Abstract
Limb regeneration requires the coordination of multiple stem cell populations to recapitulate the process of tissue formation. Therefore, bone marrow (BM) -derived cell regulation of skeletal muscle regeneration was examined in mice lacking the CC chemokine receptor 2 (CCR2). Myofiber size, numbers of myogenic progenitor cells (MPCs), and recruitment of BM-derived cells and macrophages were assessed after cardiotoxin-induced injury of chimeric mice produced by transplanting BM from wild-type (WT) or CCR2−/− mice into irradiated WT or CCR2−/− host mice. Regardless of the host genotype, muscle regeneration and recruitment of BM-derived cells and macrophages were similar in mice replenished with WT BM, whereas BM-derived cells and macrophage accumulation were decreased and muscle regeneration was impaired in all animals receiving CCR2−/− BM. Furthermore, numbers of MPCs (CD34+/Sca-1−/CD45− cells) were significantly increased in mice receiving CCR2−/− BM despite the decreased size of regenerated myofibers. Thus, the expression of CCR2 on BM-derived cells regulated macrophage recruitment into injured muscle, numbers of MPC, and the extent of regenerated myofiber size, all of which were independent of CCR2 expression on host-derived cells. Future studies in regenerative medicine must include consideration of the role of BM-derived cells, possibly macrophages, in CCR2-dependent events that regulate effective skeletal muscle regeneration.—Sun, D., Martinez, C. O., Ochoa, O., Ruiz-Willhite, L., Bonilla, J. R., Centonze, V. E., Waite, L. L., Michalek, J. E., McManus, L. M., Shireman, P. K. Bone marrow-derived cell regulation of skeletal muscle regeneration.
Keywords: CC chemokine receptor 2, CCR2, chimera, inflammation, monocyte/macrophage, myogenic progenitor cell
Tissue injury and repair are highly complex processes that involve coordinated recruitment, proliferation, and intercellular communications between multiple populations of cells including local (parenchymal) and bone marrow (BM) -derived progenitor cells, inflammatory cells, and vascular endothelial cells (1). In skeletal muscle, this dynamic process includes inflammatory cell recruitment to the injured tissue to remove necrotic debris and initiate the repair process (2), angiogenesis to establish perfusion to the injured tissues (3), and myogenic progenitor cell (MPC) activation, proliferation, and differentiation to repair or replace damaged muscle fibers (4). Although multiple cells types are essential for successful skeletal muscle regeneration, the coordinated proliferation and integrated interactions of these different cell populations are poorly understood.
In mice, monocyte/macrophage recruitment into injured tissues is promoted by chemokines, including the monocyte chemotactic protein (MCP) family members, MCP-1 (also known as CCL2), MCP-3 (CCL7), and MCP-5 (CCL12) (5,6,7). Intercellular signaling by these chemokines is transduced by a specific cell surface receptor: the CC chemokine receptor 2 (CCR2). After injury, MCP-1 (8, 9) and MCP-5 (10) are elevated in muscle tissue for up to 1 wk, and MCP-1 is localized to vascular endothelial cells and macrophages (11). Further, muscle regeneration is impaired in MCP-1−/− mice (10). Because monocytes/macrophages appear to modulate muscle regeneration (2, 12,13,14), it is conceivable that MCP-1-dependent monocyte/macrophage recruitment into injured muscle is a pivotal cellular response to support routine skeletal muscle regeneration.
CCR2, a G protein-coupled, transmembrane cell surface receptor, regulates cellular migration (chemotaxis) and initiates signal transduction cascades, leading to cell activation (15). CCR2 is expressed on a wide variety of BM-derived and non-BM-derived cells (parenchymal cells) including immune/inflammatory cells; monocytes/macrophages, neutrophils, T lymphocytes, B lymphocytes, natural killer cells, and dendritic cells (15, 16), in addition to fibrocytes (17) and endothelial progenitor cells (EPCs) (18). In skeletal muscle, CCR2 is produced by parenchymal cells such as MPCs (19), vascular smooth muscle cells (20), vascular endothelial cells, pericytes, and fibroblasts (21). Previous studies have demonstrated severe impairments in skeletal muscle regeneration in CCR2−/− mice after injury induced by freezing (22), ischemia (9), and cardiotoxin (CTX) injection (8). Although it is clear that skeletal muscle regeneration is severely impaired in CCR2−/− mice (8, 9, 22), the relative contribution of CCR2-mediated events arising from BM-derived vs. parenchymally derived cells is unknown but remains a fundamental question in our understanding of the crucial cellular processes that lead to successful skeletal muscle regeneration. Thus, in the current study we determined the importance of CCR2 expression on BM-derived vs. non-BM-derived cells.
We hypothesized that the crucial CCR2-expressing cell in skeletal muscle regeneration is a BM-derived cell and not a resident, parenchymal cell. Consequentially, CCR2-intact BM-derived cells would be expected to reverse the impaired muscle regeneration phenotype observed in CCR2−/− mice. To test this hypothesis, radiation chimeras were used and revealed that CCR2 expression on BM-derived but not host-derived cells was essential for robust macrophage recruitment and normal muscle regeneration. Surprisingly, MPCs were significantly increased in injured muscle of mice with CCR2-deficient BM cells despite impaired muscle regeneration.
MATERIALS AND METHODS
Experimental animals
CCR2−/− mice on a C57BL/6J background were derived as described previously (23) and backcrossed to C57BL/6J mice from the Jackson Laboratory (Bar Harbor, ME, USA) for 6 generations. Original CCR2−/− breeders were a kind gift from William A. Kuziel (Protein Design Laboratories, Fremont, CA, USA). Enhanced green fluorescence protein (GFP) transgenic mice [GFP-Tg, C57BL/6-Tg (ACTB-EGFP)1Osb/J] were obtained from the Jackson Laboratory; enhanced GFP was driven by a chicken β-actin promoter and cytomegalovirus enhancer, which resulted in all cells except erythrocytes and hair fluorescing green under excitation light. CCR2−/− GFP-Tg were created by crossing CCR2−/− and C56BL/6J-GFP-Tg mice. All GFP mice were heterozygous, and the genotype was confirmed by observing green fluorescence from the mice under a Woods lamp. CCR2−/−, GFP-Tg, and CCR2−/− GFP-Tg mice were bred at the Audie Murphy Veterans Hospital. C57BL/6J wild-type (WT) control mice were purchased from the Jackson Laboratory. The CCR2−/− genotype was confirmed by polymerase chain reaction (PCR) using genomic DNA isolated from tail snips and the following three primers: 1) 5′-GTG AGC CTT GTC ATA AAA CCA GTG-3′; 2) 5′-TCA GAG ATG GCC AAG TTG AGC AGA-3′; and 3) 5′-TTC CAT TGC TCA GCG GTG CT-3′.
Primers 1 and 2 were used to detect the WT gene with a PCR product of 180 bp, and primers 2 and 3 were used to detect the mutant CCR2 gene with a PCR product of 450 bp. All procedures complied with the U.S. National Institutes of Health Animal Use and Care Guidelines and were approved by the institutional animal care and use committees of the University of Texas Health Science Center at San Antonio and of the South Texas Veterans Health Care System.
Creation of radiation chimeras
Male mice 6–8 wk old were used as BM donors and hosts to create chimeric mice. Chimeric mice were constructed as described by Goodell et al. (24). Briefly, host mice (WT and CCR2−/−) were maintained on gamma-irradiated food and acidified water containing neomycin sulfate and polymyxin sulfate B (0.2 and 0.02 mg/ml, respectively; Sigma-Aldrich Corp., St. Louis, MO, USA) for 4 days before and for 4 wk after BM transplantation. A total of 1100 cGy of irradiation was given in divided doses of 620 and 480 cGy administered at least 2 h apart; radiation was generated by a 137Ce gamma ray source using a Gammacell 40 irradiator (Atomic Energy of Canada, Ltd., Ottawa, ON, Canada). BM cells were sterilely isolated from donor mice (defined hereafter as WT/GFP or CCR2/GFP) by flushing the femurs and tibiae with Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA). Marrow fragments were dissociated by triturating through a 25-gauge needle, and the resulting suspension was filtered through 40-μm cell strainers (BD Biosciences, Franklin Lakes, NJ, USA). The filtrate was centrifuged at 4°C for 5 min at 250 g, and the pellet was suspended in Hanks’ balanced salt solution (Invitrogen). For BM reconstitution, 5 × 106 cells were injected into the tail vein of host mice on the same day as the mice were irradiated.
