Abstract
A major breakthrough in systemic phosphate homeostasis regulation was achieved by the demonstration of strikingly similar physical, morphological, and biochemical phenotypes of fibroblast growth factor 23 (Fgf23) and klotho ablated mice, which led to identification of klotho as an Fgf23 signaling cofactor. Here, we generated Fgf23 and klotho double-knockout (Fgf23−/−/klotho−/−) mice to test the hypothesis whether Fgf23 has a klotho-independent function. Fgf23−/−/klotho−/− mice are viable and have high serum phosphate levels, similar to Fgf23−/− and klotho−/− single-knockout mice. In addition, the Fgf23−/−/klotho−/− mice have increased renal expression of the sodium/phosphate cotransporter NaPi2a and of 1- alpha-hydroxylase concomitant with increased serum levels of 1,25-dihydroxyvitamin-D, as also observed in the Fgf23−/− and klotho−/− mice. Moreover, Fgf23−/−/klotho−/− mice show soft tissue and vascular calcification, severe muscle wasting, hypogonadism, pulmonary emphysema, distention of intestinal wall, and skin atrophy, all of which are also seen in Fgf23−/− and klotho−/− mice. Notably, injection of bioactive FGF23 protein into Fgf23−/−/klotho−/− and klotho−/− mice does not lower serum phosphate, whereas in wild-type and Fgf23−/− mice, it reduces serum phosphate. Together, these results provide compelling evidence that Fgf23 does not have a klotho-independent role in the regulation of systemic phosphate and vitamin D homeostasis.—Nakatani, T., Sarraj, B., Ohnishi, M., Densmore, M. J., Taguchi, T., Goetz, R., Mohammadi, M., Lanske, B., Razzaque, M. S. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis.
Keywords: kidney, vitamin D, PTH, NaPi2a, calcification, survival
Maintenance of a systemic phosphate balance is crucial for a wide variety of cellular functions, which range from cellular signaling to enzymatic activities (1). An understanding of the molecular regulation of phosphate homeostasis has clinical and biological importance. An impairment of the phosphate balance can cause severe functional impairments of vital organ systems. Acute hypophosphatemia can lead to the development of myopathy, cardiac dysfunction, and hematological abnormalities, while chronic hypophosphatemia can impair bone mineralization, inducing rickets and osteomalacia. In contrast, hyperphosphatemia can cause vascular and soft tissue calcification (2, 3).
Regulation of phosphate homeostasis is a complex hormonal process that involves multiple organs, including the intestines, bone, parathyroid glands, and kidneys. Kidneys play an important role in maintaining phosphate balance by fine-tuning urinary excretion of phosphate, according to the needs of the body (1). Most chronic renal diseases, without therapeutic intervention, usually progress to irreversible renal failure (4,5,6), which affects water, electrolyte, and mineral balances.
Fibroblast growth factor 23 (Fgf23) is a master regulator of renal phosphate handling. Human studies have shown that an increased serum level of FGF23 is associated with renal phosphate wasting diseases (7), while reduced FGF23 activity leads to increased serum accumulation of phosphate due to reduced urinary excretion of phosphate (8). In a similar line of animal studies, transgenic mice overexpressing Fgf23 showed rickets and osteomalacia due to excessive urinary phosphate wasting (9). In contrast, genomic ablation of Fgf23 from mice resulted in severe hyperphosphatemia due to decreased urinary excretion of phosphate (10,11,12).
Our understanding of Fgf23-mediated regulation of phosphate homeostasis has been significantly enhanced by the identification of klotho as a cofactor in Fgf23 signaling (13, 14). The klotho gene encodes a single-pass transmembrane protein. The extracellular domain of klotho protein consists of two homologous domains that share sequence homology to the β-glucosidase protein present in bacteria and plants. The klotho gene is predominantly expressed in the kidneys, parathyroid glands and brain (15). Such restricted expression of klotho is believed to confer tissue-specificity of Fgf23 function.
Recent in vitro studies, however, have shown that Fgf23 can exert functions on certain cells in the absence of klotho. For instance, in vitro adenoviral overexpression of FGF23 in fetal rat calvaria cells has been shown to suppress osteoblast differentiation and matrix mineralization (16). Similarly, high doses of Fgf23 can weakly induce proliferation of murine bone marrow-derived pro-B cell lines overexpressing c-isoforms of FGF receptor 1–3 and FGF receptor 4 in the absence of klotho (17). Such in vitro studies raise the question of whether FGF23 can exert systemic klotho independent functions. In view of the fact that klotho is an important FGF23 signaling cofactor (18), this study was designed to assess whether FGF23 has a klotho-independent effect on regulation of systemic phosphate homeostasis. To address klotho-dependent and -independent effects of FGF23 in vivo, we generated Fgf23 and klotho double-knockout (Fgf23−/−/klotho−/−) mice and examined the phenotype of these mice in comparison to that of Fgf23-knockout (Fgf23−/−) and klotho-knockout (klotho−/−) mice.
