Abstract
Nuclear factor-κB (NF-κB) signaling is necessary for many types of muscle atrophy, yet only some of the required components have been identified. Gene transfer of a dominant negative (d.n.) IKKβ into rat soleus muscles showed complete inhibition of 7-day disuse-induced activation of a κB reporter gene, while overexpression of wild-type (w.t.) IKKβ did not. Overexpression of a d.n. IKKβ-EGFP fusion protein showed that atrophy was inhibited by 50%, indicating that IKKβ is required for the atrophy process. Overexpression of constitutively active (c.a.) IKKβ-EGFP showed a marked increase in NF-κB activity and a decrease in fiber size of weight-bearing soleus muscles, while muscles overexpressing w.t. IKKβ-HA had no effect. The same results were found for IKKα; overexpression of a d.n. form of the protein decreased unloading-induced NF-κB activation and inhibited atrophy by 50%, while overexpression of the w.t. protein had no effect. Overexpression of a c.a. IKKα–EGFP fusion protein showed that IKKα was sufficient to activate NF-κB activity and induce fiber atrophy in muscle. Overexpression of d.n. IKKβ plus d.n. IKKα showed an additive effect on the inhibition of disuse atrophy (70%), suggesting that both kinases of the IKK complex are required for muscle atrophy. These data show that both IKKα and IKKβ are necessary and sufficient for physiological muscle atrophy.—Van Gammeren, D., Damrauer, J. S., Jackman, R. W., Kandarian, S. C. The IκB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy.
Keywords: muscle wasting, inhibitor of κB kinase, IKK complex, NF-κB signaling
The intracellular signaling pathways that regulate skeletal muscle atrophy have just begun to be elucidated. In one line of work, we showed an early, robust, and sustained activation of nuclear factor-κB (NF-κB) -dependent transcription due to muscle disuse atrophy (1, 2). Identification of the specific NF-κB signaling components required for disuse and other types of muscle wasting is a complex undertaking because of the multitude of pathway components. The functional NF-κB transcription factor is composed of a dimer from various combinations of the 5-member Rel family of DNA binding proteins (3). In the case of muscle atrophy, there have been only a few studies that have investigated the requirement of specific Rel proteins or their cotransactivators in muscle wasting; we demonstrated, using knockout mice, that the Rel protein p50 (encoded by the nfkb1 gene) and a member of the inhibitor of κB (IκB) family called Bcl-3 (a NF-κB cotransactivator), were both essential for disuse muscle atrophy (2, 4), but c-Rel was not (2). RelA (p65), the most well studied of the Rel proteins and a component of classical NF-κB signaling, showed increased DNA binding or increased phosphorylation in muscles from mice with cancer (5, 6). Overexpression of RelA in skeletal muscle for 7 days was sufficient to induce fiber atrophy (7). RelB and p52 are Rel proteins known to be involved in the “alternative” NF-κB pathway, and although biochemical evidence does not support a role of these transcription factors in muscle atrophy (1, 8), they cannot yet be entirely ruled out.
Recent studies have identified a role for two of the upstream proteins that regulate NF-κB-dependent transactivation during atrophy. IκBα inactivates NF-κB dimers by binding to them in the cytosol. Genetic studies showed that inhibition of IκBα phosphorylation, and thus IκBα proteasomal degradation, produces the cytosolic retention of Rel dimers, and partially blocks muscle atrophy due to disuse (2), denervation, and cancer (6) by 40–50%, suggesting that IκBα degradation is required for normal atrophy. In addition, there have been two studies on the role of the IκB kinase (IKK) called IKKβ in muscle atrophy (6, 9). IKKβ phosphorylates IκBα, leading to its proteasomal degradation and, therefore, the nuclear translocation of Rel dimers. Mice overexpressing a constitutively active (c.a.) IKKβ transgene in muscle showed marked atrophy and increased NF-κB DNA binding, demonstrating that activated IKKβ was sufficient to induce muscle wasting (6). Recently, an IKKβ muscle-specific knockout mouse was used to determine whether IKKβ was required for denervation atrophy (9). Ablation of IKKβ inhibited denervation atrophy in soleus muscle by at least 50% at 7 and 28 days, as assessed by muscle mass. The effect was less in denervated extensor digitorum longus (EDL) and gastrocnemius, although the atrophy inhibition in gastrocnemius was more pronounced at 28 days. Overall, it appears that IKKβ is required for the normal atrophy response, particularly in the soleus. The muscle-specific IKKβ-depleted mouse, however, has more muscle fibers, a larger cross-sectional area, and greater tetanic force than IKKβ floxed mice, demonstrating that there are developmental effects of IKKβ deletion in skeletal muscle (9). Another report showed that muscle deletion of IKKβ strongly promotes myogenesis and increases fiber number in hind limb muscles (10). These developmental effects may explain the discrepancies in atrophy results among time points and between different muscle types and emphasize the desirability of creating genetic changes after the muscles have fully developed.
