Abstract
Exercise is a mechanism for maintenance of body weight in humans. Morbidly obese human patients have been shown to possess single nucleotide polymorphisms in the melanocortin-4 receptor (MC4R). MC4R knockout mice have been well characterized as a genetic model that possesses phenotypic metabolic disorders, including obesity, hyperphagia, hyperinsulinemia, and hyperleptinemia, similar to those observed in humans possessing dysfunctional hMC4Rs. Using this model, we examined the effect of voluntary exercise of MC4R knockout mice that were allowed access to a running wheel for a duration of 8 wk. Physiological parameters that were measured included body weight, body composition of fat and lean mass, food consumption, body length, and blood levels of cholesterol and nonfasted glucose, insulin, and leptin. At the termination of the experiment, hypothalamic mRNA expression levels of neuropeptide Y (NPY), agouti-related protein (AGRP), proopiomelanocortin (POMC), cocaine- and amphetamine-regulated transcript (CART), orexin, brain-derived neurotropic factor (BDNF), phosphatase with tensin homology (Pten), melanocortin-3 receptor (MC3R), and NPY-Y1R were determined. In addition, islet cell distribution and function in the pancreas were examined. In the exercising MC4R knockout mice, the pancreatic islet cell morphology and other physiological parameters resembled those observed in the wild-type littermate controls. Gene expression profiles identified exercise as having a significant effect on hypothalamic POMC, orexin, and MC3R levels. Genotype had a significant effect on AGRP, POMC, CART, and NPY-Y1R, with an exercise and genotype interaction effect on NPY gene expression. These data support the hypothesis that voluntary exercise can prevent the genetic predisposition of melanocortin-4 receptor-associated obesity and diabetes.—Haskell-Luevano, C., Schaub, J. W., Andreasen, A., Haskell, K. R., Moore, M. C., Koerper, L. M., Rouzaud, F., Baker, H. V., Millard, W. J., Walter, G., Litherland, S. A., Xiang, Z. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse.
Keywords: energy homeostasis, leptin, insulin, α-melanocyte stimulating hormone
Obesity and its associated diseases are becoming more prevalent in society. To combat this growing epidemic, scientists are looking at a myriad of treatment possibilities to both prevent and correct obesity. One well-recognized mechanism is the combination of diet and exercise. Although positive changes in diet are associated with proper cellular nutrition and changes in energy homeostasis, very little is know about the molecular mechanism by which exercise can both prevent and correct increased body weights. Energy and body weight homeostasis are extremely complex physiological mechanisms that rely on endogenous mechanisms as well as genetic and environmental inputs. Herein, we have examined how voluntary exercise can bypass the genetic predisposition for overeating, obesity, and increased serum insulin and leptin levels associated with type 2 diabetes found in the melanocortin-4 receptor (MC4R) knockout (KO) mouse model (1). MC4R gene polymorphisms also have been identified in morbidly obese humans (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17), providing translational potential between humans and the genetic MC4R KO mouse model.
The melanocortin pathway consists of the endogenous agonists derived from the proopiomelanocortin (POMC) gene and the endogenous antagonist agouti-related protein (AGRP) interacting with the melanocortin-3 receptor (MC3R) and MC4R of hypothalamic neurons. Stimulation of the MC3R and/or MC4R pathways by endogenous agonists such as α-melanocyte-stimulating hormone results in activation of the cAMP signal transduction pathway (18,19,20,21,22,23) to generate intra- and intercellular physiological responses that can be blocked or regulated by AGRP (24,25,26). An understanding of the importance of the melanocortin pathway in energy homeostasis developed from human genetic studies (27,28,29), KO and transgenic mouse models (1, 24, 30,31,32,33,34), and the discovery of human polymorphisms (2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17). Central administration of melanocortin agonists led to decreased food intake, whereas similarly administered melanocortin antagonists increased food intake and the sustained increases in food intake (days) on administration of AGRP (34,35,36,37,38,39,40). Melanocortin agonist POMC-expressing neurons have been identified as coexpressing cocaine- and amphetamine-regulated transcript (CART) (41), the MC3R (42, 43), leptin receptors (44, 45), and the neuropeptide Y (NPY) Y1 receptor (46). Melanocortin antagonist AGRP-expressing neurons have been identified as coexpressing NPY (47, 48), the MC3R (42, 43), and leptin receptors (44, 45). The POMC/CART and AGRP/NPY neurons innervate several brain nuclei containing the MC4R that have been suggested to be important for feeding and energy homeostasis, but the exact site of the MC4R feeding behavior “center” remains to be identified, although it is thought to be within the macrostructure of the hypothalamus. Leptin and insulin are adiposity factors that regulate POMC/CART and AGRP/NPY neuronal signaling and associated physiological effects. The leptin and insulin hormones signal in the hypothalamic neurons through the phosphatidylinositol 3-kinase pathway that is inhibited by phosphatase with tensin homology (Pten) (49, 50). Pten has also been identified as an important factor for pancreatic β-cell homeostasis (51). Brain-derived neurotropic factor (BDNF) is expressed in the hypothalamus (ventromedial hypothalamus, dorsomedial hypothalamus, lateral hypothalamus, and paraventricular nucleus) and has been demonstrated to reduce food intake, decrease body weight gain, modify activity (52), and function downstream of the melanocortin receptors in the agouti Ay genetic mouse model (53). The receptor for BDNF is the tyrosine kinase B (TrkB) protein that is widely expressed in the hypothalamus and brain, including the arcuate nucleus, which also contain the POMC/CART- and AGRP/NPY-expressing neurons (52). However, TrkB does not appear to colocalize with either population (53). Thus, the melanocortin pathway is a “key” neuroendocrine pathway responding to several endocrine signals (directly and indirectly) and participating in the complex physiological process of ingestive behavior and energy homeostasis.
In this study, we have examined the effects of 8 wk of voluntary exercise on the genetic MC4R KO mouse model in age- and weight-matched littermate controls. We evaluated several physiological parameters including food intake, body weight, body length, fat mass, lean mass, blood cholesterol, glucose, insulin, and leptin. To begin to search for a putative underlying molecular mechanism, we evaluated mRNA expression levels of NPY, AGRP, POMC, CART, MC3R, NPY-Y1R, orexin, BDNF, and Pten in the hypothalamus of the brain. In addition, we evaluated insulin immunohistochemical staining of pancreatic β-cells and insulin secretion to determine whether voluntary exercise affects insulin secretion capacity of the pancreas in the exercised vs. sedentary MC4R KO mice.
MATERIALS AND METHODS
All studies were performed in accord with accepted standards of humane animal care and were approved by the institutional animal care and use committee at the University of Florida.
Experimental animals
MC4R+/− heterozygous mice were generously provided by Dr. Dennis Huszar (Millennium Pharmaceuticals, Cambridge, MA, USA). The MC4R−/− homozygous KO and wild-type controls used in this experiment were obtained using a heterozygous breeding strategy and were not controlled for litter size (the observed average litter size for these heterozygous MC4R breeding pairs was similar to that of the wild-type breeding pairs in our colony). Genotyping was performed as described previously (1). Mice were housed in a 12-h light/dark cycle (lights on at 11 PM and off at 11 AM) and had ad libitum access to rodent chow and water for the duration of this study. At the initial experimental setup, the groups were littermate-matched for genotype, age, and body weight. No statistically significant differences were observed between the sedentary vs. exercised experimental groups at the initiation of these experiments. The data presented at wk 7 are after 1 wk of experimental treatment.
Running wheel vs. conventional housing experiments
Conventionally housed mice were housed in standard mouse shoebox-size cages singly or in a group of 2 mice/cage as indicated with the corresponding data. Exercising male mice were housed individually in cages containing a running wheel (Mini Mitter Co., Sunriver, OR, USA). Wheel revolutions were quantified by recording the magnetic switch closures of a magnet placed on the revolving wheel and recorded via computer as described previously (54, 55).
Cumulative food intake
Cumulative food intake was determined for singly housed mice by measuring the weight of standard chow (Harlan Teklad 8604 diet; 24% crude protein, 4% crude fat, and 4.5% maximum crude fiber, with digestible energy of 3.30 kcal/g) remaining in the cage top wire feeder 3 times weekly. The bedding was inspected for large intact pellets >0.1 g to decrease the error associated with determining food intake. Feed efficiency (amount of body weight gained relative to the caloric intake) was calculated as the total body weight change each week divided by food intake (kcal) for the respective week with the entire data averaged over the entire experiment for each group.
Body length
Body length (nose to anus, measured in cm) was determined 3 times weekly with a linear ruler after the animals were lightly anesthetized with isoflurane gas.
