Abstract
Circadian clocks regulate multiple rhythms in mammalian tissues. In most organs core clock gene expression is oscillatory, with negative components Per and Cry peaking in antiphase to Bmal1. A notable exception is the testis, where clock genes seem nonrhythmic. Earlier mammalian studies, however, did not examine clock expression patterns in accessory ductal tissue required for sperm maturation and transport. Previous studies in insects demonstrated control of sperm maturation in vas deferens by a local circadian system. Sperm ducts express clock genes and display circadian pH changes controlled by vacuolar-type H+-ATPase and carbonic anhydrase (CA-II). It is unknown whether sperm-processing rhythms are conserved beyond insects. To address this question in mice housed in a light-dark environment, we examined temporal patterns of mPer1 and Bmal1 gene expression and protein abundance in epididymis, vas deferens, seminal vesicles, and prostate. Results demonstrate variable tissue-specific patterns of expression of the two genes, with variations in levels of clock proteins and their nucleo-cytoplasmic cycling observed among examined tissues. Strikingly, mPer1 and Bmal1 mRNA and proteins oscillate in antiphase in the prostate, with similar peak-trough patterns as observed in the suprachiasmatic nuclei, the brain’s central clock. Genes encoding CA and a V-ATPase subunit, which are rhythmically expressed in sperm ducts of moths, are also rhythmic in some segments of murine sperm ducts. Our data suggest that some sperm duct segments may contain peripheral circadian systems whereas others may express clock genes in a pleiotropic manner.—Bebas, P., Goodall, C. P., Majewska, M., Neumann, A., Giebultowicz, J. M., Chappell, P. E. Circadian clock and output genes are rhythmically expressed in extratesticular ducts and accessory organs of mice.
Keywords: V-ATPase, carbonic anhydrase, prostate, sperm maturation, testis
The molecular circadian clock regulates timing of multiple cellular processes in a broad array of mammalian tissues. Clock gene oscillations are responsible for generating several endogenous daily rhythms, which are synchronized by a master cellular clock residing in the suprachiasmatic nuclei (SCN) of the hypothalamus. Core clock gene oscillations found in many peripheral tissues control rhythmic expression patterns of tissue- and cell-specific genes important for organ function (1). Recently several studies have explored the role of the circadian clock in the regulation of the reproductive axis, at the level of the hypothalamus, pituitary, and gonads (2). Indeed, several specific clock mutant or knockout mouse lines exhibit impaired fertility, demonstrating the requirement of circadian gene oscillations for reproductive patency (2). Whether the clock functionality required for fertility resides in the neuroendocrine regulation of reproduction, at the gonadal level, or both is currently unknown.
A recent study demonstrated that clock gene expression likely plays a role in testicular steroidogenesis. BMAL1 protein oscillates in mouse Leydig cells and Bmal1 KO mice exhibit decreases in StAR expression and testosterone production (3). Clock genes may play noncircadian roles in spermatogenesis, as several studies revealed constitutively elevated and nonrhythmic expression levels of Per and Cry in whole testes or within the seminiferous tubules (4,5,6). Although murine studies focused on the role of circadian clock genes within the testis, work in insects has revealed a role of a peripheral clock in regulation of sperm maturation in accessory ducts of the testis. Studies in mammals, however, have not yet examined patterns of clock gene expression in male reproductive ducts outside of the gonads.
Previous work in insects demonstrated that sperm release from testes and maturation of sperm in the vas deferens is controlled by a peripheral circadian clock. Sperm duct epithelia in Drosophila melanogaster display rhythms in dPER and dTIM proteins, and their sperm production is reduced in per-null or tim-null mutant males (7). In addition, the release of sperm in moths is controlled by the peripheral circadian system located in the testis-vas deferens complex (8,9,10,11). This circadian system also controls periodic acidification of the seminal fluid by regulating rhythmic expression of the proton pump, vacuolar-type H+-ATPase (V-ATPase) (12) and rhythmic activity of carbonic anhydrase (CA) in moth vas deferens (13). V-ATPase and CA-II are also important for the establishment of ion homeostasis in the mammalian reproductive tract (14). CA-II coexpression with V-ATPase in ductal epithelia suggests its involvement in proton secretion and bicarbonate reabsorption in semen, both crucial to maintaining low seminal fluid pH. Acidification of semen in upper sperm ducts appears to maintain sperm in an immotile stage, while simultaneously creating an appropriate environment for posttesticular sperm maturation and preventing premature acrosomal enzyme activation (15, 16). Although V-ATPase and CA-II are widely distributed in mammalian reproductive tissues, no data exist regarding their temporal expression patterns.
It is not known whether rhythms of sperm processing occur beyond insects, and whether mammals may use coordinated patterns of sperm release and maturation to optimize reproductive efficiency. Mammalian spermatozoa maturation occurs over several days in the epididymis and vas deferens, aided by paracrine androgen receptor signaling within the ductal epithelia, and involving phasic smooth muscle contractions mediated by the cGMP/nitric oxide pathway (17). Because peripheral circadian gene expression could affect any of these maturation and transport mechanisms, we wanted to determine if peripheral clock mechanisms exist in the reproductive tract of mice. We thus examined temporal patterns in gene expression and protein abundance of the core clock components Per1, Per2, and Bmal1 in the testes, different parts of the epididymis, vas deferens, seminal vesicles, and prostate and explored whether V-ATPase and CA-II expression is rhythmic in any of these tissues. Inclusion of the accessory organs prostate and seminal vesicle provides insight into potential clock regulation of seminal fluid production, comprised of enzymes and other factors critical for proper sperm maturation and capacitation.
