Abstract
Intimal hyperplasia (IH) limits the patency of all cardiovascular vein bypass grafts. We previously found the myristoylated alanine-rich C kinase substrate (MARCKS), a key protein kinase C (PKC) substrate, to be up-regulated in canine models of IH. Here, we further characterize the role of MARCKS in IH and examine the phenotypic consequences of MARCKS silencing by small interfering RNA (siRNA) transfection in human vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) in vitro and use a rapid 10-min nonviral siRNA transfection technique to determine the effects of MARCKS silencing in human saphenous vein cultured ex vivo. We demonstrate MARCKS silencing attenuates VSMC migration and arrests VSMC proliferation in part through the up-regulation of the cyclin-dependent kinase inhibitor p27kip1. Conversely, MARCKS silencing had little or no effect on EC migration or proliferation. These phenotypic changes culminated in reduced neointimal formation in cultured human saphenous vein. These data identify MARCKS as a pathogenic contributor to IH and indicate therapeutic MARCKS silencing could selectively suppress the “atherogenic,” proliferative phenotype of VSMCs without collateral harm to the endothelium. This approach could be readily translated to the clinic to silence MARCKS in vein bypass grafts prior to implantation.—Monahan, T. S., Andersen, N. D., Martin, M. C., Malek, J. Y., Shrikhande, G. V., Pradhan, L., Ferran, C., LoGerfo, F. W. MARCKS silencing differentially affects human vascular smooth muscle and endothelial cell phenotypes to inhibit neointimal hyperplasia in saphenous vein.
Keywords: bypass graft, RNA interference, small interfering RNA, gene therapy, transfection, protein kinase C
Intimal hyperplasia (IH) occurs in blood vessels following vessel injury and may be regarded as a form of wound repair. Arterial reconstruction procedures using autologous veins as conduits are limited by IH, leading to stenosis and thrombotic occlusion in 20–30% of lower extremity vein grafts within 5 yr (1). The pathogenesis of IH results from a cascade of activated cellular phenotypes triggered by endothelial cell (EC) damage and leading to the activation of contractile vascular smooth muscle cells (VSMCs) to a motile synthetic phenotype (2, 3). Activated VSMCs migrate to the neointima, where they enter a proliferative phase contributing to the cell mass of the hyperplastic lesion (3, 4). VSMC migration and proliferation are halted on the reestablishment of an intact, functional endothelium (5). Although numerous factors associated with vein grafting are known to trigger the activation of vascular cells (6,7,8), the precise molecular events coordinating IH are incompletely understood.
To gain insight into the molecular signals responsible for IH, we previously performed microarray studies of prosthetic and vein graft IH in a canine model (9, 10). This analysis identified genes that were up-regulated or down-regulated at discrete time points after creation of the bypass graft. Among the genes up-regulated in both prosthetic and vein graft models of IH was the myristoylated alanine-rich C kinase substrate (MARCKS), a downstream effector of protein kinase C (PKC).
The PKC signal transduction pathway has been directly implicated in IH following vessel injury, as well as other forms of vascular disease (11,12,13). MARCKS is a key PKC substrate thought to regulate cellular adhesion and spreading, migration, proliferation, and fusion through its interaction with the cytoskeleton (14,15,16). MARCKS translocates from the cell membrane to the cytosol in response to PKC phosphorylation (17). In the absence of PKC activity, MARCKS remains attached to the membrane, where it is thought to stabilize the cytoskeleton by cross-linking F-actin. After phosphorylation by activated PKC, MARCKS disassociates from the membrane, allowing for transient softening and remodeling of the cytoskeleton (17). In addition, MARCKS interacts with calmodulin and mitogen-activated protein kinase (MAPK) in a proposed crosstalk system between PKC, MAPK, and calcium signaling pathways (18,19,20).
In light of the MARCKS expression profiles from large animal models of IH and the known involvement of PKC signaling in vascular disease, we aimed to define the role of MARCKS in IH and characterize MARCKS as a potential target for RNA interference (RNAi) -mediated vein graft gene therapy. Here, we report the divergent consequences of MARCKS silencing by small interfering RNA (siRNA) on the migration and proliferation of human VSMCs and ECs in vitro. Migration and proliferation are the two hallmarks of the transformed “proatherogenic” VSMC phenotype and serve as indicators of EC health and survival. We then show the effects of MARCKS silencing on VSMC proliferation and neointimal formation in an organ culture model of human saphenous vein using rapid nonviral siRNA delivery methods.
MATERIALS AND METHODS
siRNA design
MARCKS siRNA (sense 5′-GGU GCC CAG UUC UCC AAG AUU-3′), control siRNA (sense 5′-CGC ACC AGA ACA AAC ACA C-3′), and Cy5 siRNA (sense 5′-Cy5- CGC ACC AGA ACA AAC ACA C - 3′) were purchased from Dharmacon (Lafayette, CO, USA), as described previously (21). Control and Cy5 siRNA sequences do not harbor any homology with the human genome.