Nonfasted glucose
Nonfasted blood glucose values were determined using a glucometer (Glucometer Elite XL, Bayer Corporation, Deerfield, IL, USA) with a sensitivity of 20 mg/dl and a range of detection to 600 mg/dl. A whole-blood sample of 1 μl was measured twice weekly. To avoid compounding error in our metabolic and molecular measurement, fasting glucose measurements were not used to prevent a potential effect on expression levels of AGRP, NPY, and other neuroendocrine pathways involved in the regulation of energy homeostasis (56).
Cholesterol
Cholesterol was determined from a 15-μl plasma sample once a week using a strip meter manufactured by Polymer Technology Systems Inc. (Indianapolis, IN, USA). We used PTS Panels Total Cholesterol test strips with a detection range of 100–400 mg/dl.
MRI
Magnetic resonance spectroscopy and imaging of the body composition of the mice in vivo were performed at the University of Florida, McKnight Brain Institute advanced MRI and spectroscopy facility. All animals were imaged on a 4.7-T Oxford magnet using a Bruker Avance console and Bruker ParaVision software (Bruker, Karlsruhe, Germany). The mice were anesthetized by reflexive inhalation of 1.5–2% isoflurane and 1 L of oxygen. A small-animal instrument monitoring and gating system was used for monitoring of and gating on respiratory rate. The mice were centered in a custom-built quadrature transmit/receive birdcage with a radiofrequency coil 5 cm in diameter and 12.5 cm in length. After shimming and rapid acquisition with relaxation enhancement (RARE) encoded pilot scans to confirm position, a single-pulse spectrum was acquired with repetition time (TR) of 4.0 s, sweep width (SW) of 8000, acquisition size of 4000, and 16 averages. The water and lipid peaks were integrated using Bruker XWinNMR software, and lipid was standardized to an integral value of 1.000. After spectral acquisition, a multislice multiecho (MSME)_TOMO RARE 3-dimensional scan was acquired with the following parameters: field of view (FOV), 7.0 × 4.0 × 2.5 cm; matrix, 256 × 128 × 96; TR, 350.0 ms; echo time (TE), 6.8 ms; number of averages (NEX), 1; and RARE factor, 16. These parameters resulted in a resolution of 273 × 313 × 260 μm inplane. Serial transverse slices were collected with a RARE-BIO T2-weighted sequence with the following parameters: FOV, 4.5 × 3.6 cm; thickness, 2.0 mm; matrix, 256 × 256; TR, 3500.0 ms; TE, 7.0 ms; and NEX, 4. These parameters resulted in an inplane resolution of 176 × 141 μm. Liver-localized spectroscopy was performed using stimulated echo acquisition mode (STEAM). Voxel location and size validation were performed on phantoms and via imaging of the voxel in vivo. Voxel size was kept at <40 mm3. All localized spectroscopy scans were respiratory gated. The sequence was triggered at expiration, and TR was kept between 4 and 4.5 s. TE and mixing time were 20 and 10 ms, respectively; 6-kHz SW with 4000 points was collected with 16 averages.
Fat and lean mass determination
The individual mice were placed weekly into a acrylic tube (polymethyl methacrylate) that was then inserted into the mouse EchoMRI-100 instrument (Echo Medical Systems LLC, Houston TX, USA) (57, 58) to determine the mass (g) of the fat vs. lean body composition. Total body weight was measured twice weekly using a standard top-loading laboratory balance.
Hormone assays in blood plasma
Blood-drop samples were drawn from the anterior facial vein into vials containing K2EDTA to prevent coagulation. Plasma was collected by centrifugation and stored at −20°C until analyzed. Plasma hormone levels (insulin and leptin) were measured from a 10-μl sample (in duplicate) using commercially available Endocrine Lincoplex kits (Linco Research, St. Charles, MO) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
At the time of sacrifice, the hypothalamus of the brain was dissected (excised with a razor blade by trimming out all of the brain structures, including the midbrain, cerebellum, and cortex; average hypothalamus weight 17.7±0.6 mg) from each mouse and placed immediately in RNAlater (Ambion Inc., Austin, TX, USA) and held at 4°C for 24 h with transfer to −20°C until RNA extraction. RNA was extracted using TRIzol reagent and quantified, and integrity was confirmed by gel electrophoresis. cDNA was generated from 2 μg of total RNA per the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). qRT-PCR reactions were performed using 100 ng of cDNA as template with TaqMan primers and reagents in a ABI 7300 system (Applied Biosystems). Samples were run in duplicate reactions on a single 96-well plate for each gene probe to confirm consistency in the amount of PCR products. The hypoxanthine guanine phosphoribosyltransferase 1 (Hprt1) housekeeping gene was used to correct for the amount of input mRNA and for data normalization. We selected this housekeeping gene because 1) it did not show any significant differences between genotype or treatment from our microarray experiments, and 2) it is recommended for a mouse housekeeping gene by Applied Biosystems (application note “Using TaqMan® Endogenous Control Assays to Select an Endogenous Control for Experimental Studies,” available at http://www.appliedbiosystems. com). Levels of mRNA are expressed as fold differences compared with the wild-type sedentary group. The fold difference over the control Hprt1 gene is calculated as 2−ΔCt, where ΔCt = Ct(gene) − Ct(Hprt1 gene). Ct is the PCR cycle in which the fluorescent signal associated with the exponential growth exceeds the threshold (10 times noise level).
Statistical analysis
Data are expressed as means ± se. Statistical differences between groups of mice (genotype and exercise) over time were determined using repeated-measures analysis of variance (ANOVA) with the Bonferroni post hoc test. If a significant time × treatment interaction term was observed, individual comparisons between groups at given time points were performed using simple main effects post hoc testing with a Bonferroni correction for the number of comparisons performed to set the experiment-wise probability at P < 0.05 (59). Simple regression analysis was used to examine the relation between phenotypes, and results were considered statistically significant if P < 0.05. For RT-PCR, a two-way ANOVA with the Bonferroni post hoc test using the ΔCt values for genotype effects, exercise effects, and genotype × exercise interactions was used. A simple Pearson correlation regression analysis was used to examine the relation between different experimental parameters. Values of P < 0.05 were considered significant.
RESULTS
Exercise overrides genetically induced obesity in MC4R KO mice
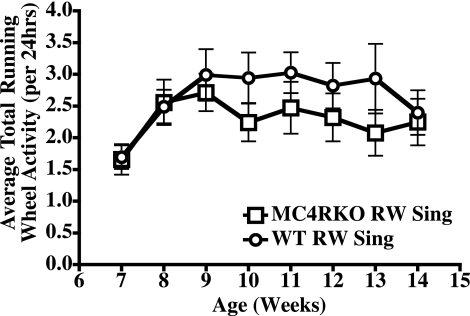
Heterozygous (+/−) breeding of the MC4R KO mice (1), resulted in the generation of the homozygous KO mice (−/−) and wild-type (+/+) littermate mice used in these experiments. Starting at 6 wk of age, 10–12 MC4R KO (−/−) and wild-type male mice (weight- and genotype-matched littermates) were singly housed in a Nalgene cage equipped with a running wheel and allowed free access to voluntary exercise for the experiment duration of 8 wk. At 6 wk of age and the inception of the experiment, the MC4R KO mice had average body weight of 23 g, and no statistically significant difference was observed for the two groups established as conventionally housed and running wheel-housed mice. The average body weight of the wild-type littermate mice was 19 g; again no statistically significant difference was observed for the two groups established as conventionally housed and running wheel housed mice. However, there was a statistically significant difference (P<0.001) between the wild-type and MC4R KO littermate body weights, consistent with previous studies (1). Figure 1 summarizes the average activity between the exercising wild-type and MC4R KO littermates in which no statistically significant differences were observed. Tables 1 and 2 summarize and analyze the physiological parameters measured in this study.
Figure 1.
Summary of the average daily running wheel (RW) activity between the wild-type (WT) and MC4R KO littermates. Values are averages ± se; n = 10–12 mice/group. □, MC4R KO RW mice; ○, wild-type littermate RW mice. No statistical difference was observed between the two groups.
TABLE 1.