MATERIALS AND METHODS
Experimental animals
Male C57Bl/6J mice (Charles River, Wilmington, MA, USA) aged 10–15 wk were fed ad libitum and maintained on a 12:12 light-dark (LD) cycle; by convention, lights-on was designated as zeitgeber time (ZT) 0, and lights-off was designated ZT12. Mice were housed for >10 d in this light environment prior to sacrifice. Reproductive tissues were collected at ZT2, ZT8, ZT14, and ZT20. All animals were briefly anesthetized with CO2, followed by cervical dislocation euthanasia. Care was in accordance with Institutional Animal Care and Use Committee guidelines and approval at Oregon State University. For real-time quantitative RT-PCR (qPCR) studies, tissues were removed, placed in TriZol (Invitrogen, Carlsbad, CA, USA), and stored at −80°C for RNA processing. For Western blotting, contralateral tissues were collected and homogenized in buffer containing 20 mM Tris-HCl, 140 mM NaCl, 0.5 mM EDTA, protease inhibitors aprotinin, pepstatin, and leupeptin (10 μg/ml each), and PMSF (1.0 mM). For immunohistochemistry (IHC), a separate cohort of mice were perfused transcardially with PBS, followed by Bouin’s fixative. Tissues were stored in Bouin’s for overnight postfixation at 4°C.
Real-time qPCR
Total RNA was extracted from TriZol homogenates. RNA was acid phenol:chloroform-purified to remove genomic DNA, precipitated with isopropanol and dissolved in RNase-free water. Equal RNA concentrations were pooled from samples obtained from 4 separate mice at each time point and converted to cDNA. As a validation step, any available remaining RNA from individual tissues was also processed in the same manner for mPer1. Gene-expression profiles were generated for Bmal1, Per1, Per2, B2 subunit of V-ATPase (Atp6v1b) and Car2 by SYBR Green (Bio-Rad, Hercules, CA, USA) real-time qPCR. Cycling conditions were the following: 94°C 2 m, followed by 40 cycles of 94°C 15 s, 59°C 30 s, 70°C 35 s, with a final dissociation curve analysis to confirm the presence of a single amplicon. Relative transcript abundance was calculated as follows. Each Ct was subtracted from the lowest Ct on the same assay plate to obtain a relative Ct (relCt) value. PCR efficiency (Ef) was calculated for each reaction using LinReg software (18). A normalization factor (Nf) was determined for each sample by GeNorm (19), which uses geometric averaging of multiple reference gene expression levels. In these studies, H2afz (H2A histone family, member Z, a nucleosome subunit), Ywhaz (tyrosine 3-monooxygenase/tryptophan 5- monooxygenase activation protein, zeta polypeptide), and Ppia (peptidylprolyl isomerase A, a homologue of cyclophilin) served as the reference genes. In preliminary time course tests in rodent tissues and cell lines, H2afz, Ywhaz, and Ppia were determined by GeNorm to be expressed at levels with the least variation over time and were thus chosen as appropriate housekeeping genes for these studies. All reactions were done in triplicate. An efficiency-corrected, normalized, relative gene-expression value (EfrelCt/Nf) is shown for the mean each reaction triplicate. Real-time qPCR gene-specific primers were designed to span an intron; sequences are shown in Supplemental Table 1. For gene expression studies on individual samples (n=3), statistical significance (at P<0.05) was calculated by one-way ANOVA followed by Bonferroni’s post hoc test, to determine significant peak-nadir differences in the means of individual expression levels, as depicted in Supplemental Fig. 1.
Western blotting
Tissue homogenates were pelleted by centrifugation, and protein concentrations in supernatants were determined by BCA Assay (Pierce, Rockford, IL, USA). Equal amounts of total protein (20 μg) were loaded onto 10% SDS-PAGE Criterion Precast Gels (Bio-Rad), separated, transferred to polyvinylidene difluoride Immobilon-FL membrane (Millipore, Bedford, MA, USA), and probed with a rabbit anti-mouse PER1 (gift from David Weaver, University of Massachusetts Medical School, Worcester, MA, USA) and hMOP3 (Bmal1; chicken anti-human, ADI, San Antonio, TX, USA). Antisera were diluted 1:1000 and 1:1500, respectively, and followed by secondary antibodies conjugated to IRDye 680 and 800 fluorophores (Rockland Immunochemicals Inc., Gilbertsville, PA, USA), both diluted 1:20,000. A monoclonal anti-β-actin antibody (clone AC-74) in dilution 1:5000 (Sigma Chemical Co., St. Louis, MO, USA) was used in Western blots to detect β-actin as a loading control. Membranes were scanned using LI-COR Odyssey Infared Imaging System (LI-COR Bioscience, Lincoln, NE, USA). As control for specificity of each antibody, Per1 antibody was incubated with excess of Per1 antigenic peptide (gift from David Weaver), and Bmal1 antibody was incubated with hMOP3 antigenic peptide (ADI) prior to its application. Membranes incubated with antigen-preabsorbed Per1 or Bmal1 antibody did not show the respective protein bands in any of the issues examined. Electrophoresis of protein and Western blotting were repeated in triplicate, with each blot using pooled tissue extracts from 2 different mice. Band intensity was quantified by densitometry using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA). Regions containing bands were selected with rectangular boxes (100×30 pixels) surrounding whole bands. Densitometric units were assigned a score of 255 U (100% opacity) for black and 0 U (0% opacity) for white. Then ratios between clock proteins and β-actin were calculated and normalized to the highest value to facilitate comparison between protein content in analyzed time points. Values from 3 blots, each representing independent samples, were averaged, and sem was calculated.
