Abstract
Peroxisome proliferator-activated receptor-α (PPARα) is a master transcriptional regulator of β-oxidation and a prominent target of hypolipidemic drugs. To gain deeper insights into the systemic consequences of impaired fat catabolism, we used quantitative, mass spectrometry-based metabolic profiling to investigate the fed-to-fasted transition in PPARα+/+ and PPARα−/− mice. Compared to PPARα+/+ animals, acylcarnitine profiles of PPARα−/− mice revealed 2- to 4-fold accumulation of long-chain species in the plasma, whereas short-chain species were reduced by as much as 69% in plasma, liver, and skeletal muscle. These results reflect a metabolic bottleneck downstream of carnitine palmitoyltransferase-1, a mitochondrial enzyme that catalyzes the first step in β-oxidation. Organic and amino acid profiles of starved PPARα−/− mice suggested compromised citric acid cycle flux, enhanced urea cycle activity, and increased amino acid catabolism. PPARα−/− mice had 40–50% lower plasma and tissue levels of free carnitine, corresponding with diminished hepatic expression of genes involved in carnitine biosynthesis and transport. One week of oral carnitine supplementation conferred partial metabolic recovery in the PPARα−/− mice. In summary, comprehensive metabolic profiling revealed novel biomarkers of defective fat oxidation, while also highlighting the potential value of supplemental carnitine as a therapy and diagnostic tool for metabolic disorders.—Makowski, L., Noland, R. C., Koves, T. R., Xing, W., Ilkayeva, O. R., Muehlbauer, M. J., Stevens, R. D., Muoio, D. M. Metabolic profiling of PPARα−/− mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation.
Keywords: β-oxidation, metabolomics, liver, skeletal muscle, acylcarnitine, organic acid, starvation
Normal physiology is characterized by periodic fluctuations in fuel supply and demand. The ability of mammalian organisms to cope with these episodes depends on finely orchestrated metabolic adjustments in tissues, such as the liver and skeletal muscle, which harbor an intrinsic capacity to switch between energy substrates based on their availability. Classic examples of physiological stresses that elicit robust shifts in substrate selection include overnight fasting and sustained exercise, during which coordinated increases in lipolysis, fat oxidation, and ketogenesis serve to prevent hypoglycemia and spare limited glucose reserves for the central nervous system. Conversely, consumption of a carbohydrate-rich meal elevates blood glucose levels while inhibiting lipolysis. Under these circumstances, glucose is plentiful and maintenance of normoglycemia relies on pancreatic secretion of insulin and its subsequent action to promote glucose uptake and utilization (1).
Emerging evidence suggests that impaired substrate switching, known as metabolic inflexibility, might contribute to the development of metabolic disease (2, 3). For example, Kelley et al. (4) reported that fat oxidation in obese and type 2 diabetic compared to lean subjects is greater during a hyperinsulinemic, euglycemic clamp (simulating the fed state) but depressed in the fasted state. Several other groups have demonstrated that the increase in fatty acid oxidation that normally accompanies fasting, exercise, or β-adrenergic stimulation is diminished in obese and/or diabetic subjects (5). Similarly, we recently reported that overnutrition leads to aberrant substrate selection in rodents. In this study, skeletal muscle from obese rodents fed a high-fat diet failed to increase glucose oxidation in the postprandial state, whereas the liver failed to increase β-oxidation in the postabsorptive state (6). Taken together, these findings suggest that healthy subjects appropriately adjust fat oxidation in response to nutrient supply, whereas obese and insulin-resistant subjects do not. Notably, our current understanding of metabolic disease is based largely on studies in which outcome parameters were measured in either the fed or fasted state, whereas the foregoing reports clearly underscore the value of capturing the transition between the two conditions.
In the present investigation, we sought to gain a comprehensive metabolic view of the fed-to-fasted transition, both in the context of normal physiology and in a state of compromised lipid catabolism. To this end, we used a targeted metabolic profiling approach to analyze wild-type mice and those harboring a null mutation in peroxisome proliferator-activated receptor (PPAR) α, a nuclear receptor that is most abundant in liver and plays a central role in the starvation response. PPARα−/− mice are viable and apparently healthy until exposed to physiological stress. On activation by lipid ligands, PPARα induces a global transcriptional program that promotes β-oxidation, ketogenesis, and gluconeogenesis (7,8,9). Because this response is largely absent in PPARα−/− mice, these animals become hypoketonemic and hypoglycemic in response to a fasting challenge, despite marked elevations in circulating nonesterified free fatty acids (NEFAs) (10,11,12,13). Although PPARα−/− mice have been characterized extensively by genome-wide expression arrays, the metabolite milieu downstream of their genomic perturbations is less understood.
Herein, we demonstrate that fasting-induced up-regulation of fatty acid oxidation in wild-type mice promotes a generalized state of metabolic homeostasis by preserving normoglycemia and defending against excessive amino acid catabolism. Conversely, an impaired shift to β-oxidation in PPARα−/− mice was accompanied by severe metabolic perturbations, including hypoglycemia, skeletal muscle depletion of tricarboxylic acid (TCA) cycle intermediates, reduced tissue levels of short-chain acylcarnitine intermediates, tissue accumulation of long-chain acyl-coenzyme As (CoAs), increased amino acid catabolism, and a corresponding fall in plasma amino acids. Our analyses also revealed a striking carnitine deficiency, apparently stemming from both direct and indirect effects of PPARα inactivity. Carnitine is a conditionally essential nutrient necessary for mitochondrial import and export of acyl-CoAs (14). Interestingly, short-term carnitine supplementation ameliorated some of the aforementioned metabolic abnormalities in PPARα−/− mice. These findings provide new insights into the potential adverse consequences of metabolic inflexibility. Our results also highlight the predictive power of comprehensive metabolic profiling, not only as a tool to diagnose primary metabolic lesions but also to identify secondary complications and prospective therapeutic avenues.
MATERIALS AND METHODS
Animals
Male 129S1/SvlmJ wild-type (Jaxlab 002448) and PPARα knockout (Ppara-tm1Gonz/J, Jaxlab 003580) mice were obtained from the colony established by Dr. Barbara Abbott at the Environmental Protection Agency (Research Triangle Park, NC, USA). These animals were originally obtained from Jackson Laboratories (Bar Harbor, ME, USA). All male mice were age-matched at 15–16 wk for the fed/fasted study (Table 1). For the carnitine supplementation study, all male mice were age-matched at 13–16.5 wk (see Supplemental Table 4).
TABLE 1.
Physiological variables in PPARα−/− and PPARα+/+ mice in the fed and fasted states
| Variable | PPARα+/+
|
PPARα−/−
|
||
|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |
| Age (wk) | 15.3 ± 0.6 | 15.1 ± 0.6 | 16.2 ± 0.1 | 16.2 ± 0.1 |
| Weight (g) | 31.7 ± 1.2 | 25.2 ± 0.8 | 28.2 ± 0.8 | 26.3 ± 0.8 |
| Glucose (mg/dl) | 141.1 ± 5.9 | 75.8 ± 2.4 | 133.6 ± 2.4 | 56.7 ± 2.1 |
| Insulin (ng/ml) | 0.593 ± 0.222 | 0.193 ± 0.075 | 0.412 ± 0.061 | 0.362 ± 0.059 |
| Insulin:glucose ratio | 4.2 ± 1.6 | 2.2 ± 0.9 | 3.1 ± 0.5 | 6.4 ± 1.0 |
| NEFA (mM) | 0.231 ± 0.032 | 0.469 ± 0.040 | 0.185 ± 0.022 | 1.145 ± 0.049 |
| Total ketones (μM) | 266.4 ± 19.2 | 1514.0 ± 111.0 | 225.4 ± 29.4 | 564.7 ± 35.1 |
Values are expressed as means ± se.
Mice were supplemented for 7 days with pH-neutralized drinking water containing 0 or 1 mg/ml l-carnitine. This regimen yielded an approximate dose of 150 mg/kg/day at an estimated consumption of 3.75 ml/day. The observed elevations in plasma carnitine are consistent with published values (15). Mice were housed in ventilated Tecniplast cages (Tecniplast, Exton, PA, USA) and provided pellet chow (LabDiet 5001; PMI Nutrition International, Brentwood, MO, USA) and tap water ad libitum. Animal facilities were controlled for temperature (20–24°C) and relative humidity (40–60%), and they were kept under a 12-h light-dark cycle. All animal studies were conducted in accordance with Duke University Institutional Animal Care and Use Committee with guidelines established by the U.S. Environmental Protection Agency Office of Research and Development/National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee. Procedures and facilities were consistent with the recommendations of the 1996 National Research Council “Guide for the Care and Use of Laboratory Animals”, the Animal Welfare Act, and Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Mice were anesthetized with Nembutal solution (80 mg/kg) prior to tissue isolation. Food was removed either 3 or 18 h before sacrifice for the fed and fasted conditions, respectively. Urine was isolated from bladder at time of sacrifice. Gastrocnemius muscle and liver tissues were isolated, clamped, and snap-frozen in liquid nitrogen for later analysis. Cardiac puncture was used to isolate blood, which was supplemented with 1.7 mg/ml EDTA (3 μM) and spun at 4000 rpm for 15 min at 4°C.