IKKβ is only one component of the functional IKK complex, and it does not act alone in the phosphorylation of IκB. The IKK complex consists of two nonredundant kinases, IKKα and IKKβ, and an essential modulator subunit (NEMO), IKKγ (reviewed by Perkins and Gilmore, ref. 11). Because the role of the IKK complex is essential in classical NF-κB signaling, we determined whether both IKKβ and IKKα are necessary and sufficient for skeletal muscle atrophy using ectopic overexpression of dominant negative (d.n.) and c.a. forms of the proteins in adult muscle, thereby obviating any developmental effects of transgenics. The role of IKKα in atrophy has not been studied until now. We used a physiological model of disuse, and our analysis focused on 7 days of atrophy, a time of the greatest rate of protein loss and when κB activation is marked. The rationale for studying both kinase subunits is that while often working together in the same complex (12, 13), IKKα and IKKβ can have distinct physiological functions (14, 15). Although there is evidence of classical pathway involvement in muscle atrophy from our lab (2, 4) and from others (6, 9), the contribution of IKKα is not known. By overexpression of d.n. forms of the two IKKs alone, or in combination, we determined whether both kinases are required for disuse atrophy. Results from this work have previously been presented in abstract form (16).
MATERIALS AND METHODS
Animals, DNA injection, electroporation, and hind limb unloading
Eight-week-old, female Wistar rats were used for all experiments. The use of animals was approved by the Boston University Institutional Animal Care and Use Committee. Plasmid DNA was injected into the soleus muscle, as described previously (17). Briefly, the DNA was ethanol precipitated and resuspended in PBS prior to injection. For luciferase experiments, one soleus muscle from each animal was coinjected (using a 28-gauge insulin syringe) with 40 μg of the NF-κB-dependent luciferase reporter plasmid (2) and 50 μg of the appropriate expression plasmid in 50 μl of PBS, and the contralateral limb was injected with the NF-κB reporter plasmid and the corresponding empty vector of the expression plasmid (pEF6/HisB for IKKβ clones or pEF4/HisB for IKKα clones; Invitrogen, Carlsbad, CA, USA). In all coinjection experiments, it is important to note that fibers taking up reporter also take up expression plasmid (18, 19). The expression plasmids used were the following: d.n. IKKα (K44M), d.n. IKKβ (K44M), c.a IKKβ (S177/S181→EE) wild type (w.t.) IKKα, and w.t. IKKβ. The d.n. IKKα and d.n. IKKβ expression plasmids encode a kinase-dead form of IKKα and IKKβ, respectively. The c.a. IKKβ plasmid was constructed in the laboratory of Steve Shoelson (Joslin diabetes center, Boston, MA, USA) (6), other plasmids were from Michael Karin (University of California, San Diego, CA, USA) (13).
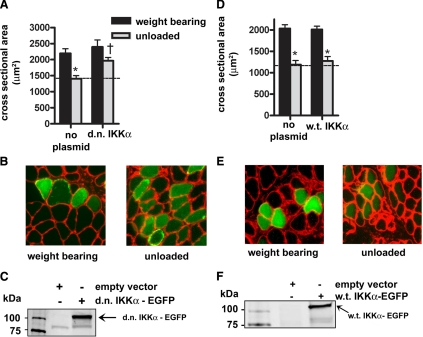
For atrophy experiments, in order to visualize the expression of exogenous proteins, the d.n. IKKα, d.n. IKKβ, w.t. IKKα, c.a. IKKβ were subcloned, inframe, into the N terminus of an EGFP expression vector (pEGFP-n1; Clontech, Palo Alto, CA, USA) to construct the d.n. IKKα-EGFP, d.n. IKKβ-EGFP, w.t. IKKα-EGFP, c.a. IKKβ-EGFP fusion plasmids. The w.t. IKKβ was subcloned, in frame, into an HA-tagged vector (pCMV-HA; Clontech). C.a. IKKα (S176/S180→EE) was constructed in the pEGFP-n1 vector by exchanging a SacI fragment from w.t. IKKα-EGFP with a SacI-ended PCR product, in which one of the primers carried coding mutations for the two serines at amino acids 176 and 180, converting them to glutamic acids (producing the plasmid c.a. IKKα-EGFP). The subclones were sequenced and expressed in C2C12 cells to verify that the correct protein was being translated and that the addition of EGFP maintained the activity of the protein. The latter was done by cotransfection with a NF-κB-dependent reporter in C2C12 cells; some cells were treated with TNF-α to show effect of d.n. IKK function (data not shown). As above, soleus muscles were injected with 50 μg of the specified expression plasmids. The fibers taking up the expression plasmid (green for EGFP fluorescence and brown after peroxidase DAB staining for HA) were compared to those fibers in the same fields that did not take up the plasmid in unloaded and weight-bearing muscles. Coinjections of the d.n. IKKα-EGFP plus the d.n. IKKβ-EGFP plasmid were done twice. In one experiment, a mixture of 50 μg of each plasmid (100 μg total) was injected into each muscle, and in a second experiment, a mixture of 25 μg of each plasmid (50 μg total) was coinjected into each muscle.