MATERIALS AND METHODS
Experimental mice
We recently generated Fgf23-null mice (Fgf23−/−) (11) and crossbred heterozygous Fgf23 mice with heterozygous klotho mutants (Lexicon Genetics; Mutant Mouse Regional Resource Centers, University of California, Davis, CA, USA) to obtain compound heterozygous animals, which were then interbred to generate the desired double-homozygous mutants (Fgf23−/−/klotho−/−). Routine polymerase chain reaction (PCR) using genomic DNA, extracted from tail clips, was performed to identify various groups of mice (11). The following primers were used for genotyping: Fgf23 wild-type (forward 5′-GGA TCC CCA CCT CAG TTC TCA-3′, reverse 5′-TAG CCG TGT ACA GGT GGG TCA-3′, 252 bp product); Fgf23 knockout (forward 5′-GGA TCC CCA CCT CAG TTC TCA-3′, reverse 5′-AGT TTG AGG GGA CGA CGA CAG TAT-3′, 366 bp product); klotho wild-type (forward 5′-GAT GGG GTC GAC GTC A-3′, reverse, 5′-TAA AGG AGG AAA GCC ATT GTC-3′, 188 bp product); klotho knockout (forward 5′-GCA GCG CAT CGC CTT CTA CT-3′, reverse 5′-ATG CTC CAG ACA TTC TCA GC-3′, 455 bp product). Mice were fed with a standard diet ad libidum (PicoLab Mouse Diet 20-5058; Purina Mills, Inc., St. Louis, MO, USA). Animals were maintained in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were employed according to protocols approved by the institution’s subcommittee on animal care.
Gross phenotype and survival
The total body weight of each of the mice was recorded every week starting at 3 wk of age until death. Survival of wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice was recorded until death.
Biochemical measurements
Blood was obtained by cheek-pouch bleeding of wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice. Serum was isolated by centrifugation at 3000 g for 10 min and stored at −80°C. Serum phosphorus and calcium were determined by colorimetric measurements using the Stanbio Phosphorus Liqui-UV Test and Calcium (Arsenazo) LiquiColor Test, respectively (Stanbio Laboratory, Boerne, TX, USA). Urinary phosphorus and creatinine were determined using Stanbio LiquiUV and creatinine kits, respectively. Differential blood cells counts were also determined by an automated device. The level of 1,25–hydroxyvitamin-D [1,25(OH)2D3] was measured in serum obtained from wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice using a kit purchased from Immunodiagnostic Systems Ltd. (Fountain Hills, AZ, USA). The serum level of parathyroid hormone (PTH) was measured using a commercial kit (Immutopics, Inc., San Clemente, CA, USA).
Histological analyses
Soft tissues obtained from wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice were fixed with 4% paraformaldehyde, 10% buffered formalin, or Carnoy’s solution and were subsequently embedded in paraffin. Paraffin sections (4–6 μm) of various tissues were mounted on SuperFrost Plus slides. Sections were then routinely stained with hematoxylin and eosin, von Kossa, periodic acid-Schiff (PAS), periodic acid-Schiff methenamine silver (PAM), and Masson’s trichrome (19, 20). Histological changes were observed by light microscopy.
Calcification analyses
To determine the effects of hyperphosphatemia on soft tissue and vascular calcification in Fgf23−/−/klotho−/− mice, sections were prepared from heart, lung, kidney, liver, spleen, gastrointestinal tract, and aorta and were stained with von Kossa to visualize mineralized tissue by light microscopy. The von Kossa-stained sections of Fgf23−/−/klotho−/− mice were compared with similarly stained sections from wild-type, Fgf23−/− and klotho−/− mice. The von Kossa staining procedure is detailed in an earlier publication (21).
Immunofluorescence staining
Immunostaining was performed as described previously (22,23,24). Briefly, kidneys obtained from wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice were stored at −80°C and embedded in optimal cutting temperature (OCT) compound. Frozen sections were incubated in a blocking solution for 30 min and then overnight with polyclonal anti-NaPi2a (sodium/phosphate cotransporter 2a protein) antibody (dilution 1:100; Alpha Diagnostic, San Antonio, TX, USA) at 4°C. The slides were washed with PBS and incubated with fluorescein isothiocyanate-labeled anti-rabbit secondary antibody (dilution, 1:100) for 30 min. After a PBS wash, coverslips were placed on slides using 4,6-diamidino-2-phenylindole (DAPI) -containing mounting media. The expression of NaPi2a was visualized under UV light, using immunofluorescence microscopy. Rabbit serum or PBS, in place of primary antibody, was used as a negative control. The staining was quantified using an image analyzer (Photoshop® software; Adobe Systems, San Jose, CA, USA) by subtracting background staining from the stained areas (25).
