Abstract
Mutations in aquaporin-2 (AQP2) that interfere with its cellular processing can produce autosomal recessive nephrogenic diabetes insipidus (NDI). Prior gene knock-in of the human NDI-causing AQP2 mutation T126M produced mutant mice that died by age 7 days. Here, we used a novel “conditional gene knock-in” strategy to generate adult, AQP2-T126M mutant mice. Mice separately heterozygous for floxed wild-type AQP2 and AQP2-T126M were bred to produce hemizygous mice, which following excision of the wild-type AQP2 gene by tamoxifen-induced Cre-recombinase gave AQP2T126M/− mice. AQP2T126M/− mice were polyuric (9–14 ml urine/day) compared to AQP2+/+ mice (1.6 ml/day) and had reduced urine osmolality (400 vs. 1800 mosmol). Kidneys of AQP2T126M/− mice expressed core-glycosylated AQP2-T126M protein in an endoplasmic reticulum pattern. Screening of candidate protein folding “correctors” in AQP2-T126M-transfected kidney cells showed increased AQP2-T126M plasma membrane expression with the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG). 17-AAG increased urine osmolality in AQP2T126M/− mice by >300 mosmol but had no effect in AQP2−/− mice. Kidneys of 17-AAG-treated AQP2T126M/− mice showed partial rescue of defective AQP2-T126M cellular processing. Our results establish an adult mouse model of NDI and demonstrate partial restoration of urinary concentration function by a compound currently in clinical trials for other indications.—Yang, B., Zhao, D., Verkman, A. S. Hsp90 inhibitor partially corrects nephrogenic diabetes insipidus in a conditional knock-in mouse model of aquaporin-2 mutation.
Keywords: AQP2, water transport, water channel, transgenic mouse, NDI, polyuria
Aquaporin-2 (AQP2) is a water-selective membrane transport protein that is responsible for the generation of a concentrated urine by the kidney in response to the antidiuretic hormone vasopressin (1,2,3,4,5). AQP2 is expressed in mammalian kidney in principal and inner medullary collecting duct epithelial cells. Vasopressin increases transepithelial water permeability in collecting duct by the exocytic insertion of AQP2-containing vesicles into the apical plasma membrane. AQP2 is of considerable clinical importance in acquired and hereditary disorders of urinary concentrating function. Mutations in AQP2 that reduce collecting duct water permeability cause the hereditary disease nephrogenic diabetes insipidus (NDI) (6,7,8,9). The more common autosomal recessive form of NDI is caused by mutations in AQP2 that interfere with its folding and cellular processing and/or its intrinsic water transport function. One such AQP2 mutation, AQP2-T126M, causes a severe form of human NDI (10) because of defective folding and retention of mutant AQP2 at the endoplasmic reticulum (11, 12). Previously, we found in cell culture models that small-molecule chemical chaperones, such as glycerol and trimethylamine oxide could rescue the defect in AQP2-T126M cellular processing, allowing its insertion into the cell plasma membrane and restoration of cell water permeability (11). As found in cystic fibrosis (13) and several other “protein-folding diseases” (14,15,16), small-molecule correctors of defective protein folding have potential therapeutic value in NDI. Small-animal models of NDI are needed to investigate such molecular therapies.
We previously generated transgenic AQP2-T126M knock-in mice by targeted gene replacement (17). The mice were polyuric soon after birth, but as found in other NDI-causing gene deletions, including NKCC2 (18), ROMK1 (19), and the vasopressin V2-receptor (20), the AQP2-T126M homozygous mutant mice died in their first week of life. It is not known why neonatal mice are particularly sensitive to polyuria-induced renal failure. Various other AQP2-related mouse models of NDI have been created, including conditional (21) and tissue-specific (22) AQP2 knockout, knock-in of a dominant-negative AQP2 mutation, causing autosomal dominant NDI (23), and ethylnitrosourea-mutagenized mice having an AQP2-F204V mutation (24).
The purpose of this study was to generate an “inducible knock-in” adult mouse model of NDI for in vivo proof-of-concept testing of a putative corrector of defective AQP2-T126M cellular processing. As diagrammed in Fig. 1, we used a novel strategy in which serial breeding of heterozygous AQP2 knock-in mice and conditional AQP2 knockout mice, each generated previously by our lab (17, 21), produced mice containing a floxed wild-type AQP2 allele, an AQP2-T126M allele, and tamoxifen-inducible Cre-recombinase elements. The resultant hemizygous mice, termed AQP2T126M/flox mice, manifest no significant phenotype because a single wild-type AQP2 gene is sufficient for normal urinary concentrating function in both mice and humans (10, 17) and because the T126M mutation does not interfere with the processing or function of wild-type AQP2 (25). Following excision of a critical portion of the wild-type AQP2 gene in the AQP2T126M/flox mice by tamoxifen-induced Cre recombinase expression, the resulting AQP2T126M/− mice express only the mutant AQP2-T126M gene. The AQP2T126M/− mice were characterized and used for in vivo testing of an Hsp90 inhibitor identified in a small screen of known protein folding “correctors” in a cell culture model of defective AQP2-T126M plasma membrane targeting. AQP2−/− (null) mice produced in parallel served as key controls.
Figure 1.
Strategy for generation of conditional AQP2-T126M mutant mice. See text for further explanation.