After plasmid injection, the muscles were electroporated using two-paddle electrodes (as shown in Dona et al., ref. 20) delivering 5 pulses at 125 V/cm for 20 ms with an interpulse interval of 200 ms (Electro Square porator ECM 830, BTX). This is a low-dose electroporation protocol that does not induce damage (21,22,23). Animals were randomly assigned to a weight-bearing control group (WB) or a hind limb unloaded group (HU). While still anesthetized, animals in the HU group were tail casted; 24 h later, the animals were suspended as described previously (24, 25). Seven days later, soleus muscles were removed from both weight-bearing and unloaded rats, weighed, and either snap frozen in liquid nitrogen for biochemical analysis or embedded in tissue-freezing medium and frozen in dry ice-cooled isopentane for histochemical analysis.
NF-κB reporter activity
Soleus muscles (8/group) were homogenized in passive lysis buffer (Promega, Madison, WI, USA), and luciferase activity was assessed as described previously (2). Relative light units (RLU) were measured on 20 μl of each muscle supernatant and reflect total RLU per muscle.
Western blot analysis
Protein concentration was determined using the detergent-compatible assay from Bio-Rad (Hercules, CA, USA). Fifty micrograms of protein was denatured and size fractionated on 4–15% SDS-polyacrylamide gels (Bio-Rad). Proteins were transferred to an Immobilon-FL polyvinylidene fluoride membrane (Millipore, Bedford, PA, USA), blocked in Odyssey blocking buffer, and incubated with the appropriate primary antibody according to the manufacturer’s instructions. Antibodies included IKKβ (2684; Cell Signaling Technology, Danvers, MA, USA), IKKα (Imgenex, San Diego, CA, USA; IMG-5477), and α-tubulin as a loading control (T6074; Sigma-Aldrich, St. Louis, MO, USA). Alexa Fluor 680 (Invitrogen) or IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA, USA) fluorescent dye-conjugated secondary antibodies were used for visualization with the Li-Cor Odyssey fluorescent detection system as described previously (25).
Immunohistochemistry
Soleus muscles were sectioned (10 μm) from the midbelly of the soleus and fixed in 4% paraformaldehyde (6 muscles per group). For the muscles injected with EGFP fusion plasmids, cross sections were incubated with anti-laminin (L9393; Sigma-Aldrich) and a Texas Red-X (T6391; Invitrogen) fluorescent dye-conjugated secondary antibody, as previously shown (2). Images were visualized using fluorescent microscopy. For the w.t. IKKβ experiment, the muscle cross sections were incubated with biotinylated anti-HA (BA-0704; Vector Laboratories, Burlingame, CA, USA), incubated with the Vectastain ABC reagent (PK-6100; Vector) and developed with the DAB substrate kit (SK-4100; Vector). All images were captured with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI, USA). Fiber cross-sectional area was calculated using the Meta-Morph Imaging System (Universal Imaging, Glendale, WI, USA). The average number of fibers measured per muscle was 150.
Statistics
A two-way ANOVA (loading condition vs. plasmid type) or a t test was used for analysis, depending on the number of groups studied (GraphPad Software, San Diego, CA, USA). All data are expressed as means ± se, and significance was established at P < 0.05.
RESULTS
Role of IKKβ in muscle atrophy
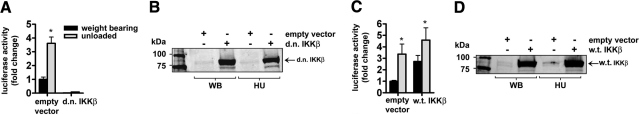
Because the activation of IKK does not always activate NF-κB-dependent transcription, we used a NF-κB activity assay to determine whether inhibition or activation of IKKβ or IKKα, in turn, inhibits κB-dependent transcriptional activity during muscle atrophy. To determine whether inhibition of IKKβ blocks unloading-induced NF-κB activity, a d.n. IKKβ plasmid was coinjected with the NF-κB reporter plasmid into soleus muscles. Seven days of muscle unloading resulted in a significant increase in NF-κB activity, while overexpression of the d.n. IKKβ plasmid inhibited κB activity in normal weight-bearing muscles and completely abolished the unloading-induced NF-κB activity (Fig. 1A). Demonstration of d.n. IKKβ overexpression is shown by immunoblotting (Fig. 1B). To confirm that the reduction in NF-κB activity was due to the ectopically expressed protein acting as a d.n., w.t. IKKβ plasmid was coinjected with the NF-κB reporter in muscles from a separate group of rats using the same design. There was an increase in NF-κB reporter activity due to overexpression of w.t. IKKβ, although it was not as large as unloading-induced activation of the reporter. Nevertheless, these data confirm the specificity of the d.n. form of IKKβ with respect to its complete inhibition of the κB reporter gene in both weight-bearing and unloaded muscle (Fig. 1C). Confirmation of w.t. IKKβ overexpression is shown by immunoblotting (Fig. 1D).
Figure 1.