Quantitative real-time PCR
Total RNA isolated from the kidneys of wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice was used to detect relative expression of 1-alpha-hydroxylase [1α(OH)ase] mRNA, as described previously (26). Real-time PCR was performed in triplicate. The quantity of mRNA was calculated by normalizing the CT (threshold cycle value) of 1α(OH)ase to the CT of the housekeeping gene GAPDH of the same sample. The sequences of the utilized primers were as follows: 1α(OH)ase (forward 5′-TCA GAT GTT TGC CTT TGC CC; reverse 5′-TGG TTC CTC ATC GCA GCT TC-3′), and GAPDH (forward 5′-ACT GAG GAC CAG GTT GTC-3′; reverse 5′-TGC TGT AGC CGT ATT CAT TG-3′).
Bioactive FGF23 protein injection
Wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice were injected intraperitoneally with vehicle, or recombinant bioactive FGF23 protein (5 μg per mouse) (27). Blood was collected by cheek-pouch bleeding prior to the injections and at 12 h postinjection. Isolated serum was used to determine phosphate levels in an animal pre- and postinjection, using the Stanbio LiquiUV kit.
Statistics
Statistically significant differences between groups were evaluated by the Student’s t test for a comparison between two groups or by one-way analysis of variance followed by Tukey’s test for multiple comparisons. All values are expressed as means ± se. Values of P < 0.05 were considered to be statistically significant. All analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 4.0 (GraphPad, San Diego, CA, USA).
RESULTS
Generation of Fgf23−/−/klotho−/− mice
Fgf23 and klotho can coordinately regulate systemic phosphate homeostasis. Genetic deletion of either Fgf23 or klotho results in hyperphosphatemia (26, 28, 29). To determine whether Fgf23 exerts klotho-independent effects on systemic phosphate homeostasis regulation, we generated a new mouse model that is deficient in both Fgf23 and klotho genes (Fgf23−/−/klotho−/−) by interbreeding heterozygous Fgf23 mice with heterozygous klotho mice. The Fgf23−/−/klotho−/− double-knockout mice were viable and appeared similar to klotho−/− mice to the naked eye. After determining the genotypes of the mice, the gross phenotypes, survival, morphological, biochemical, and molecular changes of wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/ klotho−/− mice were analyzed.
Gross phenotype and survival of Fgf23−/−/klotho−/− mice
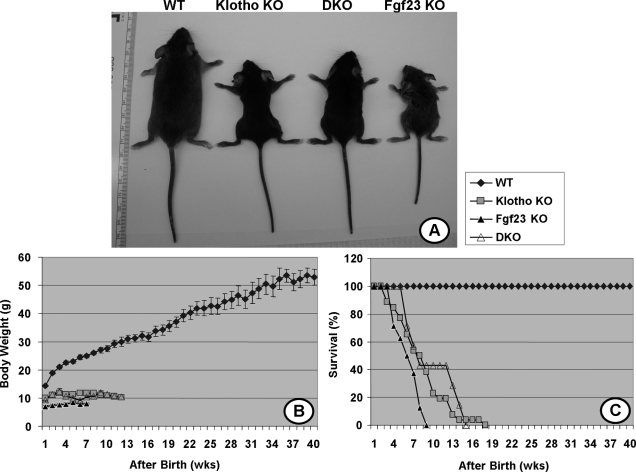
At birth, Fgf23−/−/klotho−/− double-knockout mice were indistinguishable from their littermates of other genotypes. At 3 wk of age, Fgf23−/−/klotho−/− double-knockout mice were larger in size than Fgf23−/− mice (9.4±0.86 vs. 7.0±0.37 g), smaller than wild-type (14.4±0.29 g) and similar in size to klotho−/− single-knockout animals (9.9±0.26 g). At 6 and 12 wk of age, Fgf23−/−/klotho−/− double-knockout mice were still smaller than their wild-type littermates (10.3±0.28 vs. 24.9±0.72 g at 9 wk), but their body weight was higher than that of Fgf23−/− mice (8.1±0.2 g at 9 wk). At 9 wk of age, the average body weight of klotho−/− mice was 11.8 ± 0.28 g (Fig. 1A, B). Compared to the wild-type littermates, Fgf23−/−/klotho−/− double-knockout mice were smaller in size, with sluggish movements, similar to Fgf23−/− and klotho−/− mice. Furthermore, Fgf23−/−/ klotho−/− mice had a maximum survival of around 16 wk, similar to the klotho−/− mice (Fig. 1C). It is worth mentioning that the Fgf23−/− mice also had a shorter life span compared to wild-type mice (Fig. 1C).
Figure 1.