Metabolic profiling
Blood glucose concentrations were determined on 5 μl whole blood by using gluco-analyzer blood glucose strips (Medisense, Bedford, MA, USA). Ketones and NEFAs in plasma were measured enzymatically (Wako, Richmond, VA, USA) using the Hitachi 911 clinical chemistry analyzer (Hitachi, Tokyo, Japan). For all metabolites except acyl-CoAs, the tissue was ground in a liquid nitrogen chilled mortar and pestle, and ∼50 mg was suspended in water, homogenized on ice, sonicated, and finally spun 15 min at 4°C, 14,000 rpm (16). Data are normalized to milligrams of protein in sample, as determined by BCA protein assay (Sigma). Long-chain fatty acyl-CoAs were extracted from muscle and liver using methods previously described and normalized to grams of tissue (6). Methods for sample preparation are as described previously (17). Briefly, measurement of free carnitine, acylcarnitines and amino acids in plasma, muscle, liver, and urine was completed by direct-injection electrospray tandem mass spectrometry (MS/MS), using a Micromass Quattro Micro LC-MS system (Waters-Micromass, Milford, MA, USA) equipped with a model HTS-PAL 2777 auto sampler (Leap Technologies, Carrboro, NC, USA), a model 1525 HPLC solvent delivery system (Agilent Technologies, Palo Alto, CA, USA) and a data system running MassLynx 4.0 software (Waters Corporation, Milford, MA) at the Stedman Center Mass Spectrometry Lab. Organic acids in muscle and liver were quantified using methods described previously employing Trace Ultra GC coupled to a Trace DSQ MS operating under Excalibur 1.4 (Thermo Fisher Scientific, Austin, TX, USA) (18).
mRNA quantification
Total RNA was prepared with the RNeasy Tissue Mini Kit (Qiagen, Valencia, CA, USA), quantified using RiboGreen (Molecular Probes, Eugene, OR, USA), and cDNA was synthesized using IScript (Bio-Rad, Hercules, CA, USA). Real-time quantitative (q)RT-PCR was completed using the ABI Prism 7000 Sequence Detection System instrument, software and TaqMan chemistry reagents (Applied Biosystems, Foster City, CA, USA). Sequence-specific primers and probes were purchased as predesigned/prevalidated FAM-labeled Assays-on-Demand (Applied Biosystems). Ct values were normalized using values from a duplexed reaction with VIC-labeled 18S endogenous control probes (Applied Biosystems).
Statistics
Data are shown as means ± se. Univariate analyses were performed using two-way analysis of variance (ANOVA) at a minimum P < 0.05 threshold or two-tailed Student’s t test when appropriate using SAS 9.1.3 (SAS Institute, Cary, NC, USA). A complete summary of the ANOVA is presented in Supplemental Table 2. We also performed principal component analysis (PCA) using SAS PROC PRINCOMP to reduce the dimensionality of the metabolite data. PCA is a common mathematical technique to identify patterns in a complex data set. The analysis transforms a number of correlated variables into a smaller number of uncorrelated variables called principal components, thereby identifying new variables that are linear combinations of the original variables. The first principal component (factor) accounts for as much of the variability in the data as possible, and each succeeding component accounts for as much of the remaining variability as possible. PCA analysis was performed on all metabolites across genotype and fed/fasting states. Supplemental Table 3 reports statistically significant principle components. In addition, PCA was performed separately for metabolite data obtained from PPARα+/+ and PPARα−/− animals (Table 2). Principal components (PRINC) with an eigenvalue >1 were retained. MANOVAs were used to evaluate genotype and nutritional condition (fed vs. fasted) and genotype × condition interactions. For those PRINCs in which a significant (P<0.05) difference between fed and fasted states was found, correlations were performed between PRINCs and each metabolite concentration. Significant (P<0.05) correlations were used to identify constituent metabolites. The heat map in Fig. 5A was generated using a log2 transformed ratio of PPARα−/− relative to PPARα+/+ data in Java treeview and Cluster. JMP software version 6 (SAS Institute) was used to generate heat maps of the Pearson correlation matrix of all pairwise comparisons among individual metabolites after supervised clustering based on their biochemical class.
TABLE 2.
Starvation-responsive principal components and constituent metabolites
| PPARα+/+
|
PPARα−/−
|
||
|---|---|---|---|
| Metabolite | Loading factor | Metabolite | Loading factor |
| Plasma | |||
| C10:3a | −0.124 | Arginineb | −0.193 |
| Arginineb | 0.123 | Tyrosinea | −0.185 |
| C10:1a | 0.124 | Methioninea | −0.171 |
| C14-OH/C12-DC | 0.152 | Glycinea | −0.169 |
| C12-OH/C10-DC | 0.161 | Alaninea | −0.165 |
| C6-DCb | 0.164 | C3a | −0.161 |
| Phenylalanine | 0.175 | C6-DCb | −0.148 |
| C12 | 0.175 | Prolinea | −0.127 |
| Valinea | 0.177 | Glutamate/glutaminea | −0.120 |
| Acetoacetate | 0.179 | Aspartatea | −0.103 |
| C16-OH | 0.183 | C10a | −0.102 |
| C18:1-OH | 0.185 | Phenylalanine | 0.145 |
| C14:2 | 0.196 | C2 | 0.152 |
| C2 | 0.203 | C18-DC/C20-OHa | 0.156 |
| C8-DCa | 0.204 | Leucine/isoleucine | 0.161 |
| Leucine/isoleucine | 0.207 | C12-OH/C10-DC | 0.174 |
| C18 | 0.219 | Acetoacetate | 0.175 |
| Ketones | 0.221 | C18-OHa | 0.179 |
| β-Hydroxybutyrate | 0.221 | C14-OH/C12-DC | 0.180 |
| C16 | 0.222 | C14:2 | 0.186 |
| C14 | 0.228 | β-Hydroxybutyrate | 0.186 |
| C18:2 | 0.229 | C12 | 0.186 |
| C14:1 | 0.229 | Ketones | 0.188 |
| C18:1 | 0.230 | C18 | 0.189 |
| F test | 0.0001 | C14:1 | 0.193 |
| C16-OH | 0.193 | ||
| C18:1-OH | 0.195 | ||
| C18:1 | 0.198 | ||
| C16 | 0.201 | ||
| C14 | 0.201 | ||
| C18:2 | 0.205 | ||
| F test | 0.0001 | ||
| Liver | |||
| C8a | −0.224 | C16a | −0.216 |
| C6-DCb | −0.223 | Ornithine | −0.215 |
| Ornithine | −0.211 | Citrulline | −0.203 |
| C8-DCb | −0.191 | C2 | −0.165 |
| C12-OH/C10-DCa | −0.189 | Histidinea | −0.162 |
| Citrulline | −0.186 | C14 | −0.156 |
| Tyrosinea | −0.174 | C18a | −0.149 |
| Aspartateb | −0.144 | Prolinea | −0.144 |
| C14 | −0.140 | Phenylalaninea | −0.143 |
| C2 | −0.130 | C18:1b | −0.142 |
| C18:2a | 0.144 | Leucine/isoleucinea | −0.142 |
| C18:1b | 0.148 | C16-OHa | −0.139 |
| Citrate | 0.165 | C3a | −0.126 |
| C22 | 0.180 | Methioninea | −0.110 |
| C10:2 | 0.183 | C10:2 | 0.120 |
| C5-OH/C3-DC | 0.198 | C4-DCa | 0.122 |
| Lactate | 0.198 | C12a | 0.124 |
| C18:1-OH | 0.205 | C22 | 0.124 |
| Alanine | 0.211 | C8:1a | 0.126 |
| Succinate | 0.213 | C5-OH/C3-DC | 0.137 |
| C10:1 | 0.213 | Aspartateb | 0.140 |
| Fumarate | 0.217 | Fumarate | 0.142 |
| Malate | 0.219 | C10a | 0.144 |
| F test | 0.001 | C18:1-OH | 0.144 |
| Malate | 0.146 | ||
| Citrate | 0.147 | ||
| C8-DCb | 0.156 | ||
| α-Ketoglutaratea | 0.163 | ||
| Glycinea | 0.164 | ||
| Lactate | 0.167 | ||
| Glutamate/glutaminea | 0.180 | ||
| C5-DCa | 0.193 | ||
| C10:1 | 0.203 | ||
| Alanine | 0.210 | ||
| Succinate | 0.219 | ||
| C6-DCb | 0.231 | ||
| F test | 0.001 | ||
| Muscle | |||
| Serine | −0.173 | Serine | −0.179 |
| Citrate | −0.170 | Citrullinea | −0.176 |
| Alanine | −0.145 | Ornithinea | −0.175 |
| C5:1a | −0.137 | C2a | −0.171 |
| C6-DCa | 0.134 | Alanine | −0.170 |
| C12-OH/C10-DCa | 0.138 | C4-DCa | −0.168 |
| Fumarateb | 0.145 | Glutamate/glutaminea | −0.158 |
| Valine | 0.163 | α-Ketoglutaratea | −0.149 |
| C8-DC | 0.164 | Succinatea | −0.137 |
| C8 | 0.165 | Tyrosinea | −0.134 |
| C18:2 | 0.170 | Fumarateb | −0.130 |
| Arginine | 0.171 | C5-DCa | −0.119 |
| C16-OHa | 0.176 | Citrate | −0.118 |
| C14-OH/C12-DCa | 0.194 | Malatea | −0.115 |
| C18:1 | 0.204 | C5-OH/C3-DCa | −0.109 |
| C18:1-OH | 0.205 | Lactatea | −0.108 |
| C4-OHa | 0.210 | C8:1 | 0.120 |
| C18 | 0.211 | Arginine | 0.124 |
| C4 | 0.216 | C10:1a | 0.143 |
| C16 | 0.218 | C18a | 0.143 |
| C14:1 | 0.228 | Valine | 0.144 |
| C6 | 0.236 | Prolinea | 0.144 |
| C14 | 0.244 | C8 | 0.153 |
| C14:2a | 0.155 | ||
| F test | 0.001 | C12a | 0.156 |
| C8-DC | 0.160 | ||
| Leucine/isoleucinea | 0.165 | ||
| C4 | 0.171 | ||
| C6 | 0.177 | ||
| C16 | 0.180 | ||
| Phenylalaninea | 0.183 | ||
| C14 | 0.186 | ||
| C5a | 0.192 | ||
| C14:1 | 0.196 | ||
| C18:1 | 0.203 | ||
| C18:1-OH | 0.203 | ||
| C18:2 | 0.210 | ||
| F test | 0.0001 | ||
PCA was performed separately for each genotype. A single starvation-responsive component was identified by ANOVA (F test 0 < 0.05) for each compartment. Constituent metabolites and their individual loading factors are listed.