Summary of the physiological parameters measured in this study
| Parameter | Week of experiment (age)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (7 wk) | 2 (8 wk) | 3 (9 wk) | 4 (10 wk) | 5 (11 wk) | 6 (12 wk) | 7 (13 wk) | 8 (14 wk) | ||
| Average daily food intake (g) | |||||||||
| WT Conv | 3.7 ± 0.1 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.2 ± 0.03 | 3.2 ± 0.1 | |
| WT RW | 3.9 ± 0.2 | 4.2 ± 0.1de | 4.4 ± 0.1de | 4.3 ± 0.1de | 4.2 ± 0.1de | 4.1 ± 0.1d | 4.1 ± 0.1de | 4.0 ± 0.1d | |
| MC4R KO Conv | 3.8 ± 0.1 | 3.8 ± 0.1a | 3.9 ± 0.02a | 3.6 ± 0.03a | 3.7 ± 0.1a | 3.9 ± 0.1a | 3.6 ± 0.1 | 3.7 ± 0.1a | |
| MC4R KO RW | 3.1 ± 0.2bcf | 4.2 ± 0.1bc | 4.4 ± 0.1bc | 4.3 ± 0.1bc | 4.1 ± 0.2bc | 4.2 ± 0.2c | 4.3 ± 0.1bc | 4.2 ± 0.1bc | |
| Body weight (g) | |||||||||
| WT Conv | 20.8 ± 0.3 | 21.2 ± 0.3 | 21.3 ± 0.3 | 21.6 ± 0.3 | 22.0 ± 0.3 | 22.2 ± 0.3 | 22.5 ± 0.3 | 22.8 ± 0.3 | |
| WT RW | 20.6 ± 0.4e | 20.9 ± 0.4e | 21.5 ± 0.4e | 22.0 ± 0.4e | 22.2 ± 0.4e | 22.3 ± 0.4e | 22.7 ± 0.4e | 22.6 ± 0.3e | |
| MC4R KO Conv | 25.2 ± 0.7a | 25.5 ± 0.6ab | 26.4 ± 0.6ab | 27.5 ± 0.7ab | 28.1 ± 0.7ab | 29.2 ± 0.8ab | 30.3 ± 0.8ab | 31.0 ± 0.8ab | |
| MC4R KO RW | 23.6 ± 0.7cf | 23.0 ± 0.6cf | 23.8 ± 0.6cf | 24.3 ± 0.7cf | 23.9 ± 0.8c | 24.4 ± 0.7c | 24.9 ± 0.8cf | 25.2 ± 0.8cf | |
| Body length (nose to anus, mm) | |||||||||
| WT Conv | 83.2 ± 0.5 | 82.5 ± 0.4 | 83.3 ± 0.5 | 85.2 ± 0.6 | 85.6 ± 0.6 | 86.1 ± 0.6 | 87.0 ± 0.5 | 89.3 ± 0.4 | |
| WT RW | 81.6 ± 0.5e | 83.1 ± 0.5e | 83.3 ± 0.6e | 84.4 ± 0.5 | 85.2 ± 0.5 | 85.7 ± 0.4 | 86.4 ± 0.4e | 88.0 ± 0.7e | |
| MC4R KO Conv | 86.3 ± 0.8a | 85.6 ± 0.4a | 86.8 ± 0.5a | 86.6 ± 0.6 | 87.6 ± 1.0 | 88.2 ± 0.5 | 89.9 ± 0.5a | 92.9 ± 0.4ab | |
| MC4R KO RW | 84.3 ± 1.3f | 84.6 ± 0.7 | 86.0 ± 0.9 | 85.9 ± 0.8 | 86.0 ± 1.2 | 86.6 ± 0.8 | 88.7 ± 0.8 | 89.4 ± 0.8 | |
| Fat mass (% body weight) | |||||||||
| WT Conv | 7 ± 1 | 8 ± 1 | 8 ± 1 | 8 ± 1 | 8 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 1 | |
| WT RW | 7 ± 1e | 7 ± 1e | 7 ± 1e | 7 ± 1e | 7 ± 1e | 8 ± 1e | 7 ± 1e | 7 ± 1e | |
| MC4R KO Conv | 11 ± 1a | 14 ± 2ab | 15 ± 2ab | 18 ± 2ab | 19 ± 2ab | 21 ± 2ab | 23 ± 2ab | 23 ± 2ab | |
| MC4R KO RW | 8 ± 1 | 10 ± 1 | 10 ± 1 | 10 ± 1 | 9 ± 1 | 11 ± 1 | 11 ± 1f | 11 ± 1 | |
| Lean mass (% body weight) | |||||||||
| WT Conv | 79 ± 1 | 80 ± 1 | 79 ± 1 | 79 ± 1 | 79 ± 1 | 80 ± 1 | 81 ± 1 | 78 ± 1 | |
| WT RW | 78 ± 2e | 81 ± 2e | 79 ± 1e | 80 ± 1e | 79 ± 1e | 80 ± 1e | 77 ± 1e | 78 ± 1e | |
| MC4R KO Conv | 74 ± 2a | 75 ± 2ab | 72 ± 2ab | 70 ± 1ab | 70 ± 1ab | 69 ± 1ab | 68 ± 2ab | 66 ± 2ab | |
| MC4R KO RW | 74 ± 1cf | 79 ± 1 | 77 ± 1 | 77 ± 1 | 79 ± 1 | 79 ± 1 | 78 ± 1 | 76 ± 1 | |
| Cholesterol (mg/dl) | |||||||||
| WT Conv | 169 ± 8 | 156 ± 7 | 169 ± 9 | 148 ± 8 | 158 ± 8 | 163 ± 7 | 157 ± 8 | 176 ± 9 | |
| WT RW | 137 ± 8de | 132 ± 9de | 137 ± 10de | 134 ± 9e | 126 ± 8de | 130 ± 8de | 126 ± 8de | 140 ± 11de | |
| MC4R KO Conv | 184 ± 10b | 192 ± 13ab | 185 ± 8 | 180 ± 12a | 197 ± 15ab | 200 ± 14ab | 190 ± 13a | 207 ± 11a | |
| MC4R KO RW | 156 ± 9 | 158 ± 5 | 173 ± 5f | 167 ± 10 | 165 ± 7 | 172 ± 9f | 167 ± 7f | 183 ± 12f | |
| Nonfasted glucose (mg/dl) | |||||||||
| WT Conv | 92 ± 3 | 99 ± 4 | 102 ± 2 | 94 ± 2 | 97 ± 3 | 107 ± 2 | 96 ± 3 | 113 ± 5 | |
| WT RW | 90 ± 7 | 107 ± 3 | 108 ± 4 | 109 ± 2d | 109 ± 2 | 107 ± 5 | 100 ± 2 | 115 ± 4e | |
| MC4R KO Conv | 98 ± 3 | 108 ± 3 | 116 ± 7 | 112 ± 5a | 108 ± 10 | 108 ± 5 | 108 ± 5 | 144 ± 7ab | |
| MC4R KO RW | 83 ± 3 | 114 ± 6 | 108 ± 7 | 105 ± 2 | 104 ± 3 | 104 ± 3 | 99 ± 4 | 117 ± 5 | |
| Insulin (pM) | |||||||||
| WT Conv | 104 ± 6 | 103 ± 5 | 86 ± 4 | 82 ± 5 | 83 ± 4 | 122 ± 7 | 90 ± 6 | 98 ± 9 | |
| WT RW | 136 ± 16 | 140 ± 12e | 118 ± 16e | 96 ± 10e | 135 ± 14e | 143 ± 8e | 110 ± 10e | 106 ± 8e | |
| MC4R KO Conv | 167 ± 14ab | 208 ± 13ab | 180 ± 16ab | 181 ± 15ab | 238 ± 22ab | 250 ± 20ab | 308 ± 28ab | 327 ± 45ab | |
| MC4R KO RW | 110 ± 8 | 131 ± 6c | 117 ± 6c | 112 ± 9c | 132 ± 12c | 140 ± 12 | 121 ± 11 | 131 ± 13 | |
| Leptin (pM) | |||||||||
| WT Conv | 61 ± 5 | 56 ± 7 | 65 ± 6 | 56 ± 6 | 61 ± 4 | 99 ± 8 | 64 ± 5 | 60 ± 10 | |
| WT RW | 50 ± 6e | 60 ± 9e | 47 ± 10e | 48 ± 7e | 56 ± 7e | 80 ± 3e | 54 ± 6e | 64 ± 6e | |
| MC4R KO Conv | 227 ± 29ab | 322 ± 36ab | 345 ± 42ab | 324 ± 32ab | 407 ± 40ab | 500 ± 54ab | 454 ± 45ab | 602 ± 85ab | |
| MC4R KO RW | 89 ± 11 | 114 ± 11 | 78 ± 9 | 88 ± 8 | 97 ± 8 | 140 ± 11 | 104 ± 17 | 138 ± 19 | |
Values indicate the average ± se of n = 10–12 mice/group. Conventionally housed mice were housed 2 mice/cage. Statistical analysis was performed using repeated-measures analysis with the Bonferroni post hoc test for the duration of the experimental weeks (see Table 2). Statistically significant (P<0.05) differences between groups were observed as indicated by footnotes. Conv, conventional housing; RW, running wheel housing; WT, wild type.
WT Conv vs. MC4R KO Conv.
MC4R KO Conv vs. MC4R KO RW.
MC4R KO RW vs. WT Conv.