Macroscopic phenotype of Fgf23−/−/klotho−/− double mutants. A) Gross phenotype of wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− (DKO) mice at 6 wk of age. B, C) Body weight curves (B) and survival ratios (C) for all four genotypes, showing that Fgf23−/−/klotho−/− mice are smaller in size and have shorter survival times than their wild-type counterparts.
Blood, serum, and urine analyses of Fgf23−/−/ klotho−/− mice
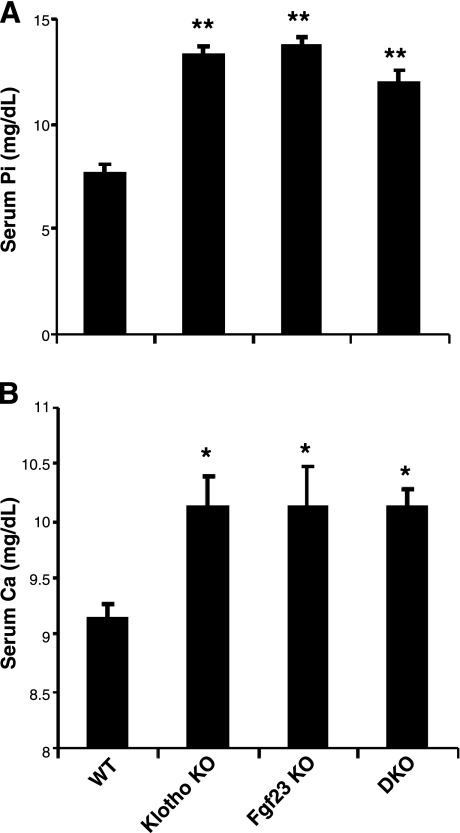
Serum phosphate, calcium, 1,25(OH)2D3, and PTH levels were measured in 3- to 6-wk-old wild-type, Fgf23−/−, klotho−/− and Fgf23−/−/klotho−/− mice. The double-knockout mice were severely hyperphosphatemic by 3 to 6 wk of age (11.2±0.5 mg/dl) when compared to wild-type mice (7.7±0.3 mg/dl). The high serum phosphate levels in Fgf23−/−/klotho−/− mice were similar to those found in age-matched Fgf23−/− mice (13.6±0.5 mg/dl) or klotho−/− mice (13.5±0.4 mg/dl) (Fig. 2A). The hyperphosphatemia in double-knockout mice was associated with decreased urinary phosphate excretion (normalized to urinary creatinine) (2.1±0.5 in Fgf23−/−/klotho−/− mice vs. 7.8±1.6 in wild-type controls). Collectively, these findings suggest that inactivation of Fgf23 and klotho function, either alone or in combination, can lead to hyperphosphatemia, due to reduced urinary excretion of phosphate.
Figure 2.
Biochemical analysis. Serum phosphate (A) and serum calcium (B) levels in control, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− (DKO) mice. Note that, compared to control mice, the serum phosphate and calcium levels are high in Fgf23−/−/klotho−/− mice; similar serum levels are noted in Fgf23−/− and klotho−/− mice. *P < 0.05, **P < 0.01 vs. control.
Serum 1,25(OH)2D3 and PTH were also markedly changed in Fgf23−/−/klotho−/− mice: serum 1,25(OH)2D3 was increased to levels resembling those found in the single-knockout mice, whereas serum PTH was decreased to low or nearly undetectable levels (Supplemental Fig. S1). Serum calcium levels were slightly higher in the double-knockout mice at around 6 wk of age, as also noted in the single-knockout animals, and remained elevated with age in all three mutant mouse lines (Fig. 2B). At around 6 wk of age, serum calcium in Fgf23−/−/klotho−/− mice (10.1±0.1 mg/dl, n=7) was similar to that found in Fgf23−/− mice (10.1±0.3 mg/dl, n=8) or klotho−/− mice (10.1±0.2 mg/dl, n=14), and higher than the levels observed in controls (9.1±0.1 mg/dl, n=22).
The total blood cell count showed increased neutrophils and decreased lymphocytes in Fgf23−/−/klotho−/− double-knockout mice, compared to the control mice. Similar neutrophilia and lymphocytopenia were also detected in Fgf23−/− and klotho−/− single-knockout animals (data not shown).