MATERIALS AND METHODS
Generation of AQP2-T126M “conditional knock-in” mice
AQP2-T126M knock-in mice (AQP2T126M/T126M) and AQP2 conditional knockout mice (AQP2−/−) were generated as described previously (17, 21). AQP2-T126M conditional knock-in mice (AQP2T126M/−) were generated by a series of intercrossing of heterozygous AQP2-T126M knock-in mice (AQP2T126M/+) and heterozygous floxed AQP2 mice (AQP2flox/+) expressing a Cre-Esr1 fusion protein. As diagrammed in Fig. 1, to generate AQP2T126M/− mice, the wild-type AQP2 allele was deleted by intraperitoneal injections of tamoxifen (4-hydroxytamoxifen; Sigma, St. Louis, MO, USA) (0.1 ml of 5 mg/ml) daily for 10 days in 8- to 10-wk-old AQP2T126M/flox mice. AQP2T126M/− mice were genotyped by polymerase chain reaction (PCR) (17) and confirmed by Southern blot analysis. All procedures were done under Institutional Animal Care and Use Committee approval.
Southern and Northern blot analysis
AQP2 gene targeting and deletion were confirmed by Southern hybridization, in which 10 μg of genomic DNA was digested with ApaI or ApaI/FseI, electrophoresed, transferred to a Nylon+ membrane (Amersham Biosciences, Piscataway, NJ), and hybridized with a 1-kb genomic fragment (indicated in Fig. 2A). For Northern blot analysis, total RNA from kidney was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNAs (10 μg/lane) were resolved on a 1.2% formaldehyde-agarose denaturing gel, transferred to a Nylon+ membrane, and hybridized at high stringency with a 32P-labeled probe corresponding to the mouse AQP2 cDNA coding sequence.
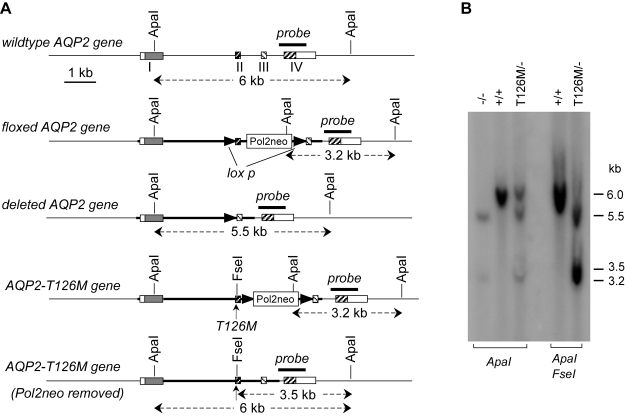
Figure 2.
Mouse AQP2 gene structure and Southern blot analysis of mutant mice. A) Organization and restriction map of wild-type AQP2 gene (top) and of the various targeted mutant AQP2 genes. Rectangles indicate exon segments, with coding regions shaded. The probe used for Southern blot analysis is indicated (labeled “probe”), along with expected sizes of hybridized fragments after ApaI and/or FseI digestions. B) Southern blot of genomic DNA digested with ApaI and ApaI/FseI with probe as indicated in A.
Fluorescence-based real-time quantitative PCR
Genomic DNA isolated from kidney was used as a template for real-time genomic PCR. Primers for amplification of the wild-type AQP2 gene were 5′-GCTCTCCACAACAATGCAACA-3′ (sense) and 5′-CCTTGGGTGTTCTGTGAGACAGTCT-3′ (antisense). For real-time reverse-transcription PCR (RT-PCR), total RNA from whole mouse kidney was isolated by homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA), and mRNA was extracted using the Oligotex mRNA mini kit (Qiagen, Valencia, CA, USA). cDNA was reverse transcribed from mRNA with oligo(dT) (SuperScript II preamplification kit; Invitrogen). Primers were 5′-GCTCTCCACAACAATGCAACA-3′ (sense) and 5′-GGTGAAATAGATCCCAAGGAGG-3′ (antisense) for wild-type AQP2, and 5′-CAGCTCAGCAACAGTACAATG-3′ (sense) and 5′-GGTGAAATAGATCCCAAGGAGG-3′ (antisense) for AQP2-T126M. AQP2 cDNA downsteam primers, 5′-TGGCTCCAGCAGTTGTCACTG-3′ (sense), and 5′-GCGTTCCTCCCAGTCAGTGTC-3′ (antisense) were used as a reference for quantifying mRNA. Fluorescence-based real-time PCR was carried out using the LightCycler instrument (Roche Diagnostics, Indianapolis, IN, USA) with FastStart DNA MasterPLUS SYBR Green I kit as described previously (26). Normalization and quantitative analysis was done by standard procedures using Roche LCS4 software.
Kidney histology
Kidney tissue was fixed overnight in Bouin’s fixative, embedded in paraffin, and cut in 3-μm-thick sections. Sections from kidney cortex, medulla, and papilla were stained with hematoxylin and eosin.
Immunofluorescence and immunoblot analysis
Kidneys were fixed with 4% paraformaldehyde in PBS for 4 h, infiltrated with 30% sucrose in PBS overnight, frozen inoptimal cutting temperature compound with liquid nitrogen, and cut in 3-μm-thick sections using a cryostat. Tissues were incubated using a rabbit polyclonal antibody against the 30-amino acid C terminus of AQP2. Cy3-conjugated sheep anti-rabbit immunoglobulin G (IgG) was used as secondary antibody (Sigma). For immunoblot analysis, tissues were homogenized with a glass Dounce homogenizer in 250-mM sucrose containing 1 mM EDTA, 20 μg/ml PMSF, 1 μg/ml pepstatin A, and 1 μg/ml leupeptin (pH 7.4), and centrifuged at 4000 g for 15 min to remove whole cells, nuclei, and mitochondria. Total protein was assayed in the supernatant fractions using the Bio-Rad DC protein assay kit (Bio-Rad, Richmond, CA, USA) and loaded on a 12% SDS-PAGE gel (10 μg/lane). Proteins were transferred to polyvinylidene difluoride membranes (Gelman Scientific, Ann Arbor, MI, USA) and immunoblotted by standard procedures. For endoglycosidase digestion, proteins from kidney homogenates (100 μg) were incubated with endoglycosidase H (0.5 U, Sigma) at 37°C for 2 h prior to immunoblot analysis.
