Abstract
The existence of a mammalian family of TRPC ion channels, direct homologues of TRP, the visual transduction channel of flies, was discovered during 1995–1996 as a consequence of research into the mechanism by which the stimulation of the receptor-Gq-phospholipase Cβ signaling pathway leads to sustained increases in intracellular calcium. Mammalian TRPs, TRPCs, turned out to be nonselective, calcium-permeable cation channels, which cause both a collapse of the cell’s membrane potential and entry of calcium. The family comprises 7 members and is widely expressed. Many cells and tissues express between 3 and 4 of the 7 TRPCs. Despite their recent discovery, a wealth of information has accumulated, showing that TRPCs have widespread roles in almost all cells studied, including cells from excitable and nonexcitable tissues, such as the nervous and cardiovascular systems, the kidney and the liver, and cells from endothelia, epithelia, and the bone marrow compartment. Disruption of TRPC function is at the root of some familial diseases. More often, TRPCs are contributing risk factors in complex diseases. The present article reviews what has been uncovered about physiological roles of mammalian TRPC channels since the time of their discovery. This analysis reveals TRPCs as major and unsuspected gates of Ca2+ entry that contribute, depending on context, to activation of transcription factors, apoptosis, vascular contractility, platelet activation, and cardiac hypertrophy, as well as to normal and abnormal cell proliferation. TRPCs emerge as targets for a thus far nonexistent field of pharmacological intervention that may ameliorate complex diseases.—Abramowitz, J., Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels.
Keywords: calcium, cardiovascular system, epithelium, lung, neuron
G protein-coupled receptor-mediated activation of the Gq-phospholipase C (PLC) -β and receptor tyrosine kinase (RTK) -mediated activation of the PLCγ signaling systems results in hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) with formation of the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). This is accompanied by activation of plasma membrane nonselective, Ca2+-permeable cation channels and is followed by IP3-induced release of Ca2+ from intracellular stores and activation of type-C protein kinases (PKCs) by the combined action of DAG and the released Ca2+. The depletion of intracellular Ca2+ stores then activates highly Ca2+-selective cation channels in the plasma membrane. Ca2+ entering through the PLC-activated channels is referred to as receptor-operated Ca2+ entry (ROCE). Ca2+ entering through the highly Ca2+-selective channels is activated when stores are depleted is referred to as store-operated Ca2+ entry (SOCE). Mammalian homologues of Drosophila transient receptor potential (TRP) channels, the canonical TRPs (TRPCs), were postulated as the pore-forming molecules through which receptor and store depletion activated Ca2+ entries takes place (1). The more recent discovery of Orai and of the functional interactions between Orai and TRPCs makes it likely that the entry channels activated by store depletion are, in fact, formed of both Orai and TRPC molecules (2, 3). In addition to receptor-mediated activation of TRPCs via PLC, hormone- and growth factor-induced increases in TRPC activity has been shown to occur also as a consequence of translocation of TRPC channels from internal organelles to the plasma membrane. Thus, depending on the receptor repertoire of a given cell or tissue, a variety of Ca2+-dependent cellular processes appear to depend, at least in part, on TRPC channels. A summary of hormones, neurotransmitters, and growth factors that have been shown to activate given TRPC channels is listed in Table 1, and Table 2 (4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88) lists agents affecting TRPC function independent of phospholipase C activation.
TABLE 1.
Agonists and receptors that have been shown to activate TRPCs
| TRPC channel | Agonist/receptor | Tissue/cell type | Reference |
|---|---|---|---|
| TRPC1 | M5 Muscarinic | COS cells | 4 |
| Purinergic P2Y (ATP) | Prostate cancer epithelial cells | 5 | |
| mGluR1 | Cerebellar Purkinje cells | 6 | |
| Thrombin (PAR-1) | Platelets | 7 | |
| Endothelin-1 | Artery | 8 | |
| Angiotensin II R AT1 | Arterial vascular smooth muscle cells | 9 | |
| Bradykinin B2 R | Superior cervical ganglion neurons | 10 | |
| Orexin (OX1R) | CHO cells | 11 | |
| B-cell Receptor | DT 40 B cells | 12 | |
| bFGF | Endothelial cells | 13 | |
| FGFR-1 | Neuronal stem cells | 14 | |
| Netrin-1 | Neurons | 15, 16 | |
| Brain-derived neurotrophic factor (BDNF) TrkB | Neurons | 16 | |
| Myelin-associated glycoprotein | Neurons | 16 | |
| TRPC2 | M5 Muscarinic | COS cells | 17 |
| Purinergic P2Y (ATP) | HEK293 cells | 18 | |
| ZP3 | Sperm | 18 | |
| Odorant V1R | Vomeronasal Organ | 19 | |
| Odorant V2R | Vomeronasal Organ | 19 | |
| Erythropoietin | Erythroid cells | 20 | |
| TRPC3 | Alpha 1A R | Prostate Smooth Muscle | 21 |
| M1 Muscarinic | Superior cervical ganglion neurons | 10 | |
| M5 Muscarinic | COS cells | 4 | |
| Purinergic P2Y (ATP) | HEK293 cells | 22 | |
| Purinergic (UTP) | Arterial vascular smooth muscle cells | 23 | |
| mGluR1 | HEK cells | 24 | |
| mGluR5 | HEK cells | 24 | |
| Angiotensin II R AT1A | CHO cells | 25 | |
| V1a-AVP R | HEK293T cells | 26 | |
| Bradykinin | Endothelial cells | 27 | |
| Bradykinin B2 R | Superior cervical ganglion neurons | 10 | |
| Substance P NK2R | HEK293 cells | 28 | |
| Histamine H1 R | CHO cells | 29 | |
| Oxytocin | PHM1 myometrial cells | 30 | |
| Orexin (OX1R) | CHO cells | 11 | |
| Melanopsin (Opn4) | Xenopus oocytes, HEK293 cells | 31, 32 | |
| Endothelin-1 | Arterial vascular smooth muscle cells | 33 | |
| Erythropoietin | HEK293 cells | 34 | |
| BDNF TrkB | Neurons | 35 | |
| B-cell Receptor | DT 40 B cells | 36, 37 | |
| T-cell Receptor | Jurkat T cells | 38 | |
| VEGF-R2 | CHO cells | 39 | |
| Vitamin D3 | ROS 17/2.8 osteoblastic cells | 40 | |
| Translocation | M3 Muscarinic | HEK293 cells | 41 |
| Translocation | EGF | HEK293 cells | 42 |
| Translocation | Insulin | Cardiomyocytes | 43 |
| Translocation | V2-AVP R | Renal collecting duct cells | 44 |
| TRPC4 | Muscarinic | Endothelial cells | 45 |
| M3 Muscarinic | HEK293 cells | 46 | |
| Purinergic P2Y (ATP) | Endothelial cells | 45 | |
| Histamine H1 | HEK293 cells | 46 | |
| Thrombin (PAR-1) | Endothelial cells | 47 | |
| Serotonin 5-HT2R | Thalamic interneurons | 48 | |
| EGF | HEK293 cells | 46 | |
| Translocation | EGF | COS-7 cells | 49 |
| TRPC5 | M1 Muscarinic | Xenopus oocytes | 50 |
| HEK293 cells | 51 | ||
| M3 Muscarinic | HEK293 cells | 46 | |
| M5 Muscarinic | HEK293 cells | 52 | |
| Prostaglandin EP1R | Xenopus oocytes | 53 | |
| V1a-AVP R | HEK293 cells | 54 | |
| Bradykinin B2R | PC12 cells | 55 | |
| Purinergic P2Y | HEK293 cells | 56 | |
| Purinergic P2y2 (UTP) | PC12 cells | 55 | |
| Histamine H1 | HEK293 cells | 57 | |
| Thrombin | CHO cells | 58 | |
| mGluR1 | Pyramidal neurons in lateral amygdala | 59 | |
| mGluR5 | Pyramidal neurons in lateral amygdala | 59 | |
| Sphingosine-1 Phosphate | HEK293 cells | 60 | |
| B-cell Receptor | DT 40 B cells | 52 | |
| IgE (FcεRI) | RBL-2H3 Mast Cells | 61 | |
| GM1 Ganglioside/α5β1 Integrin | NG108-15 cells | 62 | |
| Translocation | EGF | HEK293 cells | 63 |
| Translocation | BDNF | Hippocampal neurons | 63 |
| Translocation | NGF | Hippocampal neurons | 63 |
| Translocation | IGF-1 | Hippocampal neurons | 63 |
| TRPC6 | Alpha 1A R | Vascular smooth muscle | 64 |
| M1 Muscarinic | Superior cervical ganglion neurons | 10 | |
| M3 Muscarinic | HEK293 cells | 65 | |
| M5 Muscarinic | COS cells | 66 | |
| V1-AVP R | A7r5 vascular smooth muscle cells | 67 | |
| V1a-AVP R | HEK293 cells | 68 | |
| Serotonin R (5-HT) | A7r5 vascular smooth muscle cells | 67 | |
| Histamine H1 R | CHO cells | 29 | |
| Bradykinin B2 R | Superior cervical ganglion neurons | 10 | |
| Angiotensin II R AT1 | Cardiomyocytes | 69 | |
| Arterial vascular smooth muscle cells | 9 | ||
| Orexin (OX1R) | IMR-32 neuroblastoma cells | 70 | |
| Thrombin | Endothelial cells | 71 | |
| 20-HETE | HEK293 cells | 72 | |
| Interleukin-1β (IL-1 type 1R) | Astrocytes | 73 | |
| PDGF | A7r5 vascular smooth muscle cells | 67 | |
| T-cell Receptor | Jurkat T cells | 74 | |
| EGF | COS cells | 75 | |
| BDNF | Rat cerebellar granule cells | 76 | |
| VEGF-R2 | HEK293 cells | 77 | |
| Translocation | M3 Muscarinic | HEK293 cells | 78 |
| Translocation | Bradykinin B2 R | Endothelial cells | 79 |
| Translocation | EET | Endothelial cells | 79 |
| TRPC7 | Purinergic P2Y (ATP) | HEK293 cells | 80 |
| M3 Muscarinic | HEK cells | 81 | |
| M5 Muscarinic | SYF Cells | 66 | |
| mGluR1 | HEK cells | 24 | |
| mGluR5 | HEK cells | 24 | |
| V1a-AVP R | HEK293 cells | 68 | |
| Histamine H1 R | HEK cells | 24 | |
| Angiotensin II AT1R | HEK cells | 82 | |
| Endothelin-1 | Arterial vascular smooth muscle cells | 33 | |
| Trypsin (PAR-2) | DT 40 B cells | 83 | |
| B-cell Receptor | DT 40 B cells | 83 |
TABLE 2.
Agents affecting TRPC function without activation of phospholipase C, probably by direct action on TRPCs
| TRPC channel | Activating agent | Tissue/cell type | Reference |
|---|---|---|---|
| TRPC2 | Sulfated steroids | Vomeronasal organ | 84 |
| TRPC3 | Diacylglycerol | CHO-K1 cells | 29 |
| TRPC4 | Diacylglycerola | HEK293 and DT40 cells | 52 |
| Protons | HEK293 cells | 85 | |
| TRPC5 | Diacylglycerola | HEK293 and DT40 cells | 52 |
| Lysophospholipids | HEK293 cells | 86 | |
| Protons | HEK293 cells | 85 | |
| Nitrosylationb | HEK cells | 87 | |
| Thioredoxinb | HEK293 cells | 88 | |
| TRPC6 | Diacylglycerol | CHO-K1 cells | 29 |
| TRPC7 | Diacylglycerol | HEK cells | 80 |
Requires inhibition of PKC.
The report on activation of TRPC5 by nitrosylation of Cys553 and Cys 558 (87) conflicts with the report that TRPC5 is activated by reduced thioredoxin acting to reduce an extracellular disulfide bond between Cys 553 and Cys 558 (88).
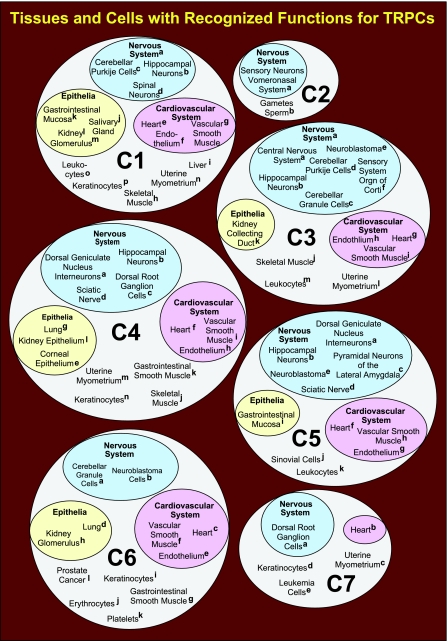
TRPCs have been shown to assemble both as homomeric and heteromeric complexes (89,90,91,92). This has made it difficult to unambiguously assign specific functions to one or the other TRPC, and in many cases, it is likley that TRPCs may functionally overlap. Nevertheless, functions and properties of different TRPCs appear to be limiting in a large variety of physiological processes. Tissues and cells in which TRPCs have been shown to play pivotal roles are summarized in Fig. 1(93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188). Because of the possibility of heteromultimerization, some or even many of these cases may involve more than one TRPC. Resolution of this quandary will require that the involvement of more than one TRPC be specifically investigated. Even though there is an example of a TRPC mutation causing a familial disease, involvement of TRPCs in disease states is more of the type, in which partial or total malfunction of a given TRPC contributes to the severity of this or that complex disease.
Figure 1.
Summary of tissues and cells in which TRPCs have been shown to play identifiable roles. C1, TRPC1; C2, TRPC2; C3, TRPC3; C4, TRPC4; C5, TRPC5; C6, TRPC6; C7, TRPC7. References to entries shown on the figure: C1: a (14), b (93), c (94), d (15, 16), e (95), f (96,97,98), g (8, 99,100,101,102,103,104,105,106,107,108), h (109), i (110, 111), j (112, 113), k (114), l (111), m (111, 115), n (30, 116,117,118,119), o (12), p (120,121,122,123); C2: a (19, 124,125,126,127,128), b (18, 129, 130); C3: a (35), b (93, 131, 132), c (76, 133), d (134), e (135), f (136), g (69, 137, 138), h (92, 139,140,141), i (23, 33, 101, 142,143,144,145), j (146), k (44), l (30, 117–119, 147), m (148,149,150,151,152); C4: a (153), b (154), c (155), d (155, 156), e (157, 158), f (159), g (47), h (45, 92, 160,161,162), i (108, 163), j (109), k (164,165,166), l (167), m (147), n (123); C5: a (153), b (154, 168, 169), c (59), d (155), e (156, 170), f (137, 159), g (87), h (60), i (114), j (88), k (150, 152); C6: a (76, 133), b (171, 172), c (69, 173, 174), d (175), e (71), f (64, 102, 106, 144, 175,176,177,178,179), g (164), h (180,181,182,183), i (121), j (184), k (185), l (5); C7: a (186), b (187), c (116, 119), d (121), e (188).
TRPCs are nonselective Ca2+-permeable cation channels that demonstrate variable Ca2+/Na+ permeability ratios ranging from 1 to 9 (reviewed in ref. 189). Responses to stimuli that activate TRPCs can be predicted to be complex and to vary not only with the complement of TRPC channels expressed in the affected cells but also on the presence of other functions. In excitable cells, which express K+ channels that keep them in a hyperpolarized state, and voltage-gated Na+ and Ca2+ channels, Na+ entry through TRPC channels would lead to membrane depolarization, activation of voltage-gated Na+ and Ca2+ channels, and additional Ca2+ entry. In nonexcitable cells, Na+ entry through TRPCs would make membrane potential more positive, alter cell volume, and affect other Na+-dependent cellular processes, which may include the Na+-K+-ATPase, Na+-anion and Na+-sugar cotransporters, and the Na+/Ca2+ exchanger. In both excitable and nonexcitable cells, activation of TRPCs results in increases in intracellular Ca2+ concentrations ([Ca2+]i) and a plethora of Ca2+-induced responses, which, depending on context, can be either positive or detrimental for the functioning of the cell, tissue, or organism.
Given the complex set of molecular responses elicited by TRPC activation, it stands to reason that TRPCs should play pivotal roles in a wide variety of cellular and tissue functions. Studies from many laboratories in all continents have borne out this prediction. Surprisingly, there exist no selective or high-affinity ligands that either activate or inhibit TRPCs. The widespread distribution of TRPCs with widely varying functional roles makes them ideal pharmacological targets for development of novel bioactive compounds with likely medicinal impact.
In this review, we shall summarize the roles played by each TRPC channel in mediating and regulating various physiological and pathophysiological processes. For readers interested in other aspects of TRPC regulation and function, including the molecular makeup of TRPC channels, mechanisms of TRPC activation and the potential roles of Orai and STIM, we recommend recent monographs and reviews on TRP channels (190,191,192,193). For further reading on involvement in diseases of not only TRPCs but also other channels of the TRP family, we also recommend the 2007 special issue on TRP Channels in Disease of Biochimica et Biophysica Acta’s series on Molecular Basis of Disease (194) and the review by Nilius et al. (189).
TRPC1
Salivary gland
TRPC1 is expressed in a wide variety of tissues, including brain, heart, smooth muscle, endothelium, liver, testis, ovaries, and salivary glands (reviewed in ref. 195), and potential physiological roles for TRPC1 have been identified in a number of different tissues. One of the first reported physiological roles for TRPC1 was its regulation of salivary fluid secretion (112). Salivary secretion is Ca2+ dependent, and increases in [Ca2+]i are associated with increased secretion. Ambudkar and colleagues demonstrated that adenovirus-mediated overexpression of human TRPC1 in rat submandibular glands results in a 5-fold increase in muscarinic-stimulated fluid secretion and in an enhanced Ca2+ entry response to carbachol and thapsigargin (112). In support of these findings, muscarinic-stimulated salivary fluid secretion is reduced by 70% in Trpc1−/− mice compared to wild-type controls, with agonist- and thapsigargin-stimulated Ca2+ entry being reduced by 60% and store-operated channel activity being reduced by 75% in salivary gland acinar cells from Trpc1−/− mice (113). Disruption of TRPC1 expression also resulted in increased salivary sodium, potassium, and chloride concentrations compared to those from wild-type controls (113).
Liver
Experiments by Chen and Barrit (110) implicate TRPC1 in the regulation of cell volume. Rat H4-IIE liver cells transfected with siRNA directed against TRPC1 demonstrated an ∼50% reduction in the expression of endogenous TRPC1. This reduction in TRPC1 expression was accompanied by an aberrant response to hypotonicity, in which cells responded to hypotonic medium with a greater immediate increase in cell volume and an enhanced regulatory cell volume decrease compared to control cells. The reduction in TRPC1 expression was also associated with a diminished Ca2+ influx in response to either thapsigargin, ATP, or maitotoxin (110). The altered response to hypotonicity may be related to the MscCA channel activity ascribed to TRPC1 in frog oocytes (196).
Endothelial cells
One of the roles of endothelial cells is to maintain the barrier function of blood vessels. Increases in endothelial cell [Ca2+]i by inflammatory agonists (e.g., thrombin, bradykinin, histamine) increase endothelial permeability by inducing endothelial cell rounding and interendothelial cell gap formation. TRPC1 has been shown to be expressed in endothelial cells isolated from several different vessels from the human, mouse, rat, and cow (reviewed in ref. 197). Heterologous expression of TRPC1 in human umbilical vein endothelial cells enhances thrombin-induced increases in endothelial permeability (96). Thrombin-induced changes in permeability were dependent on PKCα-mediated activation of and phosphorylation of TRPC1 (96). In addition to regulating endothelial permeability by activating TRPC1, inflammatory agonists increase endothelial permeability by elevating TRPC1 expression by a mechanism that involves NF-κB (97, 98).