siRNA transfection of human vascular cells
Primary human coronary artery VSMCs and ECs were purchased from Cambrex (Walkersville, MD, USA) and cultured in Clonetics (San Diego, CA, USA) smooth muscle cell (SmGM-2, 5% FBS) or EC (EGM-2MV, 5% FBS) medium. Experiments were performed between passages 5 and 8 and repeated using cells from at least two independent donors per cell type. Cells were transfected with 5–100 nmol/L siRNA in unsupplemented basal medium (SmBM or EBM-2; Cambrex, Rockland, ME, USA) for 24 h using the DharmAFECT1 lipofection reagent (Dharmacon, Abbott Park, IL, USA), as described previously (21).
siRNA transfection of human saphenous vein
Freshly harvested surplus human saphenous vein was procured from the operating room with institutional review board approval from patients undergoing cardiac or vascular surgery procedures. Vein tissue was prepared for immediate transfection in Plasmalyte A solution (140 mEq/L sodium, 5 mEq/L potassium, 3 mEq/L magnesium, 98 mEq/L chloride, 27 mEq/L acetate, 23 mEq/L gluconate, 294 mosmol/L, pH 7.4). Solutions of Cy5, control, or MARCKS siRNA were resuspended in Plasmalyte A. Vein specimens were divided into 3- or 4-cm segments, cannulated distally, secured with 3-0 silk ties, and flushed with siRNA solution. Vein segments were then clamped proximally and distended with siRNA solution for 10 min at 120 mmHg (16 kPa) using a standard angioplasty insufflator (22). After transfection, specimens were divided into 5- to 10-mm segments, rinsed, and placed in organ culture consisting of SmGM-2 medium supplemented with 30% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B, with medium changes performed every 24 h, as described previously (23). For vein segments used in proliferation experiments, culture medium was additionally supplemented with 100 μmol/L bromodeoxyuridine (BrdU; Sigma, St. Louis, MO, USA) (24).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total cellular RNA was harvested from VSMCs, ECs, and saphenous vein homogenates using RNeasy spin columns (Qiagen, Valencia, CA, USA) and qRT-PCR was performed to assess MARCKS mRNA levels, as described previously (21). MARCKS mRNA levels were normalized to 18S mRNA levels. Comparisons were made between mRNA levels from tissues transfected with MARCKS siRNA to those transfected in parallel with control siRNA.
Western blot analysis
The following antibodies were used for Western blot analysis: goat anti-MARCKS immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-p27kip1 IgG1 (Pharmingen, San Diego, CA, USA) mouse anti-glyceraldehyde-3-phosphate dehydrogenase IgG1 (GAPDH; Ambion, Austin, TX, USA) and rabbit anti-actin IgG (Sigma). Peroxidase-conjugated secondary antibodies were obtained from Bio-Rad (Hercules, CA, USA). Densitometry was performed using NIH Image J 1.34 (U.S. National Institutes of Health, Bethesda, MD, USA).
Migration assay
Cellular migration was evaluated using the Fluoroblok migration assay (BD Biosciences, San Jose, CA, USA), as described previously (25). For VSMCs, calcein-AM-labeled cells migrated through an 8-μm pore at the base of the Fluoroblok insert toward a chemoattractant stimulus of SmGM-2 medium supplemented with 30% FBS. For ECs, the assay was modified to use a 3-μm pore size and a chemoattractant stimulus of EGM-2MV medium with 5% FBS and 200 ng/ml vascular endothelial growth factor (VEGF; R&D Systems, Minneapolis, MN, USA). For VSMCs, the chemoattractant stimulus was chosen to parallel organ culture conditions, in which robust VSMC migration has been shown to occur (26). For ECs, migration was driven using the maximally effective dose of VEGF shown previously in our laboratory (27).
Proliferation assay
The Alamar Blue assay (Trek Diagnostics, Cleveland, OH, USA) was used to assess cellular proliferation, as described previously (25). VSMCs and ECs were plated and grown for 24 h in the presence of medium with 5% FBS, followed by a day 0 measurement of fluorescent intensity after 4-hour incubation with the Alamar Blue reagent. Cells were then transfected with control or MARCKS siRNA for 24 h, after which they were challenged with fresh cell medium supplemented with 30% FBS to stimulate proliferation. Alamar Blue assays were subsequently performed every 2 days to track proliferation. The stimulus for cellular proliferation was chosen to parallel organ culture conditions.
Toxicity assay
The CytoTox-ONE lactate dehydrogenase (LDH) homogenous membrane integrity assay (Promega, Madison, WI, USA) was used to determine the toxicity incurred by treatment with MARCKS siRNA. Medium aspirated from cells maintained for proliferation assays was incubated with the resazurin reagent, and fluorescence intensity was measured at excitation and emission wavelengths 560 and 590 nm, respectively (25).
Confocal microscopy
Vein segments transfected with Cy5 siRNA were snap-frozen after 24 h in organ culture, and 5-μm cryosections were mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Confocal micrographs were obtained using a Bio-Rad MRC 1024 confocal laser system at our institution’s core microscopy facility. Triple-stained micrographs with excitation wavelengths for red, green, and blue fluorescence emission were acquired to show Cy5 fluorescence (blue), as well as the vessel architecture (through green and red autofluorescence of the elastic fibers). Cy5 fluorescence in confocal micrographs was analyzed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA, USA) to quantify the percentage of pixels fluorescing in a given area, as described previously (28). Endothelial delivery was defined as fluorescence signal internal to the internal elastic lamina. Medial delivery was defined as fluorescence signal between the internal and external elastic lamina. Adventitial delivery was defined as fluorescence signal external to the external elastic lamina.