Effects of d.n. IKKβ on NF-κB reporter activity. A) Seven days of unloading induced a significant increase in NF-κB activity, while the d.n. IKKβ plasmid inhibited κB activity in normal weight-bearing muscles and completely abolished the unloading-induced NF-κB activity. B) Western blots of lysates from solei injected with the empty vector or d.n. IKKβ plasmid. C) Seven days of unloading induced a significant increase in NF-κB activity in both the empty vector and w.t. IKKβ-injected animals. D) Western blots of lysates from solei injected with the empty vector or the w.t. IKKβ plasmid. WB, weight bearing; HU, hind limb unloaded. Bars express means ± se. *P < 0.05 vs. weight-bearing control.
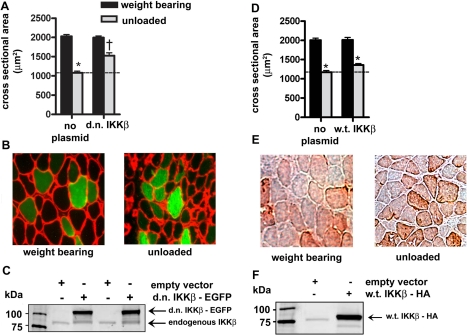
Because the d.n. IKKβ plasmid abolished NF-κB activity, we sought to determine whether it also inhibited unloading-induced muscle atrophy, which would provide evidence that IKKβ is required for atrophy. Fibers that expressed the d.n. IKKβ-EGFP fusion protein (green fibers) were compared to fibers that did not take up the plasmid (black fibers) in weight-bearing and unloaded muscles. Muscle fibers that did not express d.n. IKKβ-EGFP showed a 47% decrease in mean fiber cross-sectional area due to 7 days of unloading. On the other hand, fibers that expressed d.n. IKKβ showed a 50% inhibition of unloading-induced atrophy (Fig. 2A–C), indicating that IKKβ is required for unloading-induced loss of muscle fiber size. The fiber area frequency distribution for experiments showing differences in fiber size due to unloading and overexpression of d.n. or c.a. proteins are shown in Supplemental Fig. 1A–E. A w.t. IKKβ-HA-tagged plasmid was injected into muscles from weight-bearing and unloaded animals to show that overexpression of w.t. IKKβ did not also block atrophy (Fig. 2D–F). That is, it is specifically the d.n. form of IKKβ that inhibits atrophy by 50%.
Figure 2.
Effect of d.n. IKKβ and w.t IKKβ on muscle fiber area. A) Mean cross-sectional area of muscle fibers with and without overexpressed d.n. IKKβ-EGFP due to 7 days of unloading. B) Representative cross sections of weight-bearing and unloaded muscles. C) Western blots of lysates from solei injected with the empty vector or d.n. IKKβ-EGFP plasmid. D) Mean cross-sectional area of muscle fibers with and without w.t. IKKβ-EGFP. E) Representative cross section of weight-bearing and unloaded muscles injected with the w.t. IKKβ plasmid. F) Western blot of lysates from solei injected with the empty vector or w.t. IKKβ-HA plasmid. Bars represent means ± se. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. unloaded soleus injected with empty vector.
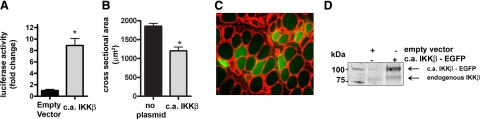
The c.a. IKKβ-EGFP plasmid was used to determine whether activated IKKβ is sufficient to induce atrophy and activate the NF-κB reporter. C.a. IKKβ-EGFP significantly activated the NF-κB reporter in weight-bearing muscles confirming its activating effect (Fig. 3A). Also in weight-bearing animals, expression of c.a. IKKβ-EGFP alone induced atrophy by 43% compared to fibers that did not express the plasmid (Fig. 3B, C). This result is similar to that shown in c.a. IKKβ transgenic mice (6).
Figure 3.
Effects of c.a. IKKβ on NF-κB reporter activity and on skeletal muscle fiber area. A) The c.a. IKKβ-EGFP plasmid induced a significant increase in NF-κB activity in weight bearing soleus muscles. B) Mean cross-sectional area of muscle fibers with and without c.a. IKKβ-EGFP. C) Representative cross section of weight-bearing muscle. D) Western blot of lysates from solei injected with the empty vector or c.a. IKKβ-EGFP plasmid. Bars represent means ± se. *P < 0.05 vs. weight-bearing control.
Role of IKKα in muscle atrophy
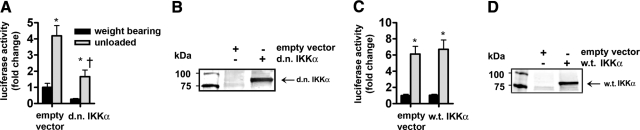
To determine whether the inhibition of IKKα blocked the unloading-induced activation of NF-κB, a d.n. IKKα plasmid was coinjected with the NF-κB reporter plasmid. Seven days of muscle unloading showed the prototypical increase in NF-κB activity; however, NF-κB activity was significantly inhibited by d.n. IKKα in both weight-bearing and unloaded muscles (Fig. 4A, B), though not quite to the same extent as d.n. IKKβ. To confirm that the reduction in NF-κB activity was due to the overexpressed protein acting as a d.n., w.t. IKKα was coinjected with the NF-κB reporter gene. Overexpression of w.t. IKKα did not affect normal weight-bearing κB activity or unloading-induced increases in NF-κB activity (Fig. 4C, D).