IHC
Tissues were removed from Bouin’s fixative and soaked in 70% ethanol for 24 h, dehydrated, and paraffin-embedded. Next, 10 μm sections were mounted on subbed slides, hydrated, and blocked with PBS containing 0.1% BSA, 5% normal goat serum, and 0.3% Triton X-100. Sections were double labeled by incubation with antisera directed against Per1 (rabbit anti-mouse; ADI) and MOP3 (BMAL1; chicken anti-human, ADI), both diluted 1:1000. For carbonic anhydrase II localization, rabbit anti-human CA-II antiserum (Abcam Inc., Cambridge, MA, USA) in final dilution 1:1000 was applied on sections cut from the same tissue samples. Specificity of staining was confirmed by incubating control sections of each tissue in primary antibody preabsorbed with the respective antigen. AlexaFluor 594 goat anti-rabbit immunoglobulin G (IgG), AlexaFluor 488 goat anti-chicken IgG, and AlexaFluor 488 donkey anti-rabbit IgG (all diluted 1:1000) were used as secondary antibodies to identify binding of anti-PER1, anti-BMAL1, and anti-CA-II antibodies, respectively (all secondary antibodies were purchased from Invitrogen). Sections were then mounted in Fluoromount G (Southern Biotech, Birmingham, AL, USA) and analyzed under a Leica DMBR fluorescent microscope equipped with a SPOT camera (Diagnostic Instruments, Waltham, MA, USA). Densitometric analysis was performed using the Histogram option of Image J software, which quantifies the average density of the desired area. Measurements were performed using a 30-pixel-diameter circle on images converted to grayscale with values between 0 (no staining) and 255 (100% opacity). Densitometric analysis of cytoplasmic and nuclear staining was performed on 25 cells from 5 different sections of each organ from 4–5 individuals. Statistical significance (P<0.05) was calculated by one-way ANOVA followed by Bonferroni’s post hoc test.
RESULTS
Clock gene mRNA, but not protein, oscillates in the testis
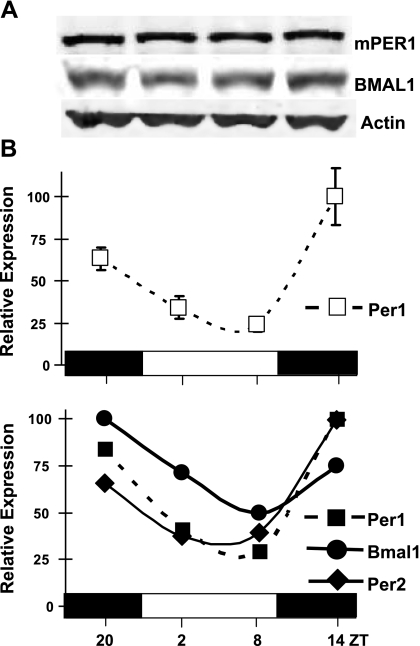
We began our investigation of core clock gene expression in the male reproductive tract by reexploring gene expression and protein abundance profiles in the testis, in part to confirm the efficacy of our methodology through comparison to previous work. Indeed, similar to previous studies (6), Western blotting of testis homogenates excluding epididymis revealed no oscillations in mPer1 and BMAL1 protein levels (Fig. 1A). Interestingly, we observed distinct rhythms of mPer1, mPer2, and Bmal1 mRNA levels using real-time qPCR of RNA pooled from testes at each time point (n=4). Transcript patterns of mPer1 and mPer2 oscillated in phase, peaking in the early evening at ZT14, followed by a peak of Bmal1 transcript at ZT20 (Fig. 1B). All 3 clock genes displayed an expression nadir in phase, at ZT8, similar to what was observed previously in the hamster (20).
Figure 1.
Core clock gene expression, but not protein abundance, is rhythmic in homogenized mouse testis. A) Representative Western blot demonstrating levels of mPER1, BMAL1, and β-actin in testis homogenates, collected at 6 h intervals, of mice entrained to 12:12 LD. B) Top panel: mean ± se relative expression levels of mPer1 transcript (open squares), derived from testes of individual mice (n=4) at each time point, as determined by real-time qPCR. Expression levels are determined by the ΔΔCt method; each time point is normalized to reference gene Ppia and compared to ZT20. Highest relative expression level is set to 100. Horizontal bars represent day (open) and night (filled), with ZT indicated below. Bottom panel: relative expression values of mPer1 (filled squares), mPer2 (filled diamonds), and Bmal1 (filled circles) at ZT20, ZT2, ZT8, and ZT14, derived from pooled testis RNA samples. Data shown are efficiency-corrected, normalized, relative gene-expression values calculated from the mean of reaction triplicates; the highest relative expression level is set to 100. Of note is the high similarity between the means of individual mPer1 expression levels in the top panel compared to mPer1 expression levels derived from pooled testis homogenates in the bottom panel.
Because samples were terminally collected at each time point, we also examined expression levels of mPer1 from testes of individual mice via real-time qPCR (Fig. 1B, top panel) and compared these patterns to those derived from pooled tissue (Fig. 1B, bottom panel). In the testis, this comparison demonstrated a high correlation in mean expression level at each time point from individual animals with that observed in pooled testes. One-way ANOVA revealed significantly (P<0.05) higher expression levels during the night phase (ZT14-20) in comparison to the day (ZT2-8) in individual samples.
However, expression profiles derived from pooled samples may not be representative of expression levels among individual tissues; therefore we also compared levels of mPer1 taken from pooled and individual samples in subsequent extratesticular tissues (shown in Supplemental Fig. 1), calculating the relative gene expression in individual samples using the ΔΔCt method with Ppia as the reference gene. Using these two different methods of analysis, we observe similar levels and patterns of expression, thereby validating both methods. Although the testis displayed very little difference between pooled and individual samples, higher individual variation in some tissues, notably the corpus epididymis and seminal vesicle, revealed a lack of a significant rhythm in comparison to what was observed from pooled samples.