Histology and immunohistochemistry
Transfected vein segments were formalin fixed and embedded in paraffin after 5 days in organ culture. To measure intimal thickening in culture, 5-μm sections were cut from the midportion of cultured vein segments and stained with Verhoeff-Van Gieson (VVG) stain for elastic fibers. NIH Image J 1.34 was used to measure intimal thickness at 5 to 10 points on two serial micrographs, 50 μm apart, from each vein segment, as described previously (29). To assess cellular proliferation, transfected vein segments cultured in 100 μmol/L BrdU were sectioned and immunostained using a BrdU-labeling kit (Invitrogen, Carlsbad, CA, USA) (24). The proportion of neointimal and medial nuclei staining positive for BrdU near the luminal interface was calculated in a masked fashion from 5 high-power fields per tissue specimen to produce a proliferation index.
Statistical methods
All experiments were performed in triplicate unless otherwise noted. Data are presented as means ± sd. Statistical analysis was performed on STATA software (STATA Corporation, College Station, TX, USA). Significance of association was assessed using one-way ANOVA with Bonferroni correction (Figs. 1 and 5B), one-way ANOVA with Dunnett’s correction (Fig. 2), repeated-measures two-way ANOVA with Bonferroni correction (Fig. 3), unpaired Student’s t test (Fig. 4), or paired Student’s t test (Figs. 5C and 6).
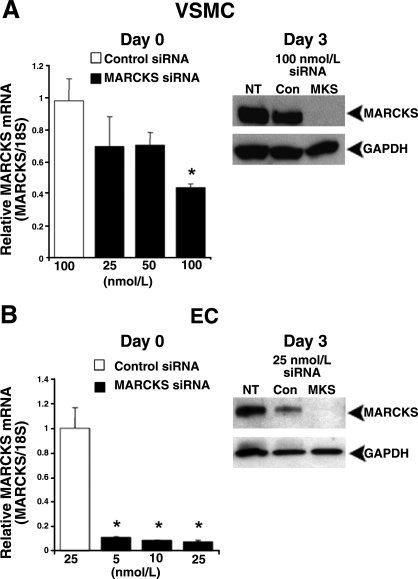
Figure 1.
Transfection of VSMCs and ECs with MARCKS siRNA silences MARCKS mRNA and protein expression. Transfection of VSMCs (A) and ECs (B) with MARCKS siRNA reduced MARCKS mRNA levels at the end of the transfection period (qRT-PCR analysis). Complete MARCKS knockdown was detected at the protein level by Western blot analysis 3 days after transfection with 100 nmol/L MARCKS siRNA (MKS) in VSMCs (A) and 25 nmol/L MARCKS siRNA in ECs (B). qRT-PCR data represent averages of 3 independent experiments. Western blots correspond to one representative experiment of 3 performed. NT, no treatment; Con, control siRNA. *P < 0.05 vs. control siRNA.
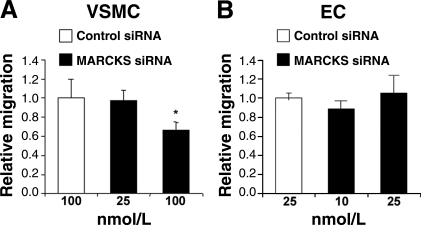
Figure 2.
MARCKS silencing inhibits migration in VSMCs but not ECs. A) VSMC migration was reduced after transfection with MARCKS siRNA. B) EC migration was unaffected after transfection with MARCKS siRNA. Data represent averages of 3 independent experiments. *P < 0.05 vs. control siRNA.
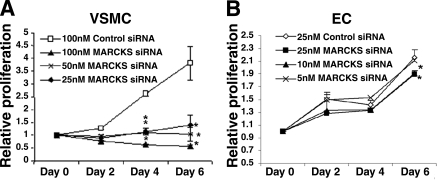
Figure 3.
MARCKS silencing arrests proliferation in VSMCs but not ECs. A) Transfection of VSMCs with MARCKS siRNA powerfully inhibited cellular proliferation at all siRNA concentrations. B) Transfection of ECs with MARCKS siRNA had a minimal effect on cellular proliferation. Data represent averages of 3 independent experiments. *P < 0.05 vs. control siRNA.
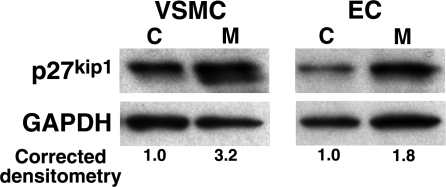
Figure 4.
MARCKS silencing preferentially up-regulates p27kip1 in proliferating VSMCs. p27kip1 expression is up-regulated to a greater extent after MARCKS silencing in proliferating VSMCs as compared to proliferating ECs (P<0.05). Western blots correspond to one representative experiment of 3 performed. C, control; M, MARCKS siRNA.
Figure 5.