Figure 4.
Effect of d.n. and w.t. IKKα on NF-κB reporter activity. A) Seven days of unloading induced a significant increase in NF-κB activity, while the d.n. IKKα plasmid significantly reduced activity in the unloaded animals. B) Western blots of lysates from solei injected with the empty vector or d.n. IKKα plasmid. C) Seven days of unloading induced a significant increase in NF-κB activity in both empty vector- and w.t. IKKα-injected animals. D) Western blots of lysates from solei injected with the empty vector or the w.t. IKKα plasmid. Bars represent means ± se. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. unloaded soleus injected with empty vector.
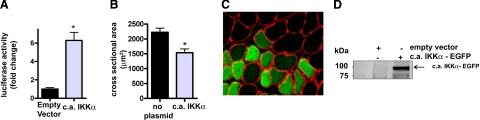
To determine whether IKKα is necessary for unloading atrophy, d.n. IKKα-EGFP was injected into the soleus muscles of weight-bearing and unloaded rats. Hind limb unloading induced a 40% decrease in mean cross-sectional area of muscle fibers that did not express the fusion protein, but fibers expressing d.n. IKKα-EGFP showed an inhibition of unloading-induced fiber atrophy by 50% (Fig. 5A–C), the same amount of fiber atrophy inhibition as seen with expression of d.n. IKKβ. Furthermore, injection of the w.t. IKKα-EGFP plasmid into muscles of animals that were weight bearing or unloaded for 7 days did not affect muscle fiber size (Fig. 5D–F), again demonstrating the specificity of the d.n. form of the kinase rather than an effect of overexpression of IKKα-EGFP. On the other hand, c.a. IKKα-EGFP overexpression elicited a significant 6-fold activation of the NF-κB reporter in weight-bearing muscle (Fig. 6A), and fiber atrophy was significantly induced (30%) by c.a. IKKα-EGFP in weight-bearing muscle (Fig. 6B, C). Thus, activated IKKα is sufficient to induce κB activity and muscle fiber atrophy in normal muscle.
Figure 5.
Effect of d.n. IKKα and w.t. IKKα on muscle fiber area. A) Mean cross-sectional area of muscle fibers with and without d.n. IKKα-EGFP. B) Representative cross sections of weight-bearing and unloaded muscles injected with the d.n. IKKα plasmid. C) Western blot of lysates from solei injected with the empty vector or d.n. IKKα-EGFP plasmid. D) Mean cross-sectional area of muscle fibers with and without w.t. IKKα-EGFP. E) Representative cross-section of weight-bearing and unloaded muscles injected with the w.t. IKKα plasmid. F) Western blot of lysates from solei injected with the empty vector or w.t. IKKα-EGFP plasmid. Bars represent means ± se. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. unloaded soleus injected with empty vector.
Figure 6.
Effect of c.a. IKKα on NF-κB reporter activity and muscle fiber area. A) The c.a. IKKα-EGFP plasmid induced a significant increase in NF-κB activity in weight-bearing soleus muscles. B) Mean cross-sectional area of muscle fibers with and without c.a. IKKα-EGFP. C) Representative cross section of weight-bearing muscle. D) Western blot of lysates from solei injected with the empty vector or c.a. IKKα-EGFP plasmid. Bars represent means ± se. *P < 0.05 vs. weight-bearing control.
Additive role of IKKα and IKKβ in muscle atrophy
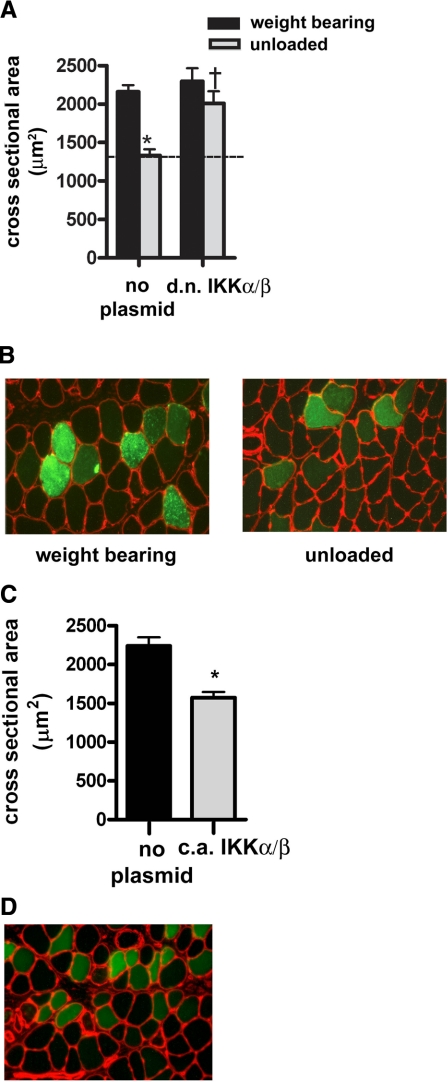
Finally, to determine whether the individual effects of IKKα and IKKβ are the same and thus redundant functions of the IKK complex, or to some extent unique and therefore additive, both d.n. IKK-EGFP plasmids were coinjected into soleus muscles. Overexpression of d.n. IKKα-EGFP and d.n IKKβ-EGFP together showed a further inhibition of atrophy (70%) (Fig. 7A, B) compared with either one alone (50%; see Figs. 2 and 5). This study was repeated using 25 μg (vs. 50 μg) of each plasmid, and the results were identical (data not shown).