Four groups of chimeric mice were created. Two host control groups (WT/GFP BM donors into WT hosts or CCR2/GFP BM donors into CCR2−/− hosts) were used to control for the effects of BM transplantation. Two experimental groups consisted of mismatched host and donor genotypes: WT/GFP BM donors into CCR2−/− hosts or CCR2/GFP BM donors into WT hosts; these experimental groups determined the effects of CCR2 expression on inflammation, numbers of MPCs, and muscle regeneration after injury in BM-derived vs. host-derived cells. For clarity, the groups will be identified with the following nomenclature: BM donor strain → host strain. Thus, host control (HC) mice were designated as WT → WT or CCR2−/− → CCR2−/− and the experimental groups were designated as WT → CCR2−/− or CCR2−/− → WT.
The percentage engraftment of donor cells, defined as the percentage of GFP+ cells in the peripheral blood of host mice, was analyzed at 4 wk after BM transplant using flow cytometry. In select experiments, animals were also studied at 8 wk after BM transplantation. Blood was collected from the retro-orbital venous complex, and leukocytes were isolated as described previously (25) with the following modifications. Blood was diluted in PBS containing heparin (0.5 U/ml; Baxter, Deerfield, IL, USA), EDTA (1.5 mg/ml; Sigma-Aldrich Corp.), and 1% dextran 500 (Amersham Biosciences, Uppsala, Sweden). Supernatants containing the white blood cells (WBCs) were collected after standing at room temperature for 25 min. The remaining red blood cells were lysed using red blood cell lysis buffer containing 0.144 M ammonium chloride and 17 mM Tris-HCl, pH 7.2. The cells were treated with monoclonal antibody (mAb) 2.4G2 (BD Biosciences, San Jose, CA, USA) for 20 min to block Fcγ II/III receptors followed by treatment with the following fluorescently labeled monoclonal antibodies (all antibodies used for flow cytometry were rat anti-mouse antibodies from BD Biosciences): phycoerythrin (PE) CD45, allophyocyanin (APC) CD3, B220, Gr-1 (clone RB6–8C5), and CD11b as well as the corresponding isotype control antibodies. CD45+ cells were defined as the total WBC population, CD3+ or B220+ cells were defined as the lymphoid lineage, and CD11b+ and/or Gr-1+ cells were defined as the myeloid lineage. The percentages of GFP+ cells within the total WBC population and subsets (lymphoid and myeloid) were determined on the basis of the innate fluorescence of GFP. Only chimeric mice that exhibited ≥80% donor cell engraftment were used. All cell analyses were performed on a FACSAria cell sorter (BD Biosciences), and data analysis was performed using FACSDiva software (BD Biosciences).
Cardiotoxin (CTX) -induced skeletal muscle injury
Chimeric mice (16–24 wk old) received intramuscular CTX (Calbiochem, San Diego, CA, USA) injections into the right hind limb muscles below the knee to induce myonecrosis at 8–16 wk after BM transplantation. Two 50-μl CTX [2.5 μM in normal saline (NS)] injections were delivered uniformly into the muscles of the anterior compartment. Similarly, the posterior compartment received four 50-μl CTX injections. The left leg was injected with identical volumes of NS and served as a control. For flow cytometry and cell sorting experiments, mice received CTX injections in both hind limbs to increase cell yields.
Flow cytometry quantitation of muscle-derived neutrophils, macrophages, and MPCs
Cells from muscle were isolated as previously described (26) with minor modifications (8, 9). Briefly, all of the muscles from the anterior and posterior compartments (muscle between the knee and ankle) received CTX injections and were removed from chimeric mice at 3 or 7 days after injury. Fat was carefully removed from the tissues. Cells were enzymatically dissociated by incubation at 37°C for 90 min in 1% collagenase type II and 2.4 U/ml Dispase (both from Invitrogen) in PBS supplemented with calcium chloride to a final concentration of 2.5 mM and passed through a 40-μm cell strainer. The filtrate was centrifuged at 350 g to harvest the dissociated cells. Trypan blue was added to an aliquot of cells that were counted on a hemocytometer to derive a total cell count and divided by the weight of the tissue. The percentage of cells from each population was determined by flow cytometry (described below) and the absolute number of each cell population was calculated by using the percentage data multiplied by the isolated cells per gram of tissue.
Macrophages and neutrophils were analyzed by a modified procedure for muscle-associated cells, as described previously (27). Neutrophils were defined as CD11b+/Gr-1+ and macrophages were defined as CD11b+/Gr-1− cells (27). Single-cell suspensions were treated with mAb 2.4G2 for 20 min to block Fcγ II/III receptors, followed by incubation with conjugated antibodies at 4°C for 25 min. The fluorescently conjugated monoclonal antibodies (all antibodies were from BD Biosciences, unless otherwise stated) were APC Gr-1 and PE CD11b and the corresponding isotype control antibodies. Isotype controls were used to titrate each antibody to minimize background staining.
MPCs were defined as CD34+/Sca-1−/CD45−, as described previously (28, 29). Briefly, the muscle-associated cell suspension was treated with mAb 2.4G2 for 20 min to block Fcγ II/III receptors, followed by incubation with biotin CD34 (eBioscience, San Diego, CA, USA), followed by streptavidin APC (BD Biosciences), PE-Cy7 Sca-1, and APC-Cy7 CD45 at 4°C for 30 min.
Histology, immunohistochemistry, and confocal microscopy
Chimeric mice were euthanized at 3, 7, and 21 days after CTX and NS injections (4–7 mice/group/time point). Hind limb tissues were collected en bloc from the anterior and posterior compartments, transected axially through the middle portion of the muscle belly, and fixed overnight in 10% neutral buffered formalin for routine paraffin embedding or 4% paraformaldehyde (PFA) in PBS (pH 7.4) at 4°C. PFA-fixed tissues were then serially washed in 10% sucrose in PBS, 20% sucrose in PBS, and 50/50 (v/v) of 20% sucrose in PBS/OCT Embedding Media (Tissue-Tek, Torrance, CA, USA) before snap-freezing in liquid nitrogen-cooled isopentane. Cultured cells were fixed in 4% PFA for 10 min at 4°C.