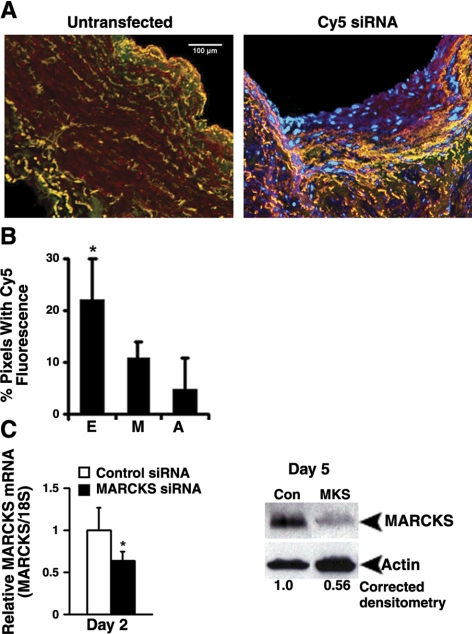
Distending pressure transfection achieves siRNA delivery and MARCKS silencing in human saphenous vein. A) Left panel: triple staining of untransfected vein segments using confocal microscopy shows overlapping green and red autofluorescence of the elastic fibers with no blue Cy5 fluorescence. Right panel: vein segments transfected with Cy5 siRNA demonstrate discrete areas of blue fluorescence within all layers, independent of the green/red autofluorescence. Micrographs (×100) correspond to one representative experiment of 3 performed. B) Quantitative analysis of Cy5 fluorescence in confocal micrographs reveals significantly greater siRNA delivery to the endothelium (E), as compared to the media (M) or adventitia (A). n = 5 to 6 vein segments per condition. C) Left panel: distending pressure transfection with MARCKS siRNA reduced MARCKS mRNA levels at day 2, as measured by qRT-PCR. Data represent averages of 5 independent experiments. Right panel: Western blot analysis of whole-vein extracts recovered 5 days after transfection showed a reduction in MARCKS protein in vein segments transfected with MARCKS siRNA (MKS) vs. control siRNA (Con). Western blots correspond to 1 representative experiment of 10 performed. *P < 0.05.
Figure 6.
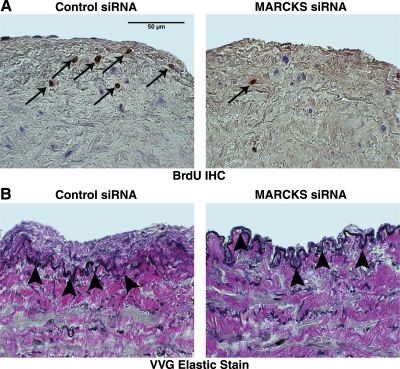
MARCKS silencing inhibits VSMC proliferation and neointimal formation in cultured human saphenous vein. A) IHC staining for BrdU revealed a reduction in proliferating nuclei (brown nuclei, arrows) measured in vein segments incubated with BrdU after transfection with MARCKS siRNA (right panel) vs. control siRNA (left panel). Micrographs (×400) correspond to one representative experiment of 4 performed. B) Intimal thickness measured in VVG-stained cross sections of transfected vein segments was reduced in patient-matched vein segments transfected with MARCKS siRNA (right panel) vs. control siRNA (left panel). Arrowheads indicate the internal elastic lamina. Micrographs (×400)correspond to one representative experiment of 5 performed.
RESULTS
MARCKS siRNA silences MARCKS expression in VSMCs and ECs
Transfection with MARCKS siRNA significantly decreased MARCKS mRNA and protein levels in VSMCs and ECs. In VSMCs, transfection with 100 nmol/L MARCKS siRNA reduced MARCKS mRNA levels by 56 ± 2% when measured immediately after the 24-h transfection period by qRT-PCR (P<0.05) and led to the complete loss of MARCKS protein by Western blot analysis at day 3 when compared to cells transfected with control siRNA (Fig. 1A). In ECs, complete MARCKS knockdown was achieved using lower concentrations of MARCKS siRNA. Transfection with 25 nmol/L MARCKS siRNA reduced MARCKS mRNA levels by 92 ± 1% after the 24-h transfection period (P<0.05) and led to the complete loss of MARCKS protein by day 3 (Fig. 1B).
MARCKS silencing inhibits migration in VSMCs but not ECs
Cellular migration was assessed in VSMCs and ECs 3 days after transfection using the previously established concentrations of MARCKS siRNA that led to complete MARCKS protein knockdown. Transfection with 100 nmol/L MARCKS siRNA reduced VSMC migration to 68 ± 7% of controls (Fig. 2A; P<0.05). In contrast, transfection with 25 nmol/L MARCKS siRNA did not affect the rate of EC migration when compared to controls (1.0±0.07 vs. 1.08±0.26, P not significant; Fig. 2B).
MARCKS silencing arrests proliferation in VSMCs but not ECs
Cellular proliferation was assessed in VSMCs and ECs for 6 days following transfection with either MARCKS or control siRNA. MARCKS knockdown inhibited VSMC proliferation. VSMCs transfected with 100 nmol/L control siRNA challenged with 30% FBS proliferated by 3.8 ± 0.6-fold after 6 days. VSMCs transfected with 25, 50, and 100 nmol/L MARCKS siRNA proliferated by 1.4 ± 0.4-, 1.0 ± 0.3-, and 0.6 ± 0.1-fold, respectively, after 6 days. Proliferation was significantly reduced compared to controls beginning 4 days after transfection for all concentrations of MARCKS siRNA (P<0.05; Fig. 3A). Serial LDH toxicity assays performed on all cell populations found no elevation in LDH release in cells treated with MARCKS siRNA compared to control siRNA (data not shown), suggesting cytotoxicity is not the cause of growth arrest.
