SUMMARY
T cell receptor engagement in the absence of costimulation induces the calcium-dependent upregulation of the expression of a specific set of genes, which leads to the establishment of T cell anergy. Caspase 3 is one of the genes activated during anergy induction. We show that upregulation of caspase 3 expression in anergic cells is also accompanied by increased activation of this protease, which does not result in apoptosis. Our results show that T cells in which caspase 3 expression is specifically blocked by RNA interference and T cells from caspase3-deficient mice show profound impairment of the induction of T cell anergy. In anergic T cells, activated caspase 3 associates to the plasma membrane, where it can cleave and inactivate proteins such as GADS and Vav1, contributing to the blockade of the T cell receptor signaling that characterizes T cell anergy.
INTRODUCTION
Random rearrangements of the gene segments at the T cell receptor (TCR) locus allow recognition of foreign antigens but also generate dangerous self-reactive T cells. Many cells carrying self-reactive TCRs are eliminated during negative selection in the thymus, however, this process has limitations and additional mechanisms of tolerance are required in the periphery to limit autoimmunity. Anergy is induced in T cells by partial or suboptimal stimulation, which results in functional inactivation of self-reactive cells. Anergic T cells show profound defects in IL-2 production and proliferation (Schwartz, 2003).
Clonal anergy is induced upon engagement of the TCR in the absence of costimulatory signals (Jenkins and Schwartz, 1987; Quill and Schwartz, 1987). Anergizing stimuli cause a sustained increase in intracellular free calcium, which induces the calcineurin-mediated dephosphorylation and nuclear translocation of members of the nuclear factor of activated T cells (NFAT) family of transcription factors, in the absence of full activation of activator protein 1 (AP-1) complexes (Jain et al., 1993; Macian et al., 2002; Macian et al., 2001). NFAT proteins play a key role in the induction of tolerance in T cells by driving the expression of anergy-inducing genes (Macian et al., 2002). The specific expression of these genes is required to impose a state of functional unresponsiveness through different mechanisms. Anergizing stimuli upregulate the expression of at least three E3-ubiquitin ligases: Cbl-b, Itch and GRAIL (Anandasabapathy et al., 2003; Heissmeyer et al., 2004; Jeon et al., 2004), with a role in the downregulation of TCR signaling by inactivation or degradation of signaling molecules (Mueller, 2004). For instance, Itch and its related protein, Nedd4, have been shown to translocate to detergent-insoluble membrane fractions where they induce ubiquitination and degradation of phospholipase C-γ and protein kinase C-θ(Heissmeyer et al., 2004).
Caspase 3 is an effector member of the caspase family that is also expressed during anergy induction. This protease recognizes proteins with a common DXXD motif, cleaving after the first aspartic residue (Rathmell and Thompson, 1999; Thornberry and Lazebnik, 1998). Caspase 3 is expressed as a proenzyme (32KDa) that contains a short prodomain, and a large (17KDa) and a small (12KDa) subunits. Activation requires proteolytic cleavage between the subunits, where linker segment is typically removed by upstream initiator caspases (Boatright and Salvesen, 2003). T and B cells from Casp3−/− mice show hyperproliferative responses, which have been attributed to reduced activation-induced cell-death (AICD) (Alam et al., 1999) and to alterations of cell cycle regulation (Woo et al., 2003), respectively. Caspase 3 also regulates many non-apoptotic cellular processes, such as cell proliferation, cell cycle regulation and cell differentiation (Algeciras-Schimnich et al., 2002; Denis et al., 1998). For instance, caspase 3 is implicated in the regulation of the late steps of T cell activation (Alam et al., 1999; Miossec et al., 1997), IL-16 processing (Zhang et al., 1998), dendritic cell maturation (Santambrogio et al., 2005), and erythrocyte (Kolbus et al., 2002) and monocyte (Sordet et al., 2002) differentiation.
In this report we characterize the activation of caspase 3 in anergic T cells and determine its role in the inhibition of TCR signaling. We show that anergizing stimuli lead to increased caspase 3 activation in the absence of apoptotic hallmarks. Activated caspase 3 associates then to the plasma membrane, where it is responsible for the cleavage of GADS and Vav1 following subsequent TCR engagement. These results support that caspase 3 plays a key role in the inhibition of TCR signaling in anergic T cells.