Vascular smooth muscle (VSM)
TRPC1 has been implicated in several functions of VSM cells. A rise in [Ca2+]i is an important stimulus for cell growth in VSM. Studies with isolated cultured human pulmonary artery smooth muscle cells (PASMCs) demonstrate that TRPC1 expression and SOCE are increased in proliferating vs. nonproliferating PASMCs (99) and that treatment of PASMCs with antisense oligonucleotides directed toward TRPC1 results in a reduction in SOCE and PASMC proliferation (100). Proliferation of VSM contributes greatly to the increased pulmonary vascular resistance and arterial pressure seen in patients with pulmonary hypertension (198). Interestingly, increasing cAMP levels with forskolin in the presence of IBMX increases TRPC1 mRNA expression in PASMCs from patients with idiopathic pulmonary hypertension but not in PASMCs from normotensive patients (101). Pulmonary hypertension is also induced by chronic hypoxia. PASMCs isolated from rats exposed to hypoxic conditions for 3 wk or PASMCs cultured under hypoxic conditions display increased TRPC1 and TRPC6 expression and increased SOCE (102). These hypoxia-induced increases in TRPC1 expression are mediated by hypoxia-inducible factor-1 (HIF-1), as these increases are absent in mice heterozygous for HIF-1 (103). Therefore, TRPC1 expression and function may play an important role in hypertensive states.
Proliferation of VSM is a component of atherosclerosis and neointimal formation following vascular injury. Beech and colleagues reported that TRPC1 expression is up-regulated in neointima from mice, rats, pigs, and humans (104). Furthermore, in vivo inhibition of proliferation in crushed rat carotid arteries reduces the response to injury and prevents the up-regulation of TRPC1 expression. These authors then demonstrated that in vitro treatment of human neointima with antibodies to TRPC1 inhibits proliferation (104). In related studies using primary cultures of human coronary artery smooth muscle cells, Takahashi et al. (105) showed that treatment of these VSMs with angiotensin-II leads to hypertrophy and increases in SOCE, as well as to increased expression of TRPC1, but not TRPC3, TRPC4, TRPC5, or TRPC6. Expression of siRNA directed against TRPC1 prevented angiotensin II-induced hypertrophy. Angiotensin II-induced hypertrophy was shown to be associated with NF-κB binding to the TRPC1 promoter and that prevention of NF-κB binding to the TRPC1 promoter prevents angiotensin II-induced hypertrophy and TRPC1 up-regulation. Thus, changes in TRPC1 expression and SOCE activity are clearly correlated with changes in VSM proliferation and hypertrophy. Interestingly, balloon dilation, a treatment for atherosclerotic arteries, increases the expression of TRPC1 and TRPC6 of dilated human internal mammary artery maintained in organ culture compared to undilated control arterial segments (106).
Vascular contraction induced by endothelin-1 (ET-1) is associated with an increase in [Ca2+]i and TRPC1 has been shown to be involved in this process. Rat caudal and cerebral arteries maintain their contractile response to ET-1 in organ culture (8, 106) and display an increase in TRPC1 and TRPC6 expression, a decrease in TRPC3 expression, and an increase in SOCE (106). Treatment of cultured arteries with an antibody to TRPC1 inhibited both ET-1- and SOCE-induced contraction (8, 106). In addition, adenovirus-mediated overexpression of human TRPC1 in rat pulmonary artery rings led to enhanced SOCE and enhanced SOCE-mediated contraction (107). Furthermore, basilar arteries isolated from a canine model of subarachnoid hemorrhage demonstrate an enhanced contractile response and enhanced ROCE response to ET-1, both of which are associated with an increase in TRPC1 and TRPC4 expression (108). Treatment of subarachnoid hemorrhage basilar arteries with an antibody to either TRPC1 or TRPC4 inhibited both ET-1-induced contraction and ROCE (108). Interestingly, despite the various proposed functions of TRPC1, cerebral arteries and aortic VSM from TRPC1-deficient mice fail to demonstrate a contractile phenotype different than wild-type mice (199).
Heart
In the heart, changes in [Ca2+]i are involved with many aspects of cardiac function and disease, including cardiac hypertrophy. Studies in the rat by Ohba et al. (95) have shown that the heart expresses TRPC1, C3, C5, and C6 and that after abdominal aortic banding to induce cardiac hypertrophy, only TRPC1 expression is elevated compared to sham controls. These authors went on to demonstrate that in primary cultures of rat neonate cardiomyocytes, ET-1-, angiotensin-II-, and phenylephrine-induced hypertrophy resulted in increased TRPC1 expression and increased SOCE. Furthermore, treatment of cardiomyocytes with siRNA targeted at TRPC1 prevented ET-1-, angiotensin-II- and phenylephrine-induced hypertrophy and increases in SOCE. These findings suggest that TRPC1 is an important regulator of cardiac hypertrophy in the rat.
Mesangial cells
Renal glomerular mesangial cells are smooth muscle-like cells located within glomerular capillary loops and contribute to the physiological regulation of glomerular hemodynamics (200). Being smooth muscle-like, [Ca2+]i play an important role in mesangial cell contraction. Du et al. (115) have shown that in cultured mesangial cells, angiotensin II-induced increases in Ca2+ influx and cell contraction are inhibited by antibodies directed at the extracellular domain of the pore region of TRPC1, as well as by down-regulation of TRPC1 expression as a result of RNAi expression. These authors went on to demonstrate that infusion of the TRPC1 antibody into rats prevented the angiotensin II- or endothelin-1-induced decrease in glomerular filtration rate (115).
Diabetic nephropathy
TRPC1 has been linked to diabetic nephropathy, a disease that targets renal glomerular function (201). The transcription factor hepatocyte nuclear factor 4α (HNF4α) regulates a number of genes involved in metabolic pathways and insulin secretion (202, 203). Niehof and Borlak (111) used a chromatin immunoprecipitation-cloning assay and identified TRPC1 and PLCβ1 as HNF4α targets. These authors demonstrated further that HNF4α, TRPC1, and PLCβ1 mRNA expression was decreased in the liver and kidney of Zucker diabetic fatty rats, that HNF4α and TRPC1 mRNA expression was reduced in the liver and kidney of streptozotocin-induced diabetic rats, and that HNF4α and TRPC1 protein expression was reduced in the kidney of patients with diabetic nephropathy. However, no clear differences in PLCβ1 protein expression could be detected. A positive correlation between mRNA for HNF4α and both TRPC1 and PLCβ1 was demonstrated in the kidney of Zucker diabetic fatty rats. HNF4α was shown to bind to the promoters of both TRPC1 and PLCβ1, and siRNA was directed against HNF4α-inhibited TRPC1 expression. It is interesting that the expression of both PLCβ1, which lies upstream of TRPC activation, and TRPC1 expression are depressed in diabetic nephropathy, creating a compound depression of TRPC1 function. Thus, TRPC1 appears to play a role in regulation of glomerular filtration rate and renal function in normal and diseased states.
Skeletal muscle
TRPC1 has been implicated in regulating skeletal muscle [Ca2+]i and in the pathology associated with Duchenne muscular dystrophy (DMD) (109). DMD results from the lack of the cytoskeletal protein dystrophin (204). Ca2+ influx is increased in muscle from mdx mice, which lack dystrophin (205). Analysis of spontaneous plasma membrane voltage-independent Ca2+ channels indicates that muscle from mdx mice has higher channel activity than that found in control mice and that the activity of this channel is enhanced by treatment with thapsigargin (109). TRPC1, TRPC4, and TRPC6 were shown to be expressed in sarcolemma from mdx mice, and a mixture of antisense oligonucleotides against TRPC1 and TRPC4 that reduced expression of TRPC1 and TRPC4 protein was effective in reducing channel activity in mdx muscle fibers. These findings suggest that TRPC1 and TRPC4 contribute to the excess Ca2+ influx observed in mdx muscle.
Other studies in mdx mice found that TRPC1 protein expression is increased in cardiac (206) and skeletal muscle (207). The increase in TRPC1 expression in mdx skeletal muscle was accompanied by increases in caveolin-3 and Src kinase expression. Similar to the reported ability of caveolin-1 to interact with TRPC1 (208, 209), muscle-specific caveolin-3 was shown to colocalize and coimmunoprecipitate with TRPC1 from mdx myocytes (207). Furthermore, reactive oxygen species (ROS) from H2O2 were shown to activate myoblast Src activity, and myoblast TRPC1 activity was shown to be dependent on caveolin-3 expression and Src phosphorylation. These increases in TRPC1 activity resulting from Src activation may be due to Src-dependent TRPC phosphorylation (68). In addition, ROS-generating pathways play an important role in amplifying the cellular damage of Ca2+ signaling seen in muscular dystrophy (210, 211). Thus, a signaling pathway exists in dystrophic muscle, encompassing ROS, Src kinase, and TRPC1 localized in caveolae, which when activated leads to aberrant Ca2+ signaling and cellular damage. Interestingly, TRPC1 contains a dystrophin homology domain (208), and TRPC1 and dystrophin coimmunoprecipitate from skeletal muscle extracts (212); thus, a lack of dystrophin would result in cytoskeletal disorganization and could result in misregulation of TRPC1, leading to increased Ca2+ influx. TRPC1 also interacts with another scaffolding protein found in muscle, Homer (213), and Homer expression is decreased in mdx muscle (214). Evaluation of skeletal muscle from Homer 1 knockout mice demonstrates that fiber cross-sectional area and force generation are decreased (214). These pathological changes were accompanied by enhanced Ca2+ influx. Knockdown of TRPC1 expression with shRNA in Homer 1−/− myocytes inhibited Ca2+ influx to a level observed in wild type myocytes. Taken together with the analysis of the mdx mice, the findings in the Homer 1 knockout mouse suggest that enhanced Ca2+ influx mediated by TRPC1 contributes to skeletal myopathy.
Lymphocyte activation
Ca2+ signaling through calcineurin and nuclear factor of activated T cells (NFAT) plays important roles in lymphocyte activation and development (215). Studies in chicken DT40 B cells, in which TRPC1 expression was eliminated by deleting the TRPC1 gene via homologous recombination, show that disruption of TRPC1 is associated with a decrease in B-cell antigen receptor- and thapsigargin-mediated Ca2+ entry and NFAT activation (12). In addition, mice deficient in TRPC1 show a compromised immune response to allergic challenge (unpublished results), supporting a role for TRPC1 in the regulation of lymphocyte-associated immune responses.
Neuronal functions
Proliferation
TRPC1 has been implicated in the control of neural function and development. Analogous to the findings in VSM, TRPC1 has been shown to be important in neuronal cell proliferation. It is well established that proliferating neuroepithelial cells are markedly dependent on Ca2+ entry (216). Studies with rat embryonic stem cells demonstrate that these cells express both fibroblast growth factor receptor-1 (FGFR-1) and TRPC1 (14). Antisense knock-down of TRPC1 expression decreased bFGF-mediated proliferation of neuronal stem cells and reduced the Ca2+ entry component of the [Ca2+]i response to bFGF (14). In addition, rat cultured H19-7 hippocampal cells can be induced to differentiate in vitro, which is manifested by elongation and development of axonal processes. When these cells are grown under differentiating conditions, TRPC1 and TRPC3 levels, as well as SOCE, are increased (93). Coexpression of siRNA for TRPC1 and TRPC3 prevented an increase in SOCE and differentiation of H19-7 cells (93). In related studies, TRPC1 has been shown to be required for netrin-1-, brain-derived neurotrophic factor (BDNF)-, and myelin-associated glycoprotein-induced turning in cultured Xenopus neurons (15, 16). Both studies used morpholino oligonucleotides to inhibit TRPC1 expression to obtain evidence for a requirement for Ca2+ entry through TRPC1 for both growth cone turning in response to neurotrophic factors (15, 16) and for the guidance of axons of commissural interneurons in the developing Xenopus spinal cord (16).
Astrocytes
Earlier, we indicated that TRPC1 is involved in the control of salivary gland secretion (112, 113). Recent evidence indicates that TRPC1 is also required for glutamate secretion from astrocytes (217). Rat frontal cortex astrocytes express protein for TRPC1, TRPC4, and TRPC5, with TRPC1 expression being restricted primarily to the plasma membrane, while the expression of TRPC4 and TRPC5 appears to be distributed throughout the cell. Mechanical stimulation of cultured astrocytes resulted in an increase in [Ca2+]i and in glutamate release, both of which could be inhibited with an antibody to TRPC1 (217). Thus, TRPC1 may be involved in the control of secretion from a variety of cell types.
Antineurotoxic activity of TRPC1
Studies using human neuroblastoma SH-SY5Y cells demonstrate that treatment with the neurotoxins 1-methyl-4-phenylpyridinium ion (218) or salsolinol (219) results in decreased TRPC1 protein levels. Overexpression and activation of TRPC1 were shown to be neuroprotective, while expression of an antisense TRPC1 cDNA enhanced neutotoxicity. Bollimuntha et al. (218, 219) went on to show that TRPC1 overexpression was also associated with inhibition of cytochrome c release and decreased Bax and Apaf-1 protein expression, suggesting that the neuroprotective actions of TRPC1 were due to inhibition of apoptosis.
Intestinal mucosa
Although TRPC1 is antiapoptotic in neuroblastoma cells, TRPC1’s role in apoptosis appears to be cell-type specific. In intestinal crypts and epithelial cells, where apoptosis plays an important role in mucosal maintenance, TRPC1 appears to increase epithelial cell sensitivity to apoptotic stimuli (220, 221). Stable overexpression of TRPC1 in IEC-6 intestinal epithelial cells increased apoptosis induced by TNF-α/cyclohexamide or staurosporine but did not increase the basal rate of apoptosis. Overexpression of TRPC1 was accompanied by an increase in SOCE and inactivation of NF-κB. Marasa et al. (220) went on to demonstrate that NF-κB activity was inversely correlated with apoptosis, that siRNA directed against TRPC1 inhibited TNF-α/cyclohexamide-induced apoptosis and enhanced NF-κB DNA binding activity, and that in the absence of extracellular Ca2+, apoptosis was inhibited and NF-κB DNA binding activity increased. In a follow-up study, this group demonstrated that increased phosphatase 2A activity in response to increased TRPC1 expression and enhanced Ca2+ influx was responsible for inhibition of NF-κB binding activity and increased sensitivity to apoptotic signals (221).
In the gastrointestinal tract, mucosal restitution is an important repair mechanism to seal superficial wounds that involves epithelial cell migration. Mucosal restitution is independent of cell proliferation and is dependent on Ca2+ entry (222). Cultured normal rat intestinal epithelial cells induced to differentiate demonstrate enhanced expression of TRPC1 and TRPC5, increased SOCE, and enhanced cell migration during restitution (114). Increased SOCE and cell migration was also observed when TRPC1 was overexpressed. In contrast, siRNA treatment targeting TRPC1 reduced SOCE and inhibited cell migration after wounding, suggesting a role for TRPC1 in both controlling SOCE and cell migration during intestinal epithelial restitution (114).
Keratinocytes
Extracellular Ca2+ is an important regulator of keratinocyte proliferation and differentiation (223), and increased [Ca2+]i that results from exposure to increased extracellular Ca2+ is required for keratinocyte differentiation (224). The first evidence for a role of TRPCs in keratinocyte differentiation came from Cai et al. (121), who studied TRPC expression in human gingival keratinocytes. Undifferentiated human gingival keratinocytes express TRPC1, TRPC5, TRPC6, and TRPC7. Placing these cells in culture under differentiating conditions in the presence of extracellular Ca2+ caused a time-dependent increase in mRNA levels for each TRPC, which reached a peak within the first 2 days in culture and decreased by day 8, while protein levels for TRPC1 and TRPC6 peaked at days 5 and 6. Protein levels of involucrin, a marker of keratinocyte differentiation, peaked at days 6–8 (121). This group went on to show that treating human gingival keratinocytes with siRNA targeting TRPC1 inhibited Ca2+-induced differentiation, as well as thapsigargin-induced Ca2+ influx (122). Similar studies by Beck et al. (123) with human HaCaT epidermal keratinocytes showed that Ca2+-induced differentiation of these cells, as assessed by increased mRNA for involurcin, cytokeratin 10, and transglutaminase 1, was associated with an increase in TRPC1 and TRPC4 protein expression, and that siRNA directed against either TRPC1 or TRPC4 inhibited Ca2+-induced differentiation (123). These authors went on to demonstrate that human basal cell carcinoma cells do not express TRPC1 or TRPC4 and fail to differentiate in culture in response to extracellular Ca2+. Thus, TRPC1 and TRPC4 appear to play important roles in the regulation of keratinocyte differentiation and may help explain the undifferentiated status of basal cell carcinoma.
Cell physiology
At the cell physiology level, TRPC1 has been implicated in regulating phospholipid asymmetry, in mediating mechanosensitivity, and in controlling motility and polarity. Regulation of plasma membrane phospholipid asymmetry and lipid raft integrity by TRPC1 was reported by Kunzelmann-Marche et al. (225). Lipid rafts are cholesterol-rich membrane domains that are involved in the recruitment of signal transduction proteins to plasma membrane microdomains. TRPC1 has been localized in caveolin-containing lipid rafts (208). In HEL human megakaryoblast cells, disruption of lipid rafts with methyl-β-cyclodextran or treatment with antibodies to TRPC1 inhibits Ca2+-induced phosphatidylserine switching to the outer membrane leaflet of the plasma membrane triggered by either norepinephrine, thrombin, or thapsigargin, implicating both receptor-operated Ca2+ entry and store-operated Ca2+ entry in regulating membrane phospholipid asymmetry (225).
Mechanosensitive cation channel
TRPC1 was reported to be a mechanosensitive cation (MscCA) channel in frog oocytes, where it was involved in translating membrane stretch into cation (Na+, K+, Ca2+, and Mg2+) currents (196). Membrane fractions enriched in MscCA activity were enriched in TRPC1. Heterologous expression of human TRPC1 increased MscCA density in membrane parches, while injection of TRPC1 antisense RNA abolished endogenous MscCA activity in frog oocytes. Further, transfection of human TRPC1 into CHO-K1 cells, which express low MscCA activity, resulted in enhanced MscCA patch density and activity, suggesting that TRPC1 is a component of the vertebrate MscCA (196). It should be noted, however, recent studies in which TRPC1 or TRPC6 were overexpressed in either COS or CHO cells were unable to detect increases in mechanosensitive current, which calls into question whether TRPC1 or TRPC6 function as MscCA channels in heterologous expression systems (226).
Cell motility and polarity
Recently, TRPC1 has been implicated in the control of cellular motility and polarity (227). Knockdown of TRPC1 expression in Madin-Darby canine kidney-focus cells with siRNA resulted in loss of cellular polarity and in loss of the ability to migrate in a given direction. The loss of polarity and directional migration was linked to the suppression of a local Ca2+ gradient at the front of the lamellipodium of the migrating cells.
Human disease
Darier’s disease is an autosomal dominant disease, which is characterized by abnormal keratinocytes and loss of cellular adhesion between epidermal cells (228) and is caused by loss-of-function mutations in the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 2b (SERCA2b; 229). Pani et al. (120) reported that epidermal cells from Darier’s disease patients demonstrate increased expression of TRPC1, enhanced SOCE, and enhanced proliferation, as compared to cells from control patients. These authors also demonstrated increased TRPC1 expression in the skin of Serca2+/− mice. Keratinocytes from Darier’s patients were resistant to the apoptotic effects of thapsigargin as were HaCaT human keratinocytes, either overexpressing TRPC1 or expressing siRNA to SERCA2. The antiapoptotic actions of overexpressed TRPC1 and down-regulation of SERCA2 were dependent on a mechanism that involves NF-κB (120). These findings are reminiscent of those showing an involvement of TRPC1 in endothelial cell permeability and neurotoxicity protection discussed above.
TRPC2
Pheromone signaling in vomeronasal sensory neurons
Unlike the other TRPCs that are widely expressed in vertebrates, TRPC2 is unique in that it exists only as a pseudogene in the genomes of Old World monkeys and humans. This loss of TRPC2 expression has been correlated with reduction of vomeronasal organ (VNO) function, reduced ability to detect pheromones, and the acquisition of female sexual swelling and male trichromatic vision in Old World Monkeys and humans (230, 231). Thus, it may not be surprising that TRPC2 is integrally involved in pheromone detection and gender-specific sexual behavior.