Cell culture
Type I MDCK cells (CCL-34; American Type Culture Collection, Manassas, VA, USA) were cultured at 37°C in a humidified 95% air/5% CO2 atmosphere in a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-12 nutrient medium supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were transfected with plasmids encoding full-length mouse AQP2-T126M or wild-type AQP2 in pcDNA3 (Invitrogen). Stably expressing cell lines were established using G418 selection medium. In some studies, AQP2-T126M cells were incubated with candidate correctors (compounds purchased from Sigma unless otherwise indicated): Congo red, chrysamine G, thioflavine S, gossypol, lactacystin, novobiocin, radcicol, trehalose, 15-deoxyspergualin, geldanamycin, 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17-DMAG), 17-AAG, (LC Laboratories, Woburn, MA, USA), Z-Leu-Leu-Leu-aldehyde (MG132; A. G. Scientific, San Diego, CA, USA), or bortezomib (Toronto Research Chemicals, North York, ON, Canada) at concentrations of 0.4, 2, and 10 μM for 20 h before analysis. DMSO was used as a vehicle control.
Analysis of surface AQP2 protein expression
MDCK cells were incubated with test compounds or DMSO vehicle for 20 h in the cell culture incubator. Surface proteins were isolated using the Pierce cell surface protein isolation kit (Pierce Biotechnology, Rockford, IL, USA). Briefly, cells were washed with PBS 3 times and biotinylated with EZ-Link Sulfo-NHS-SS-biotin for 30 min at 4°C. After the reaction was quenched, cells were harvested and lysed. Biotinylated proteins for immunoblot analysis were isolated using NeutrAvidin resin and eluted with SDS-PAGE buffer containing 50 mM dithiothreitol (DTT).
Urinary concentrating studies
Urine samples were collected by placing mice on a wire mesh platform in a clean glass beaker until spontaneous voiding was observed. In some experiments, urine samples were obtained from the same mice under basal conditions (unrestricted access to food and water), and after 18-h deprivation of food and water. Twenty-four-hour urine output and water consumption were measured in metabolic cages adapted for mice (Harvard Apparatus, Holliston, MA, USA). Blood samples were collected in heparinized glass tubes by puncture of the periorbital venous sinus. Plasma was separated from blood cells by centrifugation. Urine osmolality was measured by freezing point osmometry (micro-osmometer; Precision Systems, Natick, MA, USA). Urine and plasma chemistries were measured by the University of California, San Francisco, Clinical Chemistry Laboratory.
RESULTS
Generation of adult AQP2-T126M mutant mice
Adult AQP2-T126M mutant mice, AQP2T126M/−, were generated using a conditional gene knock-in strategy (Fig. 1). Floxed wild-type AQP2 heterozygous mice containing Cre/Esr1 gene elements (Cre/Esr1, AQP2flox/+) were bred with heterozygous AQP2-T126M mice (AQP2T126M/+) to produce hemizygous mice containing a floxed wild-type AQP2 allele and a mutant AQP2-T126M allele (AQP2T126M/flox) with Cre/Esr1 expression. The AQP2T126M/flox mice had grossly normal growth, appearance, activity and behavior, and normal renal function. Conditional deletion of the wild-type AQP2 gene, leaving the mutant AQP2-T126M gene, was accomplished by tamoxifen-inducible Cre-recombinase expression, as done previously for conditional AQP2 knockout (21).
The conditional knock-in genotype was confirmed by Southern blot analysis of genomic DNA using a 1-kb genomic fragment as probe (Fig. 2A). ApaI digestion produced three fragments in AQP2T126M/− mice with sizes of 3.2, 5.5, and 6.0 kb. The 3.2-kb fragment corresponds to the replaced gene containing an ApaI site in the Pol2neobpA cassette, confirming correct AQP2 gene targeting with the floxed targeting sequence (AQP2flox). The 5.5-kb fragment corresponds to the deleted AQP2 gene. Fragments of ∼6-kb are produced by both the wild-type AQP2 gene and the AQP2-T126M knock-in gene after excision of the Pol2neo cassette. To establish the origin of the ∼6-kb fragments, genomic DNA was digested with both ApaI and FseI. The appearance of a 3.5-kb fragment confirmed the AQP2-T126M gene allele (Fig. 2B).
To determine the efficiency of tamoxifen-induced, Cre-mediated excision of the loxP-flanked wild-type AQP2 gene fragment in the AQP2T126M/flox mice, real-time quantitative genomic and RT-PCR were done using primers corresponding to wild-type AQP2 allele. Using appropriate reference genomic primers and kidney genomic DNA, we found that the efficiency for excision of the wild-type AQP2 gene fragment was ∼95% in kidneys of AQP2T126M/− mice, and ∼ 90% in kidneys of AQP2−/− mice (Fig. 3A). RT-PCR analysis of wild-type AQP2 mRNA expression in kidneys showed ∼10% in AQP2T126M/− mice and ∼15% in AQP2−/− mice compared with wild-type mice (as 100%) (Fig. 3B). Northern blot analysis (Fig. 3C) showed a strong ∼1.7 kb and a weaker ∼3.0-kb AQP2 transcript in kidneys of wild type and AQP2T126M/− mice. In response to 18 h of water deprivation, the AQP2 transcript was significantly up-regulated.
