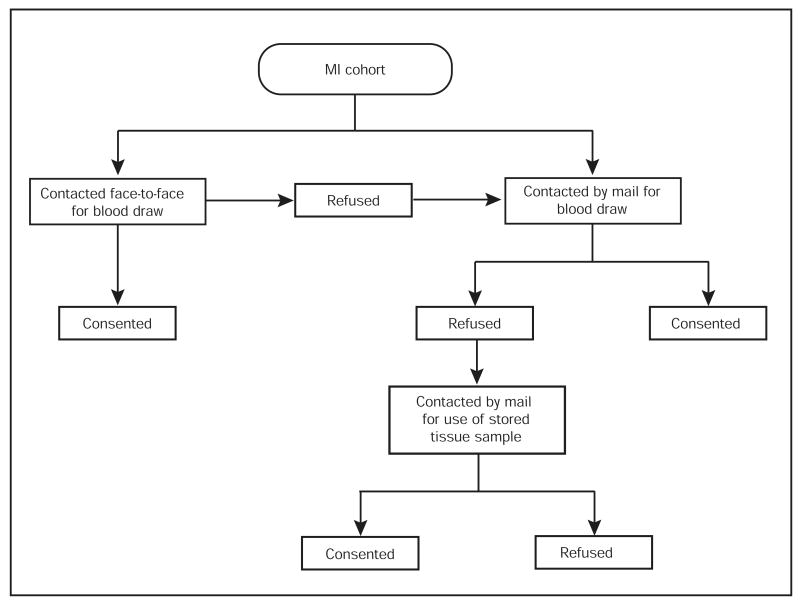
From January 1, 1979, to May 31, 2006, 3081 persons had MI in Olmsted County, MN. Face-to-face contact was used to recruit patients who were hospitalized for an acute event. Persons who had had an MI before establishment of this repository were contacted by mail. At initial contact, we sought consent to use blood samples for genetic studies. Persons who refused were contacted by mail and were asked to consent to the use of stored tissue samples. For deceased subjects, stored tissue was collected when available.
Results
Of the 3081 persons in the Olmsted County MI cohort, 1994 participated in the study; 1007 (50.5%) blood and 987 (49.5%) tissue specimens were provided. Participants were more likely to be younger men with hypertension, comorbidities, and non–ST-segment elevation MI (all, P<.05). Participants who provided blood specimens were more likely to have non–ST-segment elevation MI and lower Killip class than those who provided tissue. After adjustment for age, sex, hypertension, ST-segment elevation, Killip class, and comorbidities, participation was not associated with outcome. Participants who provided blood specimens were less likely to have heart failure (hazard ratio, 0.49; 95% confidence interval, 0.40-0.59; P<.01) or to die (hazard ratio, 0.16; 95% confidence interval, 0.12-0.21; P<.01) than those who provided tissue.
Conclusions
A variety of sources can be used to assemble community specimen repositories. Baseline characteristics differed between participants and nonparticipants and, among participants, by specimen source. Participants who provided blood specimens had better outcomes than those who provided tissue specimens. No survival advantage was observed for participants after combining blood and tissue specimens.
Participation bias occurs when comparisons are made between groups of patients who differ in determinants of outcome and when the differences are themselves related to the outcome.1 Studies that require enrollment of participants are inherently affected by participation bias.2 Because selective participation can threaten the external validity of studies, assessment of the magnitude of participation bias is essential to optimize the inference from these studies.
Few studies in the community have examined the effect of participation bias. In one population-based study of myocardial infarction (MI), nonparticipants were older, had greater comorbidity, and experienced poorer survival than participants.3 A population-based study of left ventricular function reported that participation rates were lowest among the oldest, the youngest, and those with chronic obstructive pulmonary disease.4 However, those studies did not examine the effects of participation on genetic studies of heart diseases.
Population-based studies, using both established and new cohorts, are needed to evaluate the usefulness of genetic risk factors for genetically complex diseases.5,6 However, participation bias may affect studies that require collection of biological specimens for evaluation of genetic variants that contribute to common diseases such as heart disease.7 Accordingly, we set out to examine the influence of participation on the assembly of a specimen repository within a geographically defined cohort of participants who had experienced an MI. We did so by comparing participant and nonparticipant characteristics and by determining whether participation was associated with survival outcomes.