The VNO is responsible for the detection of water-soluble pheromones. The first evidence for a role for TRPC2 in pheromone-induced VNO signaling came with the cloning of the rat TRPC2 and demonstration that TRPC2 was exclusively expressed in vomeronasal sensory neurons (124). In another study, light microscopic and ultrastructural analysis (125) revealed that TRPC2 is expressed in rat VNO sensory receptor cells and that TRPC2 is expressed at sites that express the α subunits of the heterotrimeric G proteins Gi2 and Go. These G proteins are involved in transducing receptor-mediated pheromone signaling in the VNO (reviewed in refs. 232, 233). Mice that are Trpc2−/− have a phenotype in which sensory response to pheromone cues in urine is abolished, pheromone-evoked male-male aggression is abolished, males have lost gender discrimination for mating, males demonstrate sexual behavior toward male intruders and males display defects in marking territory despite the fact that males have normal testosterone production and display normal male-female mating behavior (126, 127). Female Trpc2−/− mice display characteristic male sexual behavior, including mounting, pelvic thrust, solicitation, anogenital olfactory investigation, and emission of ultrasonic vocalizations (128). Maternal aggression against intruders and lactational behavior are also diminished in Trpc2−/− female mice despite the presence of a normal estrous cycle with normal sex hormone levels and the ability to carry a pregnancy to term (127, 128). In addition, electrophysiological analysis of the pheromone-activated ion channel in vomeronasal sensory neurons from control and Trpc2−/− mice revealed a 92% reduction in current amplitude in Trpc2−/− mice, further supporting a role for TRPC2 in pheromone-induced signaling (19).
Pheromone signaling in lower vertebrates
Although the most complete analysis for a role for TRPC2 in mediating pheromone-induced signaling comes from studies in rodents, there is also evidence for a role of TRPC2 in mediating olfactory signaling in lower vertebrates. Generation of transgenic zebrafish, Danio rerio, in which YFP is expressed in sperm cells under the control of the zebrafish TRPC2 promoter, demonstrates colocalization of TRPC2 with V2R-type receptors, which mediate pheromone signaling in olfactory sensory neurons (234). In addition, electrophysiological studies by Brann and Fadool of VNO sensory neurons in the turtle Sternotherus ordoratus (235) identified a current that was stimulated by male urine. These authors went on to demonstrate that this male urine-stimulated current could be inhibited when a peptide corresponding to amino acids 905-934 of mouse TRPC2 was included in the recording electrode.
Acrosome reaction in sperm
The sperm acrosome reaction is a Ca2+-dependent secretory event that must be completed before fertilization (reviewed in ref. 236), and Ca2+ entry through store-operated channels has been shown to drive the acrosome reaction (237). RNA for TRPC2 is expressed in mouse testis (17), and in the bovine testis TRPC2 mRNA expression is restricted to spermatocytes (238). Immunocytochemical analysis localized TRPC2 to the anterior portion of the mouse sperm head (18). In mammals, sperm head interaction with the zona pellucida protein ZP3 is the main activator of the acrosome reaction (239). Jungnickel et al. (18) demonstrated that an antibody against TRPC2 inhibited thapisgargin- and ZP3-induced Ca2+ entry in sperm cells. The final proof for the involvement of TRPC2 in the acrosome reaction came when it was demonstrated that the antibody against TRPC2 inhibited the acrosome reaction (18). IP3Rs are present in the acrosome cap (240), and the direct interaction between TRPCs and IP3Rs has been proposed as a mechanism for TRPC activation (241, 242). Junctate, an IP3R-associated protein that binds and activates TRPCs (243), is expressed in the acrosomal crescent and binds TRPC2 and TRPC5 (129). Although TRPC2 gating is downstream of phospholipase C activation in sperm, and the diacylglycerol OAG (oleylacetylglycerol) activates TRPC2 in membrane patches of VNO sensory cells (19), the acrosome reaction could not be triggered by OAG, suggesting that TRPC2 activation may result from a direct interaction among TRPC2, junctate, and IP3R (129). Enkurin is another protein that might be involved in TRPC2 activation during the acrosome reaction. Enkurin was identified as a TRPC2-interacting protein in a yeast two-hybrid screen of a mouse testis library using the N terminus of TRPC2 as bait (130). Enkurin colocalizes in the acrosomal crescent with TRPC2, as well as with TRPC1 and TRPC5. Enkurin has a functional calmodulin binding domain (130), as does TRPC2 (244), further suggesting a role for enkurin in Ca2+-mediated functions. Whether and how enkurin alters TRCP2 function must await further analysis. While TRPC2 has been shown to be important in the acrosomal reaction, it is not essential, as TRPC2-deficient mice are fertile (126, 127). As a result, the exact mechanism by which ZP3 induces Ca2+ entry and the possible involvement of other TRPCs and auxiliary proteins still needs to be determined.
TRPC3
Neuronal cell functions
IBDNF
Perhaps one of the better characterized physiological functions of TRPC3 deals with control of neuronal function and development. Both in the rat (35) and the human (245), TRPC3 expression is highly enriched in neurons of the central nervous system (CNS). Li et al. (35) went on to demonstrate that maximal TRPC3 expression in the rat CNS occurs during a narrow window just prior to and after birth. Expression of TRPC3 occurs in the same neurons that express TrkB, the neurotropin receptor that binds brain-derived nerve growth factor (BDNF), and activation of TrkB by BDNF leads to production of a PLC-dependent current (IBDNF) in pontine neurons. Colocalization of TRPC3 and TrkB could be demonstrated in neurons of the cerebral cortex, hippocampus, amygdala, cerebellum, and pons. These researchers went on to demonstrate that TRPC3 and TrkB coimmunoprecipitated and that TRPC3 contributed to IBDNF in pontine neurons (35). Using cultured cerebellar granule cells, Li et al. (76) demonstrated that TRPC channels contribute to BDNF-induced elevation of Ca2+ at growth cones and that TRPC channels are required for BDNF-induced chemoattractive turning. This was accomplished by showing that Ca2+ elevation and growth cone turning induced by BDNF were abolished by pharmacological inhibition of TRPCs, by expression of dominant-negative forms of TRPC3 or TRPC6, and by expression of RNAi directed at TRPC3. In related studies using rat hippocampal CA1 pyramidal neurons, Amaral and Pozzo-Miller (131, 132) demonstrated that RNAi-mediated TRPC3 knockdown resulted in loss of IBDNF and that IBDNF is dependent on phosphatidylinositol 3-kinase-mediated TRPC3 membrane insertion. Furthermore, functional TRPC3 channels are required for BDNF-mediated increase in dendritic spine density in CA1 neurons (131, 132). In addition, TRPC3 and TRPC6 protect cerebellar granule neurons against serum deprivation-induced cell death in vitro and promote cerebellar granule neuron survival in rat brain (133). Down-regulation of either TPRC3 or TRPC6 suppresses BDNF-mediated cell survival and induces apoptosis; both of these processes are reversed by overexpression of either TRPC3 or TRPC6 (133). Thus, many of the actions of BDNF on neuronal function and development appear to be mediated by TRPC channels. As mentioned above, TRPC3 has also been shown to be involved in the differentiation of cultured H19-7 hippocampal cells (93).
Purkinje cell function and motor coordination
TRPC1 has been reported to mediate activation of a slow mixed-cation excitatory postsynaptic conductance (sEPSC) by mGluR1 in cerebellar Purkinje cells (94). However, recent studies in Trpc1-, Trpc1/4-, and Trpc1/4/6-deficient mice failed to observe reduced mGluR1-mediated activation of sEPSC in cerebellar Purkinje cells (134). In contrast, Hartmann et al. (134) showed that TRPC3 is highly expressed in Purkinje cells and that mice null for TRPC3 fail to demonstrate mGluR1-mediated activation of sEPSC. TRPC3-null mice also showed abnormal muscle coordination leading to impaired locomotion as a result of the cerebellar defect. One explanation for the discrepancy between the results obtained by Kim et al. (94) and those of Hartmann et al. (134) may be the age of the animals studied and the presence of a developmental switch, whereby the sEPSC is carried primarily by TRPC1 in young mice and by TRPC3 in older more mature mice.
Additional evidence for a role of TRPC3 in the control of motor coordination comes from studies analyzing Δ202 mice (246). Δ202 mice carry a transgene encoding the SV40 T antigen and develop progressive paralysis starting at 35 days of age, resulting in death at 90 days of age (247). The paralysis starts in the hindlimbs and progresses to the forelimbs. The SV40 T antigen transgene was shown to be inserted into the promoter region of the mouse Trpc3 gene, resulting in the loss of TRPC3 expression (246). The neuromotor phenotype of the Δ202 mouse is more severe than that described by Hartmann et al. (134) for the TRPC3 knockout mice they developed. This is likely due to SV40 T antigen-induced glioma formation in the brains of Δ202 mice (247).
Activity of GABAergic neurons
GABAergic neurons of the substantia nigra pars reticulata fire spontaneously and are depolarized compared to other neurons. Although the tetrodotoxin-sensitive Na+ channel is involved in maintaining the depolarized state, tetrodotoxin treatment failed to completely repolarize these neurons (248). Since TRPC3 is a nonselective cation channel that demonstrates spontaneous channel activity (2, 22, 249), Zhou et al. (250) examined the role of TRPC3 in regulating GABAergic neuronal activity. TRPC3 is the only TRPC channel expressed in mouse GABAergic neurons of the substantia nigra pars reticulata. Treatment of GABAergic neurons with an antibody to TRPC3 inhibited a Na+-dependent current that resulted in hyperpolarization of the neuron and decreased spontaneous firing, suggesting that TRPC3 regulates basal neuronal activity in these cells.
Outer cochlear hair cells
TRPC3 has been suggested to be involved in regulation of [Ca2+]i in cochlear outer hair cells. TRPC3 is expressed in guinea pig and rat organ of Corti and is localized to the sensory and neural poles of the inner and outer hair cells (136). Whole-cell voltage clamp of guinea pig and rat outer hair cells show OAG-activated cation entry currents with properties similar to those of TRPC3, implicating a role for TRPC3 in the regulation of [Ca2+]i in these cells.
Human disease I
Bipolar disorder Altered TRPC function and regulation has been implicated in bipolar disorder. Basal, thrombin-, serotonin-, and thapsigargin-stimulated increases in [Ca2+]i are elevated in platelets and lymphocytes from patients with bipolar disorder (251). When B lymphoblast cell lines derived from bipolar I disorder patients were exposed to therapeutic concentrations of lithium for 7 days in vitro, both lysophosphatidic acid- and thapsigargin-stimulated increases in [Ca2+]i were decreased compared to untreated cells (252). However, lithium treatment for 24 h had no effect on either lysophosphatidic acid- or thapsigargin-stimulated increases in [Ca2+]i. Subsequently, these authors examined the effects of lithium treatment on the expression of TRPC1 and TRPC3 in B lymphoblasts from bipolar I disorder patients (148). Treatment of B lymphoblasts with lithium for 7 days reduced the levels of TRPC3 protein. However, mRNA levels for TRPC1 and TRPC3, as well as protein levels for TRPC1, were not altered. No change in TRPC3 protein levels were observed in cells treated for 24 h. Interestingly, the effects on TRPC3 protein levels were more pronounced in B lymphocytes from female patients compared to male patients. Thus down-regulation of TRPC3 expression appears to explain in part the mechanism by which lithium treatment ameliorates Ca2+ disturbances observed in bipolar disorder.
Cognition Studies on the transcription factor TFII-I have implicated a role for TRPC3 in cognition (135). Deletion of the gene that codes for TFII-I is associated with a cognitive defect in humans known as Williams-Beuren syndrome. TFII-I is a target of Burton’s tyrosine kinase (253). Knocking down TFII-I expression in PC12 cells with siRNA results in enhanced UTP- or bradykinin-mediated Ca2+ influx and in enhanced plasma membrane expression of TRPC3, but no change in TRPC3 mRNA levels (135). A biochemical explanation of the relationship between TFII-I and TRPC3 was provided by the work of Van Rossum et al. (254). These authors reported that PLCγ interacts with TRPC3 and induces plasma membrane accumulation of TRPC3, and that phosphorylation of TFII-I on Y462 results in a protein that specifically binds to PLCγ in the cytosol, interfering with its interaction with TRPC3. Thus, under normal conditions, the phosphorylation state of Y462 on TFII-I will control the pool of PLCγ that is available to interact with TRPC3 and, in this way, control plasma membrane accumulation of TRPC3 and receptor-mediated activation of TRPC3. In the absence of TFII-I, as observed in Williams-Beuren syndrome, there are abnormally high levels of TRPC3 in the plasma membrane and abnormally high receptor-mediated Ca2+ entry, which could be responsible for the cognitive defects seen in this syndrome.
Spinocerebellar ataxia type 14 (SCA14) SCA14 is an autosomal dominant neurodegenerative disorder associated with degeneration of cerebellar Purkinje cells and is due to mutations in PKCγ (255). Recent analysis shows that compared to wild-type PKCγ, heterologously expressed PKCγ mutants, in which the mutation was in the C1 domain of the protein failed to inhibit muscarinic receptor-induced Ca2+ influx (256). Although the mutants were shown to be constitutively active, they had altered DAG binding and a shorter residency time at the plasma membrane, which resulted in the failure to phosphorylate TRPC3. Because PKC-mediated phosphorylation of TRPC3 inhibits TRPC3 activity (52), the failure of mutant PKCγ to phosphorylate TRPC3 would result in sustained Ca2+ influx in Purkinje cells and may contribute to neurodegeneration associated with SCA14.
Kidney collecting duct
In the collecting duct of the kidney, arginine-vasopressin (AVP) acting via V2 AVP receptors activates Gs, leading to enhanced adenylyl cyclase activity, to increased cAMP levels, and to activation of PKA. The activated PKA phosphorylates the water channel aquaporin-2 (AQP2), which is then translocated to the apical membrane of collecting duct principal cells, resulting in water movement from the lumen of the duct into the cell (reviewed in ref. 257). Recent studies show that TRPC3 is also translocated to the apical membrane in vivo in rat kidney medullary cells and in cell lines of cortical and medullary collecting duct cells in response to AVP (44). TRPC3, but not TRPC6, which is also expressed in these renal cells, was translocated to the apical membrane along with AQP2. TRPC3 and AQP2 were shown to reside in similar subcellular vesicles and to coimmunoprecipitate. However, expression of AQP2 was shown not to be required for AVP-induced translocation of TRPC3. In addition, apical expression of TRPC3 in polarized cultured renal cells was accompanied by an increase in 45Ca2+ apical-to-basolateral transepithelial flux. This increase in transepithelial 45Ca2+ flux could be inhibited by expression of a dominant negative TRPC3. These findings suggest that TRPC3 may be responsible for transepithelial Ca2+ flux in response to AVP in principal cells of the renal collecting duct. Because the V2 AVP receptor present in rat inner medullary collecting duct cells causes increases in both cAMP and [Ca2+]i levels (258), the pathway that mediates AVP-induced translocation of TRPC3 remains to be determined. In addition, because the scaffolding protein Homer has been shown to be required for M3 muscarinic receptor-induced translocation of TRPC3 in HEK293 cells (259), the role of Homer in AVP-mediated translocation of TRPC3 and AQP2 in the kidney should be evaluated.
Endothelial cells
TRPC3 has been suggested to serve as a redox sensor, which monitors oxidative stress. Porcine aortic endothelial cells express TRPC3 and contain oxidant-induced cation currents with properties similar to those of TRPC3 (140). Expression of the N terminus of TRPC3 in endothelial cells, which acts as a dominant negative TRPC3 fragment, abolished oxidant-induced cation current and reduced membrane depolarization. TRPC3 may not be acting alone in this process. On the basis of coimmunoprecipitation experiments, it has recently been shown that heteromeric channels composed of TRPC3 and TRPC4 can associate to form redox-sensitive channels both in native endothelial cells and when expressed heterologously in HEK293 cells (92). Expression of dominant negative constructs for either TRPC3 or TRPC4 was able to suppress oxidant-induced channel activity in both systems.
During pregnancy, there are a number of cell-signaling changes that occur in uterine artery endothelial cells, including those involving [Ca2+]i (reviewed in ref. 260). SOCE and ATP-dependent ROCE are greater in uterine artery endothelial cells from pregnant ewes compared to endothelial cells from nonpregnant ewes (141, 261). Differences in TRPC3 or TRPC6 protein expression could not be detected in these cells (141). Therefore, in order to account for enhanced SOCE and ROCE in these samples, the ability of TRPC3 and type 2 IP3 receptor (IP3R2) to interact with each other was analyzed using immunoprecipitation. Treatment of endothelial cells from pregnant ewes with ATP for 8 min resulted in enhanced immunoprecipitation of TRPC3 with an antibody to IP3R2 compared to similarly treated endothelial cells from nonpregnant ewes (141). These findings suggest that a pregnancy-enhanced interaction between TRPC3 and IP3R2 in response to an agonist is responsible for enhanced ATP-dependent Ca2+ influx observed endothelial cells from pregnant ewes.
Uterine smooth muscle
Regulation of the uterine myometrium is essential for the maintenance of pregnancy and parturition. Myometrial contraction is controlled by Ca2+ (262), and a potential role of TRPCs in regulating myometrial [Ca2+]i has been investigated. Oxytocin, which causes myometrial contraction, activates TRPC3 in cultured human myometrial cells, enhancing cellular Ca2+ entry (30). Both rat and human myometrium expresses various TRPC mRNAs. The rat myometrium expresses mRNA for all TRPCs except TRPC3 (116), while the human myometrium expresses all except TRPC2 and TRPC5 (30, 117,118,119). During pregnancy in the human, the mRNA levels for TRPC1, TRPC6, and TRPC7 were increased, as were protein levels for TRPC1, TRPC3, TRPC4, and TRPC6 (119). In contrast, in the rat myometrium, no changes in mRNA levels were observed throughout pregnancy for TRPC1, TRPC2, TRPC4, or TRPC7, and the levels of TRPC5 and TRPC6 mRNA were reduced (116). The proinflammatory cytokine IL-1β has been implicated in initiation of parturition (263), and IL-1β treatment of cultured human myometrial cells has been shown to enhance SOCE (264) and to increase TRPC3 protein expression (119). This may explain, in part, the enhanced TRPC3 protein expression observed in human myometrial samples obtained during labor (119). During pregnancy, the myometrium is subjected to stretch from the developing fetus, resulting in uterine enlargement due to myometrial hypertrophy and hyperplasia, which are regulated, in part, by Ca2+ signaling. When primary cultures of human myometrial cells are subjected to stretch for up to 14 h, these cells demonstrate enhanced SOCE, increased mRNA levels for TRPC3 and TRPC4, as well as increased protein expression for TRPC3 (147). Clearly, the role of TRPCs in the regulation of myometrical function is complex and must await further investigation before a clearer picture emerges.
Skeletal muscle
In a manner analogous to the heart, skeletal muscle possesses calcium-dependent signaling pathways that result in activation of calcineurin and NFAT, inducing gene expression associated with slow oxidative myofibers that appear to be related to adaptive responses to neuromuscular activity (265). Studies designed to identify the molecular source of the Ca2+ responsible for NFAT activation in skeletal muscle in response to exercise demonstrate that extracellular Ca2+ is required for NFAT activation and translocation to the nucleus (146). These studies went on to show that TRPC3 expression is increased in slow oxidative muscle compared to fast glycolytic muscle, that exercise increased skeletal muscle TRPC3 expression compared to sedentary controls, and that constitutively activated calcineurin or NFAT enhanced TRPC3 promoter activity linked to luciferase. Finally, these studies reported that plasmid-mediated expression of TRPC3 in myocytes increased NFAT-dependent reporter gene activity, suggesting that increased TRPC3 expression increased the pool of Ca2+ responsible for regulation of calcineurin/NFAT signaling (146).