Renal expression of NaPi2a and 1α(OH)ase in Fgf23−/−/klotho−/− mice
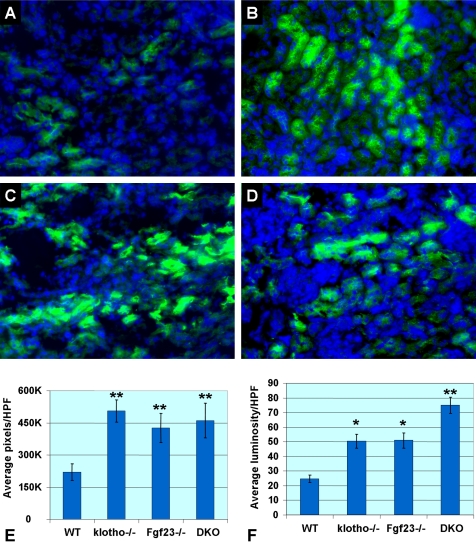
To determine the role of NaPi2a in Fgf23−/−/klotho−/− mice, we examined its expression pattern in kidney sections prepared from mice with various genotypes. Compared with wild-type mice, increased expression of NaPi2a protein in the luminal side of the proximal tubules was noted in Fgf23−/−/klotho−/− mice (Fig. 3). It is presumed that increased expression of NaPi2a is associated with increased renal reuptake of phosphate, resulting in reduced phosphate excretion, and hence hyperphosphatemia in Fgf23−/−/klotho−/− mice. Increased renal expression of NaPi2a was also noted in Fgf23−/− and klotho−/− mice (Fig. 3), suggesting that inactivation of the Fgf23-klotho axis, either alone or in combination, can up-regulate expression of NaPi2a.
Figure 3.
Immunofluorescence staining for NaPi2a in the kidneys obtained from 6-wk-old mice of various genotypes. Weak expression of NaPi2a (green fluorescence) is detected in wild-type kidney sections (A). In contrast, increased expression of NaPi2a protein is detected in the luminal side of the proximal tubular epithelial cells of the klotho−/− (B), Fgf23−/− (C), and Fgf23−/−/klotho−/− (DKO) (D) mice. Such increased renal expression of NaPi2a is believed to be associated with increased reabsorption of phosphate, which induces hyperphosphatemia in the Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice (view ×40). DAPI staining of cell nuclei is shown as blue fluorescence. Distribution (E) and staining intensity (F) of NaPi2a expression are quantified on images taken from NaPi2a-stained slides at ×40. Relative area of staining (E) is determined by calculating the number of positively stained pixels per high-power field (HPF, ×40). Relative staining intensity (F) is determined by deducting the mean luminosity value from background value. Note that both distribution and intensity of NaPi2a expression are significantly increased in Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice. *P < 0.05, **P < 0.001 vs. control.
Renal expression of 1α(OH)ase, the enzyme that converts inactive vitamin-D metabolites into active 1,25(OH)2D3, was ∼6-fold higher in Fgf23−/−/klotho−/− mice compared to wild-type mice, as determined by real-time PCR. Similarly, compared to the control kidneys, 1α(OH)ase expression was increased >10-fold in the kidneys obtained from either Fgf23−/− mice or klotho−/− mice. Increased renal expression of 1α(OH)ase was associated with an increased serum level of 1,25(OH)2D3 in all three gene-knockout mice (Supplemental Fig. S1).
Soft tissue anomalies of Fgf23−/−/klotho−/− mice
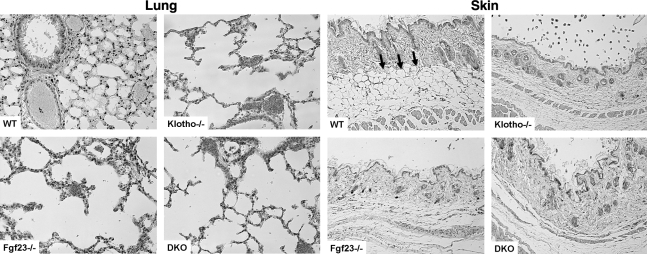
Histological examination of various soft tissues from Fgf23−/−/klotho−/− mice showed generalized atrophy of the skin (Fig. 4), skeletal muscle, reproductive organs, and intestines (Supplemental Figs. S2 and S3); such atrophy was markedly obvious by 6 wk of age and remained evident for the rest of life. Severe gonadal atrophy in Fgf23−/−/klotho−/− mice was associated with infertility of both male and female homozygous double mutants. In addition, severe lung emphysema was consistently observed in Fgf23−/−/klotho−/− mice (Fig. 4). All of these soft tissue changes noted in Fgf23−/−/klotho−/− mice were also evident in Fgf23−/− or klotho−/− mice, suggesting that abnormal soft tissue changes were the consequence of inactivation of the Fgf23-klotho axis, either alone or in combination in the single- or double-knockout mice, respectively (Table 1).
Figure 4.
Histological analysis of lung and skin tissues. Hematoxylin and eosin-stained sections of the skin and lung of 6-wk-old wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− (DKO) mice. Note that compared to wild-type mice, a marked expansion of alveolar spaces is found in Fgf23−/−/klotho−/− mice. Such pulmonary emphysematous changes are also noted in Fgf23−/− and klotho−/− mice. Compared to wild-type mice, a marked atrophy of the skin in Fgf23−/−/klotho−/− mice is apparent. The subcutaneous fat tissue layer (arrows) in the wild-type skin is mostly absent in the homozygous mutant mice, either in the single Fgf23−/− and klotho−/− mice or in the Fgf23−/−/klotho−/− double mutant mice (lung and skin, ×10).