Metabolites that are uniquely present in PPARα−/− compared to wild-type mice.
Metabolites in which the factor loading changed directions.
Figure 1.
Acylcarnitine profiles in PPARα+/+ and PPARα−/− mice. Representative subsets of individual acylcarnitines measured in plasma (a), liver (b), and gastrocnemius muscle (c) harvested in the ad libitum-fed or 18-h-fasted state from PPARα+/+ (n=8 fed, n=8 fasted) and PPARα−/− (n=10 fed, n=10 fasted) mice. Data are presented as means ± se. Main effects of starvation and genotype, as well as genotype × condition interactions, were detected by two-way ANOVA, but symbols were excluded for simplicity. Complete acylcarnitine profiles, and results of the statistical analysis are available in Supplemental Tables 1 and 2, respectively.
Figure 2.
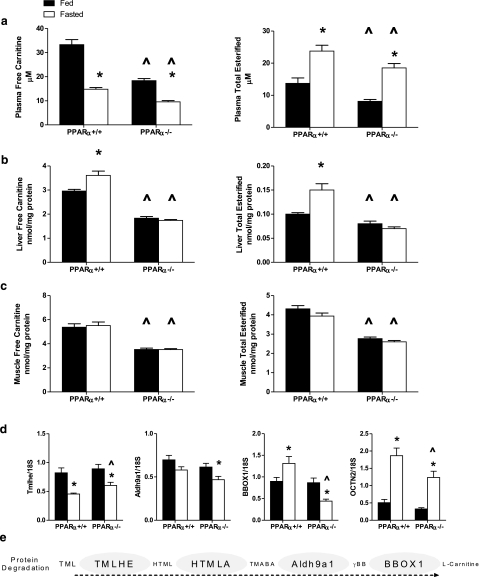
Carnitine metabolism in PPARα+/+ and PPARα−/− mice. a–c) Free carnitine and total carnitine esters were measured in plasma (a), liver (b), and gastrocnemius muscle (c) harvested in the ad libitum-fed or 18-h-fasted state from PPARα+/+ (n=8 fed, n=8 fasted) and PPARα−/− (n=10 fed, n=10 fasted) mice. d) Expression of genes involved in liver carnitine metabolism was determined by qRT-PCR: trimethyllysine hydroxylase, epsilon (TMLHE), aldehyde dehydrogenase 9 family, member A1 (Aldh9a1), gamma-butyrobetaine hydroxylase 1 (BBOX1), and carnitine/organic cation transporter (OCTN2). Data are presented as means ± se. Main effects of starvation and genotype were detected by two-way ANOVA or Student’s t test; *P < 0.05 vs. fed state, ^P < 0.05 vs. PPARα+/+ genotype. e) Pathway of carnitine biosynthesis: protein degradation generates 6-N-trimethyllysine (TML), which is hydroxylated by TMLHE to form 3-hydroxy-6-N-trimethyllysine (HTML). HTML-aldolase (HTMLA) produces 4-trimethylaminobutyraldehyde (TMABA), which is then oxidized to gamma-butyrobetaine (γBB) by Aldh9a1. Finally, BBOX1 hydroxylates γ-BB to form l-carnitine.
Figure 3.
Organic acid profiles in PPARα+/+ and PPARα−/− mice. Organic acids were measured in liver (a) and gastrocnemius muscle (b) harvested in the ad libitum-fed or 18-h-fasted state from PPARα+/+ (n=8 fed, n=8 fasted) and PPARα−/− (n=10 fed, n=10 fasted) mice. Data represent means ± se. Main effects of starvation and genotype, as well as genotype × condition interactions, were detected by two-way ANOVA, but symbols were excluded for simplicity. Complete results of the statistical analysis are available in Supplemental Table 2. αKG, alpha ketoglutarate.
Figure 4.
Amino acid profiles in PPARα+/+ and PPARα−/− mice. a–c) Amino acids were measured in plasma (a), liver (b), and gastrocnemius muscle (c) harvested in the ad libitum-fed or 18-h-fasted state from PPARα+/+ (n=8 fed, n=8 fasted) and PPARα−/− (n=10 fed, n=10 fasted) mice. Data are presented as means ± se. Alanine (ala), glutamate/glutamine (glu), aspartate/asparagine (asp), arginine (arg), citrulline (cit), leucine/isoleucine (leu/ile), and tyrosine (tyr) are reported. Amino acids reported are involved in gluconeogenesis (arg, ala, glu, asp), ureagenesis (asp, arg, cit), or ketogenesis (leu/ile, tyr). Main effects of starvation and genotype, as well as genotype × condition interactions. were detected by two-way ANOVA, but symbols were excluded for simplicity. Complete amino acid profiles and statistical analysis are provided in Supplemental Tables 1 and 2. d) Expression of genes involved in amino acid transamination was measured by qRT-PCR: Isovaleryl-CoA dehydrogenase (IVD), cytosolic glutamate-oxaloacetate transaminase (GOT1); tyrosine aminotransferase (TAT). Main effects of starvation and genotype were detected by two-way ANOVA or Student’s t test; *P < 0.05 vs. fed state; ^P < 0.05 vs. PPARα+/+ genotype.
Figure 5.
Heat map analysis of metabolite levels in PPARα+/+ and PPARα−/− mice. a) Heat map summary of the genotype effect on fasting and fed state metabolite levels in plasma (plsm), liver (liv), and gastrocnemius muscle (skm) was generated using data from PPARα−/− mice expressed relative to wild-type animals in the same condition, such that metabolites elevated in PPARα−/− mice are red and depressed metabolites are green. Plasma organic acid profiles were not measured. Heat map representations of the pairwise correlation matrix were generated using plasma metabolites and muscle organic acids measured in the fed and fasted states in PPARα+/+ (b) and PPARα−/− (c) mice. Each square represents the Pearson correlation coefficient between the metabolite of the column with that of the row. Metabolite order was determined by supervised clustering based on biochemical classification. This analysis revealed genotype-specific patterning of several hot spots, indicative of strong positive (red) or negative (blue) correlations. AC, acylcarnitines; OA, organic acid/TCA cycle intermediates; carn, carnitine; aKG, alpha ketoglutarate; NEFA, nonesterified fatty acids; ala, alanine; glu, glutamate/glutamine; gly, glycine; his, histidine; pro, proline; met, methionine; ser, serine; val, valine; asp, aspartate/asparagine; arg, arginine; cit, citrulline; orn, ornitine; leu/ile, leucine/isoleucine; phe, phenylalanine; tyr, tyrosine; sum pls LCAC, sum of plasma long chain acylcarnitines from C14 to C22.
RESULTS
Physiological variables and conventional plasma metabolites are reported in Table 1. In the fed state, most metabolic parameters including glucose, NEFAs, and total ketones were similar between genotypes. Consistent with previous reports, fasting glucose and ketone levels were lower, whereas plasma NEFAs were markedly elevated in PPARα−/− compared to PPARα+/+ mice. Plasma insulin levels and the insulin:glucose ratio in PPARα−/− mice trended lower in the fed state but was increased relative to wild-type mice in the fasted state. Thus, the absence of PPARα caused an inappropriate rise in insulin secretion during fasting-induced hypoglycemia. Interestingly, a similar response has been observed in other models of impaired β-oxidation (19).
Metabolic profiling analyses
Figures 1234 show a subset of the metabolic profiling results. A complete summary of the data is provided in Fig. 5 and Supplemental Tables 1 and 2.