TRPC3 has also been linked to changes in [Ca2+]i associated with excitation-contraction coupling in skeletal muscle. TRPC3 protein expression is increased, as myoblasts differentiate into myotubes and remains elevated in differentiated skeletal myotubes (266). Suppression of TRPC3 expression with siRNA inhibited KCl- and caffeine-induced increases in [Ca2+]i, without altering expression of ryanodine receptor 1 (RyR1). Expression of TRPC1, triadin, junctophilin type 1, and calsequestrin were increased in TRPC3 knockdown myotubes, while no changes in expression were observed for TRPC4 and TRPC6 or seven other triadic proteins analyzed. These studies also demonstrated that TRPC3 expression is decreased in RyR1−/− myocytes and that overexpression of RyR1 increased myocyte TRPC3 expression. In other studies using MALDI-TOF analysis of cross-linked triadic proteins from skeletal myotubes, it was shown that TRPC3 formed a complex with RyR1, TRPC1, junctophilin 2, Homer, mitsugumin 29, calreticulin, and calmodulin (267). However, it was not possible to demonstrate a direct interaction of TRPC3 with RyR1 in native myotubes, despite the reported coimmunoprecipitation of TRPC3 and RyR1 overexpressed in HEK 293 cells (268). It was proposed that TRPC3 and RyR1 functionally interact with each other via triadic proteins to control the gain of sarcoplasmic reticulum Ca2+ release involved in excitation-contraction coupling (266, 267). Additional evidence for a role of TRPC3 in skeletal muscle function comes from the analysis of autoantibodies found in patients with myasthenia gravis. Muscle weakness in these patients is due primarily to antibodies directed against the nicotinic acetylcholine receptor. Analysis of antibodies from antiacetylcholine receptor-positive myasthenia gravis patients indicates that they also possess antibodies to TRPC3 and RyR1 (269). TRPC3 antibodies were found in patients with more severe disease, suggesting that antibodies to TRPC3 contribute to abnormal muscle contraction seen in myasthenia gravis.
Airway smooth muscle
Airway smooth muscle is a critical effector of bronchomotor tone and plays an important role in airway remodeling and inflammation. The proinflammatory cytokine TNF-α has been implicated as a mediator in the pathophysiology of asthma and airway smooth muscle hyperresponsiveness (270), which appears to involve, in part, enhanced Ca2+ mobilization (271). Human bronchial smooth muscle cells treated with TNF-α for 18–22 h demonstrate enhanced Ca2+ influx in response to acute acetylcholine, bradykinin, or cyclopiazonic acid treatment (145). TNF-α-treated cells had increased levels of TRPC3 mRNA and protein compared to untreated cells. However, no changes in mRNA or protein levels were detected for TRPC1, TRPC4, TRPC5, or TRPC6 in response to TNF-α. Treatment of airway smooth muscle cells with siRNA directed against TRPC3 prevented the TNF-α-induced increase in TRPC3 protein and inhibited Ca2+ influx in response to either acetylcholine or cyclopiazonic acid, suggesting that increased TRPC3 expression is responsible for TNF-α-induced enhancement of Ca2+ mobilization.
Cardiovascular system and VSM
Changes in [Ca2+]i are involved in regulating many physiological functions in the cardiovascular system, and TRPC3 has been implicated in some of these. Within the vascular system, spontaneous and agonist-induced activation of TRPC3 has been reported in rat cerebral artery myocytes (23), rabbit ear artery myocytes, (142) and rabbit coronary artery myocytes (33). In cerebral arteries, UTP induces depolarization and contraction of smooth muscle, and both these responses are inhibited by treatment with antisense oliognucleotides for TRPC3, but not TRPC6, which is also expressed in these cells (23). In contrast, tail vein injection into rats of antisense oligonucleotides for TRPC3 inhibited flow- and bradykinin-induced vasodilation in small mesenteric arteries (139). Interestingly, antisense oligonucleotide treatment against TRPC3 did not alter histamine- or ATP-induced vasodilation. As treatment with antisense oligonucleotides inhibited bradykinin-induced increases in [Ca2+]i in endothelial cells, it is likely that flow- and bradykinin-mediated activation of TRPC3 in endothelial cells is responsible for the observed vasodilation (139).
Further evidence for a role of TRPC3 in regulating VSM function comes from studies in mice in which the TRPC6 gene had been knocked out (143). In this mouse model, elimination of TRPC6 expression is accompanied by an increase in TRPC3 expression in smooth muscle cells from aorta and cerebral artery. TRPC6-deficient mice demonstrated elevated blood pressure and enhanced agonist-induced contraction of isolated aortic rings and cerebral arteries. Smooth muscle cells form Trpc6−/− aorta or cerebral artery are depolarized and demonstrate enhanced spontaneous and agonist-induced Ca2+ entry, which is likely due to increased TRPC3 expression. Further evidence for a role of TRPC3 in regulating cerebral artery constriction comes from studies with endothelium- denuded rat cerebral arteries in which endothelin-1- and IP3-induced vasoconstriction was shown to depend on IP3R-mediated activation of TRPC3 (272). These last findings support a proposed mechanism of TRPC activation that is the result of the direct interaction between IP3R and TRPC (241, 242).
Hypertension and cardiac hypertrophy
Studies using monocytes from spontaneously hypertensive (SHR) rats demonstrate that compared to monocytes from normotensive Wistar-Kyoto (WKY) rats, TRPC3 but not TRPC6 expression was increased (149). Monocytes from SHR rats demonstrate elevated thapsigargin-, angiotensin II-, and OAG-induced Ca2+ influx compared to monocytes from WKY rats, and treatment of monocytes from SHR rats with siRNA targeting TRPC3 reduced thapsigargin- and OAG-induced Ca2+ influx compared to untreated monocytes (149, 151). Increased expression of TRPC3 and TRPC5 but not TRPC6 has also been observed in monocytes from patients with essential hypertension compared to monocytes from normotensive patients (150, 152). Analogous to the studies in SHR rats, monocytes from hypertensive patients demonstrate elevated thapsigargin- and OAG-induced Ca2+ influx compared to monocytes from normotensive patients and treatment of monocytes from hypertensive patients with siRNA targeting TRPC3 or TRPC5 reduced thapsigargin- and OAG-induced Ca2+ influx compared to untreated monocytes (152). It was further shown that there is a positive correlation between TRPC3 mRNA levels and systolic blood pressure, as well as mRNA levels for IL-1β and TNF-α (273), suggesting a potential role for TRPC3 and inflammation in hypertension.
Changes in [Ca2+]i are involved with many aspects of cardiac function and disease, including cardiac hypertrophy. Angiotensin II-induced cardiac hypertrophy is mediated by NFAT downstream of Ca2+-signaling through calcineurin (274). Studies with rat neonatal cardiomyocytes demonstrate that DAG, produced by activation of PLCβ downstream of angiotensin-mediated stimulation of Gqα, is required for NFAT activation and hypertrophy (69). It was demonstrated further that angiotensin II activates DAG-sensitive TRPC-like currents. Through the use of siRNA against TRPC3 and TRPC6, it was also shown that these two channels are required for angiotensin II-induced ROCE, NFAT translocation, and hypertrophy of neonatal cardiomyocytes. In related studies, Bush et al. (137) reported that culture of rat neonatal cardiomyocytes in the presence of either endothelin-1 or phenylephrine results in increased expression of both mRNA and protein for TRPC3 and that inhibition of calcineurin with either cyclosporine A or FK506 prevents the increase in TRPC3 expression. Adenovirus-mediated overexpression of TRPC3 resulted in an increase in cardiac atrial natriuretic factor expression, a marker of cardiac hypertrophy, as well as enhanced calcineurin-NFAT signaling. It was also shown that in rat models of spontaneous hypertensive heart failure, because of a mutation in the leptin receptor or transverse aortic constriction, cardiac TRPC3 expression is increased compared to control rats (137). Further evidence for a role of TRPC3 in cardiac function comes from studies in transgenic mice overexpressing TRPC3 in the heart (138). Low level overexpression of TRPC3 in the heart led to cardiac hypertrophy, left ventricle dilation, and a decrease in fractional shortening, while high-level overexpression led to premature death. Hearts overexpressing TRPC3 also demonstrated enhanced NFAT activation compared to wild-type mice, with high-TRPC3-expressing mice, showing higher levels of NFAT activation than low-TRPC3 expressing mice. Mice overexpressing TRPC3 in the heart also demonstrated enhanced cardiac hypertrophy in response to phenylephrine, angiotensin II, or transverse aortic constriction (138).
Ischemia-reperfusion of the heart is a commonly used model of cardiac injury. In cardiomyocytes from mice in which TRPC3 is overexpressed in the heart, ischemia-reperfusion resulted in increased apoptosis and calpain-mediated proteolysis of alpha-fodrin compared to cardiomyocytes from control hearts, without any changes in necrosis (275). Cardiomyocytes overexpressing TRPC3 also demonstrated increased sensitivity to high extracellular Ca2+-induced apoptosis without exhibiting increased sensitivity to TNF-α-induced apoptosis. Interestingly in the human, TRPC5 expression, but not that of TRPC3, is increased as a result of heart failure (137), raising the question as to the role of TRPC5 in the control of cardiac function in the human.
Human disease II: idiopathic pulmonary arterial hypertension (IPAH)
PASMCs of patients with IPAH express higher levels of both mRNA and protein for TRPC3 and TRPC6 compared to PASMCs from normotensive patients or patients with secondary pulmonary hypertension (144). The increased TRPC6 expression may be due to a role of TRPC6 in the control of PASMC proliferation that is associated with hypertension (see discussion below under TRPC6). Similar to what was reported for TRPC1 above, treatment of PASMCs from IPAH patients with forskolin to activate adenylyl cyclase increased the expression of both protein and mRNA for TRPC3. This increase is dependent on cAMP-dependent protein kinase (PKA) and not observed in PASMCs from control patients (101). The actions of cAMP on TRPC function in PASMCs are complex. Short-term treatment for 30 min of PASMCs with forskolin inhibited SOCE in PASMCs from control and IPAH patients. However, treatment with forskolin for 4 h did not inhibit SOCE in PASMCs from IPAH patients, whereas SOCE was inhibited in PASMCs from control subjects. The short-term actions of forskolin on SOCE inhibition were independent of PKA, suggesting an effect of cAMP on TRPC function that is either direct or dependent on an EPAC (exchange protein directly activated by cAMP) -Rap1-signaling cascade (276). The maintenance of SOCE levels in PASMCs from IPAH patients treated with forskolin is likely due to the increased expression of TRPC1 and TRPC3 observed in these cells. However, this picture is further complicated by the findings that treatment of PASMCs from IPAH patients with the prostacyclin agonist iloprost for 24 h, which would increase cAMP levels, decreased TRPC3 expression and SOCE in these cells (101). Thus, the roles played by the cAMP signaling pathway in control of TRPC function and expression remain to be fully elucidated.
TRPC4
Endothelial cells
Perhaps the best-characterized physiological role for TRPC4 is in the regulation of endothelial cell function. Primary cultures of mouse aortic endothelial cells from wild-type mice exhibit a current that is activated by store depletion—Icrac—and is associated with an increase in Ca2+ entry—SOCE. Both the store-operated current and Ca2+ entry are absent in vascular endothelial cells from Trpc4−/− mice (45). Wild-type endothelial cells also demonstrate ROCE in response to purinergic and muscarinic agonists. Agonist-induced ROCE is also absent in endothelial cells from Trpc4−/− mice. Finally, endothelial-dependent vasorelaxation of aortic rings in response to muscarinic stimulation is markedly impaired compared to wild-type mice (45). These findings indicate that TRPC4 is a required component of SOCE channels in vascular endothelial cells and that TRPC4 is part of the Ca2+ entry signal transduction pathway regulating vascular tone.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic nucleotide-regulated Cl− channel, which has been proposed to be involved in endothelial transport, pH control, and nitric oxide signaling (160). Aortic vascular endothelial cells from wild-type and TRPC4-null mice express CFTR. However, cAMP fails to activate CFTR in vascular endothelial cells from TRPC4-deficient mice. These findings suggest that TRPC4 is required for normal CFTR function in vascular endothelial cells (160).
Hypoxia sensed by endothelial cells activates transcription factors leading to the production of growth factors, which stimulate smooth muscle cell proliferation, resulting in vascular remodeling and hypertension (277). One of the transcription factors involved in this process is AP-1, which regulates Ca2+-sensitive genes. Culture of human pulmonary artery endothelial cells under hypoxic conditions results in increased TRPC4 mRNA and protein expression, enhanced SOCE, and enhanced Icrac (161). This is accompanied by enhanced binding of AP-1 to a number of AP-1-responsive genes involved in proliferation. Expression of siRNA against TRPC4 in endothelial cells prevented hypoxia-induced increases in TRPC4 expression and AP-1 binding. Thus TRPC4 appears to be involved in mediating some aspects of hypoxia-induced gene expression and cell proliferation.
Endothelial TRPC4 function is not limited to the vasculature. Lung endothelial cells respond to thrombin via the PAR-1 receptor with enhanced Ca2+ influx and increased endothelial permeability (278). Cultures of lung endothelial cells from Trpc4−/− mice fail to respond to either thrombin or a PAR-1 agonist peptide with enhanced Ca2+ influx (47). The abnormal Ca2+ influx in TRPC4-null endothelial cells was associated with a lack of thrombin-mediated actin-stress fiber formation, as well as a reduced cellular retraction response. TRPC4 knockout also inhibits PAR-1 receptor-mediated increase in vascular permeability in isolated-perfused mouse lungs, demonstrating a role for TRPC4 in mediating thrombin action in the lung (47). The inability of thrombin to induce actin-stress fiber formation in TRPC4-deficient endothelial cells may be a direct result of TRPC4 interaction with the endothelial cell cytoskeleton under normal conditions. The cytoskeleton controls SOCE channel activity in endothelial cells (279), and this appears to be the result of a direct interaction of TRPC4 with protein 4.1, an endothelial cytoskeleton protein. Thus, preventing interaction between TRPC4 and protein 4.1 prevents SOCE channel activation (162). In addition, the intracellular C-terminal region of TRPC4 has been shown to interact with cytoskeletal αII- and βV-spectrin (280). This interaction is responsible, in part, for the plasma membrane expression of TRPC4. In the absence of TRPC4, normal cytoskeletal interactions would be disrupted, resulting in the inability of thrombin to induce actin-stress fiber formation.
CNS
Within the CNS, TRPC4 is expressed in thalamic interneurons of the dorsal geniculate nucleus. Serotonin acting via 5-HT2 receptors located on interneurons of the dorsal geniculate nucleus stimulates the secretion of γ-aminobutyric acid (GABA) from F2 terminal dendrites in a Ca2+-dependent fashion (48). 5-HT2 receptor-mediated stimulation of GABA release is absent in Trpc4−/− mice, indicating that receptor-mediated Ca2+-influx via TRPC4 is required for neurotransmitter release from thalamic interneuron dendrites. In addition, in the rodent brain, TRPC4 together with TRPC5 have been shown to be highly expressed throughout the frontal cortex, lateral septum, pyramidal cell layer of the hippocampus, dentate gyrus, and ventral subiculum (153). Pyramidal neurons within the prefrontal cortex respond to mGluR1 stimulation with a burst-induced nonselective cation current-mediated slow afterdepolarization, which was correlated with expression of TRPC4 and TRPC5, suggesting that this slow afterdepolarization is mediated by TRPC4 and TRPC5 (153). Within hippocampal slices, mGluR1 stimulation results in short and long epileptiform discharges (281). The long, but not the short discharges are inhibited by SKF96365, an inhibitor of TRPC-like channels, suggesting a role for a TRPC channel in this process (154). Hippocampal mGluR1 stimulation did not alter the expression of either TRPC4 or TRPC5 but did result in a translocation of both TRPC4 and TRPC5 from the plasma membrane to the cytosol (154). The authors of these studies suggest that translocation to the cytosol is a form of cellular desensitization (154).
Neuronal regeneration
Neuronal axotomy has been used as an in vivo model to study nerve regeneration. Following sciatic nerve axotomy in the rat, neuronal levels of mRNA for TRPC4 and TRPC5 and protein levels of TRPC4 are increased (155). Axonal injection of dibutyryl cAMP, which accelerates regeneration (156), also increased TRPC4 mRNA levels. Using both cultured dorsal root ganglion cells and dorsal root ganglion cell lines, Wu et al. (155) demonstrated the presence of TRPC4 in the growth cones of these cells. These authors went on to show that treatment of these cells with shRNA directed against TRPC4 inhibited the ability of either NGF or dibutyryl cAMP to stimulate neurite outgrowth and that the overexpression of TRPC4 could overcome the inhibitory action of the shRNA. These findings suggest a role for TRPC4 in the response to neural injury and in the regulation of neurite outgrowth.
Gastrointestinal pacemaker cells
Phasic contraction of gastrointestinal (GI) smooth muscle is controlled by interstitial cells of Cajal (ICC), which are pacemaker cells that generate rhythmic oscillations in membrane potential called slow waves (282). This pacemaker activity appears to involve [Ca2+]i oscillations and the activation of nonselective cation channels. The [Ca2+]i oscillations in cultured ICC cells are insensitive to the L-type calcium channel blocker nifedipine and are blocked by SKF96365, the inhibitor of TRPC-like channels (165). RT-PCR analysis reveals that TRPC4 and TRPC6 are the only TRPC mRNA transcripts identified in ICC cells (164). However, only protein for TRPC4 could be detected by Western blot analysis and immunocytochemistry, suggesting the involvement of TRPC4 in these oscillations (165). Supporting this suggestion are studies in which the comparison of the channel properties of the native nonselective cation channel in ICC cells with that of TRPC4 expressed in HEK-293 cells revealed that both channels exhibited similar electrophysiological properties. However, when slow waves were analyzed in mouse stomachs from TRPC4-null animals, no differences were detected in the properties of these slow waves compared to those from control mice, suggesting the involvement of another TRPC-like channel in the control of slow waves of the stomach (166).
VSM
In cultured rat VSM cells, Lindsey et al. (283) showed that cyclic stretch for 6 h reduced TRPC4 protein levels and cyclopiazonic acid-induced Ca2+ entry but did not alter TRPC6 protein levels or AVP-induced Ca2+ entry. Cessation of cyclic stretch resulted in a return of TRPC4 protein expression to prestretch levels within 2 h. These authors suggested that stretch-induced down-regulation of TRPC4 expression and SOCE may be a protective mechanism to offset stretch-induced increases in [Ca2+]i.
In addition to a potential involvement in the control of smooth contraction, TRPC4 appears to be involved in the regulation of VSM proliferation. Purinergic stimulation of either rat or human PASMCs with low doses of ATP enhances proliferation (163). Studies with human PASMCs demonstrate that enhanced proliferation was associated with an increase of TRPC4 expression and an increase in SOCE induced by cyclopiazonic acid. Treatment of these cells with siRNA against TRPC4 inhibited purinergic-induced proliferation, the increase in TRPC4 expression and enhanced SOCE (163). ATP-induced increases in TRPC4 expression were preceded by increases in phospho-CREB (cyclic AMP response element-binding protein). Blockade of purinerigic receptor activation or inhibition of protein kinase activity prevented both the phosphorylation of CREB and the up-regulation of TRPC4 expression. Furthermore, transfection of these smooth muscle cells with a nonphosphorylatable CREB mutant inhibited ATP-induced increases in TRPC4 expression, suggesting that CREB phosphorylation is required for the observed increase in TRPC4 expression on ATP treatment (163).
Epithelial cells
EGF-induced proliferation of corneal epithelial cells also appears to involve TRPC4. EGF-induced stimulation of rabbit (157) and human (158) corneal epithelial cell proliferation results in enhanced Ca2+ entry. TRPC4 could be localized to the plasma membrane of rabbit corneal epithelial cells by confocal immunocytochemistry and immunogold electron microscopy (157), and siRNA knockdown of TRPC4 protein expression resulted in suppression of EGF-induced proliferation, inhibition of cyclopiazonic acid-induced Ca2+ entry and suppression of store-operated and EGF-induced channel activity in human corneal epithelial cells (158). Enhanced Ca2+ entry in response to EGF is likely due, in part, to the translocation to TRPC4 to the plasma membrane in response to EGF, which requires Src family tyrosine kinase-mediated phosphorylation of TRPC4 (49).