Figure 3.
Efficiency of Cre-induced deletion of the floxed wild-type AQP2 gene in AQP2T126M/− mice. A) Left panel: relative copy number of wild-type AQP2 gene in mice of indicated genotypes determined by real-time genomic PCR (mean±se, n=3). Right panel: schematic showing genomic PCR strategy. B) Left panel: relative copy number of mRNA encoding wild-type AQP2 determined by real-time RT-PCR (mean±se, n=3). Right panel: schematic showing RT-PCR strategy. C) Northern blot of kidney mRNA from wild-type and AQP2T126M/− mice probed with the mouse AQP2 cDNA coding sequence. Where indicated, mice were deprived of water for 18 h prior to kidney removal. D) Immunoblot analysis of total mouse kidney protein. Where indicated, protein was digested by endoglycosidase H. Blot probed by polyclonal antibody against the AQP2 C terminus. E) AQP2 immunofluorescence of kidneys of tamoxifen-treated wild-type (left panel) and AQP2T126M/− mice (right panel). White arrowhead indicates a cell expressing wild-type AQP2 protein at the apical plasma membrane.
Impaired AQP2-T126M cellular processing and urinary concentrating ability in AQP2T126M/− mice
Immunoblot analysis of whole-kidney homogenates showed that the majority of immunoreactive protein in wild-type mice migrated as a diffuse band of apparent molecular size 34–40 kDa (Fig. 3D), which represents fully processed AQP2 with complex glycosylation. A band of 29 kDa was also seen, which corresponds to nonglycosylated AQP2. These two bands were faintly seen in kidney homogenates from AQP2T126M/− mice. Instead, a band at ∼31 kDa was seen, which represents an endoplasmic reticulum-retained, core-glycosylated form of AQP2-T126M. The 31-kDa band largely disappeared following endoglycosidase H treatment, as predicted for core-glycosylated AQP2-T126M.
Figure 3E shows specific AQP2 immunofluorescence in kidneys of wild-type mice (left) and AQP2T126M/− mice (right). By confocal fluorescence microscopy, wild-type AQP2 protein was located mainly in apical membranes of kidney collecting duct epithelial cells. AQP2-T126M mutant protein was distributed mainly in an intracellular compartment that likely represents the endoplasmic reticulum. Fewer than 10% of collecting duct epithelial cells in AQP2T126M/− mice showed strong apical membrane AQP2 expression (white arrow), corresponding to cells where the wild-type AQP2 gene allele was not deleted.
Urinary concentrating function was assessed from urine osmolalities before and after tamoxifen treatment and from water deprivation studies. There was no significant difference in urine osmolality between wild-type (1840±96 mosmol) and AQP2T126M/flox mice (1767±76 mosmol). Urine osmolality in AQP2T126M/− mice decreased from ∼2000 to ∼400 mosmol with tamoxifen treatment (Fig. 4A), as did urine osmolality in AQP2−/− mice. Daily urinary output in metabolic cage collections showed marked polyuria in the AQP2T126M/− mice, which excreted ∼7-fold more urine than in litter-matched wild-type mice (Fig. 4B). Urine output in AQP2−/− mice was even greater (>20 ml/day). Fluid consumption in AQP2T126M/− and AQP2−/− mice was correspondingly greater than that in wild-type mice (data not shown).
Figure 4.
Urinary concentrating function in AQP2T126M/− mice. A) Urine osmolality in AQP2T126M/− mice given free access to food and water (mean±se, 3 mice/group). Arrows indicate tamoxifen (4OH-TM) injections. B) Twenty-four-hour urine output in wild-type mice, (tamoxifen-treated) AQP2T126M/− mice, and AQP2−/− mice. C) Urine osmolality before (H) and after (D) 18-h water deprivation. D) Body weight loss for the same mice in C after water deprivation. E) Left: relative expression of mRNAs encoding wild-type and T126M-AQP2 measured by real-time RT-PCR. Right: upstream PCR primers indicated (underlined) in partial sequence of wild-type and T126M mutant AQP2 cDNA.
Following 18 h water deprivation, urine osmolality in wild-type mice increased from 1840 ± 96 to 2872 ± 258 mosmol but was not increased in AQP2−/− mice (Fig. 4C). Urine osmolality in AQP2T126M/− mice increased to 1027 ± 118 mosmol following 18 h of water deprivation, suggesting functioning of residual wild-type AQP2 and/or partial function of mutant AQP2-T126M. Total body weight loss after water deprivation was also attenuated in AQP2T126M/− mice compared to AQP2−/− mice (Fig. 4D). As determined by quantitative RT-PCR, AQP2-T126M mRNA expression was significantly increased in kidneys of AQP2T126M/− mice in response to an 18-h water deprivation (Fig. 4E, left). Mutant-specific RT-PCR was done using a sense mutant primer (underlined in Fig. 4E, right) and a common antisense primer downstream in the AQP2 cDNA sequence. The expression of mRNA encoding wild-type AQP2 was very low in AQP2T126M/− mice under control conditions but showed a small increase after water deprivation that may account for the increased urine osmolality in AQP2T126M/− mice following water deprivation. No PCR product was amplified from wild-type mouse kidney cDNA using the mutant primer, confirming its specificity.