WT Conv vs. WT RW.
MC4R KO Conv vs. WT RW.
MC4RKO RW vs. WT RW.
TABLE 2.
Repeated measures statistical analysis of the physiological parameters measured in this study
| Parameter | T | T × G | T × E | T × G × E |
|---|---|---|---|---|
| Average daily food intake | F7,189=6.45, P<0.001 | F7,189=6.74, P<0.001 | F7,189=14.69, P<0.001 | F7,189=1.49, P=NS |
| Body weight | F7,280=118, P<0.001 | F7,280=15.82, P<0.001 | F7,280=16.16, P<0.001 | F7,280=16.41, P<0.001 |
| Body length | F7,147=40.04, P<0.001 | F7,147=0.712, P=NS | F7,147=1.74, P=NS | F7,147=0.567, P=NS |
| Fat mass | F7,280=51.86, P<0.001 | F7,280=26.28, P<0.001 | F7,280=27.94, P<0.001 | F7,280=14.85, P<0.001 |
| Lean mass | F7,280=10.45, P<0.001 | F7,280=2.81, P<0.001 | F7,280=5.13, P<0.001 | F7,280=8.18, P<0.001 |
| Cholesterol | F7,252=5.81, P<0.001 | F7,252=2.41, P<0.05 | F7,252=2.14, P<0.05 | F7,252=0.831, P=NS |
| Nonfasted glucose | F7,140=15.04, P<0.001 | F7,140=1.49, P=NS | F7,140=2.05, P=0.053 | F7,140=1.01, P=NS |
| Insulin | F7,462=11.49, P<0.001 | F7,462=10.15, P<0.001 | F7,462=9.16, P<0.001 | F7,462=5.89, P<0.001 |
| Leptin | F7,448=17.13, P<0.001 | F7,448=12.16, P<0.001 | F7,448=8.36, P<0.001 | F7,448=8.62, P<0.001 |
Statistical analysis was performed using repeated measures analysis with Bonferroni post hoc test for the duration of the experimental weeks in terms of time (T) effects, time × genotype (T×G), time × exercise (T×E), and time × genotype × exercise (T×G×E) interactions. NS, nonsignificant.
Body weight of the mice in the 4 experimental groups was measured twice weekly with averages of the 8-wk experiment. During the initial experimental setup, both the MC4R KO and wild-type littermate group body weights were not statistically different between the conventionally housed vs. running wheel-housed groups. However, as already evident at 6 wk of age, the MC4R KO mice (23.1±0.5 g) were heavier than the wild-type mice (19.9±0.3 g) (P<0.001). No statistical difference was observed between the two MC4R KO groups after 1 wk of exercise. Differences in body weight between the MC4R KO groups become apparent after 2 wk of running wheel exercise and persisted throughout the remainder of the 8-wk trial. No statistically significant differences were observed between the wild-type conventionally housed vs. running wheel-housed mice at any time. Despite the observed reduction in body weight in exercised MC4R KO mice, their body weights remained above those of the wild-type animals throughout the study (Table 1).
Exercise normalizes fat to lean body mass in MC4R KO mice
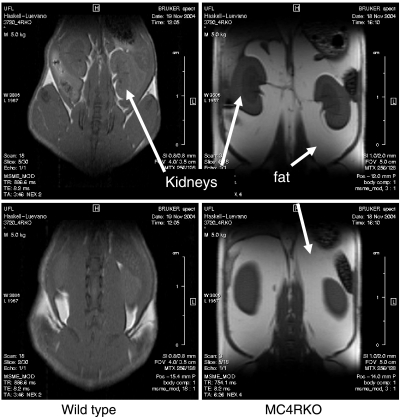
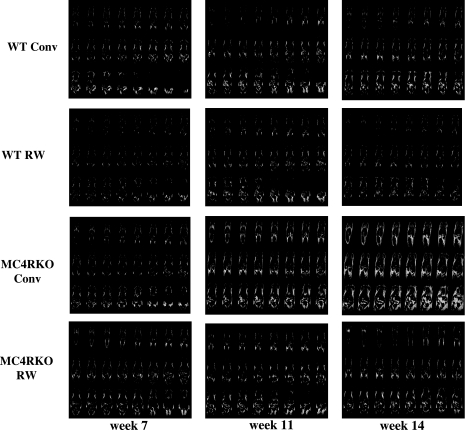
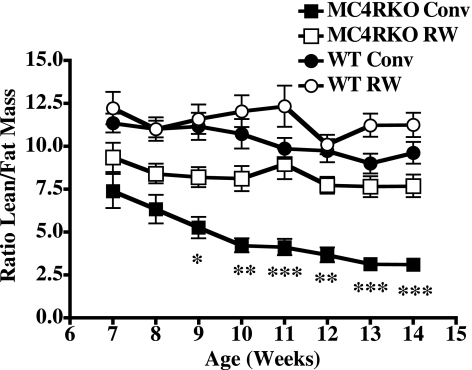
Changes in body weight could be due to a variety of factors. To visualize fat vs. lean body mass changes in mice, we performed MRI. Figure 2 indicates the comparison between a representative wild-type (24 g) and MC4R KO (57 g) sedentary mouse. Figure 3 illustrates a snapshot of representative mice from each group over time of treatment, starting at age 7 wk, after 1 wk of inclusion in the experiment, at age 11 wk, and at age 14 wk, the final week of the experiment after 8 wk of treatment. Increased body weight in the MC4R KO mice appears to be predominately associated with increased fat mass throughout the body, as postulated. MRI is a valuable technique for the visualization and distribution of fat tissue throughout the body; however, it is difficult to quantify such changes using this approach. Therefore, we used low-resolution nuclear magnetic resonance techniques and equipment for the quantitation of live animal fat vs. lean mass. Using the EchoMRI-100 system (57, 58), we analyzed the body composition of our experimental mice weekly, as summarized in Table 1. Statistically significant differences in fat mass between the MC4R KO sedentary housed mice vs. the exercising MC4R KO mice emerged after wk 2 of the experiment, correlating with total body weight (r=0.9, P<0.001). However, there were no statistically significant differences between the MC4R exercised mice vs. the wild-type conventionally housed mice, which is different from the results that were observed for total body weight. Comparison of lean mass showed statistically significant differences between the conventionally housed MC4R KO and wild-type littermate mice, the exercised MC4R KO and wild-type groups, and the MC4R KO exercised and the wild-type sedentary housed groups. However, both the MC4R KO and wild-type groups housed either in traditional conventional housing or in cages equipped with a running wheel possessed the same relative lean mass for the duration of the experiment. Interestingly, comparison of the ratio between lean and fat mass between the different groups revealed that although, as postulated, no significant changes were observed between the wild-type groups and the exercising MC4R KO mice, for the sedentary MC4R KO mice, it appears that while they increase body weight and fat mass, they are also decreasing relative lean mass (Fig. 4).
Figure 2.
MRI of a sedentary WT littermate with a body weight of 24 g and an MC4R KO mouse with a body weight of 57 g. Kidneys and fat are labeled for reference. White/light gray indicates fat mass; darker gray indicates lean mass.
Figure 3.
MRI of a representative littermate mouse from each experimental group over time to compare body fat distribution patterns (white) and the effect of exercise on genotype. Indicated wk 7 age time point is after exposure to 1 wk of experimental treatment (sedentary vs. exercise). Week 11 is after exposure to 5 wk of treatment, and wk 14 is the age of the mice at the termination of the experiment and exposure to experimental treatment for 8 wk.
Figure 4.
Effect of voluntary exercise on the ratio of lean to fat mass over time. Values are averages ± se; n = 10–12 mice/group. ▪, MC4R KO sedentary group; □, MC4R KO RW group; •, WT littermate control sedentary group; ○, WT littermate RW group. Statistical analysis was performed using repeated-measures analysis with a Bonferroni post hoc test for the duration of the experimental weeks in terms of time (F7,208=11.7, P<0.0001) effects, time × genotype (F7,208=1.69, NS), time × exercise (F7,208=3.93, P<0.0001), and time × genotype × exercise (F7,208=0.65, NS) interactions. NS, nonsignificant. Statistically significant differences were observed between the sedentary MC4R KO mice and MC4R KO exercising mice as well as both the wild-type groups as indicated. *P < 0.05, **P < 0.01, ***P < 0.001.