Daily patterns of clock gene expression in sections of the epididymis
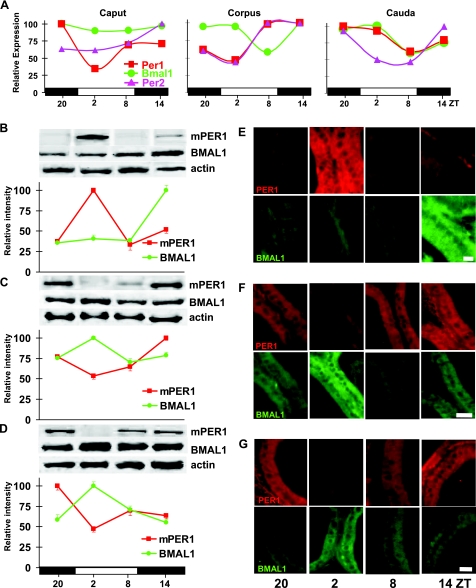
To examine expression patterns of clock genes in the ductal tissue most proximal to the testis, at multiple time points we isolated RNA from surgically excised caput (head), corpus (midsection), and cauda (tail) of the epididymis of male mice housed in a 12:12 LD environment. Real-time qPCR of mRNA from both pooled and individual tissues revealed rhythms of Per1 expression in caput, with an expression peak occurring at ZT20 (P<0.05 above ZT2 nadir of individual samples). In corpus, a low-amplitude mRNA rhythm was present in pooled tissue, with the peak expression for both mPer1 and mPer2 occurring at ZT8-14 (Fig. 2A). In addition, Bmal1 gene expression from pooled RNA was found to oscillate in corpus with a phase opposite to Per1/Per2. Analysis of Per1 levels from individual corpus samples, however, did not reveal a significant oscillation, owing to higher variability among samples (Supplemental Fig. 1A). In contrast, Per1 and Bmal1 do not appear to oscillate out of phase in the cauda; both showed higher expression levels in the dark phase, while the Per2 mRNA rhythm is more robust, but with an earlier nadir (Fig. 2A).
Figure 2.
Daily changes in clock gene expression and protein abundance in epididymis. Male mice entrained to 12:12 LD were sacrificed every 6 h at ZT20, ZT2, ZT8, ZT14; for every type of analysis in each time point, organs from 4–5 individuals were collected. A) Graphs represent expression levels, determined by real-time qPCR, of 3 genes: mPer1 (red line and squares), mPer2 (purple line and triangles), and Bmal1 (green line and circles) in epididymal caput, corpus, and cauda, respectively. Efficiency-corrected, normalized, relative gene-expression values for the mean of reaction triplicates are shown at each time point. Horizontal bars represent day (open) and night (filled), with ZT indicated below. B–D) Representative Western blots of mPER1, BMAL1, and β-actin in caput (B), corpus (C), and cauda (D). Graphs below blots represent densitometric analysis of protein bands. Clock protein/β-actin ratios were calculated and normalized to a percentage of darkest band intensity; values are averages ± sem from 3 Western blots. E–G) Representative immunohistochemical staining for mPER1 and BMAL1 in epithelia of epididymal tubules of caput (E), corpus (F), and cauda (G). A minimum of 20 images was analyzed per time point in each mouse tissue; average staining intensity is shown in Supplemental Fig. 2. Scale bars = 20 μm.
Contralateral sections of the epididymis were examined for patterns of protein abundance. Rhythmic expression of mPER1 was observed in caput with the protein peak occurring 6 h after the peak of mRNA. Interestingly, despite the lack of a Bmal1 mRNA rhythm in caput, BMAL1 protein was rhythmic in this segment of epididymis, peaking at ZT14, 12 h out of phase relative to mPER1 peak at ZT2 (Fig. 2B). In both corpus and cauda, BMAL1 protein peaked at ZT2, while mPER1 peaked at night (Fig. 2C, D). To verify and extend Western blot results, we performed IHC using anti-mPER1 and anti-BMAL1 antibodies on ductal tissues fixed at the same time points. We observed fluctuations in the levels of both proteins (Fig. 2E–G) such that the peak in BMAL1 was followed by a peak in mPER1, consistent with the role of BMAL1 as a positive regulator within the clock feedback loop. Analysis of subcellular localization of mPER1 and BMAL1 by densitometry showed that cytoplasmic and nuclear levels of each protein fluctuated in parallel (Supplemental Fig. 2). Neither protein has been predominantly nuclear at time points examined in our study (Fig. 2E–G).
Daily pattern of clock gene expression in the vas deferens, prostate, and seminal vesicles
Temporal expression patterns of clock genes were analyzed also in the vas deferens, prostate, and seminal vesicles. Real-time qPCR revealed rhythms of mPer1 expression, with significant peak (P<0.05) occurring in the afternoon at ZT8, in prostate (Fig. 3A; Supplemental Fig. 1E). In seminal vesicle, although data from pools suggested lower amplitude rhythms of mPer1 and mPer2 peaking at the same time, no significant rhythm of mPer1 was present in this tissue from individual samples (Supplemental Fig. 1F). Interestingly, antiphase oscillations of mPER1 and BMAL1 proteins were observed in all 3 tissues (Fig. 3B–D), with low-amplitude rhythms in the seminal vesicle. IHC showed that mPER1 in vas deferens was predominantly nuclear at ZT8 and predominantly cytoplasmic at ZT14 (Fig. 3E; Supplemental Fig. 2).
Figure 3.