Acylcarnitine metabolites reveal changes in fuel selection
Previous studies have shown that PPARα−/− mice have impaired β-oxidation as assessed by transcriptomics and in vitro oxidation assays (13, 20). We sought to determine whether this primary defect in lipid catabolism could be further elucidated by analyzing β-oxidative intermediates in plasma and tissues. To this end, we analyzed 37 independent acyl-carnitine species ranging in size from 2 to 22 carbons. Acylcarnitine esters are formed from their respective acyl-CoA intermediates by a family of carnitine acyltransferases that reside in subcellular organelles (primarily mitochondria), where they catalyze the exchange of CoA for carnitine. Even chain species ranging from C6 to C22 reflect incomplete fatty acid oxidation. Odd chain species, such as propionylcarnitine (C3) and isovalerylcarnitine (C5), derive primarily from amino acid catabolism, whereas butyrylcarnitine (C4) can be derived from both fatty acids and amino acids. Acetylcarnitine (C2), which is used as a proxy measure of acetyl-CoA, can arise from catabolism of fatty acids, amino acids, or glucose (21).
In the fed state, most fatty acid-derived acylcarnitine species were present at similar levels in wild-type compared to the knockout mice (Supplemental Table 1). Notable exceptions included acetylcarnitine (C2) and β-OH-butyrylcarnitine (C4OH, a strong marker of β-oxidation and ketone metabolism), both of which were markedly decreased in plasma, liver, and skeletal muscle of PPARα−/− mice regardless of feeding status (Fig. 1). Succinylcarnitine (C4DC), which arises from the TCA cycle intermediate succinyl-CoA, was reduced in plasma and liver of PPARα−/− mice, independent of condition. Skeletal muscle concentrations of this metabolite were also low in PPARα−/− compared to wild-type mice, but only in the fasted state.
In general, more dramatic and wide-ranging differences between genotypes emerged after an overnight fast. For example, in wild-type mice, fasting-induced lipolysis was accompanied by increased plasma levels of several long-chain acylcarnitine species, including C16, C18:1, and C18:2 (Fig. 1a). These same metabolites were elevated an additional 2- to 3-fold in PPARα−/− mice. Several of the hydroxylated acylcarnitine intermediates followed a similar pattern (Supplemental Table 1). Likewise, in the liver of PPARα−/− compared to wild-type mice, fasting levels of C16 and C18:1 were elevated, but medium-chain intermediates such as C8 (Fig. 1b), C10, and C12 (Supplemental Table 1) were present at lower levels. Skeletal muscle levels of C16, C18:1, and C18:2 were also elevated in fasted PPARα−/− mice, whereas the medium- and shorter-chain species were either unchanged or decreased relative to their wild-type counterparts (Fig. 1c; Supplemental Table 1). This reciprocal long- to medium/short-chain acylcarnitine profile typically reflects a metabolic block that resides distal to carnitine palmitoyltransferase 1 (CPT1), the enzyme that synthesizes long-chain acylcarnitines and that functions as the first committed step in β-oxidation. When long-chain products of this enzyme cannot be processed efficiently they accumulate within tissues and can be subsequently exported into the general circulation (22).
Also striking was the marked skeletal muscle accumulation of isovalerylcarnitine (C5), a byproduct of leucine catabolism. Whereas fasting did not alter C5 content in skeletal muscle of wild-type mice, this metabolite increased 5-fold in PPARα−/− animals (Fig. 1c). Pairwise correlation analysis revealed a strong positive relation between skeletal muscle C5 levels and plasma leucine/isoleucine (r=0.793, P<0.0001; Supplemental Fig. 1a), but only in PPARα−/− animals. Surprisingly, C5 levels were not elevated in the plasma of PPARα−/− mice, suggesting that tissue efflux of this specific metabolite might be limited for reasons that are yet unclear.
In addition to the individual carnitine esters, we also examined free carnitine and total carnitine esters (Fig. 2a–c). In comparison to wild-type animals, free carnitine levels were dramatically reduced in plasma, muscle, and liver of PPARα−/− mice; this was apparent in both the fed and fasted states. In both wild-type and PPARα−/− mice, the fasting-induced rise in plasma acylcarnitines corresponded with a fall in circulating levels of free carnitine (Fig. 2a). Wild-type mice compensated by increasing hepatic carnitine stores, whereas this regulation was lost in the knockout mice (Fig. 2b). Similarly, fasting increased the total acylcarnitine pool in liver of PPARα+/+ but not PPARα−/− mice (Fig. 2b). Muscle levels of free and total carnitine esters were unaffected by fasting but were markedly lower in PPARα−/− compared to wild-type mice regardless of feeding status (Fig. 2c). Thus, in aggregate, the acylcarnitine profiles of the PPARα−/− mice reflected impaired fatty acid and ketone catabolism (downstream of CPT1) and a compensatory increase in skeletal muscle leucine catabolism. The results also hinted of a primary defect in carnitine metabolism.
Loss of PPARα results in impaired carnitine homeostasis
l-Carnitine (beta-hydroxy-gamma-trimethylammonium butyrate) is a water-soluble quaternary amine that can be obtained from dietary sources and/or synthesized endogenously from trimethylated lysine residues derived from protein degradation (Fig. 2e) (14). Because the liver is the principal site of carnitine biosynthesis, we proceeded to examine hepatic expression of several carnitine regulatory genes (Fig. 2d). Surprisingly, overnight fasting decreased mRNA abundance of trimethyllysine hydroxylase, epsilon (Tmlhe) and aldehyde dehydrogenase 9 family, and member A1, (Aldh9a1) in both genotypes. Most remarkable, gene expression of gamma-butyrobetaine hydroxylase 1 (Bbox1), a liver-specific enzyme that catalyzes the final and rate-limiting step in carnitine synthesis, was induced 30% on fasting in PPARα+/+ mice but suppressed 65% in PPARα−/− mice. Moreover, in both the fed and fasted states, expression of the carnitine/organic cation transporter (OCTN2 or SLC22A5) was reduced by 40% in PPARα−/− compared to PPARα+/+ mice.
Loss of PPARα results in depletion of skeletal muscle TCA cycle intermediates
We next profiled organic acid metabolites to gain insight into changes in glycolysis, the TCA cycle, and urea cycle activity. In liver of wild-type mice, the fasting-induced shift from glycolysis to gluconeogenesis was accompanied by a decrease in each of the organic acids measured, with the exception of α-ketoglutarate (Fig. 3a). In the absence of PPARα, hepatic citrate levels decreased, whereas fumarate and malate increased; these changes were detected as a main effect of genotype (Supplemental Table 2). A striking interaction between genotype and feeding condition was observed for α-ketoglutarate, which fell robustly in liver of starved PPARα−/− mice (Fig. 3a; Supplemental Table 2). The organic acid profile in the knockout animals is consistent with increased amino acid catabolism, as α-ketoglutarate functions as an amino group acceptor in multiple transamination reactions, whereas fumarate and malate are produced as intermediates of the urea cycle.
In skeletal muscles of wild-type mice, lactate and citrate levels fell modestly during an overnight fast, whereas concentrations of other organic acid metabolites remained stable (Fig. 3b). Loss of PPARα resulted in reduced muscle levels of most organic acids. This was evident in the fed state and exacerbated by fasting, which caused a further drop in α-ketoglutarate, succinate, and fumarate, along with marginal declines in citrate and malate. Previous studies have shown that the muscle TCA cycle intermediate pool responds to changes in glucose availability and/or glycolytic activity (6, 23). In agreement with these reports, fasted-state skeletal muscle content of α-ketoglutarate correlated strongly with blood glucose levels (r=0.85, P=<0.0001; Supplemental Fig. 1b). Thus, the decrease in TCA cycle intermediates in muscle of the knockout mice might reflect a secondary consequence of hypoglycemia (Table 1) and/or glycogen depletion (24).
Loss of PPARα results in systemic lowering of amino acids
In Fig. 4 and Supplemental Table 1, amino acid levels in plasma, liver, and muscle were clustered according to their roles in gluconeogenesis, ureagenesis, and ketogenesis (Fig. 4a–c), although it is noteworthy that many metabolites have overlapping functions. Clear distinctions between genotypes were evident in all three pools. In plasma of wild-type mice, fasting decreased alanine and aspartate and increased the branched chain amino acids (leucine, isoleucine, and valine), whereas most other amino acids remained relatively stable (Fig. 4a; Supplemental Table 1). By contrast, in PPARα−/− mice, fasting caused a dramatic change in 10 of the 15 plasma analytes (Supplemental Table 1; Fig. 4a). Most notable were robust decreases in the gluconeogenic amino acid, alanine, and the ketogenic amino acid, tyrosine (Fig. 4a). In the liver, fasting led to a marked decrease in alanine, regardless of genotype, whereas glutamate/glutamine decreased only in the knockout mice. Amino acids linked to the urea cycle (aspartate, arginine, and citrulline) were elevated in PPARα−/− compared to wild-type mice (detected as a main effect of genotype) (Fig. 4b; Supplemental Table 2). In skeletal muscle 7 of the 15 amino acids measured were decreased in the knockout compared to wild-type mice under fasting conditions (Fig. 4c; Supplemental Table 1), possibly reflecting heavier reliance on these substrates as a mitochondrial fuel.