In contrast to total body weight, there were no statistically significant differences in body fat mass between the MC4R KO exercised mice vs. the wild-type sedentary mice. This discrepancy could be attributable to the observed change in body length seen between the MC4R KO and wild-type running wheel-housed mice (Table 1). MC4R KO conventionally housed mice are known to show increased linear growth compared with wild-type mice (1). Consistent with previous reports (1), we observed statistically significant differences in body length between the MC4R KO mice and wild-type littermates housed conventionally. Comparison of the MC4R KO sedentary vs. running wheel- housed groups showed a trend for the exercised mice possessing body lengths intermediate between those of the conventionally housed MC4R KO mice and the wild-type littermates. However, this difference was statistically significant in the MC4R KO groups only in the last week of the experiment. Analysis comparing body length (mm) with total body weight (g) and lean mass (g) correlated well for all groups (r=0.9, P<0.0001).
Consistent with results from our previous study (55), both the wild-type and MC4R KO mice housed in running wheel cages possessed similar food intake and activity patterns with the exception of wk 1 food intake, when the MC4R KO mice consumed less on average then the wild-type littermates (Table 1). These initial differences could be attributed to differences in acclimation behavior or to the stress differences observed in mice possessing disruptions in the melanocortin system (60). Consistent with previous reports, for the duration of the experiment, the sedentary MC4R KO mice were generally hyperphagic compared with the sedentary wild-type littermate mice (P<0.05). The exercising wild-type and MC4R KO littermate groups consumed more food on average then the sedentary housed control groups throughout the experiment (P<0.05). Feeding efficiency averaged over the 8-wk experiment was not statistically different for the exercising MC4R KO (14.3±1.2 g/kcal×103) and wild-type (18.2±0.5 g/kcal×103) littermate groups. However, feeding efficiency over the duration of the experiment was significantly different for the conventionally housed MC4R KO (68.8±0.7 g/kcal×103) and wild-type (25.1±0.4 g/kcal×103) (P<0.001) groups.
Blood glucose and cholesterol levels
In these studies, nonfasted whole-blood glucose levels were examined in the different experimental groups. Blood glucose data showed statistically significant changes during wk 4 and 8 of the experiment (Table 1). Plasma cholesterol values were also monitored for the duration of the experiment. Cholesterol levels were reduced from those of their sedentary controls in both exercised groups to a similar degree, and the cholesterol levels of the sedentary MC4R KO mice were similar to those of the sedentary wild-type littermate control mice.
Blood insulin and leptin concentrations are normalized with exercise in MC4R KO mice
We evaluated the average weekly plasma levels of the different experimental groups in attempts to identify the duration of exercise that becomes critical for preventing increased insulin and leptin levels. Significant differences were observed after only 1 wk of exercise between the MC4R KO mice housed conventionally or in the presence of a running wheel (P<0.01). A second notable difference in insulin and leptin values for the conventionally housed MC4R KO mice occurred at wk 5 of the experiment. Voluntary exercise by the MC4R KO mice prevented increased plasma insulin and leptin levels and maintained values similar to those observed in the wild-type littermate mice. Plasma leptin (pM) values correlated with fat mass (g) (r=0.8 for MC4R KO mice, r=0.6 for wild-type mice, P<0.0001) for all groups and with total body weight (g) (r=0.8 for MC4R KO conventionally housed, r=0.7 for MC4R KO exercised, and r=0.5 for wild-type exercised, P<0.0001; r=0.3 for the wild-type sedentary housed group, P=0.011). Plasma leptin (pM) values correlated well to lean mass (g) for the MC4R KO groups (r=0.5, P<0.0001) but not for the wild-type groups (P=0.13 and 0.58). Plasma insulin (pM) also correlated with total body weight (g) for the MC4R KO groups (r=0.6 for conventionally housed and r=0.5 for running wheel housed mice, P<0.0001) but not for the wild-type groups (P=0.42 and 0.76). Moreover, plasma insulin (pM) values correlated with fat mass (g) for both the MC4R KO groups (r=0.6 for conventionally housed and r=0.3 for running wheel housed mice, P<0.0001) and for the wild-type sedentary group (r=0.3, P=0.014) but not for the wild-type exercised group (P=0.82). In addition, comparison of plasma insulin (pM) and lean mass (g) correlated well for the MC4R KO sedentary group (r=0.3, P=0.0018) and for the MC4R KO exercised group (r=0.4, P<0.0001) but not for the wild-type sedentary (P=0.84) or wild-type exercised (P=0.43) groups. An overall correlation was observed, comparing the plasma insulin and leptin values of all groups (r=0.7, P<0.0001).
MC4R KO liver weights are normalized with exercise in MC4R KO mice
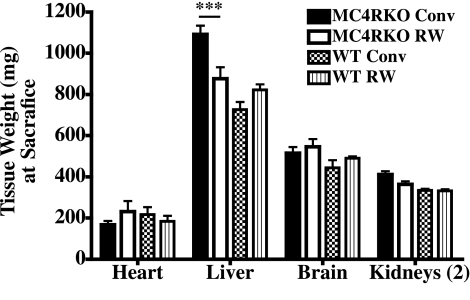
At the termination of the experiment, the heart, liver, brain, and kidneys were harvested and weighed. Only liver weights of the sedentary housed MC4R KO mice were found to be significantly different from those of the exercised MC4R KO mice and the wild-type littermate control groups (P<0.001) (Fig. 5). It is well documented for both humans and the MC4R KO mouse that increased body fat also results in increased fatty livers (61).
Figure 5.
Collected tissue weights (mg) at the termination of the experiment. ▪, MC4R KO sedentary group; □, MC4R KO RW group, ▩, WT littermate sedentary group; ▥, WT RW group. Values are averages ± se; n = 10–12 mice/group. ***P < 0.001; two-way ANOVA with Bonferroni post hoc tests.
MC4R KO prediabetic increase in pancreatic islet area and β-cell mass is normalized by exercise
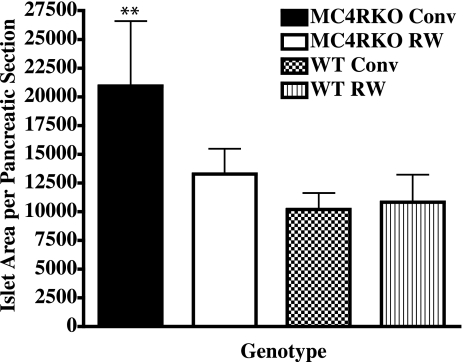
Because the MC4R KO mouse eventually develops characteristic insulin resistance and hyperinsulinemia similar to human type 2 diabetes (1), we examined the effect of voluntary exercise on the islet cell distribution and function in the pancreas of MC4R KO and wild-type littermate mice in our study. We examined the pancreatic islet cell histology and identified β-cell insulin (n=3–4 mice/group). Insulin staining indicated that all four experimental groups possessed similar β-cell numbers (data not shown) but total islet area differed between the MC4R KO conventionally housed mice (P<0.004) and the MC4R KO exercised and both wild-type groups (Fig. 6). These data support the hypothesis that voluntary exercise of the MC4R KO mouse can prevent/delay the onset of type 2 diabetes. These data are consistent with the onset of a prediabetic state with increased circulating insulin levels (Table 1) as well as increased islet size and insulin production that occurs before loss of β-cell function observed in other type 1 and type 2 diabetic mouse models (51, 62, 63).
Figure 6.
At termination of the experiment, pancreatic tissue was immediately excised from mice after death. The tissue was fixed in formalin and imbedded in paraffin for sectioning. Nonserial sections of each sample were then subjected to hematoxylin and eosin staining and insulin immunostaining. Anti-insulin-stained islets were analyzed for β-cell area, number, and endocrine to exocrine tissue ratios using Zeiss Axiophot image analysis software. Individual islet cell areas of 3 nonserial sections of each sample were analyzed. Area values for 3 to 4 animals/group were averaged and compared. Bars represent averages ± se; n = 3–4 mice/group. ▪, MC4R KO sedentary group; □, MC4R KO RW group; ▩, WT littermate sedentary group; ▥, WT RW group. **P < 0.01; two-way ANOVA with Bonferroni post hoc tests.
qRT-PCR reveals a possible signaling feedback mechanism underlying exercise effects on MC4R KO-induced obesity and predisposition for type 2 diabetes
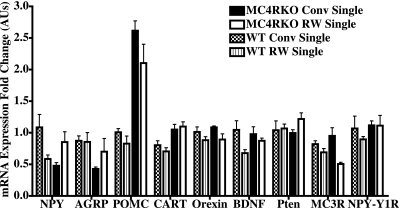
Gene candidates (Fig. 7) for these experiments were selected on the basis of a hypothalamic microarray analysis. Statistical analysis identified these genes as differentially expressed (P<0.01) in the hypothalamus and suggested that these genes in the exercised MC4R KO mice might be differentially regulated in exercised MC4R KO compared with sedentary obese MC4R KO mice. In addition, these genes have also been reported in other rodent models of voluntary exercise to be differentially regulated (64,65,66,67).
Figure 7.