Participants and Methods
From January 1, 1979, to May 31, 2006, 3081 persons experienced a validated MI in Olmsted County, MN. Of those 3081 persons, 1994 provided specimens for this study. Samples were obtained both from living participants and, via a tissue repository or autopsy, from the deceased. All aspects of this study were approved by the Mayo Foundation Institutional Review Board. Throughout the article, participation is defined as the providing of specimens for the purpose of genetic study.
Myocardial Infarction Case Ascertainment
The parent study that enabled the current investigation was an observational study of a cohort of patients with MI within the geographically defined population of Olmsted County, MN, where Mayo Clinic Rochester and Olmsted Medical Center provide medical care for all county residents. These facilities use a unified record linkage system that compiles comprehensive clinical records collected by physicians. The Rochester Epidemiology Project enables these records to be easily retrieved.8 Most residents of Olmsted County are white.
This MI cohort includes a retrospective and a prospective component. The methodologies used to assemble the retrospective cohort and to enroll participants in the prospective component have been previously reported.9,10 Briefly, for the retrospective component, lists of patients discharged from Olmsted County hospitals with diagnoses compatible with an MI were obtained from the Hospital Utilization Review Database.9 The Rochester Epidemiology Project Index of Diagnosis was also used to locate cases.8 For this study, the target codes from the International Classification of Diseases, Ninth Revision included 410 (acute MI), 411 (other acute and subacute forms of ischemic heart disease), 412 (old MI), 413 (angina pectoris), and 414 (other forms of ischemic heart disease).9 For the prospective component of the cohort, all persons who presented to an Olmsted County medical facility with a cardiac troponin T level greater than or equal to 0.03 ng/mL (reference range, ≤0.03 ng/mL) were identified within 12 hours of the blood draw through electronic files of the Department of Laboratory Medicine.10 Nurse abstractors reviewed cases that met criteria for residency and collected information to classify the MI event.9 The criteria used to diagnose MI included cardiac pain, the Minnesota Code of the electrocardiogram, and biomarker levels.11-13
Recruitment for Participation in the Specimen Repository
A stepwise process, summarized in Figure 1, was implemented to recruit living participants into the study. Participants who were hospitalized for acute MI were recruited face-to-face; if they refused, they were contacted by mail. Those who had experienced MI before the initiation of the study were also contacted by mail. For this study we used blood samples that had been initially stored for clinical need, if available. If stored blood samples were not available, we drew another blood sample in conjunction with a clinically indicated draw.
Figure 1.
Recruitment process for living participants in myocardial infarction (MI) cohort.
When persons did not consent to use of blood specimens, we reviewed the medical records to determine the availability of stored tissue specimens. Available specimens, stored at the Mayo Clinic Tissue Registry, had been previously obtained during medically indicated procedures. We contacted persons for whom tissue specimens were available and sought authorization to use those specimens for genetic studies. If stored tissue was not available, no further contact was attempted.
We reviewed the records of persons who died before the initiation of this study for availability of tissue samples stored at the Mayo Clinic Tissue Registry. When available, tissue specimens obtained from autopsy were also used.
Data Collection Among Participants
Nurse abstractors reviewed medical records to ascertain risk factors. Risk factors included smoking, current and noncurrent; diabetes mellitus; systemic hypertension; and a familial history of coronary artery disease. We defined familial coronary artery disease as parental cardiovascular disease diagnosed before the age of 55 years in a father or before the age of 65 years in a mother.14 We also recorded MI characteristics, which included ST-segment elevation MI, Q waves on electrocardiogram, MI location, and Killip class, as well as comorbid conditions, which were scored using the Charlson Index.15
Data Collection Among Nonparticipants
Since August 1997, Minnesota law has required general research authorization from every study participant before the release of information from medical records.16 For persons providing this general research authorization, extensive data are available for research irrespective of their consent to a specific study. We included patients from the MI cohort who did not provide consent but who had given general research authorization in this study and reviewed their medical records, applying the same methods and definitions used for participants who had given consent.