Above, we presented evidence for the involvement of TRPC4 in serotonin-mediated secretion of the neurotransmitter GABA (48). A role for TRPC4 in the control of secretion may be more universal. Increased angiogenesis is a hallmark of neoplasia and is a balance between proangiogenic and antiangiogenic factors. Thrombospondin-1 (TSP1) is an angiogenesis inhibitor that is synthesized and secreted by normal renal tissue (167). Although human renal cell carcinoma synthesizes TSP1, it is not secreted. Analysis of human renal cell carcinoma cells revealed reduced Ca2+ uptake in response to vascular endothelial cell growth factor (VEGF) and reduced TRPC4 expression compared to normal renal epithelium. Further, transfection of normal renal epithelial cells with siRNA against TRPC4 increased cytoplasmic retention of TSP1 and decreased TSP1 secretion, demonstrating the critical role for TRPC4 in TSP1 secretion (167).
Heart
SERCA pumps play an important role in controlling [Ca2+]i in the heart. Treatment of neonatal rat cardiomyocytes with siRNA targeting SERCA2 resulted in increased expression of TRPC4 and TRPC5 and in an enhanced Ba2+ entry in response to thapsigargin (159). Down-regulation of SERCA2 was also associated with an increase in the expression of transcription factors, SP1, MEF2, and NFAT. These findings were interpreted to indicate that down-regulation of SERCA2 leads to activation of calcineurin, and the increase in calcineurin-dependent transcription factors eventually leads to increased TRPC4 and TRPC5 expression (159). These findings are similar to those described above for Darier’s disease, in which loss of SERCA expression leads to increased TRPC1 expression (120).
TRPC5
CNS, neuronal function, and differentiation
Although many studies have been conducted on the biochemical properties of heterologously expressed TRPC5 (reviewed in ref. 284), with a few exceptions, the physiological functions of TRPC5 remain largely unknown. One area where a biological function for TRPC5 has been established is in the CNS. TRPC5 mRNA is highly expressed in hippocampal neurons in vivo (168), and TRPC5 protein has been localized throughout hippocampal neurons in vitro, including the growth cone (169). TRPC5 appears to be transported to the growth cone in synaptic-like vesicles. Transfection of hippocampal neurons with dominant-negative TRPC5 resulted in cells with longer neurites and thinner growth cones with longer filopodia (169), suggesting that Ca2+ influx via TRPC5 inhibits neurite outgrowth. TRPC5-mediated inhibition of neurite outgrowth may be due in part to TRPC5 interaction with neuronal calcium sensor-1 (NCS-1). Expression of dominant negative constructs of NCS-1 and TRPC5 in PC12 cells enhances neurite outgrowth. These enhancing actions are not additive, suggesting a common mechanism (170). Furthermore, dominant-negative NCS-1 inhibited both receptor- and thapsigargin-mediated activation of TRPC5. In addition, TRPC5 and NCS-1 were shown to coimmunoprecipitate from rat brain, suggesting the Ca2+-sensing activity of NCS-1 regulates TRPC5 function, which, in turn, controls neurite outgrowth (170).
The role of TRPC5 in the control of neurite outgrowth appears to be more complicated. Cross-linking of GM1 gangliosides by the B subunit of cholera toxin (CTX-B) promotes neuritogenesis (285) that requires Ca2+ influx via a voltage-independent Ca2+ channel (286). Axonal-like neurite outgrowth mediated by CTX-B-induced cross-linking of GM1 gangliosides is observed in undifferentiated NG108-15 (neuroblastoma- glioma hybrid) cells but not in differentiated NG108-15 cells or Neuro-2A neuroblastoma cells (62). Both differentiated and undifferentiated NG108-15 cells express mRNAs for TRPC1 and TRPC5, with TRPC5 mRNA expression being reduced in differentiated NG108-15 cells, while Neuro-2A cells express TRPC3 and TRPC6 but not TRPC5. Treatment of undifferentiated NG108-15 cells with siRNA against TRPC5 prevented CTX-B-induced neuritogenesis, as well as CTX-B-induced Ca2+ influx (62). These authors then demonstrated that the GM1 gangliosides interacted with α5β1 integrin and that TRPC5 activation was the result of a signaling cascade that involved focal adhesion kinase, phospholipase Cγ, and phosphoinositide-3 kinase. Wu et al. (62) suggest that the differences in their findings and those of Greka et al. (169) may be due, in part, to differences in the state of differentiation of the cells used in the two studies. Clearly, further studies are required to reconcile the differences.
Pyramidal neurons in the rat lateral amygdala respond to glutamate with a group I mGluR-mediated (mGluR1 and mGluR5) sESPC that is dependent on phospholipase C activation (59). The mGluR-activated current was nonselective and could be blocked by the TRPC channel inhibitors 2-aminoethoxydiphenyl borate (2-APB) and SKF96365. Lateral amygdala pyramidal neurons expressed TRPC1 and TRPC5. Intracellular application of antibodies against TRPC5, but not TRPC1, to these pyramidal neurons caused rundown of the glutamate-evoked current, suggesting that TRPC5 channels mediate the mGluR-induced response.
VSM cell migration
VSM cell migration and neointima formation following vascular injury are important components of atherosclerosis. Sphingosine-1-phosphate (S1P) binding to either Gi, Gq, or G12/13-coupled receptors stimulates VSM migration (287). SP1 induces Ca2+ entry in cultured human saphenous vein smooth muscle cells (60). Further, human saphenous vein and primary culture of VSM from human saphenous vein express both TRPC1 and TRPC5. Treatment of these cells with antibodies against TRPC5 or with 2-APB inhibited S1P-induced smooth muscle migration, as did transfection of these cells with dominant negative constructs of TRPC5. Thus, in a manner analogous to the proposed role of TRPC5 in promoting neurite outgrowth (62), TRPC5 also appears to mediate S1P-induced smooth muscle migration (60).
Endothelial cells
Nitric oxide (NO), by controlling vasoconstriction and vasorelaxation, plays an important role in regulating cardiovascular function under normal and disease states (288). Bovine aortic endothelial cells show increased expression of TRPC5 protein in culture, and NO induces Ca2+ influx in these cells (87). Treatment of endothelial cells with siRNA against TRPC5 or transfection with dominant-negative TRPC5 prevented NO-induced Ca2+ influx. These studies also demonstrated that NO caused cysteine S-nitrosylation of TRPC5 on Cys553 and Cys558 located in the S5-S6 loop region of the channel. Further, mutation of these cysteine residues to serine abrogated TRPC5 responses to NO. Cysteine residues are present at comparable positions in TRPC1 and TRPC4, and both channels are activated by NO. In addition, treatment of endothelial cells with the P2Y receptor agonist ATP, which activates endothelial nitric oxide synthase (289), induced nitrosylation of native TRPC5 channels (87). Thus, the vasodilatory action of NO may, in part, be due to NO-induced activation of endothelial TRPC5 channels. This also provides endothelial cells with a positive feedback loop in which Ca2+ influx mediated by NO-induced nitrosylation of TRPC channels leads to increased nitric oxide synthase activity, increasing NO production, resulting in enhanced smooth muscle relaxation.
Mast cells
The release of granules from activated mast cells in response to IgE is dependent not only on Ca2+ influx (290), but also on the influx of Sr2+ (291). TRPC5 has recently been suggested to be involved in IgE-mediated influx of both cations and the degranulation of mast cells (61). Rat RBL-2H3 mast cells express mRNA for TRPC1, C2, C3, C5, and C7 and protein for TRPC1, C3, and C5. These cells respond to both IgE and thapsigargin with degranulation, and enhanced Ca2+ and Sr2+ influx. Treatment of these cells with shRNA directed against TRPC5, but not TRPC3 or TRPC7, inhibited thapsigargin-induced influx of both Ca2+ and Sr2+, while shRNA directed at TRPC1 gave mixed results (61). Two additional molecules, STIM and Orai, have been identified that play an important role in store depletion-activated Ca2+ influx and the Icrac current associated with it (reviewed in refs. 193, 292), and STIM and Orai have been shown to interact with TRPC channels to enhance Ca2+ influx (2, 3, 293). Ma et al. (61) showed that shRNA directed against STIM1 and Orai1 inhibited mast cell thapsigargin-induced Ca2+ and Sr2+ influx. Inhibitory RNA against TRPC5, STIM1, and Orai1 also inhibited IgE-mediated degranulation of mast cells, as well as the influx of both Ca2+ and Sr2+. Thus, it appears that an interaction among TRPC5, STIM1, and Orai1 is responsible for mediating IgE-induced ion influx and mast cell degranulation (61).
Human disease
TRPC5 has been implicated in rheumatoid arthritis. Fibroblast-like synovial (FLS) cells secrete matrix metalloproteinases (MMPs), which play an important role in the development of arthritis (294). Concentrations of reduced thioredoxin (rTRX) found in synovial fluid of patients with rheumatoid arthritis are capable of inhibiting MMP secretion from cultured FLS cells (88). TRPC5, but not TRPC1, expressed in HEK293 cells is activated by rTRX. Activation of TRPC5 by rTRX is the result of reduction of an extracellular disulfide bond between Cys553 and Cyc558 in TRPC5. Human FLS cells express TRPC1 and TRPC5 and exhibit a nonselective current that is activated by rTRX. Transfection of FLS cells with dominant-negative TRPC5 or treatment with an antibody to TRPC5 or TRPC1 inhibited the rTRX-induced current and activated MMP secretion, suggesting that TRPC5-TRPC1 channels negatively controls MMP secretion from FLS cells (88). It should be noted that activation of TRPC5 by rTRX acting to reduce a disulfide bond between Cys553 and Cys558 (88) conflicts with the report that TRPC5 is activated by nitrosylation of Cys553 and Cys558 (87).
TRPC6
VSM
The first evidence for a role of TRPC6 in smooth muscle came from studies demonstrating that the nonselective cation channel in VSM that is activated by α1-adrenergic agonists can be blocked by suppressing TRPC6 expression using antisense oligonucleotides (64). Subsequently, a number of agonists acting through G protein-coupled receptors and receptor tyrosine kinases have been shown to activate TRPC6 in VSM (Table 1). TRPC6 has also been shown to be involved in the regulation of myogenic tone in VSM. Myogenic tone in small-resistance vessels plays an important role in the regulation of blood flow and the elevation of intravascular pressure in these vessels increases myogenic tone, a phenomenon described by Bayliss in 1902 (295). Treatment of rat cerebellar arteries with antisense oligonucleotides directed against TRPC6 inhibited vasoconstriction induced by elevating intravascular pressure and suppressed pressure-induced depolarization of VSM cells (177). The mechanism by which elevated intravascular pressure activates TRPC6 is likely related to the findings that TRPC6 is activated by stretch (296).
Vascular hypertension
TRPC6 has been implicated in animal models of vascular hypertension. The deoxycorticosterone acetate (DOCA) -salt hypertensive rat is a well-studied rat model of hypertension which demonstrates increased agonist-mediated VSM contractility associated with elevated blood pressure (297). Mesenteric artery smooth muscle cells from DOCA-salt hypertensive rats display an enhanced serotonin- and norepinephrine-induced nonselective cation current activity compared to smooth muscle cells from control rats (179). This increase in channel activity was associated with an increase in the expression of TRPC6 but not TRPC1 or TRPC3. Mineralocorticoid receptor-induced increases in TRPC6 mRNA levels could also be demonstrated in aldosterone-treated rat A7r5 VSM cells, suggesting that increased TRPC6 expression is a key component of enhanced VSM reactivity associated with mineralocorticoid-induced hypertension (179).
Cardiac hypertrophy
As mentioned above, based in part on studies using siRNA directed against TRPC3 and TRPC6, angiotensin II-induced cardiac hypertrophy has been shown to be mediated by a Ca2+-calcineurin-NFAT signaling pathway that is dependent on activation of TRPC3 and TRPC6 (69, 274). In addition, mice overexpressing calcineurin in the heart, a model of cardiac hypertrophy, demonstrate enhanced TRPC6 expression that correlated with the extent of hypertrophy (173). TRPC6 expression was also increased in mice subjected to aortic banding, in cultured rat cardiomyocytes exposed to the hypertrophic agonist endothelin-1 and in hearts from human patients with dilated cardiomyopathy. Kuwahara et al. (173) also showed that the promoter regions of mouse, rat, and human genes for TRPC6 contain two functional NFAT binding sites and that overexpression of TRPC6 in rat cardiomyocytes enhances NFAT activity, as well as NFAT-dependent transcription. These authors also showed that transgenic mice overexpressing TRPC6 in the heart develop cardiac hypertrophy, have a heightened response to cardiac stress, show enhanced NFAT-dependent transcriptional activity, and are prone to cardiac failure and premature death. Thus, TRPC6 appears to form part of a positive feedback mechanism involving the Ca2+-calcineurin-NFAT signaling pathway, which when overstimulated in cardiomyocytes, leads to pathological cardiac remodeling.
Cardiac fibroblasts
TRPC6 appears to regulate cardiac fibroblast function differently than cardiomyocyte function (174). Cardiac fibroblasts are a major cellular component of the heart, and their transformation to myofibroblasts plays an important role in the production of extracelluar matrix proteins that are responsible for cardiac fibrosis associated with heart disease (298). The α subunits of the heterotrimeric G proteins G12/13 mediate agonist-induced increases in [Ca2+]i and NFAT activity via a TRPC-like Ca2+ influx pathway in cardiac fibroblasts (299, 300). Rat cardiac fibroblasts express TRPC1, TRPC3, TRPC6, and TRPC7 (174). Only TRPC6 expression was increased by constitutively active G13α or treatment with either angiotensin-II or endothelin-1 in a tyrosine kinase-reactive oxygen species-JNK-dependent pathway. Enhanced TRPC6 expression was associated with increased NFAT activity, which could be blocked by treatment with siRNA-directed against TRPC6. In contrast to the situation in cardiomyocytes (69, 173), inhibition of calcineurin in cardiac fibroblasts increased TRPC6 mRNA levels, while constitutively active NFAT decreased TRPC6 mRNA levels (174). Furthermore, the transformation of cardiac fibroblasts to myofibroblasts and the associated increase in synthesis of extracellular matrix proteins is inhibited by siRNA directed against TRPC6. Thus, TRPC6 appears to have two opposing activities in heart disease, increasing cardiomyocyte hypertrophy, which enhances the cardiac disease state, and inhibiting myofibroblast formation and function, which suppresses cardiac fibrosis.
Vascular endothelial cells
As mentioned earlier, thrombin regulates endothelial cell function, in part, via changes in cell contraction and cell shape, resulting in increased endothelial permeability. In addition, actinomycin-mediated endothelial cell shape changes induced by thrombin are dependent on increases in [Ca2+]i and activation of RhoA and myosin light chain kinase (301). Studies with cultured human pulmonary artery endothelial cells demonstrate that siRNA directed against TRPC6 inhibits thrombin-induced Ca2+ entry, RhoA activation, and myosin light chain phosphorylation (71). Treatment of pulmonary artery endothelial cells with TRPC6 siRNA prevented thrombin-induced actin stress fiber formation and inhibited thrombin-induced reduction in transendothelial resistance. These studies suggest that not only TRPC4 (vide supra) but also TRPC6 play an important role in regulating endothelial cell permeability.
Endothelial cell migration plays an important role in vascular remodeling and regeneration. Recently, a TRPC6-TRPC5 activation cascade has been shown to regulate endothelial cell migration (302). Lysophosphatidylcholine (lysoPC) is an abundant phospholipid, which inhibits endothelial cell migration and inhibition of migration, is dependent on Ca2+-influx via a nonvoltage-gated calcium channel (303). Treatment of bovine aortic endothelial cells with lysoPC induced the translocation of both TRPC5 and TRPC6 to the plasma membrane with TRPC6 being translocated within 1 min of treatment and TRPC5 at 3 min (302). Treatment of endothelial cells with siRNA against either TRPC5 or TRPC6 reduced lysoPC-induced increases in [Ca2+]i and reduced lysoPC-induced inhibition of migration. Further, aortic endothelial cells from Trpc6−/− mice treated with lysoPC displayed reduced increases in [Ca2+]i, reduced inhibition of migration and failure to respond to lysoPC with TRPC5 translocation to the plasma membrane. Thus lysoPC-induced inhibition of endothelial cell migration appears to be the result of a signaling cascade, in which lysoPC first causes the translocation of TRPC6 to the plasma membrane followed by TRPC5, resulting in increases in [Ca2+]i and inhibition of cell migration (302).
Neuronal cell function
Within the CNS, several functions have been proposed for TRPC6. One of the earliest proposed functions of TRPC6 in the nervous system involves iron transport. Iron is required for many cellular processes and is taken up by cells via transferrin-dependent and transferrin-independent mechanisms (304). NGF-treatment of PC12 cells results in an increase in nontransferrin-bound iron uptake, which was accompanied by an increase in TRPC6 mRNA expression (171). Treatment of TRPC6-expressing PC12 cells with DAG, which activates TRPC6, stimulated iron uptake. Furthermore, overexpression of TRPC6 in HEK 293 cells resulted in enhanced agonist-mediated uptake of both ferrous and ferric iron, suggesting that TRPC6 is involved with transferrin-independent uptake of iron (171).
As summarized earlier under TRPC3, TRPC6 is required for BDNF-induced neuronal outgrowth and turning, protects cerebellar granule neurons against serum deprivation-induced cell death in vitro, and promotes BDNF-mediated cerebellar granule neuron survival in the brain (76, 133). Recent studies also suggest an important role for TRPC6 in the formation of excitatory synapses and memory (305). TRPC6 expression in the rat hippocampus peaks between postnatal day 7 and day 28, with expression being enriched in synaptosomes and excitatory postsynaptic sites. Overexpression of TRPC6 in cultured hippocampal neurons increased spine density, which was shown to be mediated by a CaM kinase IV-CREB pathway, while expression of RNAi against TRPC6, but not TRPC1, inhibited spine formation. Treatment with RNAi against TRPC6 also reduced the frequency of miniature excitatory postsynaptic currents. Zhou et al. (305) developed transgenic mice overexpressing TRPC6 in the forebrain to test the role of TRPC6 in spine formation and memory in vivo. TRPC6 transgenic mice expressed higher levels of TRPC6 in postsynaptic vesicles than wild-type mice, demonstrated increased spine density of CA1 hippocampal neurons, and showed enhanced performance in spatial learning and memory assessed by the Morris water test. TRPC6 also promotes dendritic growth in cultured rat hippocampal neurons via a CaM kinase IV-CREB pathway (306). Thus, TRPC6 appears to play an important role in neuronal differentiation, as well as in mediating synaptic and behavioral plasticity.
Eryptosis
Erythrocyte cell death, also called eryptosis, can be induced by a variety of stimuli, including oxidative stress and Cl− depletion, is characterized by phosphatidylserine exposure at the outer membrane leaflet, and is dependent on increased [Ca2+]i (307). Because erythrocytes lack internal organelles that store Ca2+, increases in [Ca2+]i depend on entry though channels located in the plasma membrane. Under resting conditions human erythrocyte ghosts leak Ca2+, resulting in increased [Ca2+]i, and undergo eryptosis (184). Human and mouse erythrocytes contain TRPC6, and treatment of human erythrocytes with antibodies to TRPC6 but not TRPC3 inhibits Ca2+ influx. Erythrocyte ghosts from Trpc6−/− mice subjected to Cl− depletion to induce eryptosis display reduced Ca2+ influx and reduced phosphatidylserine exposure at the outer membrane leaflet compared to erythrocyte ghosts from wild-type mice, suggesting that TRPC6 participates in Ca2+-induced erythrocyte cell death (184).