Table 1.
Phenotypes of various mutant mice compared to wild-type littermates
| Characteristic | Wild-type | Fgf23−/− | klotho−/− | Fgf23−/−/klotho−/− |
|---|---|---|---|---|
| Gross appearance | ||||
| Body weight | Normal | Reduced | Reduced | Reduced |
| Growth retardation | Absent | Present | Present | Present |
| Physical activity | Normal | Sluggish | Sluggish | Sluggish |
| Generalized atrophy | ||||
| Spleen atrophy | Absent | Present | Present | Present |
| Muscle wasting | Absent | Present | Present | Present |
| Skin atrophy | Absent | Present | Present | Present |
| Intestinal atrophy | Absent | Present | Present | Present |
| Hypogonadism | Absent | Present | Present | Present |
| Morphological changes | ||||
| Atherosclerosis/arteriosclerosis | Absent | Present | Present | Present |
| Ectopic calcifications | Absent | Present | Present | Present |
| Skeletal mineralization | Normal | Impaired | Impaired | Impaired |
| Emphysema | Absent | Present | Present | Present |
| Molecular changes | ||||
| Renal 1a(OH)ase expression | Normal | Increased | Increased | Increased |
| Renal NaPi2a expression | Normal | Increased | Increased | Increased |
| Biochemical changes | ||||
| Serum 1,25(OH)2D3 | Normal | High | High | High |
| Serum phosphate | Normal | High | High | High |
| Serum calcium | Normal | High | High | High |
| Serum PTH | Normal | Low | Low | Low |
| Overall affect | ||||
| Infertility | Absent | Present | Present | Present |
| Lifespan | Normal | Short | Short | Short |
Ectopic calcification of Fgf23−/−/klotho−/− mice
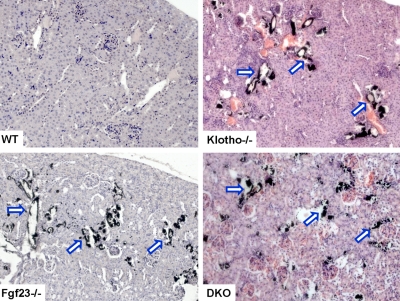
Extensive vascular and soft tissue calcifications were widely present in the lung, kidney, aorta, and other organs in Fgf23−/−/klotho−/− mice. By von Kossa staining, ectopic calcified areas were noted in the alveolar septum of the lung (Supplemental Fig. S4), tubules of the kidney, and aortic wall of Fgf23−/−/klotho−/− mice (Fig. 5). Similar calcified foci were also noted in numerous soft tissues of Fgf23−/− and klotho−/− mice. Together these data indicate that abnormal mineral metabolism due to inactivation of the Fgf23-klotho axis can induce pathological changes in the soft tissues (Fig. 5).
Figure 5.
Von Kossa staining of kidney tissues. Renal sections prepared from wild-type, Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− (DKO) mice (at 6 to 9 wk of age) showing extensive calcifications in the kidney of Fgf23−/−/ klotho−/− double-mutant mice. Similar calcification is also noted in Fgf23−/− and klotho−/− mice (view ×20).
Effects of FGF23 protein injection in Fgf23−/−/klotho−/− mice
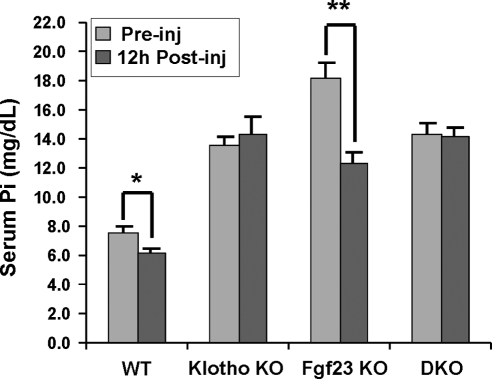
Bioactive FGF23 protein injection significantly lowered serum phosphate levels in wild-type mice (preinjection 7.61±0.5 mg/dl vs. postinjection 6.11±0.3 mg/dl; P<0.05) and Fgf23−/− mice (preinjection 16.99±0.8 mg/dl vs. postinjection 12.5±0.4 mg/dl; P<0.01). However, the treatment did not affect serum phosphate levels in Fgf23−/−/klotho−/− or klotho−/− mice (Fig. 6), which suggests that, in the absence of klotho, FGF23 cannot regulate systemic phosphate homeostasis.