Taken together, the data in Figs. 1234 suggest that impaired β-oxidation in PPARα−/− mice leads to a compensatory increase in amino acid catabolism. Predictably, this phenotype was most pronounced during fasting conditions when blood glucose fell to abnormally low levels in PPARα−/− mice. To further validate this interpretation, we assessed transcriptional regulation of a select set of genes involved in amino acid degradation. On the basis of the pronounced C5 peak in Fig. 1c, we first evaluated skeletal muscle expression of isovaleryl-CoA dehydrogenase (IVD) and HMG-CoA lyase (HMGCL), mitochondrial enzymes required for leucine catabolism to acetyl-CoA. Consistent with the metabolite profiles, a starvation-induced increase in IVD expression was observed in the PPARα null mice but not in the wild-type controls. HMGCL was unaffected by genotype (unpublished observations). We next examined hepatic transamination capacity by measuring mRNA levels of the cytosolic and mitochondrial isoforms of glutamate-oxaloacetate transaminase (GOT1 and GOT2, respectively) and tyrosine aminotransferase (TAT). In wild-type mice, expression of these genes was unaffected by fasting, implying a relatively low drive for amino acid catabolism. Conversely, in PPARα−/− mice, overnight fasting increased hepatic mRNA levels of GOT1 and TAT 3.2- and 4.4-fold, respectively, whereas expression of the mitochondrial GOT2 mRNA was unchanged (unpublished results). These results fit with the known roles of the GOT1 and TAT reactions in providing amino acid-derived carbon backbones for gluconeogenesis and ketogenesis, respectively, and might explain the marked fasting-induced depletion of α-ketoglutarate and tyrosine in PPARα−/− mice.
Heat map summaries of metabolite profiles
To gain a more global overview of how genotype affects whole body metabolic regulation, we generated heat maps of both the fed and fasted states using metabolite levels in PPARα−/− mice expressed as a ratio relative to those measured in their wild-type counterparts (Fig. 5a). The color intensities of the map show that the genotype effect was most robust in the fasted state. In general, PPARα−/− mice accumulated long-chain acylcarnitines in plasma and had elevated amino acid concentrations in liver, whereas levels of organic acids and amino acids were decreased in muscle and plasma, respectively. This representation of the data highlights instances of agreement and discordance between metabolite levels in distinct compartments. For example, C4OH was markedly lower in all three compartments of PPAR−/− mice, whereas isovalerylcarnitine was robustly elevated in muscle, but reduced in plasma and liver.
In Fig. 5b (PPARα+/+) and 5c (PPARα−/−), we generated heat maps of the correlation matrix of all pairwise comparisons of plasma metabolites and muscle organic acids in both fed and fasted states. In this analysis, supervised clustering revealed several hot spots of highly correlated metabolites in PPARα null mice that were either weaker or not present in wild-type animals. For example, in wild-type mice, the strongest relations occurred among the fatty acylcarnitines, which were positively correlated with one another and with plasma NEFAs and ketones. In PPARα−/− mice, a much different fingerprint emerged. Long-chain acylcarnitines were positively associated with several ketogenic amino acids and negatively associated with nonketogenic amino acids, muscle organic acids, and blood glucose. Muscle organic acids were positively correlated with one another, several plasma amino acids, and blood glucose. Taken together, these results reflect the metabolic stability of the wild-type mice, such that most metabolites were maintained within a relatively narrow dynamic range even on prolonged food deprivation. By contrast, metabolite levels in the PPARα−/− mice exhibited more dramatic fluctuations that appeared to be driven by fasting-induced hypoglycemia.
To better understand the unique sets of metabolites that defined the fed and fasted states for each genotype, we performed PCA for each compartment (plasma, liver, skeletal muscle). In Supplemental Table 3, PCA of the entire data set revealed several factors that were affected by genotype and/or a genotype/treatment interaction. Alternatively, Table 2 shows the results obtained when data from wild-type and knockout mice were analyzed separately. In each compartment and for both genotypes, a single and unique factor identified the fasting response. Table 2 lists the metabolite constituents and loading of these factors. Consistent with the univariate analyses, the PCA factors derived from PPARα−/− as compared to wild-type mice comprised a larger, more diverse set of metabolites. Notably, several amino and organic acids were uniquely present in the PCA of the PPARα−/− mice.
Carnitine therapy ameliorates the metabolic derangements in PPARα−/− mice
The foregoing pairwise correlation analyses revealed strong positive associations between plasma concentrations of several specific amino acids and plasma carnitine levels. These relations emerged from PPARα−/− but not PPARα+/+ animals. For example, in knockout mice, plasma carnitine levels predicted both methionine and tyrosine concentrations with Pearson correlation coefficients of 0.495 (P=0.0016) and 0.748 (P=<0.0001), respectively (Supplemental Fig. 1c, d). These findings implied that both β-oxidative capacity and amino acid homeostasis in the PPARα−/− mice might be rescued by carnitine repletion. To test this possibility, we administered oral carnitine supplementation for 1 wk; nonsupplemented control groups were run in parallel for comparison. At the end of the supplementation period, tissue and plasma specimens were harvested in the fasted state.
Carnitine supplementation did not affect body weight, blood glucose, NEFAs, ketones, or insulin in either PPARα+/+ or PPARα−/− mice (Supplemental Table 4). The supplementation regimen increased plasma levels of free carnitine ∼20–30% in both genotypes; however, the increase in total carnitine esters was more pronounced in the knockout mice (Fig. 6a). Contrary to our experimental objective, supplementation did not fully restore tissue levels of free carnitine in PPARα−/− mice (Fig. 6b, c). Likewise, tissue levels of total acylcarnitines remained substantially lower in PPARα−/− mice compared to controls. Notably, however, the robust increase in urinary carnitine provides evidence that the oral supplement was efficiently absorbed in both genotypes (Fig. 6d).
Figure 6.
Carnitine (carn) supplementation in PPARα+/+ and PPARα−/− mice. Free carnitine and total carnitine esters were measured in plasma (a), liver (b), gastrocnemius muscle (c), and urine (d) harvested in the fasted state from PPARα+/+ and PPARα−/− mice after consuming carnitine-supplemented or vehicle-treated (control) drinking water for 1 wk. Data are presented as means ± se from 6 animals per group. Main effects of carnitine supplementation were detected by Student’s t test; *P < 0.05 vs. unsupplemented group.
Whereas the intervention did not raise liver or muscle content of free carnitine, it did elicit marked increases in several carnitine esters. This effect was more robust in the PPARα-null genotype where supplementation raised plasma levels of short-, medium- and long-chain acylcarnitines, suggesting that the carnitine deficiency does impose some limit on whole body fat oxidation (Fig. 7a). In general, carnitine supplementation produced more robust metabolic changes in skeletal muscle than liver. For example, in the liver of PPARα−/− mice, supplementation caused marked elevations in C16 and C18:1 without affecting other long- and medium-chain intermediates that are generated as catabolic byproducts of β-oxidation. However, liver acylcarnitines that can arise from nonlipid substrates (C2, C3, C4, and C5) were restored to near normal levels (Fig. 7b; unpublished observations). By contrast, in skeletal muscle of the knockout animals, carnitine supplementation resulted in dramatic increases (up to 8-fold) in most long-chain and medium-chain species. Although this pattern implies some recovery of muscle fat oxidation, levels of C2 and C4OH remained low, suggesting that only a partial improvement was achieved. In addition, the marked peak in muscle C5 indicates continued abnormalities in leucine catabolism (Fig. 7c). Urinary acylcarnitine profiles were unremarkable in the control (unsupplemented) groups. After supplementation, however, wild-type mice had high urinary levels of most short-chain acylcarnitine species, whereas PPARα−/− mice exhibited massive increases in long-chain species (Fig. 7d).
Figure 7.
Acylcarnitine profiles after carnitine supplementation. Representative subsets of individual acylcarnitines measured in plasma (a), liver (b), gastrocnemius muscle (c), and urine (d) harvested in the fasted state from PPARα+/+ and PPARα−/− mice after consuming carnitine-supplemented or vehicle-treated (control) drinking water for 1 wk. Data are presented as means ± se from 6 animals per group. Main effects of supplementation were detected by two-way ANOVA, but symbols have been excluded for simplicity.
Because the acylcarnitine profiles hinted of improved lipid oxidation after carnitine supplementation, we proceeded to examine potential changes in tissue acyl-CoA concentrations (Supplemental Fig. 2). Some of the long-chain acyl-CoAs were modestly elevated in both liver and muscle of PPARα−/− mice, as compared to wild-type littermates. Carnitine supplementation tended to normalize levels of the C16 and C18:1 CoA species in liver but not muscle. Thus, in the liver, carnitine therapy appeared to alleviate lipid accumulation by promoting synthesis and efflux of palmitoyl- and oleylcarnitine (C16 and C18:1, respectively, Fig. 7b).
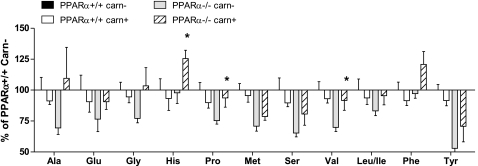
Carnitine supplementation did not impact the organic acid profiles of the PPARα−/− mice (unpublished observations). This was not surprising given that the supplementation regimen did not prevent fasting-induced hypoglycemia. Interestingly, however, the intervention did have a modest restorative effect on amino acid metabolism, such that plasma amino acid concentrations were generally higher in the PPARα−/−-supplemented group, as compared to the nonsupplemented controls (Fig. 8). Consistent with results of the first cohort of PPARα−/− mice, plasma concentrations of glycine, serine, tyrosine, proline, and alanine correlated positively with plasma carnitine levels (r>0.78, P>0.0001; unpublished observations).