In contrast, transfection with MARCKS siRNA had only a minimal affect on EC proliferation. ECs transfected with 25 nmol/L control siRNA challenged with 30% FBS proliferated by 2.2 ± 0.1-fold after 6 days. ECs transfected with 5, 10, and 25 nmol/L MARCKS siRNA proliferated by 2.1 ± 0.03-, 1.9 ± 0.05-, and 1.9 ± 0.06-fold, respectively, after 6 days (P<0.05 for 10 and 25 nmol/L; Fig. 3B).
MARCKS silencing preferentially up-regulates p27kip1 in VSMCs
To determine the molecular mechanism of proliferation arrest in VSMCs after MARCKS silencing, we screened for an interaction with the cyclin-dependent kinase inhibitor (CDKI) p27kip1, a critical cell cycle brake and inhibitor of neointimal formation (30). VSMCs and ECs were transfected with 25 nmol/L control or MARCKS siRNA then cultured for 48 h in 0.1% FBS to synchronize cells in the G0 phase of the cell cycle. Proliferation was then driven by adding 30% FBS. Cell lysates were recovered 24 h later for Western blot analysis. Western blot analysis demonstrated an average 3.2 ± 0.7-fold increase in p27kip1 protein levels in VSMCs transfected with MARCKS siRNA as compared to cells transfected with control siRNA (P<0.05, n=3) and a 1.8 ± 0.4-fold increase over controls in ECs (P<0.05, n=3; Fig. 4).
Rapid distending pressure transfection with MARCKS siRNA reduces MARCKS expression in human saphenous vein
Cy5 siRNA was used to confirm siRNA delivery to human saphenous vein using the distending pressure transfection technique. Confocal micrographs of human saphenous vein segments maintained in organ culture for 24 h following a 10-min distending pressure transfection with 25 μmol/L Cy5 siRNA demonstrated discrete areas of blue Cy5 fluorescence within all tissue layers. Quantitation of blue fluorescence using image analysis software demonstrated significantly greater uptake of Cy5 siRNA in the endothelial layer as compared to the media or adventitia [Fig. 5B; P<0.05 for the difference between endothelial (22±8% pixels fluorescing) vs. medial (11±3% pixels fluorescing) or adventitial (5±9% pixels fluorescing) tissue layers]. Blue fluorescence emission was not observed in untransfected vein (Fig. 5A).
Distending pressure transfection with 25 μmol/L MARCKS siRNA reduced MARCKS mRNA levels by 36 ± 11% in whole-vein homogenates recovered from vein segments maintained in organ culture for 2 days after transfection (P<0.05, n=5; Fig. 5C, left). MARCKS protein levels were stably reduced by 44 ± 23% in whole-vein homogenates recovered 5 days after transfection (P<0.05, n=10; Fig. 5C, right).
MARCKS silencing inhibits VSMC proliferation and neointimal formation in human saphenous vein
Transfected vein segments were maintained in organ culture for 5 days in the presence of 100 μmol/L BrdU, a nucleotide analog. Immunohistochemistry staining with an anti-BrdU antibody demonstrated positive proliferating neointimal and medial nuclei undergoing DNA replication. MARCKS knockdown reduced proliferating nuclei by 60% at day 5 in cultured vein segments as compared to vein segments transfected with control siRNA (8±6 vs. 20±4%, P<0.05, Fig. 6A).
Parallel vein segments were sectioned and stained with VVG elastic stain to delineate the internal elastic lamina and allow for quantification of intimal thickness. Measurements showed a 41% reduction in neointimal formation in patient-matched vein segments transfected with MARCKS siRNA, as compared to control siRNA (33±17 vs. 54±21 μm, P<0.05; Fig. 6B).
DISCUSSION
We previously found MARCKS transcription to be up-regulated in canine models of IH (9, 10). Here, we employ a siRNA-mediated loss-of-function approach to further explore the contribution of MARCKS to IH. Our in vitro data demonstrate MARCKS silencing arrests VSMC proliferation and attenuates VSMC migration, with only a minimal effect on EC proliferation and no effect on EC migration. Cellular proliferation after MARCKS silencing is likely inhibited in these cell types partly through the up-regulation of p27kip1. The phenotypic effects of MARCKS silencing were recapitulated in intact human saphenous vein ex vivo, yielding a reduction in VSMC proliferation and culminating in reduced neointimal formation. The siRNA transfection methods used in these experiments represent the first report to our knowledge of gene silencing in human vein by siRNA and could be adapted for use in the operating room.