For immunohistochemical analysis, 6-μm frozen sections or cultured cells were washed with PBS, blocked with 1% BSA (MP Biomedicals, Inc., Aurora, OH, USA) in PBS containing streptavidin (20 μg/ml; Vector Laboratories, Burlingame, CA, USA), and used for immunolocalization after incubation with primary antibodies for 30 min at room temperature; antigens examined included macrophage markers [with rat monoclonal antibodies against Mac3 (1:100; BD Biosciences), F4/80 (1:500; AbD Serotec, Raleigh NC, USA), and CD68, biotinylated (1:40; AbD Serotec)], muscle transcription factors [with rabbit polyclonal antibodies against MyoD (1:50) or myogenin (1:20); both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA], and myosin heavy chain [with a mouse monoclonal antibody, MF20 (1:50) developed by Donald A. Fischman, obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development, maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA]. For MyoD, myogenin, or myosin heavy chain immunolocalization, PFA-fixed specimens were treated with 0.3% Triton X-100 (FisherBiotech, Fair Lawn, NJ, USA) in PBS before the blocking step. Appropriate biotinylated secondary antibodies (mouse absorbed) included rabbit anti-rat immunoglobulin G (IgG; 1:200; Vector Laboratories) and goat anti-rabbit IgG (1:3000; Jackson ImmunoResearch, West Grove, PA, USA). Mouse IgG was detected with goat anti-mouse IgG labeled with Alexa Fluor 568 (1:400; Invitrogen); biotinylated secondary antibodies were detected after incubation with streptavidin conjugated with Alexa Fluor 633 (1:500; Invitrogen) or fluorescein isothiocyanate (FITC) (1:250; BD Biosciences, San Diego, CA, USA). All antibody dilutions were prepared in PBS containing 1% BSA. For all specimen, signals were visualized after nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) and examination with an Olympus FV500 laser scanning confocal microscope (Olympus, Center Valley, PA, USA) or with a Nikon Eclipse TE2000-U microscope equipped with a high-resolution digital camera (Nikon DXM 1200F) interfaced with a personal computer equipped with NIS-Elements software (Nikon, Melville, NY, USA).
Histomorphometric analysis of muscle fiber cross-sectional area (CSA) and injury
For morphometric analyses, 2- to 3-μm cross-sections of anterior compartment specimen were deparaffinized and stained with hematoxylin and eosin (H&E) using routine procedures as described previously (8). For these studies, the tibialis anterior (TA) muscle, the largest muscle in the anterior compartment, was easily identifiable. For histomorphometry, care was taken to avoid TA specimens with tangential or longitudinal presentation of myofibers. Within each section, 4 nonoverlapping areas of the TA were digitally captured (×20 view) using phase-contrast microscopy to enhance the identification of cell borders. Areas containing large blood vessels or fibrous tissue bands between muscle bundles were excluded. All photomicroscopy and histomorphometric analyses were performed in a blinded manner. Thus, animal group, identity, treatment, and duration of treatment were unknown to the observer.
The average CSA (μm2) of individual muscle fibers for a given animal was determined after manual outlining of individual muscle fibers in each of the digitally captured images for a given TA muscle using NIS-Elements software; fibers that were only partially present within the images were excluded from fiber size analysis. Only regenerated fibers (identified by the presence of centrally located nuclei) were measured in the post-CTX specimen, whereas in the post-NS specimen, only mature, nonregenerated fibers with peripherally located nuclei were measured.
The area of muscle regeneration and residual necrosis in the TA muscle of individual animals obtained at 21 days after CTX injection was measured on H&E stained cross-sections (described above). Individual microscope slides were scanned using a model CS ScanScope system (Aperio Technologies, Inc, Vista, CA, USA). The digitally captured slide was processed via ImageScope software (version 7.1.22.1025; Aperio Technologies, Inc) to create an electronic image of the entire anterior compartment. This image was analyzed using NIS-Elements software to measure the total area, area of regeneration, and area of necrosis of the TA. The area of regeneration/residual necrosis was defined on the basis of muscle cells with centrally located nuclei and/or the presence of necrotic myofibers. These area values were used to calculate percentage regeneration and residual necrosis with respect to the entire area of the TA or percentage necrosis with respect to the entire area of regeneration/residual necrosis.
MPC isolation and culture
MPCs were isolated from the bilateral anterior and posterior hind limb compartments of WT mice (3 or 7 days after CTX injection) after enzymatic dissociation of the muscle as described previously (30). Briefly, muscles were minced and incubated at 37°C for 60 min in 0.1% Pronase (Calbiochem) and 25 mM HEPES (Sigma-Aldrich Corp.) in PBS. The cell suspension was passed through a 100-μm cell strainer, and the filtrate was centrifuged at 700 g to harvest the dissociated cells.
Dissociated cells were treated with mAb 2.4G2 to block Fcγ II/III receptors before negative selection for Sca-1 and CD45 by magnetic separation. Cells were incubated with PE Sca-1 (BD Biosciences) and FITC CD45 (BD Biosciences) followed by incubation with anti-PE and anti-FITC microbeads (Miltenyi Biotec, Auburn, CA, USA). Cells were suspended at 104 cells/μl in PBS containing 2% FBS (HyClone, Logan, UT, USA) at pH 7.2 and fractionated into positive and negative populations by sequentially placing the cells through two LS columns using a QuadroMACs magnet (Miltenyi Biotec). The Sca-1−/CD45− cell population generated from magnetic separation was incubated with biotin-labeled CD34 followed by streptavidin APC before sorting for CD34+/Sca-1−/CD45− cells on a FACSAria cell sorter; 1 μg/ml propidium iodide (Sigma-Aldrich Corp.) was used to exclude dead cells.
Sorted cells (CD34+/Sca-1−/CD45−) averaged >96% purity and were plated at 105 cells/35-mm culture dish coated with enactin collagen IV laminin (Millipore, Danvers, MA, USA) in growth media containing F-10 media (Invitrogen), 20% FBS, 1% antibiotic-antimycotic solution (Cellgro, Manassas, VA, USA), and 10 ng/ml fibroblast growth factor-2 (Promega, Madison, WI, USA). The culture media was changed to differentiation media containing DMEM, 2% horse serum (HyClone), and 1% penicillin-streptomycin solution (Cellgro).
Data analyses
SAS software (SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses. Results from corresponding time points of each group were averaged and used to calculate descriptive statistics (mean±se). All statistical testing was two-sided with a significance level of 5%.
All data were analyzed using two-way analysis of variance; data for number of cells isolated, GFP+ cells, GFP− cells, neutrophils, macrophages, and MPCs were log-transformed to correct non-normality. Two factors (strain and donor) were included in the model along with the interaction of the two factors. The interaction term was included to allow for the possibility that one or more groups differed from the predicted result from strain and donor genotype alone or, equivalently, for the possibility that the relationship between the outcome variable and strain changed with donor genotype. Tukey’s method to correct for multiple comparisons was used on all possible pairwise comparisons among the 4 chimeric groups at each time point. In addition, data for number of cells isolated, GFP+ cells, GFP− cells, neutrophils, macrophages, and MPCs within each chimeric group at the 3- and 7-day time points were compared using unpaired t tests with a Bonferroni correction, whereas muscle CSAs of regenerated and mature fibers within each chimeric group were compared using paired t tests with a Bonferroni correction.
RESULTS
BM donor cell engraftment in chimeras was similar in all groups
Donor BM engraftment was estimated by flow cytometry as the percentage of GFP+ circulating WBCs; the entire WBC population and subsets (lymphoid and myeloid) were assessed. Overall, chimeras had 85 ± 1% GFP+ WBC. In a representative subset of each chimeric group with >80% engraftment, WBCs were further defined (Table 1). Overall, B lymphocytes (B220+) and myeloid cells (CD11b+ and/or Gr1+) were ≥87% GFP+, whereas T lymphocytes (CD3+) exhibited a lower percentage of engraftment (range 57–67%). All chimeric groups exhibited a similar percentage of engraftment of the WBC population and subsets (Table 1). This engraftment was stable over 4–8 wk; GFP+ WBCs (%) in the peripheral blood of WT → WT chimeras at 8 wk after BM transplantation were as follows: CD45+, 90 ± 0%; CD3+, 59 ± 1%; B220+, 80 ± 1%; and CD11b+ and/or Gr-1+, 92 ± 3% (mean±se, n=4). Only chimeric mice with ≥80% engraftment were used for all subsequent studies.
TABLE 1.