Variable temporal expression of clock genes and proteins in vas deferens, prostate, and seminal vesicles. Clock gene and protein expression profiles are shown analogous to Fig. 2 (compare for marking and description details), with all organs dissected from the same mice as above. A) Daily changes in the levels of mPer1, mPer2, Bmal1 mRNAs in the tissues of whole vas deferens, prostate, and seminal vesicles, respectively, based on real-time qPCR analysis. B–D) Western blots of mPER1, BMAL1, and β-actin (control) in the whole vas deferens (B), prostate (C), and seminal vesicles (D), with corresponding densitometric analysis of protein bands staining intensity shown below each Western blot. E–G) Immunohistochemical identification of mPER1 and BMAL1 in epithelia of vas deferens (E), dorso-lateral prostate (F), and seminal vesicles (G). Scale bars = 20 μm. For densitometrical analysis, see Supplemental Fig. 2.
Although the prostate exhibited a clear oscillation of clock genes, seminal vesicles displayed no significant rhythms. In the prostate, sharp peaks of mPer1 and mPer2 mRNA levels occurred at ZT8, while high levels of mPer1 and mPer2 mRNA persisted over all time points in seminal vesicles (Fig. 3A; Supplemental Fig. 1E, F). Corresponding differences were also observed at the protein level: a sharp peak in mPER1 level occurred in prostate (Fig. 3C), whereas mPER1 remained at elevated levels in seminal vesicles in 3 out of 4 examined time points (Fig. 3D). Remarkably, mPER1 was clearly nuclear at ZT8, 14, and 20 in the seminal vesicles, while in the prostate nuclear localization was detected only at ZT14 in both the dorso-lateral and ventral portions of the gland (Fig. 3F, G; Supplemental Fig. 2). Interestingly, in vas deferens and prostate, mPER1 protein peaks occur at the same point (ZT8) as mPer1 gene expression peaks, without an apparent lag. It is unclear if this is due to sampling resolution, and if at more frequent intervals expression peaks may be seen to precede peaks of protein abundance.
Expression of output genes in extratesticular ducts and accessory organs
CA and V-ATPase are important for the establishment of ion homeostasis in the mammalian reproductive tract (14). Previous studies revealed that these enzymes are rhythmically expressed in the insect sperm ducts. To determine whether similar output rhythms may exist in mice, we examined the patterns of expression of Car2 gene and corresponding CA-II protein in segments of extratesticular organs at multiple time points in 12:12 LD. Real-time qPCR from pooled samples suggest high-amplitude rhythms of Car2 mRNA levels in cauda epididymis, vas deferens, and prostate but not in other segments (Fig. 4). Interestingly, peaks in Car2 mRNA abundance displayed tissue-specific timing occurring at ZT2 in cauda, ZT14 in vas deferens, and ZT8 in prostate. The 3 tissues with strong rhythms in Car2 mRNA also showed the most striking oscillations in the level of CA-II protein, as revealed by IHC (Fig. 4). CA-II was detected in all epithelial cells of vas deferens, but only in some cells in the cauda epididymis, as well as in dorso-lateral prostate (no staining was detected in ventral prostate). The percentage of CA-II-positive cells in these tissues also fluctuated with time of day (Supplemental Fig. 3).
Figure 4.
Carbonic anhydrase II gene and protein expression patterns in specific reproductive ducts and accessory glands. Car2 gene expression in (from top to bottom) caput, corpus, and cauda epididymis, vas deferens, prostate, and seminal vesicles, as revealed by real-time qPCR analysis (left panels) and corresponding immunohistochemical identification of CA-II protein (right panels). Real-time qPCR data shown are efficiency corrected, normalized, relative gene-expression values for the mean of reaction triplicates. Horizontal bars represent day (open) and night (filled), with ZT indicated below. For immunohistochemistry, CA-II protein (green) is costained with DNA-intercalating dye Hoechst 33258 (blue). Scale bars = 20 μm. For quantitative analysis of CA-II positive cells for each time point and organ, see Supplemental Fig. 3.
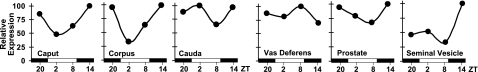
In addition to carbonic anhydrase, the proton pump V-ATPase plays an important role in ion homeostasis and the acidification of the lumen of the male reproductive tract. The B subunit of V-ATPase is rhythmic in moth vas deferens (12); thus we examined daily expression patterns of V-ATPase B2 subunit in segments of extratesticular organs. Real-time qPCR of pooled mRNA samples suggested high-amplitude rhythms of Atp6v1b2 mRNA levels in the caput and corpus sections of the epididymis, with a trough at ZT2 and a peak at ZT14 (Fig. 5). Interestingly, although core clock gene expression was not significantly rhythmic in seminal vesicles, expression of Atp6v1b2 in this gland showed high-amplitude oscillations with a trough at ZT8 followed by a peak at ZT14 (Fig. 5).
Figure 5.
Expression patterns of V-ATPase subunit B2 isoform in reproductive ducts and accessory glands. Real-time qPCR results show gene expression profiles for the B2 subunit of vacuolar-type proton pump V-ATPase (Atp6v1b2) across a 24-h period in 3 parts of epididymis (caput, corpus, cauda), vas deferens, prostate, and seminal vesicles. Data are efficiency corrected, normalized, relative gene-expression values for the mean of reaction triplicates. Total RNAs were obtained from the same individuals sacrificed for investigating clock gene and Car2 expression (see Figs. 234). Horizontal bars represent day (open) and night (filled), with ZT indicated below.
DISCUSSION
Our study demonstrates that circadian clock genes are rhythmically expressed in mammalian testis and in some accessory reproductive tissues, and suggest that tissue-specific endogenous peripheral clocks may influence the expression levels of enzymes responsible for the regulation of sperm duct luminal environment. Although this has been observed previously in insects, this study marks the first demonstration that similar timing mechanisms may operate in distant animal taxa. It should be noted that the above studies examined rhythms in mice housed in LD only, and further work in a dark-dark environment will be required to demonstrate that these rhythms persist under constant conditions.