MARCKS silencing inhibited migration in VSMCs but not ECs in vitro. Decreased VSMC migration after MARCKS silencing in cultured human saphenous vein was indirectly shown by a reduction in neointimal mass, where the predominant cell type consists of synthetic VSMCs that have migrated from the media (31). Supporting our data, a report by Disatnik et al. (15) showed antisense inhibition of MARCKS inhibited muscle cell migration in a dose-dependent fashion. MARCKS has a well-described actin binding site and participates in integrin-mediated muscle cell spreading by promoting the reorganization of the actin cytoskeleton. The initial phases of muscle cell adhesion are mediated by MARCKS phosphorylation and translocation to the cytosol after PKC activation. However, later stages of cell migration and spreading require PKC deactivation, MARCKS dephosphorylation, and the return of MARCKS to the cellular membrane, where it participates in the polymerization and cross-linking of actin and the maturation of the cytoskeleton (17). Accordingly, global reduction of MARCKS, as achieved by siRNA, is thought to inhibit VSMC migration by disrupting both cell adhesion and cytoskeletal stabilization. MARCKS involvement in EC migration has yet to be reported. However, our data suggest ECs do not require MARCKS for migration and rather rely on alternative signaling pathways and different cytoskeletal binding proteins for actin remodeling (32, 33).
MARCKS silencing arrested growth factor-driven proliferation in VSMCs in vitro and reduced VSMC proliferation by 60% in human saphenous vein. These data are in agreement with previous reports showing that PKC activation promotes VSMC proliferation (34, 35). Accordingly, interruption of PKC signaling at the level of its downstream effector MARCKS appears to block such proliferative signals.
Our data further demonstrate the antiproliferative effect of MARCKS silencing in VSMCs results in part from the up-regulation of p27kip1, a key CDKI. p27kip1 plays a critical role in vascular remodeling and arrests VSMCs in the G1 phase of the cell cycle (36). Overexpression of p27kip1 promotes a quiescent dedifferentiated VSMC phenotype in vitro (37, 38) and inhibits neointimal formation in vivo (30, 39). Our data, therefore, suggest MARCKS silencing reverts VSMCs to a quiescent cell phenotype through a p27kip1-dependent mechanism. The molecular basis for increased p27kip1 expression induced by MARCKS silencing remains undetermined and warrants further investigation. However, we speculate p27kip1 up-regulation could occur secondary to MAPK inhibition, given the known interaction of MARCKS with MAPK and the reciprocal interaction between MAPK signaling and p27kip1 expression (38).
Given that PKC signaling has also been shown to promote EC proliferation (40), we anticipated that MARCKS silencing would likewise inhibit proliferation in ECs. Our data negated this hypothesis by showing that even total MARCKS knockdown produces little effect on EC proliferation in vitro. In concert with this finding, Zhao and colleagues reported that MARCKS levels were down-regulated in proliferating pulmonary ECs (14). The differential effect of MARCKS silencing on the proliferative potential of VSMCs and ECs is poorly understood but perhaps stems from divergent interactions of MARCKS with the calmodulin and MAPK signaling pathways in these two cell types. Regardless of the mechanism of action, this differential effect is highly desirable for the safe use of MARCKS siRNA in vascular applications.
In this study, we demonstrate greater MARCKS silencing in vitro in ECs than VSMCs for a given set of transfection parameters. This finding is consistent with previously presented data (21) but should not affect experimental conclusions, as phenotypic assays were performed with siRNA concentrations that achieved total MARCKS silencing in either cell type. In VSMCs, early mRNA knockdown after the transfection/starvation period reached only 56% after transfection with 100 nmol/L MARCKS siRNA, whereas complete protein knockdown was observed at day 3. This finding is in agreement with data from our laboratory showing that mRNA knockdown levels are further enhanced after the addition of growth factors and produce a maximal effect on protein suppression in a delayed fashion (unpublished data).
Vein graft transfection with siRNA represents a model for temporally limited gene therapy with direct, local delivery only to the tissue and cells of interest. Oligonucleotide vein graft gene therapy to prevent IH formation has been attempted using multiple other methods and gene targets but has not been previously demonstrated in human vein using siRNA (41). To achieve transfection, we used a 10-min distending pressure technique used by others to transfect human vein with DNA oligonucleotides (22). The pressure level was chosen not to exceed the pressures experienced by vein grafts after implantation into the arterial circulation or the pressures shown to induce graft injury (42,43,44). Quantification of siRNA delivery achieved using this technique revealed significantly greater delivery to the endothelium as compared to the media or the adventitia. This finding further supports the notion of enhanced siRNA delivery and gene silencing in ECs and is of unique therapeutic relevance (21). However, calculation of transfection efficiency is not possible using these methods, given that siRNA localizes outside of the nucleus; therefore, colocalization of siRNA fluorescence with a nuclear counterstain cannot be used to calculate the percentage of cells demonstrating siRNA uptake. The most useful measure of efficacy, however, is to measure the degree of gene suppression achieved. Using this transfection method, global MARCKS levels were reduced in human saphenous vein by 36–44% over the following 5 days. Although this technique is convenient for intraoperative use and does not rely on transfection reagents, further optimization of transfection methods could strive to produce enhanced suppression of gene targets. Nonetheless, this study and others have shown phenotypic relief from disease processes following targeted gene silencing as low as 40% (45), suggesting RNAi-based therapeutics could be successful with <100% suppression of gene targets, depending on the pathogenic role of the targeted gene.
MARCKS silencing resulted in reduced neointimal formation in cultured human saphenous vein. Together with our in vitro findings, these data suggest MARCKS contributes to the pathogenesis of IH and could be a useful target for therapy. However, the therapeutic implications of this study are limited by the use of an ex vivo organ culture model lacking pulsatile flow through the lumen of the graft, which could augment the rate of IH formation and the duration of gene silencing. Further in vivo experimentation is needed to delineate the time course and durability of gene silencing and IH response in functioning vein graft conduits treated with MARCKS siRNA.