Partial rescue of defective AQP2-T126M cellular processing by an Hsp90 inhibitor
Stably transfected MDCK cell lines expressing AQP2-T126M and wild-type AQP2 were generated to screen known correctors of defective protein folding, with the intention of testing active compounds in AQP2T126M/− mice. As reported previously (27), immunofluorescence of cells expressing wild-type AQP2 showed AQP2 localization to intracellular vesicles under basal conditions and to the plasma membrane following cAMP stimulation by forskolin (not shown); cells expressing AQP2-T126M showed ER localization. A total of 13 compounds were tested for their ability to increase AQP2-T126M plasma membrane expression (listed in Materials and Methods), including protein misfolding and aggregation modulators, Hsp70 modulators, Hsp90 inhibitors, and proteasome inhibitors.
Figure 5A (left) shows a representative AQP2 immunoblot of cell surface proteins following surface biotinylation and avidin column purification. Cells were treated with 20 μM forskolin for 15 min prior to and during biotinylation. No specific AQP2 expression was seen in null (nontransfected) cells. Strong surface expression was seen in cells expressing wild-type AQP2. Relatively low surface expression was seen in AQP2-T126M-transfected cells under control conditions and with the test compounds. Of the test compounds, only the Hsp90 inhibitor 17-AAG consistently increased AQP2-T126M surface expression. The increased surface expression was seen without a corresponding increase in total AQP2-T126M protein (Fig. 5A, right), suggesting that the increased surface expression is not the consequence of nonspecific up-regulation of transcription or translation. Because 17-AAG has already been used in mouse models of human diseases and is in clinical trials for nonrenal indications (see Discussion), we carried out functional studies in 17-AAG-treated AQP2T126M/− mice.
Figure 5.
Partial correction of defective urinary concentrating function in AQP2T126M/− by 17-AAG. A) AQP2 immunoblot of cell surface protein (following biotinylation and avidin column purification) and total protein from MDCK cells expressing wild-type AQP2 or AQP2-T126M. Where indicated, cells were treated for 20 h with 2 μM of 17-AAG, 17-DMAG, geldanamycin, or Congo red. Arrow indicates increased cell surface AQP2-T126M in 17-AAG-treated cells. B) Urine osmolality in AQP2T126M/− mice receiving intraperitoneal injections of 17-AAG (50 mg/kg) or DMSO vehicle (mean±se, 6 mice/group). C) Urine osmolality in wild-type, AQP2T126M/−, and AQP2−/− mice before and after 17-AAG treatment (mean±se, 4 mice/group).
On the basis of prior 17-AAG studies in mice (28), we administered 50 mg/kg 17-AAG by intraperitoneal injection every 8 h for 3 days. Figure 5B shows a significant increase in urine osmolality by ∼300 mosmol in AQP2T126M/− mice, whereas identical treatment with the DMSO vehicle did not change urine osmolality. RT-PCR using wild-type AQP2-specific primers (as in Fig. 4E) indicated that 17-AAG did not affect expression of wild-type AQP2 transcript in AQP2T126M/− mice (data not shown). Further studies showed that the same 17-AAG treatment did not increase urine osmolality in wild-type or AQP2−/− mice (Fig. 5C), providing evidence for selective action on AQP2-T126M.
AQP2 immunoblot analysis of total kidney homogenate showed strong AQP2 expression in wild-type mice, with typical appearance of glycosylated and nonglycosylated protein (Fig. 6A, top). Compared with untreated AQP2T126M/− mice, AQP2-T126M immunoreactivity in 17-AAG-treated mice was greater in bands corresponding to glycosylated and nonglycosylated protein, and lower in the intermediate band (at ∼31 kDa) corresponding to core-glycosylated protein. Also, substantially less immunoreactive protein was seen at ∼23 kDa in 17-AAG-treated AQP2T126M/− mice, which probably represents an AQP2-T126M degradation product containing its C-terminal immunoreactive domain. Band intensities are quantified in Fig. 6A (bottom). AQP2 immunofluorescence in Fig. 6B shows an apical membrane pattern in kidneys of wild-type mice (left panels) and a weak intracellular pattern in AQP2T126M/− mice (middle panels). 17-AAG treatment produced a general increase in immunoreactivity, with apparent plasma membrane expression seen in many cells, likely accounting for the partial correction of the urinary concentration defect.
Figure 6.
Partial rescue of defective AQP2-T126M cellular processing by 17-AAG in kidneys of AQP2T126M/− mice. A) Top: AQP2 immunoblot of kidney homogenates from wild-type and AQP2T126M/− mice. Mice were fully treated (as in Fig. 5A) with 17-AAG were indicated. Arrow indicates the corrected AQP2-T126M protein. Bottom: averaged total AQP2 protein (se, open bars) and core glycosylation protein (solid bars). *P < 0.05 vs. total AQP2 protein from untreated AQP2T126M/− mice. **P < 0.01 vs. core-glycosylated AQP2 protein from untreated AQP2T126M/− mice. B) AQP2 immunofluorescence of kidneys from wild-type (left) and AQP2T126M/− mice, without (middle) or following (right) 17-AAG treatment. Confocal imaging done at two magnifications.
DISCUSSION
The purposes of this study were to generate and characterize an adult mouse model of autosomal recessive NDI caused by AQP2 gene mutation and to use this mouse model for in vivo testing of a putative small-molecule AQP2-T126M corrector. More than 35 NDI-producing AQP2 mutations have been identified (8, 29), most of which produce autosomal recessive NDI by impairment of cellular processing of the immature protein (30, 31). Some AQP2 mutations, including L22V, L28P, A47V, V71M, T125M, T126M, A147T, V168M, G175R, C181W, P185A, and R187C, have been characterized in Xenopus oocytes, yeast, and mammalian cells, and have been shown to have defects in ER-to-Golgi targeting with accelerated ER-associated degradation (31,32,33,34,35). These mutations probably impair AQP2 folding, resulting in recognition by the ER quality control machinery, ubiquitination, and degradation by the 26S proteasome (36, 37). Some of these AQP2 mutants, including AQP2-T126M, retain water transport function at the ER and so correction of their ER-to-plasma membrane targeting would restore cell water permeability.