Figure 6.
Bioactive FGF23 injection. Serum phosphate levels before and 12 h after injections of bioactive FGF23 protein. FGF23 protein injection resulted in significantly lowered serum phosphate levels in wild-type mice (preinjection 7.61±0.5 mg/dl vs. postinjection 6.11±0.3 mg/dl; P<0.05) and Fgf23−/− mice (preinjection 16.99±0.8 mg/dl vs. postinjection 12.5±0.4 mg/dl; P<0.01), but did not affect the serum phosphate levels in Fgf23−/−/klotho−/− (DKO) mice (preinjection 14.24±0.8 mg/dl vs. postinjection 14.2±0.3 mg/dl) or klotho−/− mice (preinjection 13.82±0.5 mg/dl vs. postinjection 14.27±1.2 mg/dl), suggesting that, in absence of klotho, FGF23 cannot regulate systemic phosphate homeostasis. *P < 0.05, **P < 0.01 vs. corresponding preinjection value.
DISCUSSION
The major finding of our in vivo genetic manipulation studies is that FGF23 does not act independently of klotho in its systemic regulation of phosphate homeostasis. Inactivation of both Fgf23 and klotho genes resulted in the development of severe hyperphosphatemia in the mutant mice. Administration of recombinant bioactive FGF23 into Fgf23+/+/klotho−/−, Fgf23+/−/klotho−/− and Fgf23−/−/klotho−/− mice had no effect on the systemic phosphate balance, demonstrating in vivo that FGF23 cannot regulate phosphate homeostasis without klotho.
Recent studies have shown that FGF23 exerts its biological effects via activation of FGF receptors in a klotho-dependent fashion. Klotho acts by enhancing binding of FGF23 to its cognate receptor, which thus enables FGF23 to activate FGFRs and induce downstream signaling events including phosphorylation of FGF receptor substrate-2a, followed by activation of extracellular signal-regulated kinase (ERK) and induction of early growth response element-1 (Egr-1) (13, 14, 30). The dependence of FGF23 on klotho for signaling in vivo is underscored by the findings that the physical, morphological and biochemical phenotypes of Fgf23−/−/ klotho−/− mice are similar to those of the Fgf23−/− mice and the klotho−/− mice, which suggests that such similarities in these genetically altered mouse models are the consequence of disruption of a common signaling pathway (18).
Note that in vitro studies have reported that FGF23 can act on certain cells that do not contain klotho. For instance, FGF23 can suppress osteoblast differentiation and bone mineralization in fetal rat calvaria cells (16). Similarly, FGF23 was shown to exhibit weak proliferative effects on a murine bone marrow-derived pro-B cell line overexpressing FGFRs in the absence of klotho (17). Such in vitro studies have raised an important question of whether FGF23 has systemic klotho-independent effects. To address this research question, we injected bioactive FGF23 protein into wild-type, single- and double-knockout mice. We did not observe any changes in systemic phosphate homeostasis in klotho−/− or Fgf23−/−/klotho−/− mice. In wild-type or Fgf23−/− mice, however, FGF23 protein injection led to a decrease in serum phosphate (27). Because both wild-type and Fgf23−/− mice have endogenous klotho, exogenous FGF23 can influence systemic phosphate homeostasis in these animals. On the other hand, in klotho−/− and Fgf23−/−/klotho−/− mice, which lack klotho, FGF23 is unable to exert any phosphaturic effect.
Our newly generated Fgf23−/−/klotho−/− mice have shortened lifespans, pulmonary emphysema, infertility, atherosclerosis, extensive soft tissue calcifications, skin atrophy, muscle wasting, and abnormal vitamin D and mineral ion metabolism. Thus, the phenotype of Fgf23−/−/klotho−/− mice is by and large identical with that of Fgf23 or klotho single-knockout mice. The overlapping phenotypes of these three mutant mouse lines suggest that such similarities are due to disruption of factors that constitute a common signaling pathway (18, 31, 32). Our results provide compelling genetic evidence of the in vivo importance of klotho in the regulation of systemic phosphate homeostasis by FGF23. Recent demonstration of a homozygous loss-of-function mutation in Klotho gene in a patient with tumoral calcinosis has provided additional evidence for the dependence of FGF23 on klotho to regulate phosphate and vitamin D homeostasis in humans (33).
Results obtained from the newly generated Fgf23−/−/ klotho−/− mice also show that renal expression of 1α-hydroxylase and serum level of 1,25(OH)2D3 were increased, whereas serum PTH was suppressed (Supplemental Fig. S1). The PTH deficit may account for the increased renal expression of NaPi2a in these double-knockout mice, as PTH can influence renal phosphate reabsorption by facilitating endocytosis of NaPi2a transporters from the brush-border membrane of proximal tubular epithelial cells and eventual lysosomal degradation of the transporters (34,35,36). A decreased level of PTH, therefore, can facilitate an increased presence of NaPi2a, which in turn induces increased renal phosphate reabsorption and, subsequently, hyperphosphatemia in Fgf23−/−/klotho−/− mice as well as in Fgf23−/− and klotho−/− mice.