Human diseases
Renal functions
Several mutations in TRPC6 are associated with the development of familial focal segmental glomerulosclerosis (FSGS; refs. 180, 181). FSGS is a disease of glomerulus dysfunction that is associated with proteinuria, hypertension, and renal insufficiency, leading to kidney failure (308). TRPC6 is expressed throughout the kidney, both in glomeruli and tubular epithelia, and has also been localized to glomerular podocytes (180, 181). The podocyte foot process together with the surrounding glomerular slit diaphragm form part of the permeability barrier in the glomerulus, whose function is disrupted in FSGS. To date, a total of six TRPC6 mutations (P111Q, R895C, E897K, A270T, N134I, and K874Ter) have been identified from six different families, which were inherited in an autosomal dominant pattern (180, 181). Three of the mutant channels (P112Q, R895C, and E897K) demonstrate enhanced receptor-mediated activation when expressed in HEK293 cells in response to angiotensin II (in the case of P112Q) or carbachol (in the case of R895C and E897K) when compared to wild-type TRPC6. The P112Q mutant channel also demonstrated enhanced plasma membrane expression in HEK293 cells compared to wild-type TRPC6. However, neither the S270T nor N134S missense mutations nor the 57-amino acid truncated (K874Ter) mutation showed evidence of altered channel function (181).
In addition to gain-of-function mutations in TRPC6-causing FSGS, changes in channel expression may also contribute to the disease. As mentioned above, the P112Q mutation demonstrates increased plasma membrane expression (180). Increased TRPC6 expression is also found in glomeruli from patients with other proteinuric renal diseases, such as membranous glomerulonephritis and minimal-change disease (182). TRPC6 expression is also increased in cultured mouse podocytes exposed to complement, and in glomeruli from two rat models of proteinuric renal disease, passive Heymann nephritis, and puromycin aminonucleoside-induced albuminuria (182). Furthermore, overexpression of TRPC6 in cultured podocytes leads to a loss of actin stress fibers, and delivery of plasmids expressing TRPC6 to the kidney of mice in vivo resulted in increased expression of TRPC6 in podocytes and the induction of proteinuria, suggesting that increased TRPC6 expression leads to disruption of podocyte cytoskeletal integrity and enhanced secretion of protein into the urine (182).
Mutations in podocin, a component of the slit diaphragm, also cause FSGS (309). Podocin is a prohibitin homology-domain protein that binds cholesterol. When coexpressed with TRPC6 in HEK293 cells, podocin and TRPC6 coimmunoprecipate, and the two proteins colocalize at the slit diaphragm of the podocyte foot-process in rat kidney (310). Podocin increases TRPC6 channel activtity when coexpressed in Xenopus oocytes. However, mutant podocin unable to bind cholesterol failed to enhance TRPC6 channel activity. Huber et al. (310) then showed that podocin, but not mutant podocin, enhanced histamine-stimulated Ca2+ influx in HeLa Cx43 cells coexpressing both proteins and that depletion of cholesterol with methyl-β-cyclodextrine abolished podocin-dependent increases in TRPC6 activity. In other studies, mice that are deficient in nephrin, another component of the slit diaphragm, display proteinuria, foot-process effacement (311), and increased TRPC6 expression (181). Thus, it appears that the components of the slit diaphragm form a complex protein filtration unit, requiring the proper interaction of each component, including the cytoskeleton, membrane lipids, membrane proteins, and TRPC6 in order to maintain the normal protein filtering function of the kidney.
Hyperglycemia: opposite changes in mesangial cells and platelets
TRPC6 has also been implicated in renal hyperfiltration associated with diabetic nephropathy. Renal hyperfiltration observed in diabetes is the result of altered contractile responsiveness of renal microvasculature and glomerular mesangial cells (201). The vasoconstrictor angiotensin-II stimulates membrane currents and Ca2+ influx in cultured human mesangial cells that are mediated by TRPC6 (183). Incubation of mesangial cells in the presence of high glucose (30 mM) for 7 days depressed angiotensin-II-mediated Ca2+ influx, which was associated with a decrease in TRPC6 mRNA and protein expression, without changes in TRPC1 or TRPC3 expression. Significant changes in TRPC6 mRNA and protein expression could be detected at 1 and 2 days of glucose treatment, the earliest time points examined. Streptozotocin-induced diabetes in rats was likewise associated with a decrease in glomerular TRPC6 but not TRPC1 expression. Thus, the reduced mesangial responsiveness to vasoactive agents seen in diabetes may be due, in part, to reduced TRPC6 expression.
In a related study, Liu et al. (185) showed that exposure of human platelets to 25 mM glucose for 60 min resulted in enhanced OAG-induced Ca2+ influx, which was inhibited by treatment with phosphatidylinositol 3-kinase (PI3K) inhibitors. Glucose treatment of platelets also resulted in an increased cell surface expression of TRPC6 but not TRPC1, TRPC3, TRPC4, or TRPC5. Significant increases in the cell surface expression of TRPC6 were observed within 40 min of glucose exposure. This increase could be prevented by treatment with PI3K inhibitors, suggesting that glucose induced a PI3K-dependent translocation of TRPC6 to the cell surface. Examination of platelets from patients with type 2 diabetes mellitus also showed an increase in cell surface TRPC6 expression. Thus, it appears that high circulating glucose levels associated with diabetes mellitus have opposite effects on TRPC6 expression in renal glomerular mesangial cells and platelets. Coincidentally, while mesangial cell responsiveness to vasoactive agents is reduced, platelets from diabetic patients are hyperreactive (reviewed in ref. 312). Elevated TRPC6 may, at least partially, be at the root of platelet hyperreactivity in diabetes mellitus.
Pulmonary hypertension
As mentioned earlier, TRPC1 and TRPC6 expression are up-regulated by hypoxia in pulmonary artery smooth muscle, and these channels have been implicated in hypoxia-induced hypertension (102, 103). Furthermore, TRPC6 expression is enhanced in pulmonary artery smooth muscle from patients with idiopathic pulmonary arterial hypertension (144). Analysis of pulmonary function in TRPC6-null mice provides additional evidence for a role for TRPC6 in response to hypoxia (175). Exposure of normal mouse lungs to hypoxic conditions produces a biphasic vasoconstrictor response, with the initial vasoconstrictor response developing within 10 min and the second response after 1 h of hypoxic exposure. In Trpc6−/− mice, the initial hypoxia-induced vasoconstrictor response is absent, while the second vasoconstrictor response is normal. The inability of TRPC6-null mice to mount the initial vasoconstrictor response results in severe hypoxemia in Trpc6−/− but not in wild-type mice after being subjected to partial occlusion of alveolar ventilation. Interestingly, a lack of TRPC6 expression did not prevent TRPC6-null animals from developing pulmonary hypertension on chronic exposure to hypoxia for 3 wk. Hypoxia also induced Ca2+ influx and a nonselective cation current in wild-type but not TRPC6-deficient PASMCs. Weissmann et al. (175) also demonstrate that hypoxic exposure of PASMCs induced an accumulation of diacylglycerol, a known activator of TRPC6 (29). Unlike the situation in the aorta and cerebral arteries, where TRPC6-null mice demonstrate a compensatory increase in TRPC3 expression in these vessels (143), no such compensatory mechanism appears to be present in pulmonary artery smooth muscle from these animals (175). Thus, TRPC6 appears to mediate hypoxia-induced pulmonary vasoconstriction.
Excessive proliferation of pulmonary artery smooth muscle is a major cause of elevated pulmonary vascular resistance in patients with pulmonary hypertension (198). Platelet-derived growth factor (PDGF) and endothelin-1 are potent smooth muscle mitogens. Stimulation of PASMC proliferation by treatment with either PDGF or endothelin-1 increases the expression of TRPC6 (176, 178). PDGF-induced up-regulation of TRPC6 expression is preceded by phosphorylation of STAT3 as well as up-regulation of c-jun (176). These studies also showed that c-jun is capable of increasing TRPC6 expression and that antisense oligonucletides directed against TRPC6 inhibit PDGF-induced proliferation of rat pulmonary artery smooth muscle. It was shown further that the endothelin-1 antagonist bosentan inhibits not only endothelin-1-induced proliferation but also PDGF-induced proliferation of human pulmonary artery smooth muscle, that inhibition of proliferation is associated with inhibition of TRPC6 expression, and that bosentan inhibits PDGF-induced phosphorylation of STAT3 (178). Finally, in studies of pulmonary artery smooth muscle from patients with various forms of pulmonary hypertension, smooth muscle from patients with IPAH was shown to have elevated levels of TRPC6 and TRPC3 protein, which correlated with increased smooth muscle proliferation. Down-regulation of TRPC6 by TRPC6-specific siRNAs resulted in attenuated proliferation of pulmonary artery smooth muscle from patients with IPAH (144). Taken together, these studies indicated TRPC6 is required for proliferation of pulmonary artery smooth muscle and that overexpression of TRPC6 may be responsible for the increased smooth muscle proliferation observed in patients with IPAH.
Proliferation of malignant cells
The involvement of TRPC6 in the control of proliferation is not limited to smooth muscle. Phenylephrine acting via α1-adrenergic receptors stimulates proliferation of human prostate cancer epithelial cells (5). Phenylephrine-induced increases in Ca2+ influx and proliferation were inhibited in these cells by antisense oligonucleotides directed against TRPC6. In addition, phenylephrine treatment of prostate cancer epithelial cells resulted in increased TRPC6 protein expression and enhanced NFAT activity. In Huh-7 human hepatoma cells, overexpression of TRPC6 increased proliferation, whereas TRPC6-siRNA inhibited proliferation (313); and in human breast adenocarcinoma, TRPC6 mRNA and protein expression are increased compared to normal breast epithelial cells (314). These findings suggest that TRPC6 plays a central role in regulating proliferation in normal and malignant cells.
Cognitive functions
TRPC6 has been implicated in the antidepressive actions of St. John’s wort (172). Hyperforin is the main active ingredient in St. John’s wort, acting as an antidepressant by inhibiting neuronal monoamine uptake as a result of increasing intracellular Na+ concentrations ([Na+]i), decreasing the Na+ gradient, which drives the monoamine transporters (315). Hyperforin also increases Ca2+ influx, and hyperforin-induced increases in both [Na+]i and [Ca2+]i are blocked by the TRPC inhibitor SKF96365 in PC12 cells (172). Treatment of PC12 cells with either siRNA directed against TRPC6 or expression of dominant-negative TRPC6 constructs inhibited hyperforin-induced Ca2+ influx. In addition, overexpression of TRPC6, but not TRPC1, TRPC3, TRPC4, or TRPC5, in HEK 293 cells resulted in enhanced hyperforin-induced Ca2+ influx. Because TRPC6 is a nonselective cation channel and as Ca2+ only contributes ∼4% to whole-cell currents in HEK293 cells stably expressing TRPC6 in the presence of extracellular Na+ (316), activation of TRPC6 by hyperforin would increase [Na+]i and inhibit neurotransmitter uptake by monoamine transporters. Leuner et al. (172) showed further that both hyperforin and NGF stimulated neurite outgrowth from PC12 cells to the same degree and that hyperforin-induced neurite outgrowth could be inhibited by treating the cells with anti-TRPC6 siRNA, suggesting a second neuroprotective function for hyperforin mediated by TRPC6.
TRPC7
TRPC7 is activated by a variety of different hormone and neurotransmitter receptors (Table 1). TRPC7 is expressed in a number of different tissues (80, 81, 245), and its expression has been shown to change during pregnancy in the uterus (116, 119), with time of culture in keratinocytes as reviewed above (121), and during development in dorsal root ganglion cells (186). Very little is known, however, about TRPC7’s role in regulating the functions of these tissues and organs. Reports have appeared, however, implicating TRPC7 in apoptosis.
Apoptosis
TRPC7 has been implicated in the induction of apoptosis in two different cell systems (187, 188). Treatment of K562 human leukemia cells with PGE2 induces apoptosis following an increase in [Ca2+]i PGE2-induced apoptosis of K562 cells was associated with cell shrinkage, depolarization of the mitochondrial potential, increased phosphatidylserine externalization, caspase activation, and DNA fragmentation (188). Treatment of K562 cells with siRNA targeting TRPC7 resulted in a decrease in TRPC7 protein and inhibited PGE2-induced phosphatidylserine externalization and DNA fragmentation, suggesting that Ca2+ entry via TRPC7 is required for PGE2-induced apoptosis (188).
Apoptosis has been implicated as a contributing factor to the development of heart disease, and, as for PGE2-induced apoptosis, angiotensin II-induced apoptosis is initiated by elevating [Ca2+]i (317). As assessed by TUNEL staining, cultured neonatal cardiomyocytes display very little apopotsis, even when exposed to angiotensin II (187). Transfecting these cells with TRPC7 increased basal and angiotensin II-induced apoptosis. Angiotensin II-induced apoptosis was also associated with increased nuclear fragmentation, destruction of actin fibers, and decreased atrial natriuretic factor expression. Treatment of TRPC7-transfected cardiomyocytes with either the TRPC inhibitor SKF96365 or the calcineurin inhibitor FK506 inhibited angiotensin II-induced apoptosis. Satoh et al. (187) went on to analyze TRPC7 expression in heart failure in the Dahl salt-sensitive rat. Hearts from Dahl salt-sensitive rats have higher rates of apoptosis and express higher levels of TRPC7 mRNA than Dahl salt-resistant rats. In addition, treatment of Dahl salt-sensitive rats with an angiotensin-converting enzyme inhibitor suppressed both myocardial apoptosis and TRPC7 expression. These findings suggest that angiotensin-II activation of TRPC7 initiates a mycardial apoptotic cascade leading to heart failure involving the calcineurin pathway. Taken together, the studies in the K562 leukemia cells (188) and cardiomyocytes (187) suggest a central role for TRPC7 in mediating apoptosis.
CONCLUSIONS
Considering that the first mammalian TRPC channel was identified and cloned in 1995 (318, 319), investigators have just started to identify and characterize the physiological functions of these channels in native tissues. We have recently summarized a number of diseases and complex syndromes that map to chromosomal regions that encode TRP channel genes (320). Analysis of the potential role played by TRPC channels in these disease states may prove useful in identifying new physiological roles for these channels. An example of the utility of this approach can be found in a recent study in which a genome-wide single nucleotide polymorphism (SNP) -based linkage analysis of infantile hypertrophic pyloric stenosis identified chromosomal loci Xq23 and 11q14-q22 that code for TRPC5 and TRPC6, respectively (321). Although further work is required to definitely ascribe roles for TRPC5 and TRPC6 in infantile hypertrophic pyloric stenosis, the abnormal GI smooth muscle phenotype associated with this disease, and the recently uncovered roles of TRPCs in the control of smooth muscle function lead the authors to choose these channel genes as candidates for this genetic disease. It is expected that with the continued development of molecular tools and the use of transgenic and knockout (classical and conditional) mouse models, combined with overexpression of specific channels in specific tissues along with the analysis of disease states, will lead to further advances in our understanding of the roles played by TRPC channels in normal physiology and disease.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (project Z01 ES101684).