Several mutant AQP2 mouse models of NDI have been established as summarized in Table 1, although for different reasons these mouse models are not suitable for the testing of corrector drug candidates. For example, AQP2-T126M knock-in (AQP2T126M/T126M) results in severe NDI and perinatal lethality (17). The various AQP2 knockout mouse models, including inducible AQP2−/−, AQP2flx/flx Hoxb7-Cre, and AQP2Δ230/Δ230, showed no AQP2 protein expression in collecting duct and no increase in urine osmolality in response to water deprivation or dDAVP administration (21, 22, 38). AQP2 mutant mouse models of autosomal dominant NDI (AQP2763−772 del and AQP2S256L/S256L) showed basolateral membrane targeting in collecting duct (23, 39). The one potentially usable model, AQP2F204V/F204V mice (24), is associated with ER retention and presumed AQP2-F204V misfolding, though this is a non-natural mutation not seen in human NDI.
TABLE 1.
Summary of published AQP2 transgenic mouse models
| Model | Strain | Basal urine osmolality (mosmol) | Maximum urine osmolality (mosmol) | Urine output (ml/day) | Hydronephrosis | AQP2 localization | Survival | Ref. |
|---|---|---|---|---|---|---|---|---|
| AQP2T126M/T126M | CD1 | 220 | 220 | High | + | ER | 1 wk | 17 |
| AQP2F204V/F204V | C57BL/6 | 161 | 470 | High | + | ER | Adult | 24 |
| Inducible AQP2−/− | CD1 | 182 | 200 | 25 ml | + | — | Adult | 21 |
| AQP2763−772 del | C57BL/6 | 240 | 1120 | 5 ml | + | Basolateral membrane | Adult | 39 |
| AQP2S256L/S256L | C57BL/6 | 480 | 540 | High | + | Basolateral membrane | Adult | 23 |
| AQP2flx/flx Hoxb7-Cre | C57BL/6 | 170 | 170 | High | + | CNT apical membrane | Adult | 22 |
| AQP2Δ230/Δ230 | B6/129/SvJ | 193 | 200 | High | + | — | Adult | 38 |
| Inducible AQP2T126M/− | CD1 | 400 | 1000 | High | — | ER | Adult | — |
The AQP2 point mutation T126M was identified in 1996 as a cause of non-X-linked recessive NDI in humans (10). Two subjects homozygous for the AQP2-T126M mutation from the same family manifested severe NDI, with high serum sodium concentration (147–186 mmol/l), low urinary osmolality (107–173 mosmol), and unresponsiveness to DDAVP. One subject died at age 13 months of brain lesions with necrosis of basal ganglia. The other subject had a fluid consumption of 2 L/day at age 2 yr. In vitro studies indicated that T126M mutation interferes with AQP2 protein folding and resulted in retention of mutant AQP2 protein at the endoplasmic reticulum (11, 12).
The Hsp90 inhibitor 17-AAG produced partial correction of the defect in AQP2-T126M cellular processing in transfected kidney cells, and kidney collecting duct in AQP2T126M/− mice, resulting in improved urinary concentrating function. The mechanisms by which Hsp90 inhibition, or off-target effects of 17-AAG, promote AQP2-T126M rescue will be challenging to establish, in part because of the large and expanding set of Hsp90-interacting proteins, which include cochaperones, protein kinases, transcription factors, and intracellular receptors involved in innate immunity (reviewed in ref. 40). Hsp90 is involved in a diverse set of cellular processes, including chaperoning of newly synthesized proteins, the stress response, signal transduction, and transcriptional regulation. 17-AAG is a semisynthetic chemical analog of the natural compound geldanamycin, which has reduced toxicity and greater therapeutic ratio (41). On the basis of its signaling and oncogene modulatory activities (42), 17-AAG is currently in phase 2 clinical trials for several cancer targets, including breast cancer, melanoma, and prostate cancer, with recent data showing promising therapeutic activity (43). Newer analogs of 17-AAG with improved pharmacological properties are in preclinical development (44).
There are several possible mechanisms by which 17-AAG might rescue the defect in AQP2-T126M protein folding and processing. Hsp90 has been found to interact with and promote ER-dependent degradation of various misfolded proteins, including the mutant cystic fibrosis protein ΔF508-CFTR (45). Disruption of an Hsp90/AQP2-T126M complex may allow AQP2-T126M exit from the ER and further processing by the Golgi. In many cell types, Hsp90 inhibition is associated with induction of Hsp70 and various modulators of the cell stress response (46, 47). There is evidence for involvement of Hsc70 in AQP2 constitutive recycling pathway. Increased association of Hsc70 to AQP2 was found following 30 min of vasopressin treatment, and functional Hsc70 knockdown caused membrane accumulation of AQP2 in transfected LLC-AQP2 cells (48). Other possible mechanisms based on the biology of Hsp90 and 17-AAG include reduced AQP2-T126M degradation with consequent increased cellular accumulation, although the data here showed comparable AQP2-T126M protein without verses with 17-AAG treatment. It is also possible that 17-AAG might target heterotetramers containing AQP2-T126M and residual wild-type AQP2. Further studies, perhaps using primary cultures of kidney collecting duct from AQP2T126M/− mice, will be needed to distinguish among these and alternative mechanisms.