Analysis of our newly generated Fgf23−/−/klotho−/− compound mutants suggest that genetic inactivation of Fgf23 and klotho activities results in severe hyperphosphatemia and impaired vitamin D metabolism, and that Fgf23 does not have a klotho-independent function in the regulation of systemic phosphate homeostasis in vivo. Furthermore, Fgf23−/−/klotho−/− mice provide a unique in vivo tool to study currently unknown systemic and local effects of FGF23 or klotho without interference from the endogenous mouse Fgf23 and klotho activities. Such studies will expand our understanding of the fundamental aspects of calcium and phosphate homeostasis (18, 37, 38) and may help in developing novel strategies to treat diseases associated with abnormal mineral ion metabolism (39).
Acknowledgments
The authors thank Ms. K. Egashira (Department of Pathology, Nagasaki University School of Biomedical Sciences, Japan) for preparing the histology sections. The research work was supported in part by U.S. National Institutes of Health grants R01-DE13686 (to M.M.), R01-DK073944 (to B.L.), and R01-DK077276 (to M.S.R.).
References
- Yu X, White K E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16:221–232. doi: 10.1016/j.cytogfr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transpl. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque M S. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008;74:566–570. doi: 10.1038/ki.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S. Does renal ageing affect survival? Ageing Res Rev. 2007;6:211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Taguchi T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med Electron Microsc. 2002;35:68–80. doi: 10.1007/s007950200009. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Razzaque M S. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol Med. 2007;13:45–53. doi: 10.1016/j.molmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- ADHR_Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- Benet-Pages A, Orlik P, Strom T M, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- Bai X, Miao D, Li J, Goltzman D, Karaplis A C. Transgenic mice overexpressing human fibroblast growth factor 23(R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque M S, St-Arnaud R, Huang W, Taguchi T, Erben R G, Lanske B. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe D W, Quarles L D. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt K P, Baum M G, Schiavi S, Hu M C, Moe O W, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin J E, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- Yu X, Ibrahimi O A, Goetz R, Zhang F, Davis S I, Garringer H J, Linhardt R J, Ornitz D M, Mohammadi M, White K E. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Nazneen A, Taguchi T, Razzaque M S. Low-dose local kidney irradiation inhibits progression of experimental crescentic nephritis by promoting apoptosis. Am J Nephrol. 2008;28:555–568. doi: 10.1159/000115290. [DOI] [PubMed] [Google Scholar]
- Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, Razzaque M S. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am J Nephrol. 2008;28:755–764. doi: 10.1159/000128607. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Soegiarto D W, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Taguchi T. Collagen-binding heat shock protein (HSP) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol. 1997;183:24–29. doi: 10.1002/(SICI)1096-9896(199709)183:1<24::AID-PATH1106>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Zha Y, Le V T, Higami Y, Shimokawa I, Taguchi T, Razzaque M S. Life-long suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–5698. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Nazneen A, Taguchi T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod Pathol. 1998;11:1183–1188. [PubMed] [Google Scholar]
- Kirkeby S, Thomsen C E. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods. 2005;301:102–113. doi: 10.1016/j.jim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz R, Beenken A, Ibrahimi O A, Kalinina J, Olsen S K, Eliseenkova A V, Xu C, Neubert T A, Zhang F, Linhardt R J, Yu X, White K E, Inagaki T, Kliewer S A, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque M S, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y I. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- Lanske B, Razzaque M S. Mineral metabolism and aging: the fibroblast growth factor 23 enigma. Curr Opin Nephrol Hypertens. 2007;16:311–318. doi: 10.1097/MNH.0b013e3281c55eca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B, Razzaque M S. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Imel E A, Kreiter M L, Yu X, Mackenzie D S, Sorenson A H, Goetz R, Mohammadi M, White K E, Econs M J. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister M F, Ruf I, Stange G, Ziegler U, Lederer E, Biber J, Murer H. Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter. Proc Natl Acad Sci U S A. 1998;95:1909–1914. doi: 10.1073/pnas.95.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int. 1998;54:1224–1232. doi: 10.1046/j.1523-1755.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007;27:503–515. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- Razzaque M S. Klotho and Na+,K+-ATPase activity: solving the calcium metabolism dilemma? Nephrol Dial Transplant. 2008;23:459–461. doi: 10.1093/ndt/gfm702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S. Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat Clin Pract Endocrinol Metab. 2007;3:788–789. doi: 10.1038/ncpendmet0667. [DOI] [PubMed] [Google Scholar]