Previous studies addressing the expression of clock genes in the male reproductive system focused entirely on testes and yielded inconsistent results. The earliest of these studies suggested rhythmic expression of mPer1 and mPer2 may be present in mice testes (21). However, subsequent studies demonstrated nonrhythmic and developmental-stage specific expression of mPer1, mCry1, and Clock in differentiating sperm cells or whole testes (4,5,6, 22, 23). A more recent study in hamsters showed low-amplitude rhythms of haPer1 and haBmal1 that appeared to oscillate in phase (20). In agreement with the latter study, we have shown in the mouse that the testis may possess a unique pattern of clock gene expression. Our results are also in agreement with a recent report demonstrating a rhythm of BMAL1 protein abundance specifically in Leydig cells (3). Those results show BMAL1 protein levels peaking at ZT3, in line with our results demonstrating Bma11 gene expression peaking in the testis in the early morning at ZT20. It is unclear if the gene expression rhythm we detected could be reflective of a Leydig cell-specific rhythm, considering the high nonrhythmic levels of PER protein present in seminiferous tubules. However, the possibility exists that elevated PER levels in the tubules, perhaps because of insufficient degradation, could act to constitutively repress clock oscillations there, while a functional clock operates in the nearby interstitial Leydig cells. Clock gene expression has been associated with the control of steroidogenesis in the adrenal gland (24) and ovary (25), and the expression levels of multiple components of normal steroidogenesis are altered in Bmal1 knockout mice (3), which suggests a role for an endogenous clock in androgen synthesis and secretion.
Our study provides the first insights into clock gene expression profiles in extratesticular accessory tissues, revealing tissue-specific variations in activity, phase, amplitude, and stability, which suggests that this specificity of clock gene and protein oscillations may be fine-tuned to the functional compartmentalization of the male reproductive ducts. Interestingly, oscillations observed in prostate were in phase with patterns observed in the SCN. This is in contrast to other peripheral oscillators, such as liver and lung, whose phases are delayed with respect to the SCN (21, 26,27,28). Considering that the previously characterized endogenous clock in the moth reproductive system oscillates independently of the brain, it will be interesting to address in future studies the degree of autonomy and entrainment of this peripheral rhythm in mice. It should be noted that in some tissues, such as the testis, caput epididymis, and prostate, clock gene expression profiles derived from pooled RNA samples closely matched a statistically significant rhythm found in RNA samples from individual mice. In contrast, expression profiles of mPer1 from individual RNA samples in other examined tissues, such as vas deferens, revealed no significant oscillations. Interestingly, while oscillations in mPer1 mRNA were not statistically significant in vas deferens, protein extracts from individual tissues showed significant oscillations and nuclear localization suggestive of clock repressor function, consistent with the emerging view that posttranscriptional modification to and degradation of clock proteins may lead to rhythms in the absence of apparent mRNA oscillations.
Our data suggest that the circadian clock may control expression levels of specific enzymes involved in the regulation of sperm duct luminal environment. We observed daily rhythms in carbonic anhydrase II at the mRNA and protein level in some segments of male reproductive tract. Specifically, high-amplitude rhythms of Car2 expression were detected in cauda epididymis. IHC demonstrated variations in cellular distribution of CA-II along the reproductive tract, from its presence in all epithelial cells of caput epidydimis to only certain cell types in corpus, and especially in cauda. This is in agreement with previous studies, showing that CA-II is expressed in clear cells but not in adjacent principal cells (29). We demonstrate here daily rhythms in both the intensity of staining in individual cells and the number of cells containing detectable levels of CA-II. It is feasible that these daily rhythms could contribute to the discrepancy in the literature regarding the presence of CA-II in the clear cells of the epididymis (14, 29, 30).
Strong rhythms in CA-II at both mRNA and protein level were also revealed in vas deferens and prostate. It should be noted that Car2 expression in this study was determined only from pooled RNA samples, and that expression profiles from individual vas deferens RNA samples revealed no significant oscillation in mPer1. Interestingly, however, changes in Car2 expression over time are closely paralleled by CA-II protein abundance patterns, as revealed by IHC. As the physiological role of CA-II is linked to the regulation of sperm motility and pH in the seminal plasma, our data suggest that circadian clock may regulate temporal aspects of luminal environment, although physiological experiments are required to test this hypothesis. Another consideration will be to determine to what extent clock control of CA-II expression and/or activity is modulated by local androgen production. The relative amount of carbonic anhydrase II in the rat epidydimis, lateral prostate, and seminal vesicles is under testosterone regulation (31, 32). In many mammals circulating testosterone has been shown to be rhythmic (33, 34); thus responsiveness of extratesticular sperm ducts may be regulated by both androgens and a cyclic sensitivity to them, potentially through clock control of receptor levels.
Our previous work in insects revealed a daily rhythm in expression and subcellular localization of V-ATPase in insect sperm ducts (12) and visual system (35). Here we report that levels of mRNA encoding the B2 subunit of murine V-ATPase change with a daily rhythm in pooled samples from some tissues, which is especially pronounced in the corpus epidydimis. V-ATPase is involved in maintenance of luminal acidic pH in murine epidiyimis (14, 15). In insect sperm ducts, pH oscillates with a daily rhythm, and acidification is prevented by V-ATPase inhibitors. Taken together our data suggest that similar clock-controlled processes could occur in both insect and murine sperm ducts. Our data may have a broader significance considering that carbonic anhydrases and V-ATPase are major players in many physiological and pathological processes (36,37,38,39,40). It remains to be determined whether night/day differences in expression of these enzymes may occur in other organs possessing a circadian clock, such as kidney. Our study points to a possibility of a completely new layer of temporal regulation of these important and ubiquitous enzymes.