Extent of BM engraftment (GFP+) of circulating white blood cells at 4 wk after BM transplantation
| Donor BM → host | GFP+ cells
|
|||
|---|---|---|---|---|
| Total WBCs: CD45+ | Lymphoid cells
|
Myeloid cells: CD11b+/Gr-1+ | ||
| CD3+ | B220+ | |||
| WT → WT (HC) | 87 ± 1 | 57 ± 2 | 90 ± 1 | 92 ± 0 |
| WT → CCR2−/− | 87 ± 1 | 62 ± 4 | 91 ± 1 | 93 ± 1 |
| CCR2−/− → CCR2−/− (HC) | 86 ± 1 | 60 ± 2 | 92 ± 0 | 87 ± 1 |
| CCR2−/− → WT | 87 ± 1 | 67 ± 2 | 93 ± 1 | 87 ± 2 |
Data presented as mean ± se %GFP+ cells; n = 10–22 mice/group. CD45+ cells were defined as total WBCs, CD3+ cells were defined as T lymphocytes, B220+ cells were defined as B lymphocytes, and CD11b+ and/or Gr-1+ cells were defined as myeloid cells (neutrophils or monocytes). HC mice were used to control for the effects of BM transplantation.
Histological evaluation of muscle injury, inflammation, and regeneration after CTX injury
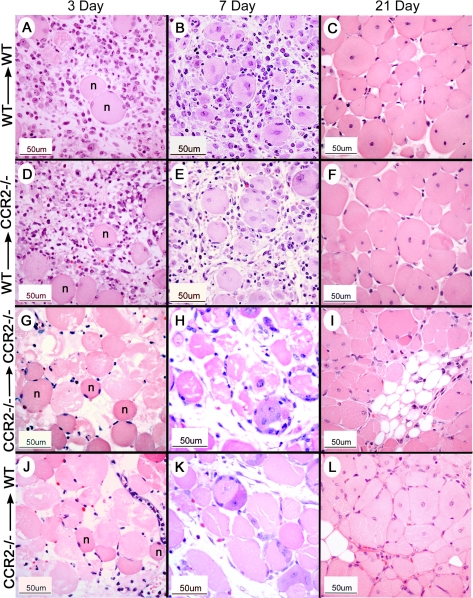
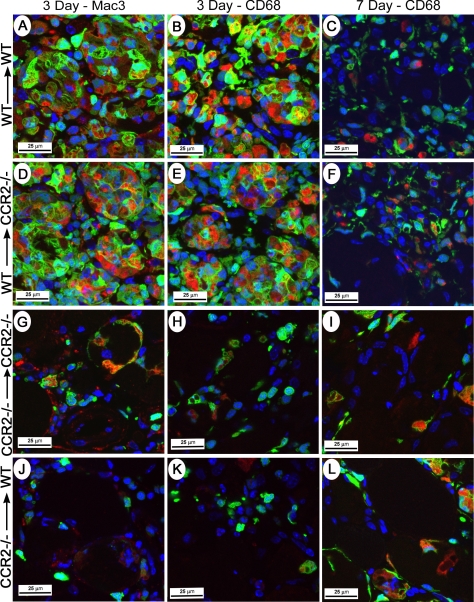
In the control, NS-injected muscle of all groups, there were rare foci of skeletal muscle regeneration, a pattern consistent with muscle damage from the needle used for injection. After CTX injury, however, the genotype of the BM donor determined the histological appearance of the muscle; that is, both groups of host mice receiving WT BM had a similar histological appearance, whereas both groups of host mice receiving CCR2−/− BM were comparable. Of importance, there were distinctly different histological patterns, depending on the genotype of the BM donor. Thus, at 3 days after CTX injury, a robust, predominately mononuclear, inflammatory infiltrate was evident within injured muscle in all host mice receiving WT BM (Fig. 1A, D). The majority of these mononuclear cells were of donor BM origin as indicated by the presence of GFP and were strongly positive for multiple macrophage markers, Mac3 and CD68 (Fig. 2A, B, D, E) as well as F4/80 (not shown). The histological patterns were similar using all 3 macrophage markers, and cells positive for macrophage markers were generally also positive for GFP. Although still abundant, cells positive for macrophage markers were decreased at day 7 (Fig. 2C, F) compared with day 3 after CTX injury. Small, regenerated muscle fibers were present at day 7 (Fig. 1B, E). In contrast, host mice receiving CCR2−/− BM exhibited minimal mononuclear cell infiltrates at both day 3 (Fig. 1G, J) and day 7 (Fig. 1H, K) after CTX treatment; consistent with this histological pattern, few GFP+, Mac3+, or CD68+ cells were present at day 3 (Fig. 2G, H, J, K) or day 7 (Fig. 2I, L). At both days 3 and 7 after CTX treatment, the extent of infiltrating cells in mice receiving CCR2−/− BM remained substantially less than that of mice receiving WT BM. In all chimeric groups at 21 days after injury, regenerated fibers were widespread and identified by the presence of centrally located nuclei; however, muscle fiber size appeared to be larger in host mice receiving WT BM (Fig. 1C, F) than in host mice receiving CCR2−/− BM (Fig. 1I, L).
Figure 1.
BM-derived cells define the histopathological appearance of early inflammation and subsequent regeneration in injured skeletal muscle. Representative results were derived from CTX-injured skeletal muscle from each chimeric group; BM donor and recipient strains are indicated as the BM donor strain → host mouse strain. A–C, G–I) host control groups. D–F, J–L) experimental groups at 3, 7, or 21 days after CTX, as indicated at the top of each column. H&E stain. n, normal muscle fibers.
Figure 2.
BM-derived cells define early inflammation after skeletal muscle injury. Representative results were derived from injured skeletal muscle from chimeric mice at 3 or 7 days after CTX; BM donor and recipient strains are indicated as the BM donor strain → host mouse strain. A–C, G–I) host control groups. D–F, J–L) experimental groups. Most Mac3+ and CD68+ positive cells identified by immunohistochemical analysis (red signal) were also GFP+ (green signal) and in combination, appeared as an orange signal; these cells were abundant in host mice receiving WT BM (A–F). Few Mac3+, CD68+, or GFP+ cells were present in host mice receiving CCR2−/− BM (G–L). Confocal image with nuclei identified by DAPI (blue).
Expression of the myogenesis markers, MyoD and myogenin, was minimal at day 3 after CTX treatment in all groups with only rare positive cells identified in areas of injury. At day 7 after CTX injury, however, both MyoD+ and myogenin+ cells were widespread within the regenerating muscle of mice that received WT BM (Fig. 3A, B, E, F) and were reduced within the poorly regenerated muscle of mice that received CCR2−/− BM (Fig. 3C, D, G, H). Fluorescent signals for MyoD or myogenin did not colocalize with GFP, indicating that MyoD+ and myogenin+ cells originated from the host mouse.
Figure 3.
MyoD and myogenin expression in chimeric mice and in vitro myogenic potential of CD34+/Sca-1−/CD45− cells. Representative results were derived from injured skeletal muscle of chimeric mice at 7 days after CTX; BM donor and recipient strains are indicated as the BM donor strain → host mouse strain. MyoD-positive cells (red signal) (A–D) and myogenin-positive cells (red signal) (E–H) cells were more abundant in host mice receiving WT BM compared with mice receiving CCR2−/− BM. Whereas MyoD- and myogenin-positive cells were present in areas containing BM-derived cells (green signal), the myogenic markers did not colocalize with the GFP signal. I, J) Myotube formation in cultured CD34+/Sca-1−/CD45− cells isolated from hind limb muscle 3 days after CTX injection: phase contrast (I) and corresponding epifluorescent colocalization of MyoD (green) and myosin heavy chain (red) (J). All nuclei (identified with DAPI) were MyoD-positive. Myosin-negative mononucleated cell adjacent to myotube (arrowhead); myosin-positive mononucleated cell between mature myotubes (arrow).