Figure 7.
Effect of d.n. IKKβ and d.n. IKKα coinjections on unloading atrophy, and c.a. IKKb and c.a. IKKa coinjections on atrophy in weight bearing muscle. A) Mean fiber cross-sectional area from weight-bearing and unloaded muscle injected with d.n. IKKβ-EGFP and d.n. IKKα-EGFP. B) Representative cross sections of weight-bearing and unloaded muscles coinjected with d.n. IKKβ-EGFP and d.n. IKKα-EGFP. C) Mean fiber area from weight-bearing muscle coinjected with c.a. IKKβ-EGFP and c.a. IKKα-EGFP. D) Representative cross section of normal weight-bearing muscle injected with c.a. IKKβ-EGFP and c.a. IKKα-EGFP; green fibers express the transgenes and black fibers do not. Bars represent means ± se. *P < 0.05 vs. weight-bearing control. †P < 0.05 vs. unloaded soleus injected with empty vector.
When c.a. IKKα and c.a. IKKβ were coinjected (25 μg each) into soleus muscles that remained weight bearing, muscle fiber atrophy was again induced at 7 days. However, there was no additive effect of atrophy due to coexpression of these c.a. kinases, suggesting that their substrates are overlapping when activated in weight-bearing muscle (Fig. 7C, D).
Endogenous expression of IKK complex proteins
Overexpression of c.a. IKKα or c.a. IKKβ did not affect the protein expression of endogenous IKKβ or IKKα, respectively, nor did the overexpression of these c.a. proteins alter the expression of endogenous IKKγ (i.e., NEMO) (Supplemental Fig. 2).
DISCUSSION
The NF-κB (Rel) family of transcription factors and their immediate upstream regulators make up a ubiquitous cellular signaling network, and this is reflected in the large number of cellular processes regulated by these proteins in virtually all cell types (26,27,28). NF-κB signaling regulates processes as diverse as immune function, cell proliferation, and apoptosis. We showed several years ago that both NF-κB binding and transcriptional activity was markedly increased in skeletal muscles that were atrophying due to disuse (1, 2, 4). Other labs have shown NF-κB activation in muscle atrophy elicited by denervation, sepsis, cancer, and aging (6, 9, 29,30,31,32). However, only a few studies have identified specific Rel proteins or Rel cotransactivators that are required (p50 and Bcl-3, but not c-Rel) for muscle atrophy (2, 4). Muscles from cachectic animals have greater p65 DNA binding or phospho-p65 levels (5, 6), but confirmation of the requirement of p65 in the atrophy process is not yet available. Overexpression of w.t. p65 seems to be sufficient to induce atrophy in fibers of weight-bearing rat muscles (7).
There are several genetic studies on the upstream regulators of Rel proteins. IκBα, which binds Rel dimers in the cytosol, limiting their binding to DNA, is needed for normal atrophy. This has been shown using overexpression of a superrepressor form of IκBα in muscles, of either transgenic mice or ectopic expression in rat muscle, which blocks atrophy due to disuse and cancer by 40–50% (2, 6). Activated IKKβ, an upstream kinase that phosphorylates IκBα, leading to its proteasomal degradation and the release of bound Rel transcription factors, is sufficient to induce atrophy in muscles of weight-bearing mice. That is, a c.a. form of the protein overexpressed in muscles of transgenic mice elicited atrophy without any other atrophy stimulus (6), a finding that was confirmed in the present study using ectopic overexpression of c.a. IKKβ in adult rat muscle.
In the present study, we found that both kinases of the IKK complex, IKKα and IKKβ, are necessary and sufficient for muscle atrophy, and that simultaneous inhibition of both kinases has an additive effect on blocking disuse atrophy. Using a d.n. form of IKKβ, we found that this kinase was necessary for the atrophy-induced activation of NF-κB transcriptional activity and for muscle atrophy because it completely blocked κB activity and blocked fiber atrophy by 50% at 7 days of disuse. These data are comparable to those published by Mourkioti et al. (9), who showed atrophy inhibition in 7-day denervated soleus muscles (assessed by muscle mass) of transgenic mice with muscle-specific IKKβ ablation.