Follow-Up
Inpatient and outpatient records were reviewed by nurse abstractors who collected clinical data and validated the diagnosis of heart failure after MI using the Framingham criteria.17 In our experience, use of these criteria is excellent for verification of heart failure.18
Follow-up was completed by passive surveillance of the community medical records. Death was ascertained through death certificates filed in Olmsted County, autopsy reports, obituary notices, and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics.19
Statistical Analyses
Persons were classified as participants or nonparticipants. Logistic regression was used to examine the association between participation and demographics, cardiovascular risk factors, MI characteristics, and comorbidities. A nonlinear effect of age was examined by including a quadratic term in the model. The effect of age on participation was compared between sexes by including an interaction term between age and sex. Cox proportional hazards regression models were constructed to evaluate the unadjusted and covariate-adjusted hazard ratios and 95% confidence intervals for death and post-MI heart failure associated with participation. For participants only, all analyses were repeated to compare the specimen source (blood vs tissue). The assumption of proportionality was met after examining Schoenfeld residuals. The threshold for statistical significance was P=.05, except when testing for quadratic effects and interactions, in which case P=.10.
Results
Characteristics Associated With Participation
Of the 3081 persons who had experienced MI in Olmsted County from January 1, 1979, to May 31, 2006, 1470 (48%) were deceased at the start of our study. Of those, 852 (58%) had stored tissue available. The study enrolled 1994 participants, 1171 (59%) of whom were men; the mean ± SD age of study participants was 68±14 years. Of the 1087 nonparticipants, 569 (52%) were men; the mean ± SD age for nonparticipants was 68±15 years.
Overall, men were more likely to participate than women (Table 1). The effect of sex on participation differed according to age (P=.04 for the sex-by-age interaction term). Men aged 60 to 79 years were more likely to participate than their younger or older counterparts. Among women, participation did not change significantly until the age of 80 years or greater, when a decrease in participation occurred.
Table 1. Participation Rates and OR, Stratified by Age and Sex*.
| Men | Women | |||
|---|---|---|---|---|
| Age (y) | Participation rate (%) | OR (95% CI) | Participation rate (%) | OR (95% CI) |
| <60 | 425/678 (63) | 1.00 | 142/222 (64) | 1.06 (0.77-1.45) |
| 60-69 | 299/420 (71) | 1.47 (1.13-1.91) | 138/212 (65) | 1.11 (0.80-1.53) |
| 70-79 | 277/380 (73) | 1.60 (1.22-2.11) | 261/401 (65) | 1.11 (0.86-1.44) |
| ≥80 | 170/262 (65) | 1.10 (0.82-1.48) | 282/506 (56) | 0.75 (0.59-0.95) |
CI = confidence interval; OR= odds ratios.
Baseline characteristics differed by participation (Table 2). Participants were more likely to have a history of hypertension and smoking. Adjustment for age and sex attenuated the association with smoking and strengthened the association with hypertension. Participants were more likely to have non–ST-segment elevation MI. Adjustment for age and sex did not affect the association between participation and non–ST-segment elevation MI status. The comorbidity burden was higher among participants, despite adjustment for age and sex.
Table 2. Comparison by Participation Status*†.
| Participation | Unadjusted | Adjusted for age and sex | ||||
|---|---|---|---|---|---|---|
| No | Yes | OR (95% CI) | P value | OR (95% CI) | P value | |
| Cardiovascular risk factors | ||||||
| Hypertension | 54 | 58 | 1.18 (1.01-1.39) | .04 | 1.28 (1.08-1.52) | .004 |
| Diabetes mellitus | 20 | 21 | 1.07 (0.88-1.31) | .48 | 1.11 (0.91-1.36) | .32 |
| Familial CAD | 18 | 21 | 1.19 (0.97-1.47) | .10 | 1.22 (0.98-1.51) | .07 |
| Smoking | 57 | 62 | 1.23 (1.05-1.45) | .01 | 1.15 (0.96-1.38) | .13 |
| MI characteristics | ||||||
| Q waves | 49 | 51 | 1.08 (0.90-1.28) | .41 | 1.06 (0.89-1.26) | .54 |
| Non–ST-segment elevation | 61 | 67 | 1.29 (1.08-1.54) | .005 | 1.29 (1.08-1.55) | .005 |
| Anterior MI | 38 | 37 | 0.96 (0.80-1.14) | .64 | 0.98 (0.82-1.16) | .78 |
| Killip class >1 | 37 | 33 | 0.84 (0.71-1.00) | .05 | 0.86 (0.72-1.02) | .08 |
| Comorbidities | ||||||
| Charlson index | ||||||
| 0 | 42 | 38 | 1.00 | 1.00 | ||
| 1-2 | 37 | 36 | 1.07 (0.89-1.28) | .05‡ | 1.16 (0.95-1.40) | .002‡ |
| 3+ | 21 | 25 | 1.29 (1.05-1.60) | 1.41 (1.12-1.78) | ||
Values are number (percentage) unless stated otherwise. CAD = coronary artery disease; CI = confidence interval; MI = myocardial infarction; OR= odds ratios.