Figure 8.
Effect of carnitine (carn) supplementation on plasma amino acids. Fasting amino acid concentrations were measured in plasma from PPARα+/+ and PPARα−/− mice after consuming carnitine-supplemented or vehicle-treated (control) drinking water for 1 wk. Data are presented as means ± se from 6 animals per group and are expressed relative to metabolite concentrations measure in unsupplemented (control) PPARα+/+ mice (set at 100%). Main effects of the carnitine supplementation were detected by Student’s t test and MANOVA. *P < 0.05 vs. unsupplemented.
DISCUSSION
Recent advances in “metabolomic” technologies afford a new opportunity to expand our understanding of how biological systems adapt to metabolic challenges, both in healthy and diseased states. In the present investigation, we used a targeted metabolic profiling approach to examine the impact of impaired β-oxidation on intermediary metabolism during the fed to fasted transition. In wild-type mice, steady-state levels of a broad range of intermediary metabolites remained relatively stable during an 18-h period of food withdrawal, with the predictable exception of fatty acids and their catabolic byproducts. A much different scenario occurred, however, when adaptive β-oxidation was genetically disallowed in PPARα−/− mice. In these mice, fasting-induced hypoglycemia was accompanied by marked depletion of the skeletal muscle TCA cycle intermediate pool. This was reflected not only in the organic acid profile but also by a drop in succinyl-carnitine (C4DC) levels. As a result, the PPARα−/− animals advanced quickly to a state of protein catabolism, evidenced by decreased levels of plasma amino acids, increased skeletal muscle production of isovalerylcarnitine and expression of IVD, elevated urea cycle intermediates in liver (citrulline, aspartate, arginine, fumarate, and malate), and increased hepatic mRNA expression of the aminotransferase enzymes TAT and GOT1 (Fig. 9). Amino acids are unique in that they can provide carbon backbones for the TCA cycle, gluconeogenesis, and ketogenesis. Typically, however, physiological adaptations to acute energy deprivation are designed to protect lean body mass, and only during prolonged fasting is protein degradation accelerated as a last resort to prevent hypoglycemia. The results presented herein, which are in general agreement with other recent metabolomic studies of the PPARα null mouse (25, 26), highlight the essential role of β-oxidation in limiting amino acid utilization, even during relatively brief periods of energy stress.
Figure 9.
Pathways of intermediary metabolism in liver and muscle. In the fed state, glucose-derived pyruvate serves as a principal source of acetyl-CoA. In the fasted state, pyruvate is conserved for alanine synthesis (muscle), anaplerotic entry into the TCA cycle (muscle), and gluconeogenesis (liver). Long-chain fatty acids, branched-chain amino acids, and ketones provide alternative sources of acetyl-CoA. Transport of fatty acids into the mitochondria requires CPT1-mediated conversion of cytosolic fatty acyl-CoAs to acylcarnitines, which are converted back to acyl-CoA on the matrix side of the inner mitochondrial membrane. Long-, medium- and short-chain acyl-CoA intermediates of fuel oxidation give rise to acylcarnitine metabolites [e.g., C16, C8, C5, C4OH, C4-DC, C2 (in green)] that are recycled or exported from the tissue. Use of amino acids as oxidative or gluconeogenic substrates requires aminotransferases such as GOT and TAT, which use αKG to generate glutamate, glutamine, and NH4+ as nitrogen precursors of liver ureagenesis (yellow box). Liver-specific pathways are denoted in blue. Dashed lines represent multistep reactions. Pathways: β-ox, β-oxidation; TCA, tricarboxylic acid cycle. Enzymes: CPT1, carnitine palmitoyltransferase 1; GOT1, cytosolic aspartate aminotransferase; GOT2, mitochondrial aspartate aminotransferase; IVD, isovaleryl-CoA dehydrogenase; TAT, tyrosine aminotransferase. Metabolites: aKG, alpha ketoglutarate; βHB, β-hydroxybutyrate; leu/isoleu, leucine/isoleucine; OAA, oxaloacetate; suc-CoA, succinyl coA.
In the context of this discussion, it is important to emphasize that steady-state metabolite concentrations in tissues and plasma represent the net balance between production, consumption, import, and export. In addition, many intermediates participate in multiple metabolic pathways. For these reasons, static measurements do not provide definitive information regarding substrate flux. Still, the comprehensive nature of our approach does enable us to infer metabolic activity at specific regulatory sites. Notably, the foregoing interpretation of our results is consistent with data derived from complementary methods such as isotopic tracer studies and transcriptomics. For example, a recent study used 2H/13C tracers and nuclear magnetic resonance spectroscopy to show that pharmacological activation of PPARα in rats caused robust increases in hepatic TCA cycle flux and gluconeogenesis, implying that loss of function might provoke the opposite effects (27). Similar studies seeking to understand the etiology of hypoglycemia in PPARα−/− mice found that these animals had elevated rates of peripheral glucose uptake and diminished hepatic lactate conversion to glucose, whereas glucose production from glycerol was increased (28, 29). Moreover, cDNA microarray analyses have shown that fasting activates a global transcriptional program that promotes amino acid catabolism and urea synthesis in liver of PPARα−/− mice, but not in their wild-type counterparts (30,31,32). Taken together, these findings support our conclusion that PPARα inactivity necessitates the use of amino acids as a requisite carbon source during periods of dietary carbohydrate restriction.
Still unknown is whether PPARα acts directly through a PPAR response element (PPRE) to suppress protein degradation. Alternatively, impaired β-oxidation and the corresponding fall in blood glucose levels might trigger a metabolic stress signal that initiates amino acid catabolism. An intriguing candidate for such signal is α-ketoglutarate, a critical metabolic intermediate that also plays a role in stress response via its regulation of the hypoxia-inducible factors (HIF) 1 and 2 (33, 34). These transcription factors can be activated by hypoxia, glucose deprivation, or cellular depletion of α-ketoglutarate, and, in turn, regulate a program of genes that compensate for oxygen deficit and/or other metabolic crises (35, 36). Thus, the low α-ketoglutarate levels in tissues of starved PPARα−/− mice might activate HIF or other similar metabolic sensors to elicit compensatory adjustments in amino acid metabolism.
Similar to the phenotype of the PPARα−/− mice, metabolic disorders such as obesity, type 2 diabetes and nonalcoholic fatty liver disease are associated with liver steatosis, low hepatic β-oxidative capacity, carnitine depletion, and impaired substrate switching in response to physiological stresses, such as fasting and exercise. Our observation that PPARα inactivity leads to increased amino acid catabolism leads us to reason that less severe defects in liver fat oxidation and/or carnitine homeostasis might likewise give rise to secondary complications in protein homeostasis. For example, sarcopenic obesity has been described as a form of muscle wasting that occurs with aging, obesity, and type 2 diabetes. This condition is characterized by central adiposity, insulin resistance, reduced muscle mass, and frailty (37,38,39,40,41). Carnitine depletion has also been shown to correlate negatively with muscle mass and function (42). Muscle atrophy occurring in the setting of central obesity has been linked to proinflammatory mediators such as TNF-α, interleukin-6 (IL-6), C-reactive protein, interleukin-1 receptor antagonist, and soluble IL-6 receptor (43, 44). Interestingly, IL-6 is decreased by PPARα activation and elevated in PPARα−/− mice (45,46,47,48). IL-6 is produced abundantly by skeletal muscle and has been shown to stimulate protein degradation, lipolysis, and gluconeogenesis (49,50,51). These findings have raised speculation that IL-6 functions as a catabolic messenger that mediates metabolic crosstalk between the liver, skeletal muscle, and adipose tissue. A clearer understanding of the role of IL-6 as a potential link between impaired fat oxidation and protein wasting now awaits further investigation.
One of the most striking features of the PPARα−/− mice was their marked depletion of whole body carnitine reserves. This deficiency corresponded with low hepatic expression of genes involved in carnitine synthesis (BBOX1) and transport (OCTN2). In addition, PPARα−/− mice had low levels of methionine and α-ketoglutarate in plasma and tissues, respectively, both of which contribute to carnitine biosynthesis. Methionine serves as a methyl donor during post-translational assembly of methylated proteins. Degradation of these proteins supplies the essential carnitine precursor, trimethyllysine, whereas α-ketoglutarate serves as a cofactor for both Tmlhe and BBOX1 (52). Thus, impaired carnitine synthesis in PPARα-null mice might stem from systemic depletion of requisite biosynthetic precursors and enzymatic cofactors as well as the aforementioned lesions in gene regulation. Previous studies have shown that carnitine regulatory genes are activated by fasting, fibrates, and other PPARα ligands (53,54,55,56,57). During preparation of the present article, Van Vlies et al. (58) likewise demonstrated that fasting-induced up-regulation of hepatic BBOX1 and OCTN2 expression requires PPARα. In this study, diminished BBOX1 mRNA expression corresponded with a 45% reduction in enzyme activity. Together with this earlier report, our results now establish an essential role for PPARα in maintaining carnitine homeostasis. In light of these findings, we speculate that improvements in systemic carnitine metabolism might contribute to the clinical efficacy of PPARα activators.