Our data add to the growing body of evidence that clocks are important in regulating mammalian reproduction at multiple points within the hypothalamic-pituitary-gonadal axis. In addition to Bmal1KO mice, which are infertile, Clock/Clock mutant mice are subfertile (41) and do not display preovulatory LH surges (42). In addition, studies have shown that normal clock expression rhythms are required for pulsatile GnRH neurosecretion in vitro (41). Interestingly, circadian patterns of gene expression have been observed in the steroidogenic granulosa cells surrounding the developing follicle in the rat, quail, and chicken ovary (25, 43, 44). Core clock gene expression can modulate expression patterns of enzymes and proteins required for ovarian steroidogenesis, including StAR and 3β-HSD (25, 44). Per1 is also regulated by ovarian steroids in rat uterus (45).
A recent study suggests that some core clock transcripts may exhibit ultradian rhythms in the testis, which cannot be determined in this study because of lower sampling resolution (46). Our studies in combination with previous work (3) suggest that the clock likely plays a role in testicular steroidogenesis, which could exert downstream effects on spermatogenesis as well as sperm maturation. Tissues examined in this study widely express androgen receptors (ARs), required for normal fertility even in mice with ARs knocked out in extratesticular tissues only (47). Potential clock regulation in sperm ducts may also impact smooth muscle contractions required to advance sperm through the lumen. In mice peritubular myocyte activity requires nitric oxide-mediated signaling via the cyclic GMP pathway (17), similar to clock input mechanisms found in retina and SCN (48), and in insects sperm movement is associated with daily rhythms of myogenic contractions in the vas deferens (49). Based on our earlier insect studies (50, 51), we speculate that the circadian clock could regulate protein secretion from the murine sperm duct epithelia. Interestingly, noting clock rhythms in prostate epithelia, future studies will focus on any clock dysregulation potentially underlying the high prevalence of prostatic cancers suggested by epidemiological studies and recent demonstrations of clock proteins acting as tumor suppressors (52).
Acknowledgments
We thank Dr. David Weaver (University of Massachusetts Medical School, Worcester, MA, USA) for the gift of anti-mPER1 antibody, antigen, and tissues of mPER1 K/O mice. This research was supported by National Institutes of Health-National Institute of General Medical Studies (NIH-NIGMS) grant GM073792 to J.M.G., Polish Science Foundation (MNiSzW) grant NN303342235 to P.B., and NIH grants R01 DK075357 and K01 DK064919 to P.E.C.
References
- Davidson A J, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121. discussion 121–125, 281–284. [PubMed] [Google Scholar]
- Boden M J, Kennaway D J. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- Alvarez J D, Hansen A, Ord T, Bebas P, Chappell P E, Giebultowicz J M, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J D, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod. 2003;69:81–91. doi: 10.1095/biolreprod.102.011833. [DOI] [PubMed] [Google Scholar]
- Bittman E L, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol. 2003;285:R561–R569. doi: 10.1152/ajpregu.00783.2002. [DOI] [PubMed] [Google Scholar]
- Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17:141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- Beaver L M, Gvakharia B O, Vollintine D S, Hege D M, Stanewsky R, Giebultowicz J M. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:2134–2139. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebas P, Cymborowski B. Effects of constant light on male sterility in Spodoptera litttoralis (Lepidoptera: Noctuidae) Physiol Entomol. 1999;24:165–170. [Google Scholar]
- Giebultowicz J M, Ridgway R L, Imberski R B. Physiological basis for sterilizing effects of constant light in Lymantria dispar. Physiol Entomol. 1990;15:149–156. [Google Scholar]
- Bebas P, Cymborowski B, Giebultowicz J M. Circadian rhythm of sperm release in males of the cotton leafworm, Spodoptera littoralis: in vivo and in vitro study. J Insect Physiol. 2001;47:859–866. [Google Scholar]
- Giebultowicz J M, Riemann J G, Raina A K, Ridgway R L. Circadian system controlling release of sperm in the insect testes. Science. 1989;245:1098–1100. doi: 10.1126/science.245.4922.1098. [DOI] [PubMed] [Google Scholar]
- Bebas P, Cymborowski B, Giebultowicz J M. Circadian rhythm of acidification in insect vas deferens regulated by rhythmic expression of vacuolar H+-ATPase. J Exp Biol. 2002;205:37–44. doi: 10.1242/jeb.205.1.37. [DOI] [PubMed] [Google Scholar]
- Kotwica J, Ciuk M A, Joachimiak E, Rowinski S, Cymborowski B, Bebas P. Carbonic anhydrase activity in the vas deferens of the cotton leafworm—Spodoptera littoralis (Lepidoptera: Noctuidae) controlled by circadian clock. J Physiol Pharmacol. 2006;57:107–123. [PubMed] [Google Scholar]
- Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 2005;20:417–428. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- Breton S, Smith P J, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470–472. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- Brown D, Smith P J, Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol. 1997;200:257–262. doi: 10.1242/jeb.200.2.257. [DOI] [PubMed] [Google Scholar]
- Mewe M, Bauer C K, Muller D, Middendorff R. Regulation of spontaneous contractile activity in the bovine epididymal duct by cyclic guanosine 5′-monophosphate-dependent pathways. Endocrinology. 2006;147:2051–2062. doi: 10.1210/en.2005-1324. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter J M, Deprez R H, Moorman A F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Guo H, Brewer J M, Lee H, Lehman M N, Bittman E L. Expression of haPer1 and haBmal1 in Syrian hamsters: heterogeneity of transcripts and oscillations in the periphery. J Biol Rhythms. 2004;19:113–125. doi: 10.1177/0748730403262871. [DOI] [PubMed] [Google Scholar]