Distributions of the clinical characteristics were obtained from a sample of 1673 participants and 924 nonparticipants.
P value from the likelihood ratio test, 2 df.
Role of Specimen Source
Blood specimens were obtained from 1007 participants (50.5%), tissue specimens from 987 (49.5%). Baseline characteristics differed by specimen source (Table 3). Participants who provided blood were more likely to have familial coronary disease and non–ST-segment elevation MI. Participants who provided blood specimens were less likely to have Killip class greater than 1. The comorbidity burden was less for participants who provided blood specimens. Adjustment for age and sex strengthened the association between blood specimens and hypertension but attenuated the association between blood specimens and familial coronary disease and comorbidity burden. The associations between blood specimens and non–ST-segment elevation MI and Killip class remained unchanged.
Table 3. Comparison by Specimen Source*†.
| Source | Unadjusted | Adjusted for age and sex | ||||
|---|---|---|---|---|---|---|
| Blood | Tissue | OR (95% CI) | P value | OR (95% CI) | P value | |
| Cardiovascular risk factors | ||||||
| Hypertension | 58 | 58 | 0.97 (0.80-1.18) | .76 | 1.41 (1.13-1.74) | .002 |
| Diabetes mellitus | 19 | 23 | 0.82 (0.65-1.04) | .10 | 0.95 (0.74-1.21) | .65 |
| Familial CAD | 23 | 19 | 1.30 (1.02-1.66) | .03 | 1.01 (0.78-1.31) | .92 |
| Smoking | 64 | 60 | 1.20 (0.98-1.46) | .08 | 0.79 (0.64-0.99) | .04 |
| MI characteristics | ||||||
| Q waves | 52 | 50 | 1.07 (0.87-1.31) | .55 | 1.05 (0.85-1.31) | .64 |
| Non–ST-segment elevation | 71 | 62 | 1.49 (1.20-1.85) | <.001 | 1.70 (1.36-2.14) | <.001 |
| Anterior MI | 35 | 40 | 0.82 (0.66-1.01) | .06 | 0.90 (0.72-1.12) | .34 |
| Killip class >1 | 22 | 42 | 0.39 (0.31-0.48) | <.001 | 0.47 (0.38-0.59) | <.001 |
| Comorbidities | ||||||
| Charlson index | ||||||
| 0 | 47 | 32 | 1.00 | 1.00 | ||
| 1-2 | 33 | 40 | 0.56 (0.44-0.70) | <.001‡ | 0.77 (0.60-0.97) | <.001‡ |
| 3 | 21 | 29 | 0.48 (0.37-0.62) | 0.78 (0.59-1.03) | ||
Values are number (percentage) unless stated otherwise. CAD = coronary artery disease; CI = confidence interval; MI = myocardial infarction; OR= odds ratio.
Distributions of the clinical characteristics were obtained from a sample of 1673 participants.
P value from the likelihood ratio test, 2 df.
Outcomes
Outcomes were associated with specimen source but not participation (Table 4). Participation was not associated with heart failure or death after MI, despite adjustment for age, sex, hypertension, ST-segment elevation, Killip class, and Charlson Index. Participants who provided blood specimens had better outcomes after MI for both heart failure and death vs subjects who provided tissue specimens. The strength of the associations did not change after adjustment for age, sex, hypertension, ST-segment elevation, Killip class, and Charlson Index for either heart failure or death.
Table 4. Outcomes by Participation Status and Tissue Source Status*.
| Heart failure | Death | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted† | Unadjusted | Adjusted† | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Participants vs nonparticipants | 0.89 (0.79-1.00) | .06 | 0.92 (0.80-1.04) | .19 | 0.93 (0.83-1.05) | .23 | 0.92 (0.81-1.04) | .20 |
| Blood vs tissue | 0.34 (0.29-0.41) | <.01 | 0.49 (0.40-0.59) | <.01 | 0.10 (0.08-0.14) | <.01 | 0.16 (0.12-0.21) | <.01 |
CI = confidence interval; HR = hazard ratio.