The metabolic manifestations of PPARα deficiency are reminiscent of those observed in several inborn errors of metabolism, including genetic mutations in carnitine-acylcarnitine translocase, carnitine palmitoyltransferase 2, and the very long, long-, medium-, and short-chain acyl-CoA dehydrogenases (59,60,61). Patients affected by these disorders present with nonketotic hypoglycemia, muscle weakness, elevated plasma levels of long-chain acylcarnitines and carnitine depletion. On the basis of reports that carnitine therapy can alleviate these symptoms, we employed a similar strategy in the PPARα−/− mice. In this model, carnitine administration did not prevent fasting-induced hypoglycemia or hypoketonemia, indicating that carnitine insufficiency does not play a principal role in driving these perturbations. Still, the intervention produced several interesting outcomes that could prove broadly relevant to a variety of chronic metabolic disorders. Carnitine supplementation partially restored plasma amino acid concentrations in PPARα-null mice, suggesting decreased reliance on amino acid catabolism. Fitting with this interpretation, the intervention also increased muscle and plasma levels of short- and medium-chain acylcarnitine intermediates that are produced as byproducts of fatty acid oxidation. Notably, however, this effect was not evident in liver, consistent with the previous finding that PPARα inactivity causes a more severe β-oxidative block in liver as compared to skeletal muscle (24).
Carnitine supplementation also produced robust elevations in long-chain acylcarnitine species in muscle, liver, plasma, and urine, and a corresponding fall in liver levels of some long-chain acyl-CoAs, but only in the PPAR-null background. These findings support the notion that supplemental carnitine facilitates efflux of excess acyl units from lipid-stressed tissues, and by doing so, might prove useful as an adjunct therapy for disorders of lipid dysregulation (62, 63). Indeed, emerging evidence suggests that carnitine confers therapeutic benefits in patients with end-stage renal disease, cancer, and type 2 diabetes (62, 64, 65). Carnitine supplementation has also been shown to limit leucine oxidation, suppress branched-chain ketoacid dehydrogenase activity, increase protein anabolism, and improve muscle strength (64, 66,67,68). The mechanisms underlying these effects are yet unclear, but recent evidence points toward improvements in intratissue lipid balance, mitochondrial function, and changes in leucine-mediated activation of the mTOR signaling pathway (6, 62, 69).
In addition to its therapeutic potential, we reason that carnitine could have value as a diagnostic tool. Thus, acute carnitine administration to patients presenting with various forms of the metabolic syndrome might amplify plasma and urinary acylcarnitine concentrations to a threshold that exposes and/or pinpoints subtle defects in intermediary metabolism. Whereas acylcarnitine profiling is used commonly to screen for inborn metabolic errors in infants, only recently has this approach been employed experimentally to evaluate chronic metabolic diseases (6). These reports, together with the current study, provide impetus for considering more widespread clinical application of this technology.
In summary, acylcarnitine profiles of PPARα−/− mice revealed defects in fatty acid catabolism, carnitine homeostasis and fasting-induced substrate selection, whereas amino acid and organic profiles were consistent with impaired TCA cycle flux, elevated protein catabolism, and increased urea cycle activity. Plasma and urinary metabolite profiles partly reflected the perturbations observed in liver and skeletal muscle. Carnitine therapy ameliorated some of the metabolic abnormalities caused by PPARα deficiency and enhanced systemic detection of the null genotype. Considering that lesions in hepatic PPARα activity and β-oxidation have been implicated as early and fundamental factors in the development of chronic metabolic disorders, biomarkers of impaired lipid and amino metabolism, including those highlighted by this study, could prove useful as diagnostic indicators.
Acknowledgments
We thank Barbara Abbott (National Institute of Environmental Health Sciences, Raleigh, NC, USA) for sharing her mouse colony and Chris Newgard for his thoughtful evaluation of this article. We thank the dedicated staff of the Metabolomics and Biomarker Core of the Sarah W. Stedman Nutrition and Metabolism Center. This work was supported by National Institutes of Health grants P30-AG028716 (D.M.M.), K99-AA017376-01 (L.M.), and F32-DK080609 (R.N.); the American Diabetes Association (D.M.M.); and a John A. Hartford Duke Center for Excellence award (T.R.K).
References
- Cahill G F., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- Muoio D M, Newgard C B. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- Thyfault J P, Rector R S, Noland R C. Metabolic inflexibility in skeletal muscle: a prelude to the cardiometabolic syndrome? J Cardiometab Syndr. 2006;1:184–189. doi: 10.1111/j.1559-4564.2006.05629.x. [DOI] [PubMed] [Google Scholar]
- Kelley D E, Goodpaster B, Wing R R, Simoneau J A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Blaak E E. Basic disturbances in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc. 2004;63:323–330. doi: 10.1079/PNS2004361. [DOI] [PubMed] [Google Scholar]
- Koves T R, Ussher J R, Noland R C, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck J R, Newgard C B, Lopaschuk G D, Muoio D M. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J K, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Patsouris D, Reddy J K, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006;147:1508–1516. doi: 10.1210/en.2005-1132. [DOI] [PubMed] [Google Scholar]
- Finck B N, Kelly D P. Peroxisome proliferator-activated receptor alpha (PPARα) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol. 2002;34:1249–1257. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Cook W S, Qi C, Yeldandi A V, Reddy J K, Rao M S. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- Leone T C, Weinheimer C J, Kelly D P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S S, Gonzalez F J. Targeted disruption of the peroxisome proliferator-activated receptor alpha gene, PPAR alpha. Ann N Y Acad Sci. 1996;804:524–529. doi: 10.1111/j.1749-6632.1996.tb18642.x. [DOI] [PubMed] [Google Scholar]
- Ramsay R R, Gandour R D, van der Leij F R. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta. 2001;1546:21–43. doi: 10.1016/s0167-4838(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Primassin S, Ter Veld F, Mayatepek E, Spiekerkoetter U. Carnitine supplementation induces acylcarnitine production in tissues of very long-chain acyl-CoA dehydrogenase-deficient mice, without replenishing low free carnitine. Pediatr Res. 2008;63:632–637. doi: 10.1203/PDR.0b013e31816ff6f0. [DOI] [PubMed] [Google Scholar]
- Stevens R D, Hillman S L, Worthy S, Sanders D, Millington D S. Assay for free and total carnitine in human plasma using tandem mass spectrometry. Clin Chem. 2000;46:727–729. [PubMed] [Google Scholar]
- An J, Muoio D M, Shiota M, Fujimoto Y, Cline G W, Shulman G I, Koves T R, Stevens R, Millington D, Newgard C B. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Jensen M V, Joseph J W, Ilkayeva O, Burgess S, Lu D, Ronnebaum S M, Odegaard M, Becker T C, Sherry A D, Newgard C B. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem. 2006;281:22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- Hardy O T, Hohmeier H E, Becker T C, Manduchi E, Doliba N M, Gupta R K, White P, Stoeckert C J, Jr, Matschinsky F M, Newgard C B, Kaestner K H. Functional genomics of the beta-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol. 2007;21:765–773. doi: 10.1210/me.2006-0411. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters J M, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez F J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Bhuiyan A K, Bartlett K, Sherratt H S, Agius L. Effects of ciprofibrate and 2-[5-(4-chlorophenyl)pentyl]oxirane-2-carboxylate (POCA) on the distribution of carnitine and CoA and their acyl-esters and on enzyme activities in rats. Relation between hepatic carnitine concentration and carnitine acetyltransferase activity. Biochem J. 1988;253:337–343. doi: 10.1042/bj2530337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlies N, Tian L, Overmars H, Bootsma A H, Kulik W, Wanders R J, Wood P A, Vaz F M. Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse. Biochem J. 2005;387:185–193. doi: 10.1042/BJ20041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyfault J P, Cree M G, Zheng D, Zwetsloot J J, Tapscott E B, Koves T R, Ilkayeva O, Wolfe R R, Muoio D M, Dohm G L. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2007;292:C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- Muoio D M, MacLean P S, Lang D B, Li S, Houmard J A, Way J M, Winegar D A, Corton J C, Dohm G L, Kraus W E. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- Atherton H J, Bailey N J, Zhang W, Taylor J, Major H, Shockcor J, Clarke K, Griffin J L. A combined 1H-NMR spectroscopy- and mass spectrometry-based metabolomic study of the PPAR-α null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiol Genomics. 2006;27:178–186. doi: 10.1152/physiolgenomics.00060.2006. [DOI] [PubMed] [Google Scholar]