Effect of CCR2 expression by BM-derived cells on regenerated muscle fiber size and area of injury
The CSAs (μm2) of mature muscle fibers with peripherally located nuclei in NS control TA muscle and the CSA of regenerated fibers with centrally located nuclei were measured in injured muscle at day 21 after injection. In each group of chimeras, regenerated fiber size was significantly (P≤0.009) decreased compared with mature fiber size (Table 2). Although mature fiber size was similar in all chimeric groups after NS injection, regenerated fiber size was significantly (P<0.001) dependent on the genotype of the BM donor but independent of the host mouse genotype. In both groups of host mice receiving WT BM (WT→WT and WT→CCR2−/−), fiber size was comparable and significantly (P<0.001) greater than that of host mice receiving CCR2−/− BM. Thus, WT BM reversed the impaired muscle regeneration that occurred in CCR2−/− host mice, whereas CCR2−/− BM resulted in impaired muscle regeneration in WT host mice.
TABLE 2.
Effect of BM donor genotype on regenerated muscle fiber size and area of regeneration and residual necrosis
| Donor BM → host | CSA (μm2)
|
Area of regeneration/necrosis: % TA (CTX) | |
|---|---|---|---|
| Regenerated fibers (CTX) | Mature fibers (NS) | ||
| WT → WT (HC) | 1463 ± 34‡ | 2605 ± 105* | 81 ± 6 |
| WT → CCR2−/− | 1367 ± 57‡ | 2665 ± 39* | 83 ± 9 |
| CCR2−/− → CCR2−/− (HC) | 908 ± 65† | 2855 ± 180* | 86 ± 7 |
| CCR2−/− → WT | 940 ± 52† | 2412 ± 148* | 83 ± 7 |
Regenerated (centrally located nuclei) and mature (peripherally located nuclei) muscle fiber cross-sectional area and the area of muscle regeneration and residual necrosis were measured in a TA muscle specimen obtained at 21 days after CTX or NS injection. HC mice were used to control for the effect of BM transplantation. Data presented as means ± se; n = 4–7 mice/group.
P ≤ 0.009 vs. regenerated fibers.
P < 0.001 vs. WT → WT (HC).
P < 0.001 vs. CCR2−/− → CCR2−/− (HC). There were no significant differences among the four groups for mature fiber size or the area of regeneration and residual necrosis.
The area of TA regeneration and residual necrosis at day 21 was similar in all 4 chimeric groups (Table 2). Residual myofiber necrosis did not persist in mice that received WT BM. In contrast, focal areas of necrosis were present in 4 of 6 CCR2−/− → CCR2−/− mice and 2 of 7 CCR2−/− → WT mice with the average area of necrosis being 0.7 ± 0.3 and 1.1 ± 1.0% of the area of regeneration/residual necrosis, respectively.
Tissue accumulation of BM-derived cells after CTX injury
The number of cells isolated from the injured tissue of mice (Table 3) receiving WT BM was approximately double that of mice receiving CCR2−/− BM at day 3 (P<0.001). However, by day 7, there were no significant differences in number of cells isolated between the 4 chimeric groups. Within each chimeric group, the total number of cells isolated at day 3 after CTX injection was comparable with that of day 7. The decreased number of isolated cells from mice receiving CCR2−/− BM was attributable to fewer BM-derived GFP+ cells (P<0.001 at both days 3 and 7 for chimeric groups receiving WT BM vs. CCR2−/− BM) as the numbers of cells lacking GFP, defined as host-derived, were similar in all 4 groups at days 3 and 7 after CTX injection (Table 3). Thus, BM-derived cell recruitment was severely impaired in mice receiving CCR2−/− BM.
TABLE 3.
Effect of BM donor genotype on BM cell recruitment to injured muscle
| Donor BM → host | Number of cells isolated cells × 106/g tissue
|
GFP+ cells × 106/g tissue (% cells)
|
GFP− cells × 106/g tissue (% cells)
|
|||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 3 | Day 7 | Day 3 | Day 7 | |
| WT → WT (HC) | 66 ± 7† | 53 ± 7 | 44 ± 7† | 24 ± 3†‡ | 22 ± 2 | 28 ± 6 |
| (65 ± 4) | (48 ± 5) | (34 ± 3) | (52 ± 5) | |||
| WT → CCR2−/− | 58 ± 2† | 49 ± 7 | 39 ± 1† | 23 ± 4† | 18 ± 3 | 26 ± 5 |
| (68 ± 4) | (47 ± 4) | (31 ± 4) | (53 ± 4) | |||
| CCR2−/− → CCR2−/− (HC) | 29 ± 1* | 37 ± 5 | 8 ± 2* | 4 ± 1* | 21 ± 2 | 33 ± 5 |
| (28 ± 7) | (10 ± 1) | (71 ± 7) | (90 ± 1) | |||
| CCR2−/− → WT | 26 ± 2* | 33 ± 5 | 9 ± 1* | 4 ± 1* | 17 ± 2 | 29 ± 5 |
| (34 ± 4) | (13 ± 3) | (65 ± 4) | (87 ± 3) | |||
Single-cell suspensions from muscle were obtained on the indicated day after CTX-induced injury and analyzed by flow cytometry. HC mice were used to control for the effect of BM transplantation. GFP+ cells originated from the BM donor, whereas GFP− cells were defined as originating from the host mouse. Data are presented as means ± se; n = 4–6 mice/group.
P < 0.001 vs. WT → WT (HC).
P < 0.001 vs. CCR2−/− → CCR2−/− (HC).
P = 0.05 vs. day 3. Results for GFP− cells did not differ significantly within or between groups.
Flow cytometry was also used to determine the accumulation of neutrophils (CD11b+/Gr-1+) and macrophages (CD11b+/Gr-1−) in injured muscle after CTX injection (Fig. 4A). Among all chimeric groups, intramuscular neutrophils were increased and were significantly (P≤0.008) higher at day 3 compared with day 7 (Table 4). The BM donor genotype but not the host mouse genotype affected the magnitude of neutrophil recruitment into injured muscle. Thus, at day 3 after CTX, tissue neutrophils in CCR2−/− → CCR2−/− mice were significantly (P=0.05) increased compared with WT → CCR2−/− mice, and at day 7 after CTX, neutrophils were significantly (P≤0.03) higher in host mice that received CCR2−/− BM than in host mice that received WT BM.
Figure 4.
Flow cytometry identification of tissue macrophages/neutrophils and tissue MPCs at 7 days after CTX-induced injury. Representative results were derived from CTX-injured skeletal muscle from chimeric mice; BM donor and recipient strains are indicated as the BM donor strain → host mouse strain. A) Monocytes/macrophages were defined as CD11b+/Gr-1− cells (left top quadrant) and neutrophils were defined as CD11b+/Gr-1+ cells (right top quadrant). MPCs were defined as CD34+/Sca-1−/CD45− cells. B) Representative histograms in chimeric mice; events contained within the white curve represent cells before addition of antibodies (i.e., contained GFP fluorescence only), whereas events contained within the gray curve were obtained after the addition of CD34, Sca-1, and CD45 antibodies, CD34+ cells gated in R3. C) Dot plot analysis of Sca-1 and CD45 expression on CD34+ cells derived from R3, above. Bottom lower quadrant (Sca-1−/CD45−) represents MPCs.
TABLE 4.