References
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong D L, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu M X, Armstrong D L, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen'kyi V, Slomianny C, Beck B, Mariot P, Bonnal J L, Mauroy B, Shuba Y, Capiod T, Skryma R, Prevarskaya N. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–2047. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci M C, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Rosado J A, Brownlow S L, Sage S O. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez M F, Dreja K, Xu S Z, Adner M, Beech D J, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- Saleh S N, Albert A P, Peppiatt C M, Large W A. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol (Lond) 577:479–495, 2006. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie F C, Mistry M, Brown D A. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Larsson K P, Peltonen H M, Bart G, Louhivuori L M, Penttonen A, Antikainen M, Kukkonen J P, Akerman K E. Orexin-A-induced Ca2+ entry: evidence for involvement of trpc channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, Hirose K, Mizushima A, Kurosaki M, Mori E, Gotoh K, Okada T, Fleig A, Penner R, Iino M, Kurosaki T. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J Exp Med. 2002;195:673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniotti S, Lovisolo D, Fiorio Pla A, Munaron L. Expression and functional role of bTRPC1 channels in native endothelial cells. FEBS Lett. 2002;510:189–195. doi: 10.1016/s0014-5793(01)03256-2. [DOI] [PubMed] [Google Scholar]
- Pla A F, Maric D, Brazer S C, Giacobini P, Liu X, Chang Y H, Ambudkar I S, Barker J L. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G X, Poo M M. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Shim S, Goh E L, Ge S, Sailor K, Yuan J P, Roderick H L, Bootman M D, Worley P F, Song H, Ming G L. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci U S A. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel M K, Marrero H, Birnbaumer L, Lemos J R, Florman H M. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Chu X, Cheung J Y, Barber D L, Birnbaumer L, Rothblum L I, Conrad K, Abrasonis V, Chan Y M, Stahl R, Carey D J, Miller B A. Erythropoietin modulates calcium influx through TRPC2. J Biol Chem. 2002;277:34375–34382. doi: 10.1074/jbc.M205541200. [DOI] [PubMed] [Google Scholar]
- Thebault S, Zholos A, Enfissi A, Slomianny C, Dewailly E, Roudbaraki M, Parys J, Prevarskaya N. Receptor-operated Ca2+ entry mediated by TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell line. J Cell Physiol. 2005;204:320–328. doi: 10.1002/jcp.20301. [DOI] [PubMed] [Google Scholar]
- Hurst R S, Zhu X, Boulay G, Birnbaumer L, Stefani E. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Reading S A, Earley S, Waldron B J, Welsh D G, Brayden J E. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- Berg A P, Sen N, Bayliss D A. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Obukhov A G, Strübing C, Zobel A, Kalkbrenner F, Lückhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Brown D M, Qin N, Jiang M, Dietrich A, Zhu M X, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E J, Gover T D, Cordoba-Rodriguez R, Weinreich D. Substance P evokes cation currents through TRP channels in HEK293 cells. J Neurophysiol. 2003;90:2069–2073. doi: 10.1152/jn.00026.2003. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov A G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Shlykov S G, Yang M, Alcorn J L, Sanborn B M. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 2003;69:647–655. doi: 10.1095/biolreprod.103.015396. [DOI] [PubMed] [Google Scholar]
- Panda S, Nayak S K, Campo B, Walker J R, Hogenesch J B, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson S M, Wong K Y, Krishna V, Provencio I, Berson D M. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Peppiatt-Wildman C M, Albert A P, Saleh S N, Large W A. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol (Lond) 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Hirschler-Laszkiewicz I, Zhang W, Conrad K, Neagley D W, Barber D L, Cheung J Y, Miller B A. TRPC3 is the erythropoietin-regulated calcium channel in human erythroid cells. J Biol Chem. 2008;283:10385–10395. doi: 10.1074/jbc.M710231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H S, Xu X Z, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Lievremont J P, Bird G, Putney J W. Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Natl Acad Sci U S A. 2001;98:11777–11782. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Ma H T, Ford D L, Gill D L. Expression of functional receptor-coupled TRPC3 channels in DT40 triple receptor InsP3 knockout cells. J Biol Chem. 2001;276:33980–33985. doi: 10.1074/jbc.C100321200. [DOI] [PubMed] [Google Scholar]
- Philipp S, Strauss B, Hirnet D, Wissenbach U, Mery L, Flockerzi V, Hoth M. TRPC3 mediates T-cell receptor-dependent calcium entry in human T-lymphocytes. J Biol Chem. 2003;278:26629–26638. doi: 10.1074/jbc.M304044200. [DOI] [PubMed] [Google Scholar]
- Cheng H W, James A F, Foster R R, Hancox J C, Bates D O. VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1768–1776. doi: 10.1161/01.ATV.0000231518.86795.0f. [DOI] [PubMed] [Google Scholar]
- Baldi C, Vazquez G, Calvo J C, Boland R. TRPC3-like protein is involved in the capacitative cation entry induced by 1α,25-dihydroxy-vitamin D3 in ROS 17/2.8 osteoblastic cells. J Cell Biochem. 2003;90:197–205. doi: 10.1002/jcb.10612. [DOI] [PubMed] [Google Scholar]
- Singh B B, Lockwich T P, Bandyopadhyay B C, Liu X, Bollimuntha S, Brazer S C, Combs C, Das S, Leenders A G, Sheng Z H, Knepper M A, Ambudkar S V, Ambudkar I S. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Smyth J T, Lemonnier L, Vazquez G, Bird G S, Putney J W. Dissociation of regulated trafficking of TRPC3 channels to the plasma membrane from their activation by phospholipase C. J Biol Chem. 2006;281:11712–11720. doi: 10.1074/jbc.M510541200. [DOI] [PubMed] [Google Scholar]
- Fauconnier J, Lanner J T, Sultan A, Zhang S J, Katz A, Bruton J D, Westerblad H. Insulin potentiates TRPC3-mediated cation currents in normal but not in insulin-resistant mouse cardiomyocytes. Cardiovasc Res. 2007;73:376–385. doi: 10.1016/j.cardiores.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins W G, Zuo C D, Hopfer U, Schilling W P. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol. 2007;293:F1476–F1488. doi: 10.1152/ajprenal.00186.2007. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh S H, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant T D, Obukhov A G, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Freichel M, Vogel S M, Paria B C, Mehta D, Flockerzi V, Malik A B. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- Munsch T, Freichel M, Flockerzi V, Pape H C. Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc Natl Acad Sci U S A. 2003;100:16065–16070. doi: 10.1073/pnas.2535311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell A F, Scott J L, Van Helden D F. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–37987. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- Kanki H, Kinoshita M, Akaike A, Satoh M, Mori Y, Kaneko S. Activation of inositol 1,4,5-trisphosphate receptor is essential for the opening of mouse TRP5 channels. Mol Pharmacol. 2001;60:989–998. doi: 10.1124/mol.60.5.989. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham D E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Zheng F, Gill D L. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278:29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- Tabata H, Tanaka S, Sugimoto Y, Kanki H, Kaneko S, Ichikawa A. Possible coupling of prostaglandin E receptor EP(1) to TRP5 expressed in Xenopus laevis oocytes. Biochem Biophys Res Commun. 2002;298:398–402. doi: 10.1016/s0006-291x(02)02455-5. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Yildirim E, Liao Y, Abramowitz J. Molecular and functional diversity of the TRPC family of ion channels. TRPC channels and their role in ROCE/SOCE. Conn M, Kordon C, Christen Y, editors. Berlin, Germany: Springer-Verlag; Insights into Receptor Function and New Drug Development. 2006:1–22. [Google Scholar]
- Ohta T, Morishita M, Mori Y, Ito S. Ca2+ store-independent augmentation of [Ca2+]i responses to G-protein coupled receptor activation in recombinantly TRPC5-expressed rat pheochromocytoma (PC12) cells. Neurosci Lett. 2004;358:161–164. doi: 10.1016/j.neulet.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:20279–20287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant T D. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu M X, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005;280:30788–30796. doi: 10.1074/jbc.M504745200. [DOI] [PubMed] [Google Scholar]
- Faber E S, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience. 2006;137:781–794. doi: 10.1016/j.neuroscience.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Xu S Z, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman A M, Flemming P K, McHugh D, Naylor J, Cheong A, Bateson A N, Munsch C M, Porter K E, Beech D J. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H T, Peng Z, Hiragun T, Iwaki S, Gilfillan A M, Beaven M A. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu Z H, Obukhov A G, Nowycky M C, Ledeen R W. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with α5β1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides V J, Ramsey I S, Kotecha S, Greka A, Clapham D E. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Estacion M, Li S, Sinkins W G, Gosling M, Bahra P, Poll C, Westwick J, Schilling W P. Activation of human TRPC6 channels by receptor stimulation. J Biol Chem. 2004;279:22047–22056. doi: 10.1074/jbc.M402320200. [DOI] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant T D. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Kawasaki B T, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci U S A. 2006;103:335–340. doi: 10.1073/pnas.0508030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Akerman K E. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Knezevic N, Ahmmed G U, Kini V, Malik A B, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, Payet M D. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003;278:31709–31716. doi: 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- Beskina O, Miller A, Mazzocco-Spezzia A, Pulina M V, Golovina V A. Mechanisms of interleukin-1β-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol. 2007;293:C1103–C1111. doi: 10.1152/ajpcell.00249.2007. [DOI] [PubMed] [Google Scholar]
- Tseng P H, Lin H P, Hu H, Wang C, Zhu M X, Chen C S. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia Y C, Cui K, Li N, Zheng Z Y, Wang Y Z, Yuan X B. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Pocock T M, Foster R R, Bates D O. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1015–H1026. doi: 10.1152/ajpheart.00826.2003. [DOI] [PubMed] [Google Scholar]
- Cayouette S, Lussier M P, Mathieu E L, Bousquet S M, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck J R, Morisseau C, Hammock B D, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential and protein homologue, TRP7. A Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27570. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Riccio A, Mattei C, Kelsell R E, Medhurst A D, Calver A R, Randall A D, Davis J B, Benham C D, Pangalos M N. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Nakanishi Y, Walsh E J, Wilson D P, Welsh D G, Cole W C. Heteromultimeric TRPC6-TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ Res. 2006;98:1520–1527. doi: 10.1161/01.RES.0000226495.34949.28. [DOI] [PubMed] [Google Scholar]
- Lievremont J P, Numaga T, Vazquez G, Lemonnier L, Hara Y, Mori E, Trebak M, Moss S E, Bird G S, Mori Y, Putney J W. The role of canonical transient receptor potential 7 in B-cell receptor-activated channels. J Biol Chem. 2005;280:35346–35351. doi: 10.1074/jbc.M507606200. [DOI] [PubMed] [Google Scholar]
- Nodari F, Hsu F F, Fu X, Holekamp T F, Kao L F, Turk J, Holy T E. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant T D. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Flemming P K, Dedman A M, Xu S Z, Li J, Zeng F, Naylor J, Benham C D, Bateson A N, Muraki K, Beech D J. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Xu S Z, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan C J, Bahnasi Y M, Al-Shawaf E, Porter K E, Jiang L H, Emery P, Sivaprasadarao A, Beech D J. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier W F, Romanin C, Zhu M X, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem. 275:27799–277805, 2000. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham D E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu M X, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya T K, Gurda G T, Eves E M, Villereal M L. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19–7 hippocampal neuronal cells. J Biol Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Kim S J, Kim Y S, Yuan J P, Petralia R S, Worley P F, Linden D J. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Ahmmed G U, Mehta D, Vogel S, Holinstat M, Paria B C, Tiruppathi C, Malik A B. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Paria B C, Vogel S M, Ahmmed G U, Alamgir S, Shroff J, Malik A B, Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1303–L1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- Paria B C, Bair A M, Xue J, Yu Y, Malik A B, Tiruppathi C. Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-κB activation in endothelial cells. J Biol Chem. 2006;281:20715–20727. doi: 10.1074/jbc.M600722200. [DOI] [PubMed] [Google Scholar]
- Golovina V A, Platoshyn O, Bailey C L, Wang J, Limsuwan A, Sweeney M, Rubin L J, Yuan J X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel S S, Yuan J X. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Zhang S, Patel H H, Murray F, Remillard C V, Schach C, Thistlethwaite P A, Insel P A, Yuan J X. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1202–L1210. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- Lin M J, Leung G P, Zhang W M, Yang X R, Yip K P, Tse C M, Sham J S. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Wang J, Weigan D, Lu W, Sylvester J T, Semenza G L, Shimoda L A. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- Kumar B, Dreja K, Shah S S, Cheong A, Xu S Z, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston P A, Heagerty A M, Munsch C M, Bergdahl A, Hultgardh-Nilsson A, Gomez M F, Porter K E, Hellstrand P, Beech D J. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195:287–296. doi: 10.1016/j.atherosclerosis.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez M F, Wihlborg A K, Erlinge D, Eyjolfson A, Xu S Z, Beech D J, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–C880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- Kunichika N, Yu Y, Remillard C V, Platoshyn O, Zhang S, Yuan J X. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:L962–L969. doi: 10.1152/ajplung.00452.2003. [DOI] [PubMed] [Google Scholar]
- Xie A, Aihara Y, Bouryi V A, Nikitina E, Jahromi B S, Zhang Z D, Takahashi M, Macdonald R L. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2007;27:1692–1701. doi: 10.1038/sj.jcbfm.9600471. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Barritt G J. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca2+-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem J. 2003;373:327–336. doi: 10.1042/BJ20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehof M, Borlak J. HNF4 alpha and the Ca-channel TRPC1 are novel disease candidate genes in diabetic nephropathy. Diabetes. 2008;57:1069–1077. doi: 10.2337/db07-1065. [DOI] [PubMed] [Google Scholar]
- Singh B B, Zheng C, Liu X, Lockwich T, Liao D, Zhu M X, Birnbaumer L, Ambudkar I S. Trp1-dependent enhancement of salivary gland fluid secretion: role of store-operated calcium entry. FASEB J. 2001;15:1652–1654. doi: 10.1096/fj.00-0749fje. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng K T, Bandyopadhyay B C, Pani B, Dietrich A, Paria B C, Swaim W D, Beech D, Yildrim E, Singh B B, Birnbaumer L, Ambudkar I S. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J N, Platoshyn O, Golovina V A, Liu L, Zou T, Marasa B S, Turner D J, Yuan J X, Wang J Y. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G782–G792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- Du J, Sours-Brothers S, Coleman R, Ding M, Graham S, Kong D H, Ma R. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol. 2007;18:1437–1445. doi: 10.1681/ASN.2006091067. [DOI] [PubMed] [Google Scholar]
- Babich L G, Ku C Y, Young H W, Huang H, Blackburn M R, Sanborn B M. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov S G, Corrigan R, Tsujimoto S, Sanborn B M. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod. 2002;67:988–994. doi: 10.1095/biolreprod.102.004119. [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Slater D M, Beech D, Poston L, Tribe R M. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2002;8:946–951. doi: 10.1093/molehr/8.10.946. [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Slater D M, Poston L, Tribe R M. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1 beta. J Clin Endocrinol Metab. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- Pani B, Cornatzer E, Cornatzer W, Shin D M, Pittelkow M R, Hovnanian A, Ambudkar I S, Singh B B. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier’s disease. Mol Biol Cell. 2006;17:4446–4458. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland R B, Belton C M, Izutsu K T. TRPC channel expression during calcium-induced differentiation of human gingival keratinocytes. J Dermatol Sci. 2005;40:21–28. doi: 10.1016/j.jdermsci.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland R B, Belton C M, Roberts F A, Goodwin P C, Schubert M M, Izutsu K T. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflügers Arch. 2006;452:43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Beck B, Lehen'kyi V, Roudbaraki M, Flourakis M, Charveron M, Bordat P, Polakowska R, Prevarskaya N, Skryma R. TRPC channels determine human keratinocyte differentiation: New insight into basal cell carcinoma. Cell Calcium. 2008;43:492–505. doi: 10.1016/j.ceca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Liman E R, Corey D P, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci U S A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menco B P, Carr V M, Ezeh P I, Liman E R, Yankova M P. Ultrastructural localization of G-proteins and the channel protein TRP2 to microvilli of rat vomeronasal receptor cells. J Comp Neurol. 2001;438:468–489. doi: 10.1002/cne.1329. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy T E, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Leypold B G, Yu C R, Leinders-Zufall T, Kim M M, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Stamboulian S, Moutin M J, Treves S, Pochon N, Grunwald D, Zorzato F, De Waard M, Ronjat M, Arnoult C. Junctate, an inositol 1,4,5-triphosphate receptor associated protein, is present in rodent sperm and binds TRPC2 and TRPC5 but not TRPC1 channels. Dev Biol. 2005;286:326–337. doi: 10.1016/j.ydbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Sutton K A, Jungnickel M K, Wang Y, Cullen K, Lambert S, Florman H M. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev Biol. 2004;274:426–435. doi: 10.1016/j.ydbio.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Amaral M D, Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci. 2007;27:5179–5189. doi: 10.1523/JNEUROSCI.5499-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral M D, Pozzo-Miller L. BDNF induces calcium elevations associated with IBDNF, a nonselective cationic current mediated by TRPC channels. J Neurophysiol. 2007;98:2476–2482. doi: 10.1152/jn.00797.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning H A, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G, van Rossum D B, Patterson R L, Snyder S H, Desiderio S. Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science. 2006;314:122–125. doi: 10.1126/science.1127815. [DOI] [PubMed] [Google Scholar]
- Raybould N P, Jagger D J, Kanjhan R, Greenwood D, Laslo P, Hoya N, Soeller C, Cannell M B, Housley G D. TRPC-like conductance mediates restoration of intracellular Ca2+ in cochlear outer hair cells in the guinea pig and rat. J Physiol. 2007;579:101–113. doi: 10.1113/jphysiol.2006.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush E W, Hood D B, Papst P J, Chapo J A, Minobe W, Bristow M R, Olson E N, McKinsey T A. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Wilkin B J, Bodi I, Molkentin J D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C L, Huang Y, Ngai C Y, Leung Y K, Yao X Q. TRPC3 is involved in flow- and bradykinin-induced vasodilation in rat small mesenteric arteries. Acta Pharmacol Sin. 2006;27:981–990. doi: 10.1111/j.1745-7254.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res. 1999;42:543–549. doi: 10.1016/s0008-6363(99)00025-5. [DOI] [PubMed] [Google Scholar]
- Gifford S M, Yi F X, Bird I M. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-inositol 1,4,5-trisphosphate receptor 2 interaction. J Endocrinol. 2006;190:385–395. doi: 10.1677/joe.1.06773. [DOI] [PubMed] [Google Scholar]
- Albert A P, Pucovsky V, Prestwich S A, Large W A. TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol. 2006;571:361–369. doi: 10.1113/jphysiol.2005.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos Y, Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft F C, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard C V, Landsberg J W, Kunichika N, Platoshyn O, Tigno D D, Thistlethwaite P A, Rubin L J, Yuan J X. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T A, Xue A, Chini E N, Thompson M, Sieck G C, Wylam M E. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P, Hawkins A, Stiber J, Shelton J M, Hutcheson K, Bassel-Duby R, Shin D M, Yan Z, Williams R S. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci U S A. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe R M. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13:171–179. doi: 10.1093/molehr/gal110. [DOI] [PubMed] [Google Scholar]
- Andreopoulos S, Wasserman M, Woo K, Li P P, Warsh J J. Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenomics J. 2004;4:365–373. doi: 10.1038/sj.tpj.6500266. [DOI] [PubMed] [Google Scholar]
- Liu D, Scholze A, Zhu Z, Kreutz R, Wehland-von-Trebra M, Zidek W, Tepel M. Increased transient receptor potential channel TRPC3 expression in spontaneously hypertensive rats. Am J Hypertens. 2005;18:1503–1507. doi: 10.1016/j.amjhyper.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, Streffer K, Holz S, Harteneck C, Zidek W, Tepel M. Transient receptor potential channels in essential hypertension. J Hypertens. 2006;24:1105–1014. doi: 10.1097/01.hjh.0000226201.73065.14. [DOI] [PubMed] [Google Scholar]
- Liu D Y, Scholze A, Kreutz R, Wehland-von-Trebra M, Zidek W, Zhu Z M, Tepel M. Monocytes from spontaneously hypertensive rats show increased store-operated and second messenger-operated calcium influx mediated by transient receptor potential canonical Type 3 channels. Am J Hypertens. 2007;20:1111–1118. doi: 10.1016/j.amjhyper.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Liu D Y, Thilo F, Scholze A, Wittstock A, Zhao Z G, Harteneck C, Zidek W, Zhu Z M, Tepel M. Increased store-operated and 1-oleoyl-2-acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. J Hypertens. 2007;25:799–808. doi: 10.1097/HJH.0b013e32803cae2b. [DOI] [PubMed] [Google Scholar]
- Fowler M A, Sidiropoulou K, Ozkan E D, Phillips C W, Cooper D C. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS ONE. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Bianchi R, Chuang S C, Zhao W, Wong R K. Group I metabotropic glutamate receptor-dependent TRPC channel trafficking in hippocampal neurons. J Neurochem. 2007;101:411–421. doi: 10.1111/j.1471-4159.2006.04377.x. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang W, Richardson P M, Priestley J V, Liu M. TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem. 2008;283:416–426. doi: 10.1074/jbc.M703177200. [DOI] [PubMed] [Google Scholar]
- Wu D, Zhang Y, Bo X, Huang W, Xiao F, Zhang X, Miao T, Magoulas C, Subang M C, Richardson P M. Actions of neuropoietic cytokines and cyclic AMP in regenerative conditioning of rat primary sensory neurons. Exp Neurol. 2007;204:66–76. doi: 10.1016/j.expneurol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Yang H, Sun X, Wang Z, Ning G, Zhang F, Kong J, Lu L, Reinach P S. EGF stimulates growth by enhancing capacitative calcium entry in corneal epithelial cells. J Membr Biol. 2003;194:47–58. doi: 10.1007/s00232-003-2025-9. [DOI] [PubMed] [Google Scholar]
- Yang H, Mergler S, Sun X, Wang Z, Lu L, Bonanno J A, Pleyer U, Reinach P S. TRPC4 knockdown suppresses epidermal growth factor-induced store-operated channel activation and growth in human corneal epithelial cells. J Biol Chem. 2005;280:32230–32237. doi: 10.1074/jbc.M504553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth M, Sumbilla C, Mullen S P, Lewis D, Klein M G, Hussain A, Soboloff J, Gill D L, Inesi G. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci U S A. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Freichel M, Jaspers M, Cuppens H, Cassiman J J, Droogmans G, Flockerzi V, Nilius B. Functional interaction between TRP4 and CFTR in mouse aorta endothelial cells. BMC Physiol. 2001;1:3. doi: 10.1186/1472-6793-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi I, Zhang S, Platoshyn O, Remillard C V, Cowling R T, Yuan J X. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–L1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- Cioffi D L, Wu S, Alexeyev M, Goodman S R, Zhu M X, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005;97:1164–1172. doi: 10.1161/01.RES.0000193597.65217.00. [DOI] [PubMed] [Google Scholar]