Adjusted for age, sex, Charlson Index, ST-segment elevation, Killip class, and hypertension.
Discussion
The current study documents the feasibility of assembling a specimen repository for the purpose of conducting genetic studies among community-dwelling subjects who experienced an MI. Baseline characteristics differed between participants and nonparticipants; among participants, they differed by specimen source. Whereas participants who provided blood specimens had better outcomes than those who provided tissue specimens, no survival difference was associated with participation when all specimens from all sources were combined in one single cohort. This finding underscores the importance of examining how the composition of specimen-based cohorts can be associated with outcomes, thereby affecting the inferences that can be drawn from these studies.
Feasibility of Assembling a Community Specimen Repository
When a collection of blood samples is necessary, initial face-to-face contact achieves higher recruitment rates than mail contact.20 Thus, we first attempted to recruit patients via face-to-face contact and then, if necessary, made contact by mail.
Half of the specimens included in the study were obtained from the Mayo Clinic Tissue Registry, allowing the inclusion of participants who were deceased at the initiation of this study and thereby reducing the effect of survival bias. For living participants who initially did not consent to the use of blood specimens, the tissue repository also provided the option to use stored tissue specimens, thereby increasing participation.
With this recruitment strategy, we achieved a participation rate of 65%, which is well within the range of participation rates for studies that did not involve the use of biological specimens and higher than those that did. Participation rates varied: epidemiological studies that used questionnaires had participation rates from 49% to 82%21-23; studies that used interviews, from 56% to 67%24,25; and studies that required a physical examination, from 51% to 71%.4,26-28 Our study obtained higher participation rates than other studies that required collection of specimens from buccal swabs (53%) or blood lymphocytes (58%).29
Because all nucleated cells are suitable specimens for DNA analysis,5,30 noninvasive methods that minimize participant burden should be the first choice for sample collection.30 Thus, for this repository, we chose to rely on stored specimens before resorting to blood draws, a strategy that contributed to our high participation rates.
Participation, Clinical Characteristics, and Outcome
Whereas epidemiological studies increasingly rely on the collection of biological specimens, participation in such studies has been declining, possibly because of study design, recruitment methods, participant burden, and societal factors.2,31 The progressive decline in participation rates underscores the importance of examining how participation influences cohort composition and subsequent outcomes results. Such an assessment is needed to properly draw inferences to the population as a whole. This methodological step, although crucial, is often omitted.2
In our study, which included both men and women, we observed lower participation rates for women than for men, particularly for older women. Lower participation rates of older4,25 and younger persons20 have been reported. Studies that included only men 28 or only women26,32 reported lower participation rates among older participants.
Participants had more hypertension, non–ST-segment elevation MI, and comorbidities than nonparticipants. Participation-associated differences in such characteristics vary across studies. For example, in a study of persons with osteoporosis, nonparticipants had higher systolic blood pressure than participants.33 The impact of such clinical differences on outcomes likely will also vary across cohorts. Indeed, in our parent community-based cohort of participants with MI, those with non–ST-segment elevation MI had similar survival to subjects with ST-segment elevation MI, thereby providing reassurance that the prevalence of ST-segment elevation MI in participants and nonparticipants did not affect outcome.34 Because participation-associated characteristics may vary across studies, they should be systematically examined in each study to determine how they affect outcome and inference.
In our study, participants who provided blood specimens had better outcomes than their counterparts who provided tissue specimens. However, when both groups of participants were combined, participation was not associated with outcomes despite differences in baseline characteristics. This finding emphasizes the importance of complementary approaches to specimen collection when feasible to optimize external validity.
Implications for Future Studies
Studies should use strategies that minimize participant burden and obtain specimens by the least invasive approach. The choice of specimen source will vary according to the resources available in each institution. When possible, it is important to assess the influence of nonparticipation on the robustness of the inference that is drawn.
Limitations and Strengths
Some differences between participants and nonparticipants may not have been detected because of sample size. Given the demographic homogeneity of the study population, ie, mainly white, our findings require confirmation in other cohorts and in more diverse racial and ethnic groups.