Effect of BM donor genotype on neutrophil, macrophage, and MPC accumulation in injured muscle
| Donor BM → host | Neutrophils × 106/g tissue (% cells)
|
Macrophages × 106/g tissue (% cells)
|
MPC × 106/g tissue (% cells)
|
|||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 3 | Day 7 | Day 3 | Day 7 | |
| WT → WT (HC) | 6.3 ± 0.9 | 0.4 ± 0.1*† | 43.0 ± 5.1† | 18.4 ± 2.7*† | 2.4 ± 0.1† | 5.5 ± 1.7† |
| (9 ± 1) | (1 ± 0) | (65 ± 2) | (37 ± 6) | (4 ± 0) | (10 ± 2) | |
| WT → CCR2−/− | 5.0 ± 0.5† | 0.4 ± 0.2*† | 37.2 ± 1.5† | 18.7 ± 3.7*† | 1.8 ± 0.3† | 4.1 ± 0.9† |
| (9 ± 1) | (1 ± 0) | (65 ± 3) | (38 ± 4) | (4 ± 1) | (8 ± 2) | |
| CCR2−/− → CCR2−/− (HC) | 9.4 ± 1.5 | 1.6 ± 0.4*‡ | 2.0 ± 0.6‡ | 1.6 ± 0.5‡ | 7.3 ± 1.2‡ | 22.2 ± 3.3*‡ |
| (32 ± 4) | (4 ± 1) | (7 ± 2) | (4 ± 1) | (19 ± 2) | (55 ± 4) | |
| CCR2−/− → WT | 8.5 ± 1.5 | 1.7 ± 0.5*‡ | 1.5 ± 0.4‡ | 1.5 ± 0.6‡ | 5.1 ± 0.8‡ | 17.8 ± 2.3 |
| (32 ± 4) | (5 ± 1) | (6 ± 1) | (4 ± 1) | (25 ± 5) | (61 ± 5)*‡ | |
Single-cell suspensions from muscle were obtained on the indicated day after CTX treatment and analyzed by flow cytometry. HC mice were used to control for the effect of BM transplantation. Data are presented as means ± se; n = 4–6 mice/group.
P ≤ 0.03, day 3 vs. day 7.
P ≤ 0.05 vs. CCR2−/− → CCR2−/− (HC).
P ≤ 0.02 vs.WT → WT (HC).
Tissue macrophages were significantly (P≤0.03) increased at day 3 compared with day 7 in both groups of host mice receiving WT BM but were similar in both groups of mice receiving CCR2−/− BM (Table 4). Tissue macrophages were significantly (P<0.001) dependent on the genotype of the BM donor strain and independent of the host mouse genotype. In both groups of host mice receiving WT BM (WT→WT and WT→CCR2−/−) macrophage accumulation was similarly robust and significantly (P<0.001) increased compared with both groups of host mice receiving CCR2−/− BM at both 3 and 7 days after CTX injury. Thus, macrophage recruitment was severely impaired in the absence of CCR2 expression on BM-derived cells, whereas the absence of CCR2 expression on host-derived cells did not influence the extent of macrophage recruitment.
Increased MPCs in host mice receiving CCR2−/− BM
Numbers of MPCs (CD34+/Sca-1−/CD45−) in injured muscle were determined in the chimeric groups at days 3 and 7 after CTX injection by flow cytometry (Fig. 4B, C). Numbers of MPCs were significantly (P≤0.006) increased at day 7 compared with day 3 in both groups of host mice receiving CCR2−/− BM but not in host mice receiving WT BM (Table 4). Perhaps more important, numbers of MPCs were significantly increased in host mice receiving CCR2−/− BM compared with host mice receiving WT BM at both day 3 (P≤0.02) and day 7 (P≤0.005) after injury; this finding was surprising given the subsequent reduced size of regenerated myofibers in both groups of host mice receiving CCR2−/− BM (Table 2). Thus, in the absence of CCR2 expression on BM-derived cells, numbers of MPCs increased; a paradoxical observation given the muscle regeneration impairments in host mice receiving CCR2−/− BM.
Myogenic potential of CD34+/Sca-1−/CD45− cells
Three and 7 days after CTX treatment, CD34+/Sca-1−/CD45− cells were isolated from the injured muscle of WT mice and cultured in conditions to induce myogenic differentiation (30). The cells formed myotubes that expressed myosin heavy chain and MyoD (Fig. 3I, J), confirming the myogenic potential of CD34+/Sca-1−/CD45− cells.
DISCUSSION
In the present study, we determined whether CCR2 expression by cells originating in the BM regulated skeletal muscle regeneration after injury. This investigation was prompted by observations that muscle regeneration was impaired in animals lacking the CCR2 chemokine receptor (8, 9, 22) and that CCR2 was expressed by cells of both BM and non-BM (parenchymal) origin. We observed that impaired muscle regeneration in CCR2−/− mice was reversed with WT BM transplantation; conversely, muscle regeneration was attenuated in WT mice that received CCR2−/− BM. Macrophage and BM-derived cell recruitment into injured muscle was also dependent on CCR2 expression by BM-derived cells. Somewhat surprisingly, however, numbers of MPCs were increased in the absence of CCR2 expression by BM-derived cells. Taken altogether, our results demonstrate that BM-derived cells were strategically involved in CCR2-dependent intramuscular events that had a profound effect on skeletal muscle regeneration.
In the current study, radiation chimeras were used to delineate the consequences of CCR2 expression by BM-derived vs. host-derived (parenchymal) cells on muscle regeneration. For these investigations, HC mice were included to determine the effects of BM transplantation on reparative events. We determined that the patterns of impaired macrophage recruitment and muscle regeneration in CCR2 host control mice (CCR2−/−→CCR2−/−) were analogous to that of nonchimeric CCR2−/− mice (8, 9). Similarly, the robust macrophage recruitment and larger regenerated muscle fiber size in WT host control mice (WT→WT) was parallel to that in nonchimeric WT mice (8, 9). Thus, host control chimeric mice recapitulated a nonchimeric phenotype.
Previous studies using nonchimeric mice have uniformly demonstrated severe impairments in skeletal muscle regeneration in CCR2−/− mice after injury (8, 9, 22). Macrophage recruitment in CCR2−/− mice, however, was severely decreased after ischemic (9) and CTX (8) injury; in contrast, after freeze injury (22), macrophage accumulation was similar at early time points and increased in CCR2−/− mice at day 14 compared with WT mice. In the freeze injury model, macrophage accumulation was estimated using thresholding of Mac-3 immunolocalized sections. In the current study and in previous reports demonstrating impaired macrophage recruitment, (8, 9), flow cytometry was used to quantitate cells. Flow cytometry reflects a larger, more representative sampling of tissue macrophage content, whereas histological approaches are prone to sampling error. Furthermore, freeze injury exhibits different zones of injury (22), which may influence macrophage quantitation. Thus, different injury models as well as macrophage enumeration methods may explain the disparities in macrophage recruitment in CCR2−/− mice. Nevertheless, the observations in the current study are consistent with extensive reports of impaired macrophage recruitment in CCR2−/− mice in many different models (23, 31,32,33,34,35,36,37).
BM-derived inflammatory cells are integral to the coordinated cellular events leading to the successful repair of injured tissues. Indeed, the mere presence of inflammatory cells within muscle is recognized as a hallmark of injury (2). Nevertheless, a role for specific inflammatory cells in directly promoting tissue regeneration remains to be established. For instance, neutrophils could contribute to CCR2-dependent modulation of muscle regeneration. However, in the present study, neutrophils accumulated within injured muscle among all chimeric groups (Table 4) despite decreased myofiber growth only in animals receiving CCR2−/− BM. The presence of neutrophils among all chimeric groups was not unexpected because the chemoattraction and activation of neutrophils does not generally depend on CCR2 (15). However, the modest persistence of neutrophils in animals with CCR2−/− BM may reflect a reduction in macrophage-mediated neutrophil clearance (38, 39). Given that neutrophils were significantly increased in chimeras with CCR2−/− BM, it is conceivable that the continued presence of neutrophils may have had a negative impact on myofiber growth. Parenthetically, neutrophils can extend muscle injury and membrane damage (2, 40). Additional studies are required to define the basis for delayed neutrophil clearance and its relationship to CCR2-dependent events in muscle regeneration.