RT-PCR hypothalamic mRNA fold changes in gene expression of individually housed experimental mice. Values are means ± se; 5–6 mice/group. Statistical analysis was performed using two-way ANOVA with the Bonferroni post hoc test using the ΔCt values for genotype effects (G), exercise effects (E), and G × E interactions. Genes found to possess statistical significance include NPY (G×E: F1,17=8.63, P=0.009), AGRP (G: F1,18=11.54, P=0.003), POMC (G: F1,19=22.04, P=0.002; E: F1,19=4.68, P=0.04), CART (G: F1,18=21.6, P=0.0002), orexin (E: F1,18=10.63, P=0.004), MC3R (E: F1,19=12.69, P=0.002), and the NPY-Y1R (G: F1,17=6.87, P=0.01).
POMC/CART and AGRP/NPY mRNAs are expressed primarily in parallel hypothalamic arcuate nucleus neurons and are postulated to be in a regulatory loop by which AGRP/NPY neurons regulate POMC/CART-expressing neurons via the MC3R and NPY-Y1 receptor but not visa versa (26, 68). Results for the RT-PCR mRNA fold expression levels are summarized in Fig. 7. We observed a statistically significant interaction between genotype and exercise for hypothalamic NPY expression (F1,17=8.63, P=0.009). A genotype effect (F1,18=11.54, P=0.003) was observed for AGRP mRNA expression between the wild-type and MC4R KO littermate mice. Both a genotype (F1,19=22.04, P=0.002) and exercise effect (F1,19=4.68, P=0.04) was observed for POMC mRNA expression levels, whereas only a genotype effect was observed for the CART expression (F1,18=21.6, P=0.0002). The MC3R mRNA expression levels were significantly reduced by exercise (F1,19=12.69, P=0.002), whereas the NPY-Y1 receptor expression levels were affected by genotype (F1,17=6.87, P=0.01).
Orexin A, also known as hypocretin 1, has been reported previously to have spontaneous activity in rats when injected into the paraventricular nucleus of the hypothalamus (66). We examined orexin hypothalamic mRNA expression levels (Fig. 7) to determine whether this neurohormone pathway might be involved in the mechanism by which voluntary exercise of the MC4R KO mouse can prevent onset of its phenotypic metabolic syndrome. We found that orexin levels were affected by exercise (F1,18=10.63, P=0.004) but not by genotype. The BDNF gene has been associated with modifying food intake, body weight, and locomotor activity, is up-regulated in the brain by voluntary wheel running in rats (52, 69), and has been linked to the melanocortin system and the MC4R (53). Therefore, we examined the hypothalamic BDNF mRNA expression in our experimental paradigm. However, we did not observe any statistically significant difference in comparing genotype or exercise in our experiment. Similar to BDNF, hypothalamic Pten mRNA expression was not found to be significantly different between genotype or exercise in this study.
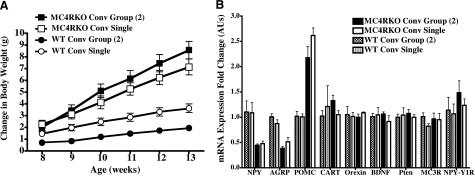
Comparisons of conventionally group housed vs. singly housed phenotypes
To investigate whether differences between paired or singly housed sedentary MC4R KO or wild-type littermate mice possessed similar or distinctly different physiological parameters or gene expression patterns in our experimental paradigm, we compared physiological parameters as well as hypothalamic gene expression patterns (Fig. 8). We observed that although the absolute values were slightly different, comparisons such as changes in body weight (and other measured physiological parameters discussed in our experimental paradigm) over time between singly or paired conventionally housed mice resulted in the same trends and were not statistically significantly different among the same genotype. These data are consistent with previous reports of the MC4R KO phenotype (1, 55, 70). Comparisons of hypothalamic mRNA gene expression levels also were not statistically different between the singly or group conventionally housed mice.
Figure 8.
Comparison of Conv sedentary mice housed either in pairs or singly. A) Changes in body weight during the experiment, starting at age 7 wk after exposure to 1 wk of experimental treatment. Statistical analysis was performed using repeated-measures analysis with the Bonferroni post hoc test. No statistical significance was observed between the paired or singly housed mice per genotype. B) Summary of the RT-PCR hypothalamic mRNA fold changes in gene expression of paired or singly housed Conv mice. Statistical analysis was performed using two-way ANOVA with the Bonferroni post hoc test. No statistical significance was observed between the paired or singly housed mice per genotype.
DISCUSSION
Obesity, including obesity linked to genetic factors and human single nucleotide polymorphisms, is becoming more prevalent in human societies. Exercise is a well-established method to prevent and/or reduce body weight and its effect is enhanced in the presence of a proper diet. Herein, we examined the MCR4R KO mouse genetic model for obesity and type 2 diabetes and discovered that voluntary exercise at a young age can prevent the physiological metabolic syndrome observed under sedentary housing conditions (1, 55). Several models for studying voluntary exercise of male rodents have been reported and reviewed (71). These models include the agouti Ay heterozygous mouse (72, 73), which ectopically overexpresses the MC3R and MC4R antagonist agouti protein (74), the β-endorphin knockout mouse (which is derived from the POMC gene transcript) (75), the obese carboxypeptidease E knockout mice (Cpefat/fat) mouse (76, 77), diet-induced obesity or diet-resistant Sprague-Dawley rats (67, 78), Otsuka Long-Evans Tokushima fatty (OLETF) rats that lack CCK-1 receptors (64, 79), wild-type C57BL/6N mice (80), wild-type Sprague-Dawley rats (65, 78, 81), and Syrian Golden hamsters (82).
Exercise has been previously reported to slow the weight gain of the Ay mouse (72, 73, 83). Chiu et al. (73) examined the effects of diet restriction and voluntary exercise for the obese agouti Ay [B6.Cg-Ay (Ay)] genetic mouse model starting at 15 wk of age for up to 11 wk. Plasma leptin concentrations were ∼3-fold higher in the sedentary Ay mice compared with the sedentary B6 control mice but were not decreased on exercise in either the Ay or control groups. After 8 wk of different experimental housing conditions, as anticipated, the sedentary MC4R KO mice possessed 10-fold increased plasma leptin values compared with the sedentary wild-type littermate controls (P<0.001) and possessed 4-fold higher leptin plasma levels compared with the exercised MC4R KO mice (P<0.001), which possessed 2-fold higher levels than the sedentary control group (Table 1). No difference was observed between the sedentary and exercised wild-type littermate control groups. Thus, the agouti Ay mouse, which is arguably the same as an MC3R/MC4R double knockout mouse by the ectopic expression of the agouti antagonist for both these receptors (34, 74, 84) has a different response of leptin to exercise. Ay mice are longer (anal-nasal length) then control B6 mice. Exercise was reported to increase body length in both models. At the conclusion of this study, the sedentary MC4R KO mice were 4% longer (nasal-anal length) then the sedentary wild-type littermate mice (P<0.001) and 4% longer then the exercised MC4R KO mice (P<0.01), which had the same body length as the sedentary wild-type control group (Table 1). Sedentary Ay mice had higher total cholesterol levels then B6 mice (64%) and the exercised Ay mice (14%). After 8 wk of voluntary exercise, the sedentary MC4R KO mice had 18% increased total cholesterol levels compared with the sedentary wild-type littermate mice and 12% decreased cholesterol levels, similar to the sedentary wild-type control level, compared with the exercised MC4R KO mice (Table 1). Exercised Ay and wild-type B6 mice had similar running wheel activity profiles and consumed approximately equivalent amounts of food, similar to the findings observed in this study for the MC4R KO and wild-type littermate control mice.