Our study addressed the advantages of combining different sources of DNA to assemble a repository. However, DNA extraction from tissue may lead to DNA fragmentation.35 To overcome this limitation, genotyping (Golden Gate assay; Illumina, San Diego, CA) may be used with fragmented DNA.35
Strengths of our study include its community-based nature, whereby all consecutive patients in a geographically defined population were considered for participation; its cohort design7; and its ability to examine the influence of nonparticipation. The research authorization required by Minnesota law enabled the review of medical records of persons who were unwilling to provide biological specimens for our study, allowing participants and nonparticipants to be compared. The rigorous ascertainment of MI, the comprehensive nature of the clinical and outcome data, and the unique ability to retrieve tissue specimens all strengthened our study.
Conclusions
A variety of sources can be used to assemble a community specimen repository. Baseline characteristics of participants differ from nonparticipants, and among participants, those characteristics differ by specimen source. Whereas participants who provided blood specimens had better outcomes than participants who provided tissue specimens, no survival advantage was detected for participants after combining both specimens. This finding underscores the importance of analyzing clinical characteristics and survival according to participation and specimen source.
Acknowledgments
This study was supported by a Clinician Investigator Fellowship Award from Mayo Clinic and grants from the Public Health Service and the National Institutes of Health (AR30582, R01 HL59205, and R01 HL72435). Dr Roger is an Established Investigator of the American Heart Association.
We are indebted to Susan Stotz, BS, for assistance in data collection and Kristie Shorter for preparation of the submitted manuscript.
References
- 1.Fletcher RH, Fletcher SW. Clinical Epidemiology: The Essentials. 4th. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2005. [Google Scholar]
- 2.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006 Feb 1;163(3):197–203. doi: 10.1093/aje/kwj036. Epub 2005 Dec 7. [DOI] [PubMed] [Google Scholar]
- 3.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Participation bias assessment in a community-based study of myocardial infarction, 2002-2005. Mayo Clin Proc. 2007;82(8):933–938. doi: 10.4065/82.8.933. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14(8):579–584. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg K, Beck J, Nickerson D, et al. DNA banking for epidemiologic studies: a review of current practices. Epidemiology. 2002;13(3):246–254. doi: 10.1097/00001648-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Godard B, Schmidtke J, Cassiman JJ, Ayme S. Data storage and DNA banking for biomedical research: informed consent, confidentiality, quality issues, ownership, return of benefits: a professional perspective. Eur J Hum Genet. 2003;11(suppl 2):S88–S122. doi: 10.1038/sj.ejhg.5201114. [DOI] [PubMed] [Google Scholar]
- 7.Manolio TA, Bailey-Wilson JE, Collins FS. Genes, environment and the value of prospective cohort studies. Nat Rev Genet. 2006;7(10):812–820. doi: 10.1038/nrg1919. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136(5):341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, Killian JM, Weston SA, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006 Aug 22;114(8):790–797. doi: 10.1161/CIRCULATIONAHA.106.627505. Epub 2006 Aug 14. [DOI] [PubMed] [Google Scholar]
- 11.Kors JA, van Herpen G, Wu J, Zhang Z, Prineas RJ, van Bemmel JH. Validation of a new computer program for Minnesota coding. J Electrocardiol. 1996;29(suppl):83–88. doi: 10.1016/s0022-0736(96)80025-2. [DOI] [PubMed] [Google Scholar]
- 12.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention. AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003 Nov 18;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. Epub 2003 Nov 10. [DOI] [PubMed] [Google Scholar]
- 13.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/s0735-1097(00)00804-4. published correction appears in. [DOI] [PubMed] [Google Scholar]; J Am Coll Cardiol. 2001;37(3):973. [Google Scholar]
- 14.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Yawn BP, Yawn RA, Geier GR, Xia Z, Jacobsen SJ. The impact of requiring patient authorization for use of data in medical records research. J Fam Pract. 1998;47(5):361–365. [PubMed] [Google Scholar]