Perhaps the most studied inflammatory cell relative to muscle regeneration is the macrophage, a BM-derived cell with established activities that could facilitate tissue regeneration after injury. For example, macrophages clear necrotic myofibers (2, 41), promote angiogenesis (42), produce growth factors, chemokines, and MPC chemotactic factors (43), and decrease MPC apoptosis (44, 45). Macrophages are clearly involved in the recovery of injured muscle as the selective ablation of macrophages resulted in impaired muscle regeneration (12,13,14). Consistent with these findings and together with the established role of CCR2 and MCP-1 in the chemotaxis of monocytes/macrophages, there was a virtual absence of macrophages within the injured muscles of animals that received CCR2−/− BM in the present study. Although these results provide additional evidence in support of an essential role for macrophages in skeletal muscle regeneration, definitive macrophage-mediated mechanisms of skeletal muscle regeneration remain to be established.
Other BM-derived cells, including EPCs (46) and other progenitor cells (47), could also contribute to muscle regeneration. EPCs express CCR2 and the inhibition of CC chemokine receptors decreased EPC homing in vivo (18). In addition, multipotent adult progenitor cells that can differentiate in vitro and in vivo into cells/tissues of all three germinal layers may emanate from the BM (48). Thus, it is conceivable that the absence of BM-derived progenitor cells may have resulted in impaired muscle regeneration in chimeric mice reconstituted with CCR2−/− BM.
CCR2 expression by host-derived cells was not essential for muscle regeneration in the present study. Previous investigations with chimeric mice documented that although MPCs could originate from BM, the vast majority of MPCs were host-derived (49). On this basis, it seems unlikely that BM-derived MPCs account for the observations in our study.
Similarly, CCR2 expression by host-derived cells was also not required for macrophage accumulation within injured muscle in the present study. Rather, CCR2 expression only on BM-derived cells was essential for macrophage accumulation within injured muscle (Table 4). Despite the possibility that CCR2 expression on vascular endothelial cells may have been required for the transendothelial migration of monocytes in response to MCP-1 (50), it seems unlikely that BM transplantation had repopulated the vascular endothelium in chimeric animals with CCR2−/− herein. Although tissue endothelial cells could be BM-derived, there is considerable variation in the incorporation of BM-derived cells into the capillary endothelium. Thus, variable, but significant, incorporation of BM-derived cells into the vascular endothelium during angiogenesis has been described (51,52,53,54) as has the lack thereof (55,56,57). Nevertheless, in all reports, the majority of endothelial cells during angiogenesis were not BM-derived. Thus, it seems unlikely that BM-derived endothelial cells expressing CCR2 could account for macrophage recruitment in chimeric (WT→CCR2−/−) mice in the present study.
MPCs in this study were defined as CD34+/Sca-1−/CD45− cells as this subpopulation has exhibited myogenic differentiation potential when isolated from both normal (28, 29) and CTX-injured (28) muscle; the myogenic potential of CD34+/Sca-1−/CD45− cells from CTX-injured muscle was confirmed in the present study. CD34 is expressed on many cell populations including hematopoietic cells (58), MPCs (59), and endothelial cells (59, 60); thus, CD45 and Sca-1 were used to exclude immune cells and endothelial cells (60, 61), respectively. Although Sca-1+ MPCs have been reported (61, 62), these cells probably represent a rare MPC population. Given the diversity of cells that could potentially express CD34, cell populations present in injured muscle other than MPCs may also be CD34+/Sca-1−/CD45−, further work to define the in vivo MPC markers during muscle regeneration is ongoing.
An unexpected finding of the current study was the substantial increase in MPCs after injury in chimeric mice receiving CCR2−/− BM in conjunction with impaired muscle regeneration (Tables 2 and 4). Consistent with the impaired muscle regeneration, immunohistochemical studies suggested that MyoD+ and myogenin+ cells were decreased in the injured muscle of mice receiving CCR2−/− BM (Fig. 3). Although the in vivo kinetics of CD34 expression in regenerating muscle is poorly understood, nonchimeric mice exhibit increased CD34+/Sca-1−/CD45− cells after CTX injury compared with baseline specimen (unpublished data). Isolated single muscle fibers demonstrated that newly isolated preparations containing predominately quiescent satellite cells were mostly CD34+ but transiently expressed both CD34 and MyoD in conditions that promoted satellite cell activation and proliferation (59). After 48 h in culture, however, proliferating satellite cells continued to express MyoD but not CD34 (59). Furthermore, the immortal myogenic C2C12 cell line expressed CD34 on a minority of proliferating cells but not on differentiating cells (59). Taken altogether, the increased number of CD34+/Sca-1−/CD45− cells in the presence of impaired muscle regeneration suggests that in the absence of CCR2-expression on BM-derived cells, MPCs may exhibit impairments in proliferation, differentiation, and/or fusion to repair and form new muscle fibers.
With regard to the above, previous in vitro studies have documented the complex, bidirectional interactions between macrophages and MPCs (43, 63,64,65,66,67). Our data support the possibility that in vivo interactions between MPC and CCR2-expressing macrophages may be critical for the normal progression of satellite cell activation, proliferation, and differentiation needed to repair damaged muscle. Alternatively, as previous studies have demonstrated that macrophages decreased MPC apoptosis (44, 45), it is conceivable that reductions in macrophage recruitment may have caused increased MPC apoptosis. Further experiments to resolve these intriguing findings are ongoing.
Increased numbers of MPCs in chimeric mice receiving CCR2−/− BM may also reflect alterations in growth factor and chemokine expression. Multiple growth factors, chemokines, and chemokine receptors are elevated in CTX-injured tissue (68). Although MPC chemotaxis, proliferation, and differentiation have been documented for a variety of growth factors (4), chemokine-MPC interactions and the role of chemokines in muscle regeneration have not been thoroughly studied (reviewed in ref. 69). Finally, alterations in chemokine (8, 70) and cytokine (8, 71) expression have been documented in CCR2−/− mice. Thus, it is conceivable that the increased tissue MPC in chimeric mice receiving CCR2−/− BM may have resulted from changes in cytokine/chemokine expression.
In conclusion, the results of the present study document that normal muscle regeneration is dependent on CCR2 expression by BM-derived cells. Furthermore, macrophages are a likely BM-derived cell type responsible for modulating muscle regeneration, although other BM-derived progenitor cells or inflammatory/immune cells may also contribute. CCR2 may be critical for recruiting essential bone marrow-derived cells important in creating a microenvironment necessary for normal MPC function and muscle regeneration. Further studies are required to understand the relationship between decreased macrophage recruitment and impaired muscle regeneration despite increased MPC within injured muscle.
Acknowledgments
We acknowledge the expert technical assistance of Michael D. Wetzel and Jimmy Wewer in these studies. A portion of the statistical analyses was expertly performed by Lee Ann Zarzabal. The flow cytometry portion of this work was performed at the Institutional Flow Cytometry Core of the University of Texas Health Science Center, San Antonio (UTHSCSA). Images were generated in the UTHSCSA Core Optical Imaging Facility, which is supported by UTHSCSA, CA54174 (San Antonio Cancer Institute), AG013319 (Nathan Shock Center), and AG19316. These studies were supported, in part, by a Veterans Administration Merit Review grant, the San Antonio Area Foundation (Sun-UTHSCSA-060104), and the National Institutes of Health (R01HL074236 and F32HL090196) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no relationships that pose a conflict of interest for these studies.