In conclusion, this inquiry into the effects of MARCKS silencing in human vascular tissues has uncovered novel information about the divergent contributions of MARCKS to VSMC and EC phenotypes and revealed the powerful therapeutic potential of MARCKS silencing by siRNA for the prevention of IH in vein grafts. Given that any vein graft siRNA intervention would likely subject all cells in the vessel wall to gene silencing, MARCKS siRNA appears ideally suited to inhibit pathological VSMC phenotypes without negatively affecting EC health and function. Furthermore, the therapeutic utility of MARCKS as a target appears relatively obtainable given the favorable results achieved with even modest levels of MARCKS inhibition and the adaptability of the transfection methods for the clinical setting.
Acknowledgments
The authors are grateful to Ian Driscoll, Faramarz Edalat, Monica Jain, and Lena Caron for help with transfection experiments, and Scott Damrauer for careful reading of the manuscript. This work was supported by National Institutes of Health (NIH) T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734 (to T.S.M., M.C.M., J.Y.M., and G.V.M.); a Howard Hughes Medical Institute Research Training Fellowship for Medical Students (to N.D.A.); NIH R01 grants HL021796, HL086741 (to C.F., and F.W.L.), and HL080130 (to C.F.); and the von Liebig Foundation (to L.P. and F.W.L.).
References
- Davies M G, Hagen P O. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9:7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- Allaire E, Clowes A W. Endothelial cell injury in cardiovascular surgery: the intimal hyperplastic response. Ann Thorac Surg. 1997;63:582–591. doi: 10.1016/s0003-4975(96)01045-4. [DOI] [PubMed] [Google Scholar]
- Schwartz S M. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–S89. [PubMed] [Google Scholar]
- Hoofnagle M H, Thomas J A, Wamhoff B R, Owens G K. Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- Clowes A W, Reidy M A, Clowes M M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- Stegemann J P, Nerem R M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- Jovinge S, Hultgardh-Nilsson A, Regnstrom J, Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:490–497. doi: 10.1161/01.atv.17.3.490. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy M A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D J, Kalish J A, Li C, Deutsch E R, Contreras M A, LoGerfo F W, Quist W C. Temporal gene expression following prosthetic arterial grafting. J Surg Res. 2004;120:27–36. doi: 10.1016/j.jss.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Kalish J A, Willis D J, Li C, Link J J, Deutsch E R, Contreras M A, Quist W C, Logerfo F W. Temporal genomics of vein bypass grafting through oligonucleotide microarray analysis. J Vasc Surg. 2004;39:645–654. doi: 10.1016/j.jvs.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Andrassy M, Belov D, Harja E, Zou Y S, Leitges M, Katus H A, Nawroth P P, Yan S D, Schmidt A M, Yan S F. Central role of PKCbeta in neointimal expansion triggered by acute arterial injury. Circ Res. 2005;96:476–483. doi: 10.1161/01.RES.0000156903.37007.d1. [DOI] [PubMed] [Google Scholar]
- Hall J L, Matter C M, Wang X, Gibbons G H. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.res.87.7.574. [DOI] [PubMed] [Google Scholar]
- Raptis A E, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109:S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Neltner B S, Davis H W. Role of MARCKS in regulating endothelial cell proliferation. Am J Physiol Cell Physiol. 2000;279:C1611–C1620. doi: 10.1152/ajpcell.2000.279.5.C1611. [DOI] [PubMed] [Google Scholar]
- Disatnik M H, Boutet S C, Lee C H, Mochly-Rosen D, Rando T A. Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J Cell Sci. 2002;115:2151–2163. doi: 10.1242/jcs.115.10.2151. [DOI] [PubMed] [Google Scholar]
- Dulong S, Goudenege S, Vuillier-Devillers K, Manenti S, Poussard S, Cottin P. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase Calpha and calpain proteolytic cleavage. Biochem J. 2004;382:1015–1023. doi: 10.1042/BJ20040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik M H, Boutet S C, Pacio W, Chan A Y, Ross L B, Lee C H, Rando T A. The bi-directional translocation of MARCKS between membrane and cytosol regulates integrin-mediated muscle cell spreading. J Cell Sci. 2004;117:4469–4479. doi: 10.1242/jcs.01309. [DOI] [PubMed] [Google Scholar]
- Schonwasser D C, Palmer R H, Herget T, Parker P J. p42 MAPK phosphorylates 80 kDa MARCKS at Ser-113. FEBS Lett. 1996;395:1–5. doi: 10.1016/0014-5793(96)00991-x. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz A A, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant C, You J Y, Sasaki Y, Grabarek Z, Morgan K G. MARCKS is a major PKC-dependent regulator of calmodulin targeting in smooth muscle. J Cell Sci. 2005;118:3595–3605. doi: 10.1242/jcs.02493. [DOI] [PubMed] [Google Scholar]