- 17.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 18.Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 19.Roger VL, Killian J, Henkel M, et al. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55(6):593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 20.Eastwood BJ, Gregor RD, MacLean DR, Wolf HK. Effects of recruitment strategy on response rates and risk factor profile in two cardiovascular surveys. Int J Epidemiol. 1996;25(4):763–769. doi: 10.1093/ije/25.4.763. [DOI] [PubMed] [Google Scholar]
- 21.Eaker S, Bergstrom R, Bergstrom A, Adami HO, Nyren O. Response rate to mailed epidemiologic questionnaires: a population-based randomized trial of variations in design and mailing routines. Am J Epidemiol. 1998;147(1):74–82. doi: 10.1093/oxfordjournals.aje.a009370. [DOI] [PubMed] [Google Scholar]
- 22.Eagan TM, Eide GE, Gulsvik A, Bakke PS. Nonresponse in a community cohort study: predictors and consequences for exposure-disease associations. J Clin Epidemiol. 2002;55(8):775–781. doi: 10.1016/s0895-4356(02)00431-6. [DOI] [PubMed] [Google Scholar]
- 23.Jousilahti P, Salomaa V, Kuulasmaa K, Niemela M, Vartiainen E. Total and cause specific mortality among participants and non-participants of population based health surveys: a comprehensive follow up of 54 372 Finnish men and women. J Epidemiol Community Health. 2005;59(4):310–315. doi: 10.1136/jech.2004.024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahar E, Folsom AR, Jackson R. Atherosclerosis Risk in Communities (ARIC) Study Investigators. The effect of nonresponse on prevalence estimates for a referent population: insights from a population-based cohort study. Ann Epidemiol. 1996;6(6):498–506. doi: 10.1016/s1047-2797(96)00104-4. [DOI] [PubMed] [Google Scholar]
- 25.Boshuizen HC, Viet AL, Picavet HS, Botterweck A, van Loon AJ. Non-response in a survey of cardiovascular risk factors in the Dutch population: determinants and resulting biases. Public Health. 2006 Apr;120(4):297–308. doi: 10.1016/j.puhe.2005.09.008. Epub 2005 Dec 20. [DOI] [PubMed] [Google Scholar]
- 26.Lissner L, Skoog I, Andersson K, et al. Participation bias in longitudinal studies: experience from the Population Study of Women in Gothenburg, Sweden. Scand J Prim Health Care. 2003;21(4):242–247. doi: 10.1080/02813430310003309-1693. [DOI] [PubMed] [Google Scholar]
- 27.Bergstrand R, Vedin A, Wilhelmsson C, Wilhelmsen L. Bias due to non-participation and heterogenous sub-groups in population surveys. J Chronic Dis. 1983;36(10):725–728. doi: 10.1016/0021-9681(83)90166-2. [DOI] [PubMed] [Google Scholar]
- 28.Benfante R, Reed D, MacLean C, Kagan A. Response bias in the Honolulu Heart Program. Am J Epidemiol. 1989;130(6):1088–1100. doi: 10.1093/oxfordjournals.aje.a115436. [DOI] [PubMed] [Google Scholar]
- 29.Begg CB, Orlow I, Hummer AJ, et al. Environment and Melanoma Study Group. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst. 2005;97(20):1507–1515. doi: 10.1093/jnci/dji312. [DOI] [PubMed] [Google Scholar]
- 30.Holland NT, Pfleger L, Berger E, Ho A, Bastaki M. Molecular epidemiology biomarkers—sample collection and processing considerations. Toxicol Appl Pharmacol. 2005;206(2):261–268. doi: 10.1016/j.taap.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Hartge P. Raising response rates: getting to yes [editorial] Epidemiology. 1999;10(2):105–107. doi: 10.1097/00001648-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Corbie-Smith G, Viscoli CM, Kernan WN, Brass LM, Sarrel P, Horwitz RI. Influence of race, clinical, and other socio-demographic features on trial participation. J Clin Epidemiol. 2003;56(4):304–309. doi: 10.1016/s0895-4356(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 33.Heilbrun LK, Ross PD, Wasnich RD, Yano K, Vogel JM. Characteristics of respondents and nonrespondents in a prospective study of osteoporosis. J Clin Epidemiol. 1991;44(3):233–239. doi: 10.1016/0895-4356(91)90034-7. [DOI] [PubMed] [Google Scholar]
- 34.Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106(18):2309–2314. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- 35.Shen R, Fan JB, Campbell D, et al. High-throughput SNP genotyping on universal bead arrays. Mutat Res. 2005;573(12):70–82. doi: 10.1016/j.mrfmmm.2004.07.022. [DOI] [PubMed] [Google Scholar]