Levin et al. (78) studied the effects of voluntary exercise (4 wk) as well as dietary fat composition on Sprague-Dawley rats that developed diet-induced obesity (DIO) or diet resistance (DR). In this study, they reported that exercising rats ate the same amounts as the sedentary rats for both the DIO and DR phenotypes but that exercising rats gained less weight over the 4-wk period in which they were allowed access to running wheels and the exercising DIO rats had a significant 63% reduction in weight gain over this period. Exercising decreased leptin levels by 12–18% and insulin levels by 21 and 14%, respectively in the DR and DIO phenotypes and resulted in decreased total fat pad weights. In the studies reported herein with the MC4R KO mouse model, we observed similar food intake quantities and kilocalories between the exercising wild-type and MC4R KO littermate groups and a decrease in body weight gain, comparing the exercised MC4R KO vs. the sedentary MC4R KO mice. Consistent with previous reports (1), at the conclusion of this study, sedentary MC4R KO mice increased body weight by 36%. Exercise in the MC4R KO mice resulted in a 74% reduction in weight gain compared to sedentary controls, whereas both the exercised and sedentary wild-type mice had identical weight gain profiles. Compared with the sedentary wild-type mice at the termination of the 8-wk experiment, the sedentary MC4R KO mice had a 10-fold, 900% increase (P<0.001) in plasma leptin concentration and exercising MC4RKO mice had 4-fold, 77% (P<0.001) reduced leptin levels compared with those of the sedentary MC4R KO mice. Comparison of the exercising wild-type and MC4R KO littermate mice showed a 54% (P<0.001) increase in plasma leptin values. In another study, Patterson et al. (67) examined the effects of selected gene expression in several hypothalamic nuclei, studying either voluntary exercise or food restriction in diet-induced obese rats. These authors, using the experimental paradigm of 3 wk of voluntary exercise (and sedentary controls) postweaning, reported 27% lower MC3R and 28% lower NPY-Y1R arcuate expression levels associated with exercising. With use of the experimental paradigm of 8 wk of voluntary exercise postweaning, decreases of 16% (wild-type) and 46% (MC4R KO) were observed for MC3R hypothalamic gene expression (Fig. 8). The hypothalamic NPY-Y1R expression levels were modified by wild-type vs. MC4R KO genotypes (4–19% increase) between the sedentary and exercising littermate mice.
Voluntary running wheel exercise of the obese and hyperphagic OLETF rats that lack CCK-1 receptors has been reported to normalize food intake and body weight compared with sedentary control animals (64, 79). These studies resulted in the discovery that in response to exercise, hypothalamic arcuate NPY mRNA levels were increased and arcuate POMC levels were not elevated (64). Interestingly, food intake and body weight are normal in CCK-1 receptor knockout mice (85). An interesting difference between the OLETF rats and CCK-1R knockout mice is that the mice do not have increased dorsomedial hypothalamus NPY mRNA expression whereas the OLETF rats do (79), indicating the possibility of both species-specific and genetic types of differences between these two models.
Thus, there are several similar trends among the different genetic and diet-induced obesity rat models and the MC4R KO genetic mouse model used herein in that several common and general observations can be seen with the different models. However, using the agouti Ay and MC4R KO obese genetic models, as well as other genetic rodent models such as the OLETF rats, genotype-specific differences have also been revealed. These data provide supporting experimental evidence that genetic rodent models, possessing phenotypes similar to the same human genetic modifications, are important for studying the effects of voluntary exercise and changes in physiology and gene expression profiles that might be genotype-specific and important for development of human therapeutic agents. In addition, because the voluntary exercise field is relatively young and research is increasing at a fast pace with rapidly improving technologies, the determination of mechanisms associated with exercise cause and/or effect are still being identified and the parameters that define these factors are still being developed. Toward these goals, the study presented herein provides further physiological characterization of the genetic MC4R KO obese mouse model and the effects of voluntary exercise over a 2-month duration and supports the hypothesis that voluntary exercise can prevent the genetic predisposition of MC4R-associated obesity and diabetes.
Acknowledgments
This work was supported by an American Diabetes Association Research Award and National Institutes of Health grant RO1DK057080 (C.H.L.). We thank Dr. Dennis Huszar and Millennium Pharmaceuticals (Cambridge, MA, USA) for providing the MC4RKO mice. We thank Dr. Maureen Keller-Wood, Dr. Charles Wood, and Dr. Hendrik Luesch for the expert technical assistance and discussions regarding the RT-PCR experiments, Dr. Maureen Keller-Wood for assistance with the statistical analysis, and Dr. Martha Campbell-Thompson and the Pathology Molecular Core for help with pancreatic islet cell staining and quantitation.
References
- Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Smith F J, Kesterson R A, Boston B A, Fang Q, Berkemeir L R, Gu W, Cone R D, Campfield L A, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Yeo G S, Farooqi I S, Aminian S, Halsall D J, Stanhope R G, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Krude H, Gruters A. Implications of proopiomelanocortin (POMC) mutations in humans: the POMC deficiency syndrome. Trends Endocrinol Metab. 2000;11:15–22. doi: 10.1016/s1043-2760(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Hinney A, Schmidt A, Nottebom K, Heibult O, Becker I, Ziegler A, Gerber G, Sina M, Gorg T, Mayer H, Siegfried W, Fichter M, Remschmidt H, Hebebrand J. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 1999;84:1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- Hinney A, Remschmidt H, Hebebrand J. Candidate gene polymorphisms in eating disorders. Eur J Pharmacol. 2000;410:147–159. doi: 10.1016/s0014-2999(00)00812-8. [DOI] [PubMed] [Google Scholar]
- Sina M, Hinney A, Ziegler A, Neupert T, Mayer H, Siegfried W, Blum W F, Remschmidt H, Hebebrand J. Phenotypes in three pedigrees with autosomal dominant obesity caused by haploinsufficiency mutations in the melanocortin-4 receptor gene. Am J Hum Genet. 1999;65:1501–1507. doi: 10.1086/302660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I S, Yeo G S, Keogh J M, Aminian S, Jebb S A, Butler G, Cheetham T, O'Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergen M, Mergen H, Ozata M, Oner R, Oner C. A novel melanocortin 4 receptor (MC4R) gene mutation associated with morbid obesity. J Clin Endocrinol Metab. 2001;86:3448–3451. doi: 10.1210/jcem.86.7.7809. [DOI] [PubMed] [Google Scholar]
- Gu W, Tu Z, Kleyn P W, Kissebah A, Duprat L, Lee J, Chin W, Maruti S, Deng N, Fisher S L, Franco L S, Burn K, Yagaloff K, Nathan J, Heymsfield S, Albu J, Pi-Sunyer F X, Allison D B. Identification and functional analysis of novel human melanocortin-4 receptor variants. Diabetes. 1999;48:635–639. doi: 10.2337/diabetes.48.3.635. [DOI] [PubMed] [Google Scholar]
- Dubern B, Clement K, Pelloux V, Froguel P, Girardet J P, Guy-Grand B, Tounian P. Mutational analysis of melanocortin-4 receptor, agouti-related protein, and α-melanocyte-stimulating hormone genes in severely obese children. J Pediatr. 2001;139:204–209. doi: 10.1067/mpd.2001.116284. [DOI] [PubMed] [Google Scholar]
- Vink T, Hinney A, van Elburg A A, van Goozen S H, Sandkuijl L A, Sinke R J, Herpertz-Dahlmann B M, Hebebrand J, Remschmidt H, van Engeland H, Adan R A. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry. 2001;6:325–328. doi: 10.1038/sj.mp.4000854. [DOI] [PubMed] [Google Scholar]
- Yeo G S, Lank E J, Farooqi I S, Keogh J, Challis B G, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P, Vaisse C. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12:145–153. doi: 10.1093/hmg/ddg016. [DOI] [PubMed] [Google Scholar]
- Farooqi I S, Keogh J M, Yeo G S, Lank E J, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy K G, Robbins L S, Mortrud M T, Low M J, Tatro J B, Entwistle M L, Simerly R B, Cone R D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy K G, Mortrud M T, Low M J, Simerly R B, Cone R D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson S J, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson S J, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- Konda Y, Gantz I, DelValle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual signal transduction pathways activated by the melanocortin-3 receptor. J Biol Chem. 1994;269:13162–13166. [PubMed] [Google Scholar]
- Daniels D, Patten C S, Roth J D, Yee D K, Fluharty S J. Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res. 2003;986:1–11. doi: 10.1016/s0006-8993(03)03162-7. [DOI] [PubMed] [Google Scholar]
- Ollmann M M, Wilson B D, Yang Y-K, Kerns J A, Chen Y, Gantz I, Barsh G S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck E K. Agouti-related protein (AGRP) functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- Proneth B, Xiang Z, Pogozheva I D, Litherland S A, Gorbatyuk O S, Shaw A M, Millard W J, Mosberg H I, Haskell-Luevano C. Molecular mechanism of the constitutive activation of the L250Q human melanocortin-4 receptor polymorphism. Chem Biol Drug Des. 2006;67:215–229. doi: 10.1111/j.1747-0285.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Barsh G S, Farooqi S, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Commuzzie A G, Hixson J E, Almasy L, Mitchell B D, Mahaney M C, Dyer T D, Stern M P, MacCluer J W, Blangero J. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 1997;15:273–276. doi: 10.1038/ng0397-273. [DOI] [PubMed] [Google Scholar]
- Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P. A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet. 1998;20:304–308. doi: 10.1038/3123. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan M B, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Chen A S, Marsh D J, Trumbauer M E, Frazier E G, Guan X M, Yu H, Rosenblum C I, Vongs A, Feng Y, Cao L, Metzger J M, Strack A M, Camacho R E, Mellin T N, Nunes C N, Min W, Fisher J, Gopal-Truter S, MacIntyre D E, Chen H Y, Van Der Ploeg L H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Butler A A, Kesterson R A, Khong K, Cullen M J, Pelleymounter M A, Dekoning J, Baetscher M, Cone R D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Graham M, Shutter J R, Sarmiento U, Sarosi I, Stark K L. Overexpression of AGRT leads to obesity in transgenic mice. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel I R, Kadwell S, Overton L, Kost T, Luther M, Chen W, Yowchik R P, Wilkison W O, Cone R D. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston B A, Kesterson R A, Hruby V J, Cone R D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim M S, Morgan D G, Small C J, Edwards C M, Sunter D, Abusnana S, Goldstone A P, Russell S H, Stanley S A, Smith D M, Yagaloff K, Ghatei M A, Bloom S R. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Hagan M M, Rushing P A, Pritchard L M, Schwartz M W, Strack A M, Van Der Ploeg L H, Woods S C, Seeley R J. Long-term orexigenic effects of AGRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Marsh D J, Hollopeter G, Huszar D, Laufer R, Yagaloff K A, Fisher S L, Burn P, Palmiter R D. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Marsh D J, Miura G I, Yagaloff K A, Schwartz M W, Barsh G S, Palmiter R D. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- Grill H J, Ginsberg A B, Seeley R J, Kaplan J M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C F, Lee C, Kelly J, Aschkenasi C, Ahima R S, Couceyro P R, Kuhar M J, Saper C B, Elmquist J K. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Bagnol D, Lu X Y, Kaelin C B, Day H E, Ollmann M, Gantz I, Akil H, Barsh G S, Watson S J. Anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou S, Boutelet I, Vaudry H. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurons of the rat arcuate nucleus. J Neuroendocrinol. 2000;12:501–505. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- Baskin D, Breininger J, Schwartz M W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- Cheung C C, Clifton D K, Steiner R A. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Broberger C, Landry M, Wong H, Walsh J N, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- Hahn T M, Breininger J F, Baskin D G, Schwartz M W. Coexpression of AGRP and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning K, Miller L C, Laidlay H A, Burgess L A, Perera N M, Downes C P, Leslie N R, Ashford M L J. A novel leptin signalling pathway via PTEN inhibition in hypothalamic cell lines and pancreatic β-cells. EMBO J. 2006;25:2377–2387. doi: 10.1038/sj.emboj.7601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M F. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Nguyen K T, Tajmir P, Lin C H, Liadis N, Zhu X D, Eweida M, Tolasa-Karaman G, Cai F, Wang R, Kitamura T, Belsham D D, Wheeler M B, Suzuki A, Mak T W, Woo M. Essential role of Pten in body size determination and pancreatic β-cell homeostasis in vivo. Mol Cell Biol. 2006;26:4511–4518. doi: 10.1128/MCB.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie S G, Liebl D J, Parada L F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Goulding E H, Zang K, Cepoi D, Cone R D, Jones K R, Tecott L H, Reichardt L F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A A, Marks D L, Fan W, Kuhn C M, Bartolome M, Cone R D. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Irani B G, Xiang Z, Moore M C, Mandel R J, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- Li J Y, Lescure P A, Misek D E, Lai Y M, Chai B X, Kuick R, Thompson R C, Demo R M, Kurnit D M, Michailidis G, Hanash S M, Gantz I. Food deprivation-induced expression of minoxidil sulfotransferase in the hypothalamus uncovered by microarray analysis. J Biol Chem. 2002;277:9069–9076. doi: 10.1074/jbc.M110467200. [DOI] [PubMed] [Google Scholar]
- Taicher G Z, Tinsley F C, Reiderman A, Heiman M L. Quantitative magnetic resonance (QMR) method for bone and whole body composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Tinsley F C, Taicher G Z, Heiman M L. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Kirk R E. Belmont, CA, USA: Brooks and Cole; Experimental DesignProcedures for the Behavioral Sciences. 1968 [Google Scholar]
- De Souza J, Butler A A, Cone R D. Disproportionate inhibition of feeding in A(y) mice by certain stressors: a cautionary note. Neuroendocrinology. 2000;72:126–132. doi: 10.1159/000054579. [DOI] [PubMed] [Google Scholar]
- Sutton G M, Trevaskis J L, Hulver M W, McMillan R P, Markward N J, Babin M J, Meyer E A, Butler A A. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetens D, Stefan Y, Ravazzola M, Malaisse-Lagae F, Coleman D L, Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978;27:1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- Garris D R, Garris B L. Cytochemical analysis of pancreatic islet hypercytolipidemia following diabetes (db/db) and obese (ob/ob) mutation expression: influence of genomic background. Pathobiology. 2004;71:231–240. doi: 10.1159/000080056. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott K A, Hyun J, Ladenheim E E, Moran T H. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Scott K A, Moran T H, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1800–R1805. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kotz C M, Wang C, Lanningham-Foster K, Levine J A. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–E559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- Patterson C M, Bunn-Meynell A A, Levin B E. Three weeks of early onset exercise prolongs obesity-resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R290–R301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- Cowley M A, Smart J L, Rubinstein M, Cerdan M G, Diano S, Horvath T L, Cone R D, Low M J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Oliff H S, Berchtold N C, Isackson P, Cotman C W. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Chen A S, Metzger J M, Trumbauer M E, Guan X, Yu H, Fraizer E G, Marsh D, Forrest M J, Gopal-Truter S, Fisher J, Camacho R E, Strack A, Mellin T N, MacIntyre D E, Chen H Y, Van der Ploeg L. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Dishman R K, Berthoud H R, Booth F W, Cotman C W, Edgerton V R, Fleshner M R, Gandevia S C, Gomez-Pinilla F, Greenwood B N, Hillman C H, Kramer A F, Levin B E, Moran T H, Russo-Neustadt A A, Salamone J D, Van Hoomissen J D, Wade C E, York D A, Zigmond M J. Neurobiology of exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Goodrick C L. Effect of voluntary wheel exercise on food intake, water intake, and body weight for C57BL/6J mice and mutations which differ in maximal body weight. Physiol Behav. 1978;21:345–351. doi: 10.1016/0031-9384(78)90093-8. [DOI] [PubMed] [Google Scholar]
- Chiu S, Fisler J S, Espinal G M, Havel P J, Stern J S, Warden C H. The yellow agouti mutation alters some but not all responses to diet and exercise. Obes Res. 2004;12:1243–1255. doi: 10.1038/oby.2004.158. [DOI] [PubMed] [Google Scholar]
- Bultman S J, Michaud E J, Woychick R P. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Koehl M, Meerlo P, Gonzales D, Rontal A, Turek F W, Abrous D N. Exercise-induced promotion of hippocampal cell proliferation requires β-endorphin. FASEB J. 2008;22:2253–2262. doi: 10.1096/fj.07-099101. [DOI] [PubMed] [Google Scholar]
- Che F Y, Yuan Q, Kalinina E, Fricker L D. Peptidomics of Cpefat/fat mouse hypothalamus: effect of food deprivation and exercise on peptide levels. J Biol Chem. 2005;280:4451–4461. doi: 10.1074/jbc.M411178200. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Fontenele-Neto J D, Fricker L D. Effect of voluntary exercise on genetically obese Cpefat/fat mice: quantitative proteomics of serum. Obes Res. 2004;12:1179–1188. doi: 10.1038/oby.2004.147. [DOI] [PubMed] [Google Scholar]
- Levin B E, Dunn-Meynell A A. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int J Obes (Lond) 2006;30:722–727. doi: 10.1038/sj.ijo.0803192. [DOI] [PubMed] [Google Scholar]
- Moran T H, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci. 2006;361:1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste S K, Gesing A, Ulbricht S, Muller M B, Linthorst A C, Reul J M. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Narath E, Skalicky M, Viidik A. Voluntary and forced exercise influence the survival and body composition of ageing male rats differently. Exp Gerontol. 2001;36:1699–1711. doi: 10.1016/s0531-5565(01)00145-0. [DOI] [PubMed] [Google Scholar]
- Coutinho A E, Fediuc S, Campbell J E, Riddell M C. Metabolic effects of voluntary wheel running in young and old Syrian Golden hamsters. Physiol Behav. 2006;87:360–367. doi: 10.1016/j.physbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Carpenter K, Mayer J. Physiologic observations on yellow obesity in the mouse. Am J Physiol. 1958;193:499–504. doi: 10.1152/ajplegacy.1958.193.3.499. [DOI] [PubMed] [Google Scholar]
- McNulty J C, Jackson P J, Thompson D A, Chai B, Gantz I, Barsh G S, Dawson P E, Millhauser G L. Structures of the agouti signaling protein. J Mol Biol. 2005;346:1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Kopin A S, Mathes W F, McBride E W, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]