The requirement of IKKα in muscle atrophy has not been studied until now. This is important because IKKα and IKKβ are known to operate in a complex with NEMO (IKKγ), the essential modulator subunit. Both IKKα and IKKβ can phosphorylate IκBα, and thus both kinases are involved in classical NF-κB signaling (12,13,14, 33). However, IKKα also has kinase activity, leading to NF-κB activation independent from IKKβ and NEMO (27, 33). In the present study, we have shown that IKKα is necessary and sufficient for muscle atrophy and for increased NF-κB transcriptional activity due to disuse. Overexpression of d.n. IKKα inhibited unloading-induced κB activation, and it blocked atrophy by 50%. Moreover, the combination of d.n. IKKα plus d.n. IKKβ showed a partially additive inhibition (70%) of unloading atrophy, indicating either that both kinases need to be inhibited to abolish the phosphorylation of the substrates of the entire IKK complex, or that the two kinases have some distinct targets. The literature indicates that inhibition of IKKβ is more effective on the IKK complex than inhibition of IKKα (13). Our data on the inhibitory effects of d.n. IKKβ vs. d.n. IKKα on the κB reporter support this idea. It is important to remember that the target genes in atrophying muscle are not solely regulated by NF-κB, and so the reporter serves only to indicate the direction of the κB-dependent component of atrophy, but in reality, the amount of atrophy will be the sum of the NF-κB component together with other signaling pathways. That is, the d.n. IKKβ inhibited κB activity by 100%, while atrophy was inhibited by 50%.
On the other hand, the 70% inhibition of atrophy due to both kinases being inhibited compared to either one alone (50%) is an indication that the kinases are not mutually redundant and that combined, their effects are more a part of the total atrophy response than either alone. One reason for this additive effect on atrophy could be related entirely to a stronger inhibition on the IKK complex that is not reflected by the κB reporter activity, but that is reflected in the proteins or genes that elicit the atrophy phenotype. That is, there is something about both kinases being inhibited that eliminates some aspect of the single d.n. It could be that when one kinase is d.n., the other still has some kinase activity that is lost when both are kinase dead. Alternatively, it could be that one of the kinases has κB targets other than those of the IKK complex. IKKα could be operating via the alternative pathway (27, 34, 35). For instance, d.n. IKKα could partly inhibit (e.g., 30%) the kinase activity of IKK complex and partly inhibit (e.g., 20%) the alternative pathway. Therefore, when the two kinases are inhibited, the sum of effect on the atrophy is the 50% from IKKβ (for which the 30% of d.n. IKKα is redundant) and an extra 20% from the alternative pathway. Our previous work did not show changes in RelB and p52, components of the alternative pathway, but small effects could have gone undetected (1). In addition, IKKα appears to have several nuclear roles of NF-κB regulation. First, it can phosphorylate SMRT, thereby promoting nuclear export of HDAC3 and acetylation of p65 leading to κB transcription (36, 37). IKKα was also found to be recruited with p65 and CBP to NF-κB target genes where it phosphorylates histone H3 leading to its acetylation and subsequent NF-κB-dependent transactivation (38,39,40). The extra 20% of atrophy inhibition found in the combination of d.n. IKKα and d.n. IKKβ could be related to these nuclear effects. Finally, there is still the potential that either kinase may have non-NF-κB roles and that these are contributing to the extra atrophy inhibition when they are both eliminated.
We currently have no definitive support for the requirement of NF-κB alternative pathway in muscle atrophy. Our previous work demonstrated that “an alternative pathway” was strongly linked to unloading atrophy (1). However, we were not referring to what is now widely known as the alternative pathway. Since that time, the pathway we referred to, involving an absolute requirement for p50 and Bcl-3 (1, 4), has been termed “Pathway 3” by some authors (37). Our data presented here on IKKα plus the literature on κB signaling and atrophy indicate that either all NF-κB signaling pathways are involved in disuse atrophy or, at a minimum, the classical pathway and Pathway 3 are required.
The results of overexpression of both c.a. IKKs together in weight-bearing muscle show no additive effect on inducing atrophy. Therefore, it appears that while d.n. forms of the two kinases added together can interfere with the signals of unloading-induced atrophy more than either one alone, the active versions of the kinases together are not more effective than either one alone at regulating the genes that are sufficient to induce atrophy in weight-bearing muscle.
Taken together, the present data add to the number of proteins found to be required for NF-κB signaling and the associated muscle atrophy. IKKα and IKKβ are both required for NF-κB activation and atrophy. More work is needed to determine how the two kinases produce additive atrophy inhibition when they are simultaneously inhibited. In addition, it is not clear whether the requirement of Bcl-3, which we previously showed (4), indicates the involvement of the “third” NF-κB pathway (27) or whether multiple κB signals converge to activate the NF-κB-dependent genes that regulate muscle atrophy. Our ongoing studies are aimed at understanding the way in which specific Rel proteins, and Bcl-3, activate NF-κB-dependent transcription on the atrophy target genes.
Acknowledgments
This work was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) to D.V.G. (F32 AR054265) and an R01 award from NIAMS to S.K. (AR41705). We thank Mary Rhoads and Angie Cornwell for technical assistance.