- Zylka M J, Shearman L P, Weaver D R, Reppert S M. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Sancar A. Circadian regulation of cryptochrome genes in the mouse. Brain Res Mol Brain Res. 1999;71:238–243. doi: 10.1016/s0169-328x(99)00192-8. [DOI] [PubMed] [Google Scholar]
- Lambert C M, Weaver D R. Peripheral gene expression rhythms in a diurnal rodent. J Biol Rhythms. 2006;21:77–79. doi: 10.1177/0748730405281843. [DOI] [PubMed] [Google Scholar]
- Oster H, Damerow S, Hut R A, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006;21:350–361. doi: 10.1177/0748730406293053. [DOI] [PubMed] [Google Scholar]
- Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp P J, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148:3031–3038. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block G D, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo S H, Yamazaki S, Lowrey P L, Shimomura K, Ko C H, Buhr E D, Siepka S M, Hong H K, Oh W J, Yoo O J, Menaker M, Takahashi J S. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Hammar K, Smith P J, Brown D. Proton secretion in the male reproductive tract: involvement of Cl-independent HCO-3 transport. Am J Physiol. 1998;275:C1134–C1142. doi: 10.1152/ajpcell.1998.275.4.C1134. [DOI] [PubMed] [Google Scholar]
- Hermo L, Chong D L, Moffatt P, Sly W S, Waheed A, Smith C E. Region- and cell-specific differences in the distribution of carbonic anhydrases II, III, XII, and XIV in the adult rat epididymis. J Histochem Cytochem. 2005;53:699–713. doi: 10.1369/jhc.4A6575.2005. [DOI] [PubMed] [Google Scholar]
- Harkonen P L, Vaananen H K. Androgen regulation of carbonic anhydrase II, a major soluble protein in rat lateral prostate tissue. Biol Reprod. 1988;38:377–384. doi: 10.1095/biolreprod38.2.377. [DOI] [PubMed] [Google Scholar]
- Kaunisto K, Fleming R E, Kneer J, Sly W S, Rajaniemi H. Regional expression and androgen regulation of carbonic anhydrase IV and II in the adult rat epididymis. Biol Reprod. 1999;61:1521–1526. doi: 10.1095/biolreprod61.6.1521. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Nieschlag E. Circadian rhythm of plasma testosterone in the male Djungarian hamster (Phodopus sungorus) Acta Endocrinol (Cph) 1977;86:193–199. doi: 10.1530/acta.0.0860193. [DOI] [PubMed] [Google Scholar]
- Winters S J, Medhamurthy R, Gay V L, Plant T M. A comparison of moment to moment and diurnal changes in circulating inhibin and testosterone concentrations in male rhesus monkeys (Macaca mulatta) Endocrinology. 1991;129:1755–1761. doi: 10.1210/endo-129-4-1755. [DOI] [PubMed] [Google Scholar]
- Pyza E, Borycz J, Giebultowicz J M, Meinertzhagen I A. Involvement of V-ATPase in the regulation of cell size in the fly’s visual system. J Insect Physiol. 2004;50:985–994. doi: 10.1016/j.jinsphys.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Breton S. The cellular physiology of carbonic anhydrases. J Pancreas. 2001;2:159–164. [PubMed] [Google Scholar]
- Herak-Kramberger C M, Breton S, Brown D, Kraus O, Sabolic I. Distribution of the vacuolar H+ atpase along the rat and human male reproductive tract. Biol Reprod. 2001;64:1699–1707. doi: 10.1095/biolreprod64.6.1699. [DOI] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Sennoune S R, Bakunts K, Martinez G M, Chua-Tuan J L, Kebir Y, Attaya M N, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol. 2004;286:C1443–C1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N M, Hallows K R, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. A J Physiol. 2008;294:C488–C494. doi: 10.1152/ajpcell.00537.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell P E, White R S, Mellon P L. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B H, Olson S L, Turek F W, Levine J E, Horton T H, Takahashi J S. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776. doi: 10.1210/en.2006-0305. [DOI] [PubMed] [Google Scholar]
- Karman B N, Tischkau S A. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- He P J, Hirata M, Yamauchi N, Hattori M A. Up-regulation of Per1 expression by estradiol and progesterone in the rat uterus. J Endocrinol. 2007;194:511–519. doi: 10.1677/JOE-07-0172. [DOI] [PubMed] [Google Scholar]
- Liu S, Cai Y, Sothern R B, Guan Y, Chan P. Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice. Chronobiol Int. 2007;24:793–820. doi: 10.1080/07420520701672556. [DOI] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Davey R A, Zajac J D, Handelsman D J. Severe subfertility in mice with AR inactivation in sex accessory organs, but not in testis. Endocrinology. 2008;149:3330–3338. doi: 10.1210/en.2007-1805. [DOI] [PubMed] [Google Scholar]
- Ko G Y, Ko M, Dryer S E. Circadian and cAMP-dependent modulation of retinal cone cGMP-gated channels does not require protein synthesis or calcium influx through L-type channels. Brain Res. 2004;1021:277–280. doi: 10.1016/j.brainres.2004.05.072. [DOI] [PubMed] [Google Scholar]
- Giebultowicz J M, Blackburn M B, Thomas-Laemont P A, Weyda F, Raina A K. Daily rhythm in myogenic contractions of vas deferens associated with sperm release cycle in a moth. J Comp Physiol A. 1996;178:629–636. [Google Scholar]
- Bebas P, Kotwica J, Joachimiak E, Giebultowicz J M. Yolk protein is expressed in the insect testis and interacts with sperm. BMC Dev Biol. 2008;8:64. doi: 10.1186/1471-213X-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebas P, Maksimiuk E, Gvakharia B O, Cymborowski B, Giebultowicz J M. Circadian rhythm of glycoprotein secretion in the vas deferens of moth, Spodoptera littoralis. BMC Physiol. 2002;2:15. doi: 10.1186/1472-6793-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler H P. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]