In summary, we have generated and characterized an inducible adult mouse model of human recessive NDI caused by AQP2-T126M gene mutation and discovered a novel corrector that partially rescued defective AQP2-T126M cellular processing. Because the mutant phenotype can be induced at a specified age, the AQP2T126M/− mice are suitable as an in vivo model to study candidate AQP2 therapies. 17-AAG or one of its analogs, or, pending further mechanistic studies, other Hsp90 inhibitors may be useful for therapy of some forms of NDI.
Acknowledgments
We thank Liman Qian for mouse breeding and genotype analysis. This work was supported by U.S. National Institute of Health (NIH) grants DK35124, HL59198, EY13574, EB00415, DK72517, and HL73856, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation (to A.S.V.), NIH grant DK66194, American Heart Association grant 0365027Y and Polycystic Kidney Disease Foundation grant 147a2r (to B.Y.).
References
- Deen P M, Knoers N V. Physiology and pathophysiology of the aquaporin-2 water channel. Curr Opin Nephrol Hypertens. 1998;7:37–42. doi: 10.1097/00041552-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Frøkiaer J, Marples D, Kwon T H, Agre P, Knepper M A. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- Noda Y, Sasaki S. Trafficking mechanism of water channel aquaporin-2. Biol Cell. 2005;97:885–892. doi: 10.1042/BC20040120. [DOI] [PubMed] [Google Scholar]
- Verkman A S. Dissecting the roles of aquaporins in renal pathophysiology using transgenic mice. Semin Nephrol. 2008;28:217–226. doi: 10.1016/j.semnephrol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen P M, Verdijk M A, Knoers N V, Wieringa B, Monnens L A, van Os C H, van Oost B A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Bichet D G. Hereditary polyuric disorders: new concepts and differential diagnosis. Semin Nephrol. 2006;26:224–233. doi: 10.1016/j.semnephrol.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Fujiwara T M, Bichet D G. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005;16:2836–2846. doi: 10.1681/ASN.2005040371. [DOI] [PubMed] [Google Scholar]
- Kamsteeg E J, Deen P M, van Os C H. Defective processing and trafficking of water channels in nephrogenic diabetes insipidus. Exp Nephrol. 2000;8:326–331. doi: 10.1159/000020686. [DOI] [PubMed] [Google Scholar]
- Mulders S M, Knoers N V, Van Lieburg A F, Monnens L A, Leumann E, Wuhl E, Schober E, Rijss J P, Van Os C H, Deen P M. New mutations in the AQP2 gene in nephrogenic diabetes insipidus resulting in functional but misrouted water channels. J Am Soc Nephrol. 1997;8:242–248. doi: 10.1681/ASN.V82242. [DOI] [PubMed] [Google Scholar]
- Tamarappoo B K, Verkman A S. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarappoo B K, Yang B, Verkman A S. Misfolding of mutant aquaporin-2 water channels in nephrogenic diabetes insipidus. J Biol Chem. 1999;274:34825–34831. doi: 10.1074/jbc.274.49.34825. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Lukacs G L, Du K, Caci E, Zegarra-Moran O, Galietta L J, Verkman A S. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F E, Kelly J W. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- Gregersen N. Protein misfolding disorders: pathogenesis and intervention. J Inherit Metab Dis. 2006;29:456–470. doi: 10.1007/s10545-006-0301-4. [DOI] [PubMed] [Google Scholar]
- Luheshi L M, Crowther D C, Dobson C M. Protein misfolding and disease: from the test tube to the organism. Curr Opin Chem Biol. 2008;12:25–31. doi: 10.1016/j.cbpa.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Yang B, Gillespie A, Carlson E J, Epstein C J, Verkman A S. Neonatal mortality in an aquaporin-2 knock-in mouse model of recessive nephrogenic diabetes insipidus. J Biol Chem. 2001;276:2775–2779. doi: 10.1074/jbc.M008216200. [DOI] [PubMed] [Google Scholar]
- Flagella M, Clarke L L, Miller M L, Erway L C, Giannella R A, Andringa A, Gawenis L R, Kramer J, Duffy J J, Doetschman T, Lorenz J N, Yamoah E N, Cardell E L, Shull G E. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Lorenz J N, Baird N R, Judd L M, Noonan W T, Andringa A, Doetschman T, Manning P A, Liu L H, Miller M L, Shull G E. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter’s syndrome. J Biol Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- Yun J, Schoneberg T, Liu J, Schulz A, Ecelbarger C A, Promeneur D, Nielsen S, Sheng H, Grinberg A, Deng C, Wess J. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J Clin Invest. 2000;106:1361–1371. doi: 10.1172/JCI9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhao D, Qian L, Verkman A S. Mouse model of inducible nephrogenic diabetes insipidus produced by floxed aquaporin-2 gene deletion. Am J Physiol Renal Physiol. 2006;291:F465–F472. doi: 10.1152/ajprenal.00494.2005. [DOI] [PubMed] [Google Scholar]
- Rojek A, Fuchtbauer E M, Kwon T H, Frokiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDill B W, Li S Z, Kovach P A, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci U S A. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D J, Hall F W, Tarantino L M, Gekakis N. Diabetes insipidus in mice with a mutation in aquaporin-2. PLoS Genet. 2005;1:171–178. doi: 10.1371/journal.pgen.0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck T M, Eledge J, Skach W R. Evidence for stabilization of aquaporin-2 folding mutants by N-linked glycosylation in endoplasmic reticulum. Am J Physiol Cell Physiol. 2004;287:C1292–C1299. doi: 10.1152/ajpcell.00561.2003. [DOI] [PubMed] [Google Scholar]