- Atherton H J, Jones O A, Malik S, Miska E A, Griffin J L. A comparative metabolomic study of NHR-49 in Caenorhabditis elegans and PPAR-α in the mouse. FEBS Lett. 2008;582:1661–1666. doi: 10.1016/j.febslet.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S, He T, Inagaki T, Potthoff M, Merritt M E, Esser V, Mangelsdorf D J, Kliewer S A, Browning J D, Burgess S C. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes. 2008;57:2012–2021. doi: 10.2337/db08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xiao G, Trujillo C, Chang V, Blanco L, Joseph S B, Bassilian S, Saad M F, Tontonoz P, Lee W N, Kurland I J. Peroxisome proliferator-activated receptor alpha (PPARα) influences substrate utilization for hepatic glucose production. J Biol Chem. 2002;277:50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- Xu J, Chang V, Joseph S B, Trujillo C, Bassilian S, Saad M F, Lee W N, Kurland I J. Peroxisomal proliferator-activated receptor alpha deficiency diminishes insulin-responsiveness of gluconeogenic/glycolytic/pentose gene expression and substrate cycle flux. Endocrinology. 2004;145:1087–1095. doi: 10.1210/en.2003-1173. [DOI] [PubMed] [Google Scholar]
- Sheikh K, Camejo G, Lanne B, Halvarsson T, Landergren M R, Oakes N D. Beyond lipids, pharmacological PPARα activation has important effects on amino acid metabolism as studied in the rat. Am J Physiol Endocrinol Metab. 2007;292:E1157–E1165. doi: 10.1152/ajpendo.00254.2006. [DOI] [PubMed] [Google Scholar]
- Kersten S, Mandard S, Escher P, Gonzalez F J, Tafuri S, Desvergne B, Wahli W. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- Edgar A D, Tomkiewicz C, Costet P, Legendre C, Aggerbeck M, Bouguet J, Staels B, Guyomard C, Pineau T, Barouki R. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicol Lett. 1998;98:13–23. doi: 10.1016/s0378-4274(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Semenza G L. Hypoxia-inducible factor 1 (HIF-1) pathway (Online) Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- MacKenzie E D, Selak M A, Tennant D A, Payne L J, Crosby S, Frederiksen C M, Watson D G, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung N H, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten S K, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris R A, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Aminova L R, Ratan R R. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32:931–946. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer A A, Syddall H E, Dennison E M, Martin H J, Phillips D I, Cooper C, Byrne C D. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. QJM. 2007;100:707–713. doi: 10.1093/qjmed/hcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–557. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- Villareal D T, Banks M, Siener C, Sinacore D R, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- Anderson S R, Gilge D A, Steiber A L, Previs S F. Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metabolism. 2008;57:347–354. doi: 10.1016/j.metabol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S W, Goodpaster B H, Strotmeyer E S, Kuller L H, Broudeau R, Kammerer C, de Rekeneire N, Harris T B, Schwartz A V, Tylavsky F A, Cho Y W, Newman A B. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- Steiber A L, Weatherspoon L J, Spry L, Davis A T. Serum carnitine concentrations correlated to clinical outcome parameters in chronic hemodialysis patients. Clin Nutr. 2004;23:27–34. doi: 10.1016/s0261-5614(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Pedersen B K, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13:56–62. doi: 10.1034/j.1600-0838.2003.20218.x. [DOI] [PubMed] [Google Scholar]
- Schrager M A, Metter E J, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri R, Bauge E, Staels B, Gervois P. Systemic and distal repercussions of liver-specific PPARα control of the acute phase response. Endocrinology. 2008;149:3215–3223. doi: 10.1210/en.2007-1339. [DOI] [PubMed] [Google Scholar]
- Smeets P J, Teunissen B E, Willemsen P H, van Nieuwenhoven F A, Brouns A E, Janssen B J, Cleutjens J P, Staels B, van der Vusse G J, van Bilsen M. Cardiac hypertrophy is enhanced in PPARα−/− mice in response to chronic pressure overload. Cardiovasc Res. 2008;78:79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- Murakami K, Bujo H, Unoki H, Saito Y. Effect of PPARα activation of macrophages on the secretion of inflammatory cytokines in cultured adipocytes. Eur J Pharmacol. 2007;561:206–213. doi: 10.1016/j.ejphar.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Zambon A, Gervois P, Pauletto P, Fruchart J C, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-α activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart J C, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Blumberg D, Hochwald S, Brennan M F, Burt M. Interleukin-6 stimulates gluconeogenesis in primary cultures of rat hepatocytes. Metabolism. 1995;44:145–146. doi: 10.1016/0026-0495(95)90255-4. [DOI] [PubMed] [Google Scholar]
- Barton B E. IL-6-like cytokines and cancer cachexia: consequences of chronic inflammation. Immunol Res. 2001;23:41–58. doi: 10.1385/IR:23:1:41. [DOI] [PubMed] [Google Scholar]
- Vaz F M, Wanders R J. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich J, van Vlies N, Jansen G A, Denis S, Ruiter J P, van Werkhoven M A, Duran M, Vaz F M, Wanders R J, Ferdinandusse S. A phytol-enriched diet induces changes in fatty acid metabolism in mice both via PPARα-dependent and -independent pathways. J Lipid Res. 2005;46:716–726. doi: 10.1194/jlr.M400337-JLR200. [DOI] [PubMed] [Google Scholar]
- Koch A, Konig B, Stangl G I, Eder K. PPAR α mediates transcriptional upregulation of novel organic cation transporters-2 and -3 and enzymes involved in hepatic carnitine synthesis. Exp Biol Med (Maywood) 2008;233:356–365. doi: 10.3181/0706-RM-168. [DOI] [PubMed] [Google Scholar]
- Moody D E, Reddy J K. Increase in hepatic carnitine acetyltransferase activity associated with peroxisomal (microbody) proliferation induced by the hypolipidemic drugs clofibrate, nafenopin, and methyl clofenapate. Res Commun Chem Pathol Pharmacol. 1974;9:501–510. [PubMed] [Google Scholar]
- Ringseis R, Posel S, Hirche F, Eder K. Treatment with pharmacological peroxisome proliferator-activated receptor alpha agonist clofibrate causes upregulation of organic cation transporter 2 in liver and small intestine of rats. Pharmacol Res. 2007;56:175–183. doi: 10.1016/j.phrs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Paul H S, Gleditsch C E, Adibi S A. Mechanism of increased hepatic concentration of carnitine by clofibrate. Am J Physiol Endocrinol Physiol. 1986;251:E311–E315. doi: 10.1152/ajpendo.1986.251.3.E311. [DOI] [PubMed] [Google Scholar]
- Van Vlies N, Ferdinandusse S, Turkenburg M, Wanders R J, Vaz F M. PPAR alpha-activation results in enhanced carnitine biosynthesis and OCTN2-mediated hepatic carnitine accumulation. Biochim Biophys Acta. 2007;1767:1134–1142. doi: 10.1016/j.bbabio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kelly D P, Whelan A J, Ogden M L, Alpers R, Zhang Z F, Bellus G, Gregersen N, Dorland L, Strauss A W. Molecular characterization of inherited medium-chain acyl-CoA dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1990;87:9236–9240. doi: 10.1073/pnas.87.23.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull D M, Bartlett K, Stevens D L, Alberti K G, Gibson G J, Johnson M A, McCulloch A J, Sherratt H S. Short-chain acyl-CoA dehydrogenase deficiency associated with a lipid-storage myopathy and secondary carnitine deficiency. N Engl J Med. 1984;311:1232–1236. doi: 10.1056/NEJM198411083111906. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Uchida Y, Kelley R I, Marble M, Hofman K, Tonsgard J H, Rhead W J, Hashimoto T. A novel disease with deficiency of mitochondrial very-long-chain acyl-CoA dehydrogenase. Biochem Biophys Res Commun. 1993;191:1369–1372. doi: 10.1006/bbrc.1993.1368. [DOI] [PubMed] [Google Scholar]
- Power R A, Hulver M W, Zhang J Y, Dubois J, Marchand R M, Ilkayeva O, Muoio D M, Mynatt R L. Carnitine revisited: potential use as adjunctive treatment in diabetes. Diabetologia. 2007;50:824–832. doi: 10.1007/s00125-007-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Okumura K, Takahashi R, Matsui H, Numaguchi Y, Murakami H, Murakami R, Murohara T. Combined therapy with PPARα agonist and L-carnitine rescues lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res. 2006;70:566–577. doi: 10.1016/j.cardiores.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Biolo G, Stulle M, Bianco F, Mengozzi G, Barazzoni R, Vasile A, Panzetta G, Guarnieri G. Insulin action on glucose and protein metabolism during L-carnitine supplementation in maintenance haemodialysis patients. Nephrol Dial Transplant. 2008;23:991–997. doi: 10.1093/ndt/gfm664. [DOI] [PubMed] [Google Scholar]
- Gramignano G, Lusso M R, Madeddu C, Massa E, Serpe R, Deiana L, Lamonica G, Dessi M, Spiga C, Astara G, Maccio A, Mantovani G. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–145. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Owen K Q, Jit H, Maxwell C V, Nelssen J L, Goodband R D, Tokach M D, Tremblay G C, Koo S I. Dietary L-carnitine suppresses mitochondrial branched-chain keto acid dehydrogenase activity and enhances protein accretion and carcass characteristics of swine. J Anim Sci. 2001;79:3104–3112. doi: 10.2527/2001.79123104x. [DOI] [PubMed] [Google Scholar]
- Siami G, Clinton M E, Mrak R, Griffis J, Stone W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron. 1991;57:306–313. doi: 10.1159/000186280. [DOI] [PubMed] [Google Scholar]
- Giovenali P, Fenocchio D, Montanari G, Cancellotti C, D'Iddio S, Buoncristiani U, Pelagaggia M, Ribacchi R. Selective trophic effect of L-carnitine in type I and IIa skeletal muscle fibers. Kidney Int. 1994;46:1616–1619. doi: 10.1038/ki.1994.460. [DOI] [PubMed] [Google Scholar]
- Vary T C, Lynch C J. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–1843. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]