References
- Grounds M D. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Tidball J G. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Carlson B M, Faulkner J A. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc. 1983;15:187–198. [PubMed] [Google Scholar]
- Hawke T J, Garry D J. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Charo I F, Taubman M B. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem. 1996;271:11603–11607. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- Sarafi M N, Garcia-Zepeda E A, MacLean J A, Charo I F, Luster A D. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa O, Sun D, Reyes-Reyna S M, Waite L L, Michalek J E, McManus L M, Shireman P K. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2007;293:R651–R661. doi: 10.1152/ajpregu.00069.2007. [DOI] [PubMed] [Google Scholar]
- Contreras-Shannon V, Ochoa O, Reyes-Reyna S M, Sun D, Michalek J M, Kuziel W A, McManus L M, Shireman P K. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol. 2007;292:C953–C967. doi: 10.1152/ajpcell.00154.2006. [DOI] [PubMed] [Google Scholar]
- Shireman P K, Contreras-Shannon V, Ochoa O, Karia B P, Michalek J M, McManus L M. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- Shireman P K, Contreras-Shannon V, Reyes-Reyna S M, Robinson S C, McManus L M. MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J Surg Res. 2006;134:145–157. doi: 10.1016/j.jss.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Summan M, Warren G L, Mercer R R, Chapman R, Hulderman T, Van Rooijen N, Simeonova P P. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- Tidball J G, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fiber growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi R K, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I F, Ransohoff R M. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Murphy P M, Baggiolini M, Charo I F, Hebert C A, Horuk R, Matsushima K, Miller L H, Oppenheim J J, Power C A. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Moore B B, Murray L, Das A, Wilke C A, Herrygers A B, Toews G B. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring H, Schuler T, Arnold B, Hammerling G J, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci U S A. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C, Civatte M, Pellissier J F, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol (Berl) 2001;102:385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- Hayes I M, Jordan N J, Towers S, Smith G, Paterson J R, Earnshaw J J, Roach A G, Westwick J, Williams R J. Human vascular smooth muscle cells express receptors for CC chemokines. Arterioscler Thromb Vasc Biol. 1998;18:397–403. doi: 10.1161/01.atv.18.3.397. [DOI] [PubMed] [Google Scholar]
- Carulli M T, Ong V H, Ponticos M, Shiwen X, Abraham D J, Black C M, Denton C P. Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: evidence for autocrine regulation of myofibroblast differentiation. Arthritis Rheum. 2005;52:3772–3782. doi: 10.1002/art.21396. [DOI] [PubMed] [Google Scholar]
- Warren G L, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel W A, Simeonova P P. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- Kuziel W A, Morgan S J, Dawson T C, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell M A, Brose K, Paradis G, Conner A S, Mulligan R C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter M J, Norman K E, Hellewell P G, Ridger V C. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am J Pathol. 2001;159:473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando T A, Blau H M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Weissman I L. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Sherwood R I, Christensen J L, Conboy I M, Conboy M J, Rando T A, Weissman I L, Wagers A J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Lee S, Shin H S, Shireman P K, Vasilaki A, Van Remmen H, Csete M E. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Schober A, Zernecke A, Liehn E A, von Hundelshausen P, Knarren S, Kuziel W A, Weber C. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ Res. 2004;95:1125–1133. doi: 10.1161/01.RES.0000149518.86865.3e. [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel W A, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- Maus U, von Grote K, Kuziel W A, Mack M, Miller E J, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med. 2002;166:268–273. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- Maus U A, Wellmann S, Hampl C, Kuziel W A, Srivastava M, Mack M, Everhart M B, Blackwell T S, Christman J W, Schlondorff D, Bohle R M, Seeger W, Lohmeyer J. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol. 2005;288:L350–L358. doi: 10.1152/ajplung.00061.2004. [DOI] [PubMed] [Google Scholar]
- Yang X, Lu P, Ishida Y, Kuziel W A, Fujii C, Mukaida N. Attenuated liver tumor formation in the absence of CCR2 with a concomitant reduction in the accumulation of hepatic stellate cells, macrophages and neovascularization. Int J Cancer. 2006;118:335–345. doi: 10.1002/ijc.21371. [DOI] [PubMed] [Google Scholar]
- Belperio J A, Keane M P, Burdick M D, Lynch J P, 3rd, Xue Y Y, Berlin A, Ross D J, Kunkel S L, Charo I F, Strieter R M. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel W A, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol. 2004;172:398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- Li P, Garcia G E, Xia Y, Wu W, Gersch C, Park P W, Truong L, Wilson C B, Johnson R, Feng L. Blocking of monocyte chemoattractant protein-1 during tubulointerstitial nephritis resulted in delayed neutrophil clearance. Am J Pathol. 2005;167:637–649. doi: 10.1016/S0002-9440(10)62039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H X, Lusis A J, Tidball J G. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J Physiol. 2005;565:403–413. doi: 10.1113/jphysiol.2005.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimorady-Esfahani A, Grounds M D, McMenamin P G. Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve. 1997;20:158–166. doi: 10.1002/(sici)1097-4598(199702)20:2<158::aid-mus4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Robertson T A, Maley M A, Grounds M D, Papadimitriou J M. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993;207:321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- Sonnet C, Lafuste P, Arnold L, Brigitte M, Poron F, Authier F J, Chretien F, Gherardi R K, Chazaud B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci. 2006;119:2497–2507. doi: 10.1242/jcs.02988. [DOI] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol A C, Poron F, Authier F J, Dreyfus P A, Gherardi R K. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano K F, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo D W, Isner J M, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Jin D K, Shido K, Kopp H G, Petit I, Shmelkov S V, Young L M, Hooper A T, Amano H, Avecilla S T, Heissig B, Hattori K, Zhang F, Hicklin D J, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett N R, Crystal R G, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Li S, Foraker J, Kimura E, Chamberlain J S. Donor origin of multipotent adult progenitor cells in radiation chimeras. Blood. 2005;106:3646–3649. doi: 10.1182/blood-2004-12-4603. [DOI] [PubMed] [Google Scholar]
- Dreyfus P A, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, Butler-Browne G, Gherardi R K. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzenko K A, Song L, Ge S, Kuziel W A, Pachter J S. CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvasc Res. 2005;70:53–64. doi: 10.1016/j.mvr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett N R, Crystal R G, Moore M A, Hajjar K A, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Ruzinova M B, Schoer R A, Gerald W, Egan J E, Pandolfi P P, Rafii S, Manova K, Mittal V, Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- Li H, Gerald W L, Benezra R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res. 2004;64:6137–6143. doi: 10.1158/0008-5472.CAN-04-1287. [DOI] [PubMed] [Google Scholar]
- Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler C M. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung. Cancer J Clin Pathol. 2004;57:965–969. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri M A, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- Wilson A, Oser G M, Jaworski M, Blanco-Bose W E, Laurenti E, Adolphe C, Essers M A, Macdonald H R, Trumpp A. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- Beauchamp J R, Heslop L, Yu D S, Tajbakhsh S, Kelly R G, Wernig A, Buckingham M E, Partridge T A, Zammit P S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P, Beauchamp J. The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation. 2001;68:193–204. doi: 10.1046/j.1432-0436.2001.680407.x. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson J B, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini M, Massimino M L, Bruson A, Catani C, Dalla Libera L, Carraro U. Macrophages regulate proliferation and differentiation of satellite cells. Biochem Biophys Res Commun. 1994;202:1688–1696. doi: 10.1006/bbrc.1994.2129. [DOI] [PubMed] [Google Scholar]
- Massimino M L, Rapizzi E, Cantini M, Libera L D, Mazzoleni F, Arslan P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol. 1995;54:121–128. doi: 10.1097/00005072-199501000-00014. [DOI] [PubMed] [Google Scholar]
- Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut M F. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve. 1999;22:724–732. doi: 10.1002/(sici)1097-4598(199906)22:6<724::aid-mus9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Yan Z, Choi S, Liu X, Zhang M, Schageman J J, Lee S Y, Hart R, Lin L, Thurmond F A, Williams R S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem. 2003;278:8826–8836. doi: 10.1074/jbc.M209879200. [DOI] [PubMed] [Google Scholar]
- Shireman P K. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45:48A–56A. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing M D, Lyon A, Inglis C, Giannoni F, Charo I, Sarawar S R. Chemokine regulation of the inflammatory response to a low-dose influenza infection in CCR2−/− mice. J Leukoc Biol. 2007;81:793–801. doi: 10.1189/jlb.0506299. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic O B, Stamatovic S M, Keep R F, Andjelkovic A V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]