- Klein J D, Sands J M, Qian L, Wang X, Yang B. Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol. 2004;15:1161–1167. doi: 10.1097/01.asn.0000125617.19799.72. [DOI] [PubMed] [Google Scholar]
- Deen P M, Rijss J P L, Mulders S M, Errington R J, van Baal J, van Os C H. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J Am Soc Nephrol. 1997;8:1493–1501. doi: 10.1681/ASN.V8101493. [DOI] [PubMed] [Google Scholar]
- Sydor J R, Normant E, Pien C S, Porter J R, Ge J, Grenier L, Pak R H, Ali J A, Dembski M S, Hudak J, Patterson J, Penders C, Pink M, Read M A, Sang J, Woodward C, Zhang Y, Grayzel D S, Wright J, Barrett J A, Palombella V J, Adams J, Tong J K. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci U S A. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Al-Mojalli H, Al-Abbad A, Al-Hassoun I, Al-Hamed M, Al-Amr R, Butt A I, Meyer B F. Novel mutations underlying nephrogenic diabetes insipidus in Arab families. Genet Med. 2006;8:443–447. doi: 10.1097/01.gim.0000223554.46981.7a. [DOI] [PubMed] [Google Scholar]
- Morello J P, Bichet D G. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607–630. doi: 10.1146/annurev.physiol.63.1.607. [DOI] [PubMed] [Google Scholar]
- Marr N, Bichet D G, Hoefs S, Savelkoul P J, Konings I B, De Mattia F, Graat M P, Arthus M F, Lonergan M, Fujiwara T M, Knoers N V, Landau D, Balfe W J, Oksche A, Rosenthal W, Müller D, Van Os C H, Deen P M. Cell-biologic and functional analyses of five new Aquaporin-2 missense mutations that cause recessive nephrogenic diabetes insipidus. J Am Soc Nephrol. 2002;13:2267–2277. doi: 10.1097/01.asn.0000027355.41663.14. [DOI] [PubMed] [Google Scholar]
- Deen P M, Croes H, van Aubel R A, Ginsel L A, van Os C H. Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes insipidus are impaired in their cellular routing. J Clin Invest. 1995;95:2291–2296. doi: 10.1172/JCI117920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goji K, Kuwahara M, Gu Y, Matsuo M, Marumo F, Sasaki S. Novel mutations in aquaporin-2 gene in female siblings with nephrogenic diabetes insipidus: evidence of disrupted water channel function. J Clin Endocrinol Metab. 1998;83:3205–3209. doi: 10.1210/jcem.83.9.5074. [DOI] [PubMed] [Google Scholar]
- Knoers N V, Deen P M. Molecular and cellular defects in nephrogenic diabetes insipidus. Pediatr Nephrol. 2001;16:1146–1152. doi: 10.1007/s004670100051. [DOI] [PubMed] [Google Scholar]
- Boccalandro C, De Mattia F, Guo D C, Xue L, Orlander P, King T M, Gupta P, Deen P M, Lavis V R, Milewicz D M. Characterization of an aquaporin-2 water channel gene mutation causing partial nephrogenic diabetes insipidus in a Mexican family: evidence of increased frequency of the mutation in the town of origin. J Am Soc Nephrol. 2004;15:1223–1231. doi: 10.1097/01.asn.0000125248.85135.43. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Hasler U, Greasley P J, von Heijne G, Geering K. Determinants of topogenesis and glycosylation of type II membrane proteins. Analysis of Na, K-ATPase beta 1 AND beta 3 subunits by glycosylation mapping. J Biol Chem. 2000;275:29011–29022. doi: 10.1074/jbc.M002867200. [DOI] [PubMed] [Google Scholar]
- Shi P, Cao X, Qu J, Volk K A, Kirby P A, Williamson R A, Stokes J B, Yang B. Nephrogenic diabetes insipidus in mice caused by deleting C-terminal tail of aquaporin-2. Am J Physiol Renal Physiol. 2007;292:F1334–F1344. doi: 10.1152/ajprenal.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohara E, Rai T, Yang S S, Uchida K, Nitta K, Horita S, Ohno M, Harada A, Sasaki S, Uchida S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci U S A. 2006;103:14217–14222. doi: 10.1073/pnas.0602331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl L H, Prodromoli C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res. 2006;95:323–348. doi: 10.1016/S0065-230X(06)95009-X. [DOI] [PubMed] [Google Scholar]
- Drysdale M J, Brough P A, Massey A, Jensen M R, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel. 2006;9:483–954. [PubMed] [Google Scholar]
- Solit D B, Ivy S P, Kopil C, Sikorski R, Morris M J, Slovin S F, Kelly W K, DeLaCruz A, Curley T, Heller G, Larson S, Schwartz L, Egorin M J, Rosen N, Scher H I. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13:1775–1782. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Fang S L, Xiao Y F, O'Connor S P, Nadler S G, Lee D W, Jefferson D M, Kaplan J M, Smith A E, Cheng S H. Partial restoration of cAMP-stimulated CFTR chloride channel activity in ΔF508 cells by deoxyspergualin. Am J Physiol Cell Physiol. 1998;275:C171–C178. doi: 10.1152/ajpcell.1998.275.1.C171. [DOI] [PubMed] [Google Scholar]
- Shen H Y, He J C, Wang Y, Huang Q Y, Chen J F. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- Stravopodis D J, Margaritis L H, Voutsinas G E. Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem. 2007;14:3122–3138. doi: 10.2174/092986707782793925. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper M A, Wang W, Giebisch G, Shull G E, Hebert S C. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter’s) knockout mice. J Biol Chem. 2002;277:37881–37887. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]