- Andersen N D, Monahan T S, Malek J Y, Jain M, Daniel S, Caron L D, Pradhan L, Ferran C, Logerfo F W. Comparison of gene silencing in human vascular cells using small interfering RNAs. J Am Coll Surg. 2007;204:399–408. doi: 10.1016/j.jamcollsurg.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mann M J, Gibbons G H, Hutchinson H, Poston R S, Hoyt E G, Robbins R C, Dzau V J. Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues. Proc Natl Acad Sci U S A. 1999;96:6411–6416. doi: 10.1073/pnas.96.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyombo A A, Angelini G D, Bryan A J, Jasani B, Newby A C. Intimal proliferation in an organ culture of human saphenous vein. Am J Pathol. 1990;137:1401–1410. [PMC free article] [PubMed] [Google Scholar]
- Mann M J, Whittemore A D, Donaldson M C, Belkin M, Conte M S, Polak J F, Orav E J, Ehsan A, Dell'Acqua G, Dzau V J. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- Monahan T S, Andersen N D, Panossian H, Kalish J A, Daniel S, Shrikhande G V, Ferran C, Logerfo F W. A novel function for cadherin 11/osteoblast-cadherin in vascular smooth muscle cells: modulation of cell migration and proliferation. J Vasc Surg. 2007;45:581–589. doi: 10.1016/j.jvs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- George S J, Lloyd C T, Angelini G D, Newby A C, Baker A H. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- Stone D, Phaneuf M, Sivamurthy N, LoGerfo F W, Quist W C. A biologically active VEGF construct in vitro: implications for bioengineering-improved prosthetic vascular grafts. J Biomed Mater Res. 2002;59:160–165. doi: 10.1002/jbm.1229. [DOI] [PubMed] [Google Scholar]
- Kirkeby S, Thomsen C E. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods. 2005;301:102–113. doi: 10.1016/j.jim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Soyombo A A, Angelini G D, Newby A C. Neointima formation is promoted by surgical preparation and inhibited by cyclic nucleotides in human saphenous vein organ cultures. J Thorac Cardiovasc Surg. 1995;109:2–12. doi: 10.1016/S0022-5223(95)70415-9. [DOI] [PubMed] [Google Scholar]
- Chen D, Krasinski K, Sylvester A, Chen J, Nisen P D, Andres V. Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997;99:2334–2341. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K E, Varty K, Jones L, Bell P R, London N J. Human saphenous vein organ culture: a useful model of intimal hyperplasia? Eur J Vasc Endovasc Surg. 1996;11:48–58. doi: 10.1016/s1078-5884(96)80134-1. [DOI] [PubMed] [Google Scholar]
- Holderfield M T, Hughes C C. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- Itoh H, Yamamura S, Ware J A, Zhuang S, Mii S, Liu B, Kent K C. Differential effects of protein kinase C on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–H370. doi: 10.1152/ajpheart.2001.281.1.H359. [DOI] [PubMed] [Google Scholar]
- Herbert J M, Clowes M, Lea H J, Pascal M, Clowes A W. Protein kinase C alpha expression is required for heparin inhibition of rat smooth muscle cell proliferation in vitro and in vivo. J Biol Chem. 1996;271:25928–25935. doi: 10.1074/jbc.271.42.25928. [DOI] [PubMed] [Google Scholar]
- Tanner F C, Yang Z Y, Duckers E, Gordon D, Nabel G J, Nabel E G. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res. 1998;82:396–403. doi: 10.1161/01.res.82.3.396. [DOI] [PubMed] [Google Scholar]
- Izzard T D, Taylor C, Birkett S D, Jackson C L, Newby A C. Mechanisms underlying maintenance of smooth muscle cell quiescence in rat aorta: role of the cyclin dependent kinases and their inhibitors. Cardiovasc Res. 2002;53:242–252. doi: 10.1016/s0008-6363(01)00444-8. [DOI] [PubMed] [Google Scholar]
- Castro C, Diez-Juan A, Cortes M J, Andres V. Distinct regulation of mitogen-activated protein kinases and p27Kip1 in smooth muscle cells from different vascular beds. A potential role in establishing regional phenotypic variance. J Biol Chem. 2003;278:4482–4490. doi: 10.1074/jbc.M204716200. [DOI] [PubMed] [Google Scholar]
- Abid M R, Yano K, Guo S, Patel V I, Shrikhande G, Spokes K C, Ferran C, Aird W C. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- Tsopanoglou N E, Pipili-Synetos E, Maragoudakis M E. Protein kinase C involvement in the regulation of angiogenesis. J Vasc Res. 1993;30:202–208. doi: 10.1159/000158995. [DOI] [PubMed] [Google Scholar]
- Conte M S. Molecular engineering of vein bypass grafts. J Vasc Surg. 2007;45:A74–A81. doi: 10.1016/j.jvs.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Ramos J R, Berger K, Mansfield P B, Sauvage L R. Histologic fate and endothelial changes of distended and nondistended vein grafts. Ann Surg. 1976;183:205–228. doi: 10.1097/00000658-197603000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom L E, Olinger G N, Bonchek L I, Gunay I I, Kissebah A H, Rodriguez E R, Ferrans V J. The relative influence of arterial pressure versus intraoperative distention on lipid accumulation in primate vein bypass grafts. J Thorac Cardiovasc Surg. 1985;90:756–764. [PubMed] [Google Scholar]
- LoGerfo F W, Quist W C, Crawshaw H M, Haudenschild C. An improved technique for preservation of endothelial morphology in vein grafts. Surgery. 1981;90:1015–1024. [PubMed] [Google Scholar]
- Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt F J, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]