References
- Hunter R B, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig D A, Kandarian S C. Activation of an alternative NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- Judge A R, Koncarevic A, Hunter R B, Liou H C, Jackman R W, Kandarian S C. Role for IκBα, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292:C372–C382. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- Hayden M S, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hunter R B, Kandarian S C. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya S, Butchbach M E, Sahenk Z, Wang H, Saji M, Carathers M, Ringel M D, Skipworth R J, Fearon K C, Hollingsworth M A, Muscarella P, Burghes A H, Rafael-Fortney J A, Guttridge D C. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz J D, Tawa N E, Jr, Melendez P A, Oh B C, Lidov H G, Hasselgren P O, Frontera W R, Lee J, Glass D J, Shoelson S E. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Polkinghorne E, Lau Q, Cooney G J, Kraegen E W, Cleasby M E. Local activation of the IκK-NF-κB pathway in muscle does not cause insulin resistance. Am J Physiol Endocrinol Metab. 2008;294:E316–E325. doi: 10.1152/ajpendo.00537.2007. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker S H, Goldberg A L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkioti F, Kratsios P, Luedde T, Song Y H, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116:2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N, Wang J, Ladner K J, Wang H, Dahlman J M, Carathers M, Acharyya S, Rudnicki M A, Hollenbach A D, Guttridge D C. IKK/NF-κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N D, Gilmore T D. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Huynh Q K, Boddupalli H, Rouw S A, Koboldt C M, Hall T, Sommers C, Hauser S D, Pierce J L, Combs R G, Reitz B A, Diaz-Collier J A, Weinberg R A, Hood B L, Kilpatrick B F, Tripp C S. Characterization of the recombinant IKK1/IKK2 heterodimer. Mechanisms regulating kinase activity. J Biol Chem. 2000;275:25883–25891. doi: 10.1074/jbc.M000296200. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Van Gammeren D, Judge A, Kandarian S C. The role of IKKα and IKKβ in the activation of NF-κB during skeletal muscle atrophy. Pine Mountains, GA, USA: Frontiers in Myogenesis. 2006:28. (meeting abstr.) [Google Scholar]
- Mitchell-Felton H, Kandarian S C. Normalization of muscle plasmid uptake by Southern blot: application to SERCA1 promoter analysis. Am J Physiol Cell Physiol. 1999;277:C1269–C1276. doi: 10.1152/ajpcell.1999.277.6.C1269. [DOI] [PubMed] [Google Scholar]
- Alzghoul M B, Gerrard D, Watkins B A, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18:221–223. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- Rana Z A, Ekmark M, Gundersen K. Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand. 2004;181:233–238. doi: 10.1111/j.1365-201X.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- Dona M, Sandri M, Rossini K, Dell'Aica I, Podhorska-Okolow M, Carraro U. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem Biophys Res Commun. 2003;312:1132–1138. doi: 10.1016/j.bbrc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Rossini K, Sandri M, Dona M. High level of gene transfer into adult skeletal muscle bi in vivo direct electroporation. Basic Appl Myol. 2002;12:97–100. [Google Scholar]
- Taylor J, Babbs C F, Alzghoul M B, Olsen A, Latour M, Pond A L, Hannon K. Optimization of ectopic gene expression in skeletal muscle through DNA transfer by electroporation. BMC Biotechnol. 2004;4:11–18. doi: 10.1186/1472-6750-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer J D, Plant D R, Lynch G S. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol Ther. 2006;13:795–803. doi: 10.1016/j.ymthe.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Peters D G, Mitchell-Felton H, Kandarian S C. Unloading induces transcriptional activation of the sarco(endo)plasmic reticulum Ca2+-ATPase 1 gene in muscle. Am J Physiol. 1999;276:C1218–C1225. doi: 10.1152/ajpcell.1999.276.5.C1218. [DOI] [PubMed] [Google Scholar]
- Koncarevic A, Jackman R W, Kandarian S C. The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J. 2007;21:427–437. doi: 10.1096/fj.06-6665com. [DOI] [PubMed] [Google Scholar]
- Perkins N D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Gilmore T D. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Hasselgren P O, Menconi M J, Fareed M U, Yang H, Wei W, Evenson A. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–2168. doi: 10.1016/j.biocel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Guttridge D C, Mayo M W, Madrid L V, Wang C Y, Baldwin A S., Jr NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia [see comments] Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Bar-Shai M, Carmeli E, Ljubuncic P, Reznick A Z. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-κB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor P M, Rennie M J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Gloire G, Dejardin E, Piette J. Extending the nuclear roles of IκB kinase subunits. Biochem Pharmacol. 2006;72:1081–1089. doi: 10.1016/j.bcp.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Dejardin E. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Xiao G, Rabson A B, Young W, Qing G, Qu Z. Alternative pathways of NF-κB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 2006;17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Hoberg J E, Yeung F, Mayo M W. SMRT derepression by the IκB kinase alpha: a prerequisite to NF-κB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hoberg J E, Popko A E, Ramsey C S, Mayo M W. IκB kinase α-mediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol Cell Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anest V, Hanson J L, Cogswell P C, Steinbrecher K A, Strahl B D, Baldwin A S. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma U N, Prajapati S, Kwak Y T, Gaynor R B. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- Park G Y, Wang X, Hu N, Pedchenko T V, Blackwell T S, Christman J W. NIK is involved in nucleosomal regulation by enhancing histone H3 phosphorylation by IKKα. J Biol Chem. 2006;281:18684–18690. doi: 10.1074/jbc.M600733200. [DOI] [PMC free article] [PubMed] [Google Scholar]