- Zhang S, Remillard C V, Fantozzi I, Yuan J X. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C1192–C1201. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]
- Epperson A, Hatton W J, Callaghan B, Doherty P, Walker R L, Sanders K M, Ward S M, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol Cell Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- Lee K P, Jun J Y, Chang I Y, Suh S H, So I, Kim K W. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cell. 2005;20:435–441. [PubMed] [Google Scholar]
- Veliceasa D, Ivanovic M, Hoepfner F T, Thumbikat P, Volpert O V, Smith N D. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalié A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham D E. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu S Z, Khan S U, Levenson R, Beech D J, Weiss J L. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572:165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanjewe J, Grover A K. Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochem J. 2004;378:975–982. doi: 10.1042/BJ20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Müller M, Essin K, Henke B, Gollasch M, Harteneck C, Müller W E. Hyperforin, a key constituent of St John’s wort, specifically activates TRPC6 channels. FASEB J. 2007;21:4101–4111. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson J A, Bassel-Duby R, Hill J A, Olson E N. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Gα12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani H A, Schermuly R T, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan J X. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Welsh D G, Morielli A D, Nelson M T, Brayden J E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Kunichika N, Landsberg J W, Yu Y, Kunichika H, Thistlethwaite P A, Rubin L J, Yuan J X. Bosentan inhibits transient receptor potential channel expression in pulmonary vascular myocytes. Am J Respir Crit Care Med. 2004;170:1101–1107. doi: 10.1164/rccm.200312-1668OC. [DOI] [PubMed] [Google Scholar]
- Bae Y M, Kim A, Lee Y J, Lim W, Noh Y H, Kim E J, Kim J, Kim T K, Park S W, Kim B, Cho S I, Kim D K, Ho W K. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25:809–817. doi: 10.1097/HJH.0b013e3280148312. [DOI] [PubMed] [Google Scholar]
- Winn M P, Conlon P J, Lynn K L, Farrington M K, Creazzo T, Hawkins A F, Daskalakis N, Kwan S Y, Ebersviller S, Burchette J L, Pericak-Vance M A, Howell D N, Vance J M, Rosenberg P B. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Reiser J, Polu K R, Möller C C, Kenlan P, Altintas M M, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith P L, Clapham D E, Pollak M R. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C C, Wei C, Altintas M M, Li J, Greka A, Ohse T, Pippin J W, Rastaldi M P, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland S J, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- Graham S, Ding M, Sours-Brothers S, Yorio T, Ma J X, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol. 2007;293:F1381–F1390. doi: 10.1152/ajprenal.00185.2007. [DOI] [PubMed] [Google Scholar]
- Foller M, Kasinathan R S, Koka S, Lang C, Shumilina E, Birnbaumer L, Lang F, Huber S M. TRPC6 contributes to the Ca2+ leak of human erythrocytes. Cell Physiol Biochem. 2008;21:183–192. doi: 10.1159/000113760. [DOI] [PubMed] [Google Scholar]
- Liu D, Maier A, Scholze A, Rauch U, Boltzen U, Zhao Z, Zhu Z, Tepel M. High glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathway. Arterioscler Thromb Vasc Biol. 2008;28:746–751. doi: 10.1161/ATVBAHA.108.162222. [DOI] [PubMed] [Google Scholar]
- Elg S, Marmigere F, Mattsson J P, Ernfors P. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J Comp Neurol. 2007;503:35–46. doi: 10.1002/cne.21351. [DOI] [PubMed] [Google Scholar]
- Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, Mori Y, Makino N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem. 2007;294:205–215. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- Foller M, Kasinathan R S, Duranton C, Wieder T, Huber S M, Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell Physiol Biochem. 2006;17:201–210. doi: 10.1159/000094125. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters J A. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Flockerzi V, Nilius B. Berlin, Germany: Springer-Verlag; Transient Receptor Potential (TRP) ChannelsHandbook of Experimental Pharmacology. 2007 [PubMed] [Google Scholar]
- Liedtke WB, Heller S. Boca Raton, FL, USA: CRC Press; TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. 2007 [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009 doi: 10.1146/annurev.pharmtox.48.113006.094928. In press. [DOI] [PubMed] [Google Scholar]
- TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–1032. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Beech D J, Xu S Z, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood T G, Kurosky A, Martinac B, Hamill O P. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Ahmmed G U, Malik A B. Functional role of TRPC channels in the regulation of endothelial permeability. Pflügers Arch. 2005;451:131–142. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- Rubin L J. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos Y, Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Stockand J D, Sansom S C. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- Raptis A E, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109:S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver and function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol Rev. 2002;54:129–158. doi: 10.1124/pr.54.1.129. [DOI] [PubMed] [Google Scholar]
- Wang H, Maechler P, Antinozzi P A, Hagenfeldt K A, Wollheim C B. Hepatocyte nuclear factor 4α regulates the expression of pancreatic β-cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem. 2000;275:35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- Hoffman E P, Brown R H, Jr, Kunkel L M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Tutdibi O, Brinkmeier H, Rüdel R, Föhr K J. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol. 1999;515:859–868. doi: 10.1111/j.1469-7793.1999.859ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I A, Allen D G. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- Gervásio O L, Whitehead N P, Yeung E W, Phillips W D, Allen D G. TRPC1 binds to caveolin-3 and is regulated by Src kinase—role in Duchenne muscular dystrophy. J Cell Sci. 2008;121:2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- Lockwich T P, Liu X, Singh B B, Jadlowiec J, Weiland S, Ambudkar I S. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- Brazer S C, Singh B B, Liu X, Swaim W, Ambudkar I S. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I A, Allen D G. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- Jung C, Martins A S, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signaling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21:608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Yuan J P, Kiselyov K, Shin D M, Chen J, Shcheynikov N, Kang S H, Dehoff M H, Schwarz MK, Seeburg P H, Muallem S, Worley P F. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Stiber J A, Zhang Z S, Burch J, Eu J P, Zhang S, Truskey G A, Seth M, Yamaguchi N, Meissner G, Shah R, Worley P F, Williams R S, Rosenberg P B. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal TRP channel activity. Mol Cell Biol. 2008;28:2637–2647. doi: 10.1128/MCB.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K, Graef I A, Neilson J R, Park S H, Crabtree G R. Signaling through calcium, calcineurin, and NF-AT in lymphocyte activation and development. Cold Spring Harb Symp Quant Biol. 1999;64:505–516. doi: 10.1101/sqb.1999.64.505. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Barker J L. Developmental changes in cell calcium homeostasis during neurogenesis of the embryonic rat cerebral cortex. Cereb Cortex. 2000;10:561–573. doi: 10.1093/cercor/10.6.561. [DOI] [PubMed] [Google Scholar]
- Malarkey E B, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Singh B B, Shavali S, Sharma S K, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S, Ebadi M, Singh B B. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006;1099:141–149. doi: 10.1016/j.brainres.2006.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasa B S, Rao J N, Zou T, Liu L, Keledjian K M, Zhang A H, Xiao L, Chen J, Turner D J, Wang J Y. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-κB activation through Ca2+ influx. Biochem J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasa B S, Xiao L, Rao J N, Zou T, Liu L, Wang J, Bellavance E, Turner D J, Wang J Y. Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-κB activation. Am J Physiol Cell Physiol. 2008;294:C1277–C1287. doi: 10.1152/ajpcell.90635.2007. [DOI] [PubMed] [Google Scholar]
- Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–229. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa S H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Li L, Tucker R W, Hennings H, Yuspa S H. Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J Cell Physiol. 1995;163:105–114. doi: 10.1002/jcp.1041630112. [DOI] [PubMed] [Google Scholar]
- Kunzelmann-Marche C, Freyssinet J M, Martinez M C. Loss of plasma membrane phospholipid asymmetry requires raft integrity. Role of transient receptor potential channels and ERK pathway. J Biol Chem. 2002;277:19876–19881. doi: 10.1074/jbc.M200324200. [DOI] [PubMed] [Google Scholar]
- Gottlieb P, Folgering J, Maroto R, Raso A, Wood T G, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill O P, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Fabian A, Fortmann T, Dieterich P, Riethmüller C, Schön P, Mally S, Nilius B, Schwab A. TRPC1 channels regulate directionality of migrating cells. [E-pub ahead of print] Pflügers Arch. 2008 doi: 10.1007/s00424-008-0515-4. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- Burge S M, Wilkinson J D. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40–50. doi: 10.1016/0190-9622(92)70154-8. [DOI] [PubMed] [Google Scholar]
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro C S, O'Donovan M, Craddock N, Kucherlapati R, Rees J L, Owen M, Lathrop G M, Monaco A P, Strachan T, Hovnanian A. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- Liman E R, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci U S A. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Webb D M. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci U S A. 2003;100:8337–8341. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Birnbaumer L. TRPC2: molecular biology and functional importance. Handb Exp Pharmacol. 2007;179:53–75. doi: 10.1007/978-3-540-34891-7_3. [DOI] [PubMed] [Google Scholar]
- Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17:675–683. doi: 10.1016/j.conb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann J H, Fadool D A. Vomeronasal sensory neurons from Sternotherus odoratus (stinkpot/musk turtle) respond to chemosignals via the phospholipase C system. J Exp Biol. 2006;209:1914–1927. doi: 10.1242/jeb.02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B M, Schackmann R W, Tombes R M, Kazazoglou T. Coupled ionic and enzymatic regulation of sperm behavior. Curr Top Cell Regul. 1985;26:97–113. doi: 10.1016/b978-0-12-152826-3.50015-2. [DOI] [PubMed] [Google Scholar]
- O'Toole C M, Arnoult C, Darszon A, Steinhardt R A, Florman H M. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Mol Biol Cell. 2000;11:1571–1584. doi: 10.1091/mbc.11.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissenbach U, Schroth G, Philipp S, Flockerzi V. Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett. 1998;429:61–66. doi: 10.1016/s0014-5793(98)00561-4. [DOI] [PubMed] [Google Scholar]
- Bleil J D, Wassarman P M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Walensky L D, Snyder S H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J Cell Biol. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson J D, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu M X. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu M X, Mikoshiba K, Girard T, Smida-Rezgui S, Ronjat M, Zorzato F. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Dietrich A, Birnbaumer L. The mouse C-type transient receptor potential 2 (TRPC2) channel: alternative splicing and calmodulin binding to its N terminus. Proc Natl Acad Sci U S A. 2003;100:2220–2225. doi: 10.1073/pnas.0438036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Medhurst A D, Mattei C, Kelsell R E, Calver A R, Randall A D, Benham C D, Pangalos M N. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Santiago M, Mendoza-Torres M, Jiménez- Bremont J F, López-Revilla R. Knockout of the trcp3 gene causes a recessive neuromotor disease in mice. Biochem Biophys Res Commun. 2007;360:874–879. doi: 10.1016/j.bbrc.2007.06.150. [DOI] [PubMed] [Google Scholar]
- López-Revilla R, Soto-Zárate C, Ridaura C, Chávez-Dueñas L, Paul D. Progressive paralysis associated with diffuse astrocyte anaplasia in delta 202 mice homozygous for a transgene encoding the SV40 T antigen. Neuropathology. 2004;24:30–37. doi: 10.1111/j.1440-1789.2003.00536.x. [DOI] [PubMed] [Google Scholar]
- Atherton J F, Bevan M D. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci. 2005;25:8272–8281. doi: 10.1523/JNEUROSCI.1475-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- Zhou F W, Matta S G, Zhou F M. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci. 2008;28:473–482. doi: 10.1523/JNEUROSCI.3978-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough C, Lu S J, Davis C L, Chuang D M, Post R M. Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol Psychiatry. 1999;46:247–255. doi: 10.1016/s0006-3223(98)00308-4. [DOI] [PubMed] [Google Scholar]
- Wasserman M J, Corson T W, Sibony D, Cooke R G, Parikh S V, Pennefather P S, Li P P, Warsh J J. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology. 2004;29:759–769. doi: 10.1038/sj.npp.1300400. [DOI] [PubMed] [Google Scholar]
- Perez Jurado L A, Wang Y K, Peoples R, Coloma A, Cruces J, Francke U. A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of BTK. Hum Mol Genet. 1998;7:325–334. doi: 10.1093/hmg/7.3.325. [DOI] [PubMed] [Google Scholar]
- Van Rossum D B, Patterson R L, Sharma S, Barrow R K, Kornberg M, Gill D L, Snyder S H. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- Chen D H, Brkanac Z, Verlinde C L, Tan X J, Bylenok L, Nochlin D, Matsushita M, Lipe H, Wolff J, Fernandez M, Cimino P J, Bird T D, Raskind W H. Missense mutations in the regulatory domain of PKC gamma: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet. 2003;72:839–849. doi: 10.1086/373883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N. Enzymological analysis of mutant protein kinase Cγ causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem. 2008;283:19854–19863. doi: 10.1074/jbc.M801492200. [DOI] [PubMed] [Google Scholar]
- Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology. 2005;146:5063–5070. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- Ecelbarger C A, Chou C L, Lolait S J, Knepper M A, DiGiovanni S R. Evidence for dual signaling pathways for V2 vasopressin receptor in rat inner medullary collecting duct. Am J Physiol. 1996;270:F623–F633. doi: 10.1152/ajprenal.1996.270.4.F623. [DOI] [PubMed] [Google Scholar]
- Kim J Y, Zeng W, Kiselyov K, Yuan J P, Dehoff M H, Mikoshiba K, Worley P F, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- Bird I M, Zhang L, Magness R R. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Gifford S M, Yi F X, Bird I M. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling. J Endocrinol. 2006;190:373–384. doi: 10.1677/joe.1.06635. [DOI] [PubMed] [Google Scholar]
- Wray S, Kupittayanant S, Shmygol A, Smith R D, Burdyga T. The physiological basis of uterine contractility: a short review. Exp Physiol. 2001;86:239–246. doi: 10.1113/eph8602114. [DOI] [PubMed] [Google Scholar]
- Steinborn A, Kühnert M, Halberstadt E. Immunmodulating cytokines induce term and preterm parturition. J Perinat Med. 1996;24:381–390. doi: 10.1515/jpme.1996.24.4.381. [DOI] [PubMed] [Google Scholar]
- Tribe R M, Moriarty P, Dalrymple A, Hassoni A A, Poston L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68:1842–1849. doi: 10.1095/biolreprod.102.011403. [DOI] [PubMed] [Google Scholar]
- Olson E N, Williams R S. Calcineurin signaling and muscle remodeling. Cell. 2000;101:689–692. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Lee E H, Cherednichenko G, Pessah I N, Allen P D. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J Biol Chem. 2006;281:10042–10048. doi: 10.1074/jbc.M600981200. [DOI] [PubMed] [Google Scholar]
- Woo J S, Kim-do H, Allen P D, Lee E H. TRPC3-interacting triadic proteins in skeletal muscle. Biochem J. 2008;411:399–405. doi: 10.1042/bj20071504. [DOI] [PubMed] [Google Scholar]
- Kiselyov K I, Shin D M, Wang Y, Pessah I N, Allen P D, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell. 2000;6:421–431. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Takamori M. Autoantibodies against TRPC3 and ryanodine receptor in myasthenia gravis. J Neuroimmunol. 2008;200:142–144. doi: 10.1016/j.jneuroim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Babu K S, Davies D E, Holgate S T. Role of tumor necrosis factor alpha in asthma. Immunol Allergy Clin North Am. 2004;24:583–597. doi: 10.1016/j.iac.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Amrani Y, Martinet N, Bronner C. Potentiation by tumour necrosis factor-alpha of calcium signals induced by bradykinin and carbachol in human tracheal smooth muscle cells. Br J Pharmacol. 1995;114:4–5. doi: 10.1111/j.1476-5381.1995.tb14896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Adebiyi A, Zhao G, Chapman K E, Waters C M, Hassid A, Jaggar J H. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo F, Scholze A, Liu D Y, Zidek W, Tepel M. Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch Biochem Biophys. 2008;471:57–62. doi: 10.1016/j.abb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Wilkins B J, Molkentin J D. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- Shan D, Marchase R B, Chatham J C. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C833–C841. doi: 10.1152/ajpcell.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Stenmark K R, Fagan K A, Frid M G. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- Sandoval R, Malik A B, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2001;280:L239–L247. doi: 10.1152/ajplung.2001.280.2.L239. [DOI] [PubMed] [Google Scholar]
- Wu S, Sangerman J, Li M, Brough G H, Goodman S R, Stevens T. Essential control of an endothelial cell ISOC by the spectrin membrane skeleton. J Cell Biol. 2001;154:1225–1233. doi: 10.1083/jcb.200106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell A F, Van Helden D F, Scott J L. The spectrin cytoskeleton influences the surface expression and activation of human transient receptor potential channel 4 channels. J Biol Chem. 2008;283:4395–4407. doi: 10.1074/jbc.M709729200. [DOI] [PubMed] [Google Scholar]
- Merlin L R, Wong R K. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Sanders K M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Lindsey S H, Tribe R M, Songu-Mize E. Cyclic stretch decreases TRPC4 protein and capacitative calcium entry in rat vascular smooth muscle cells. Life Sci. 2008;83:29–34. doi: 10.1016/j.lfs.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Beech D J. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007;179:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- Fang Y, Wu G, Xie X, Lu Z H, Ledeen R W. Endogenous GM1 ganglioside of the plasma membrane promotes neuritogenesis by two mechanisms. Neurochem Res. 2000;25:931–940. doi: 10.1023/a:1007596223484. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xie X, Ledeen R W, Wu G. Characterization of cholera toxin B subunit-induced Ca2+ influx in neuroblastoma cells: evidence for a voltage-independent GM1 ganglioside-associated Ca2+ channel. J Neurosci Res. 2002;69:669–680. doi: 10.1002/jnr.10333. [DOI] [PubMed] [Google Scholar]
- Fegley A J, Tanski W J, Roztocil E, Davies M G. Sphingosine-1-phosphate stimulates smooth muscle cell migration through Gαi and PI3-kinase-dependent p38(MAPK) activation. J Surg Res. 2003;113:32–41. doi: 10.1016/s0022-4804(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Yetik-Anacak G, Catravas J D. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45:268–276. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kimura C, Park S J, Oike M, Ito Y. Functional implications of Ca2+ mobilizing properties for nitric oxide production in aortic endothelium. Life Sci. 2002;72:511–520. doi: 10.1016/s0024-3205(02)02246-4. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat and basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Foreman J C, Hallett M B, Mongar J L. Movement of strontium ions into mast cells and its relationship to the secretory response. J Physiol. 1977;271:233–251. doi: 10.1113/jphysiol.1977.sp011998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Ong H L, Cheng K T, Liu X, Bandyopadhyay B C, Paria B C, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh B B, Gill D L, Ambudkar I S. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage P S, Mix K S, Brinckerhoff C E. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Bayliss W M. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M A, Hewavitharana T, Xu W, Soboloff J, Gill D L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Y M, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39:77–88. doi: 10.1016/s0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- Brown R D, Ambler S K, Mitchell M D, Long C S. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Fujii T, Onohara N, Maruyama Y, Tanabe S, Kobayashi H, Fukutomi M, Nagamatsu Y, Nishihara N, Inoue R, Sumimoto H, Shibasaki F, Nagao T, Nishida M, Kurose H. Gα12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J Biol Chem. 2005;280:23041–23047. doi: 10.1074/jbc.M409397200. [DOI] [PubMed] [Google Scholar]
- Nagamatsu Y, Nishida M, Onohara N, Fukutomi M, Maruyama Y, Kobayashi H, Sato Y, Kurose H. Heterotrimeric G protein G α13-induced induction of cytokine mRNAs through two distinct pathways in cardiac fibroblasts. J Pharmacol Sci. 2006;101:144–150. doi: 10.1254/jphs.fp0051036. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik A B. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Colles S M, Bhat M, VanWagoner D R, Birnbaumer L, Graham L M. Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement. Mol Biol Cell. 2008;19:3203–3211. doi: 10.1091/mbc.E07-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Colles S M, Damron D S, Graham L M. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arterioscler Thromb Vasc Biol. 2003;23:218–223. doi: 10.1161/01.atv.0000052673.77316.01. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–606. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, Wang Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121:2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- Lang F, Lang K S, Lang P A, Huber S M, Wieder T. Mechanisms and significance of eryptosis. Antioxid Redox Signal. 2006;8:1183–1192. doi: 10.1089/ars.2006.8.1183. [DOI] [PubMed] [Google Scholar]
- Daskalakis N, Winn M P. Focal and segmental glomerulosclerosis. Cell Mol Life Sci. 2006;63:2506–2511. doi: 10.1007/s00018-006-6171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler M C, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- Huber T B, Schermer B, Müller R U, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstädt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Ferroni P, Basili S, Falco A, Davì G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskay A N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–2077. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- Guilbert A, Dhennin-Duthille I, Hiani Y E, Haren N, Khorsi H, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer. 2008;8:125. doi: 10.1186/1471-2407-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W E. Current St. John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47:101–109. doi: 10.1016/s1043-6618(02)00266-9. [DOI] [PubMed] [Google Scholar]
- Estacion M, Sinkins W G, Jones S W, Applegate M A, Schilling W P. Human TRPC6 expressed in HEK 293 cells forms non-selective cation channels with limited Ca2+ permeability. J Physiol. 2006;572:359–377. doi: 10.1113/jphysiol.2005.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P M, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med. 2003;9:177–182. doi: 10.1016/s1471-4914(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chu P B, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- Wes P D, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramowitz J, Birnbaumer L. Know thy neighbor: a survey of diseases and complex syndromes that map to chromosomal regions encoding TRP channels. Handb Exp Pharmacol. 2007;179:379–408. doi: 10.1007/978-3-540-34891-7_23. [DOI] [PubMed] [Google Scholar]
- Everett K V, Chioza B A, Georgoula C, Reece A, Capon F, Parker K A, Cord-Udy C, McKeigue P, Mitton S, Pierro A, Puri P, Mitchison H M, Chung E M, Gardiner R M. Genome-wide high-density SNP-based linkage analysis of infantile hypertrophic pyloric stenosis identifies loci on chromosomes 11q14-q22 and Xq23. Am J Hum Genet. 2008;82:756–762. doi: 10.1016/j.ajhg.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]