RESULTS
Caspase 3 is activated during anergy induction in T cells
We had previously shown that the expression of caspase 3 mRNA was induced in T cells that became unresponsive after being treated with the calcium ionophore ionomycin (Macian et al., 2002). We observed similar results when Th1 cells were anergized with antiCD3. A clear upregulation of caspase 3 mRNA expression was detected in cells stimulated with antiCD3 when compared to resting cells or cells stimulated with antiCD3 and antiCD28 (Fig 1A). Interestingly, caspase 3 mRNA was still clearly upregulated even when cells received a costimulatory signal in the form of antiCD28 antibodies after T cells had already been exposed for two hours to antiCD3, which suggests that once the transcriptional complexes that induce caspase 3 transcription are formed, engagement of CD28 might not be sufficient to disrupt them (Figure 1B). Similar results were obtained when D011.10 Th1 cells were stimulated with CHO cells expressing MHC class II IAd molecules (CHO-IAd) loaded with the ovalbumin Ova323–339 peptide (OVA), and compared with resting cells or cells activated using CHO-IAd cells that also expressed B7.2 (CHO-IAd-B7) (Fig. 1C). Stimulated cells also showed some level of caspase 3 mRNA upregulation, although much smaller than anergic cells. In order to assess whether caspase 3 proteolytic activity played any role in the induction of T cell anergy, we determined if increased gene expression correlated with activation. Anergy was induced co-culturing CD4+ T cells isolated from DO11.10 mice and fully differentiated into Th1 cells, with OVA–loaded CHO-IAd cells. As controls, we cocultured Th1 cells with unloaded CHO-IAd cells or with OVA-loaded CHO-IAd-B7 cells. T cells were then recovered, rested for 3 days and re-stimulated with antiCD3 and antiCD28. Induction of anergy was confirmed by decreased proliferation and IL-2 production in Th1 cells that had been previously stimulated with OVA-loaded CHO-IAd, compared to control cells (Fig. 1D and E). We then determined caspase 3 processing by western blot, detected by the presence of a 17/19 KDa band. Proteolytic activation of caspase 3 was only observed in T cells that had received an anergic stimulus, but not in resting or fully stimulated T cells (Fig. 1F). To better characterize the activation of caspase 3, we determined the kinetics of processing during the induction of T cell anergy. Cleavage was evident 6 hours post anergizing stimulation and increased with time (Fig.1G, upper panel). As we had previously shown for caspase 3 expression, activation also seemed to be dependent on calcium signaling, as proteolytic processing was observed with similar kinetics in response to ionomycin (Fig. 1G, lower panel). In an attempt to identify the mechanism responsible for the activation of caspase 3 in anergic T cells, we determined whether caspase 8 and caspase 9 were also activated in response to anergizing stimuli. We could not detect activation of these proteases in anergic cells (Suppl. Fig. 1A). Similarly, the use of biotin-ZVAD-fmk to pull down activated caspases in anergic cells revealed that only caspase 3, but not caspase 8 or caspase 9, was activated in response to an anergizing stimulus (Suppl. Fig. 1B). Recent reports had indicated that calpain, a calcium regulated protease, could be involved in the activating cleavage of caspase 3 (McCollum et al., 2002; McGinnis et al., 1999). In order to investigate this possibility in anergic T cells, we anergized Th1 cells with ionomycin in the presence of different concentrations of the calpain inhibitor calpeptin. Calcium-induced caspase 3 cleavage was greatly reduced in the presence of calpeptin, suggesting that calpain may be responsible, at least in part, for the activation of caspase 3 in anergic T cells (Fig. 1H). Supporting the important role of calpain in the activation of caspase 3 in anergic cells, ionomycin-induced hyporesponsiveness in Th1 cells was compromised by the inhibition of calpain with calpeptin (Suppl. Fig 2).
Figure 1. Anergic cells activate caspase 3.
Th1 cells were stimulated with antiCD3 and/or antiCD28 for 6 hours and caspase 3 mRNA measured by (A) RNase protection assay or (B) qPCR. Where indicated, cells treated with antiCD3 received antiCD28 with a two-hour delay (delαCD28). Results (mean+SEM) are fold induction of mRNA expression relative to the control resting cells from at least 4 experiments. C. DO11.10 Th1 cells were cocultured for 6 hours with CHO-IAd cells loaded or not with OVA, or fully stimulated with OVA-loaded CHO-IAd-B7 cells. Levels of caspase 3 mRNA were analyzed by qPCR. Results are mean+SEM of 3 experiments. D and E. DO11.10 Th1 cells were cocultured for 12 hours with CHO-IAd cells, loaded or not with OVA or with OVA-loaded CHO-IAd–B7 cells. After a 3-day resting period, cells were stimulated with antiCD3+antiCD28 and (D) BrdU incorporation and (E) IL-2 production measured. Bars show mean+SEM from at least 3 experiments. F. Th1 cells were treated as described in (D) and caspase 3 processing detected by western blot. G. Th1 cells were stimulated with OVA-loaded CHO-IAd (upper panel) or ionomycin (lower panel). Processing of caspase 3 was measured at different time points using a specific antibody for its active form. Actin was detected as a loading control. H. Th1 cells were treated with ionomycin for 6 hours in the presence of calpeptin or control vehicle (DMSO). Caspase 3 activation was detected by western blot. Actin was detected as a loading control. Densitometric quantifications of two independent experiments are shown (mean+SEM).
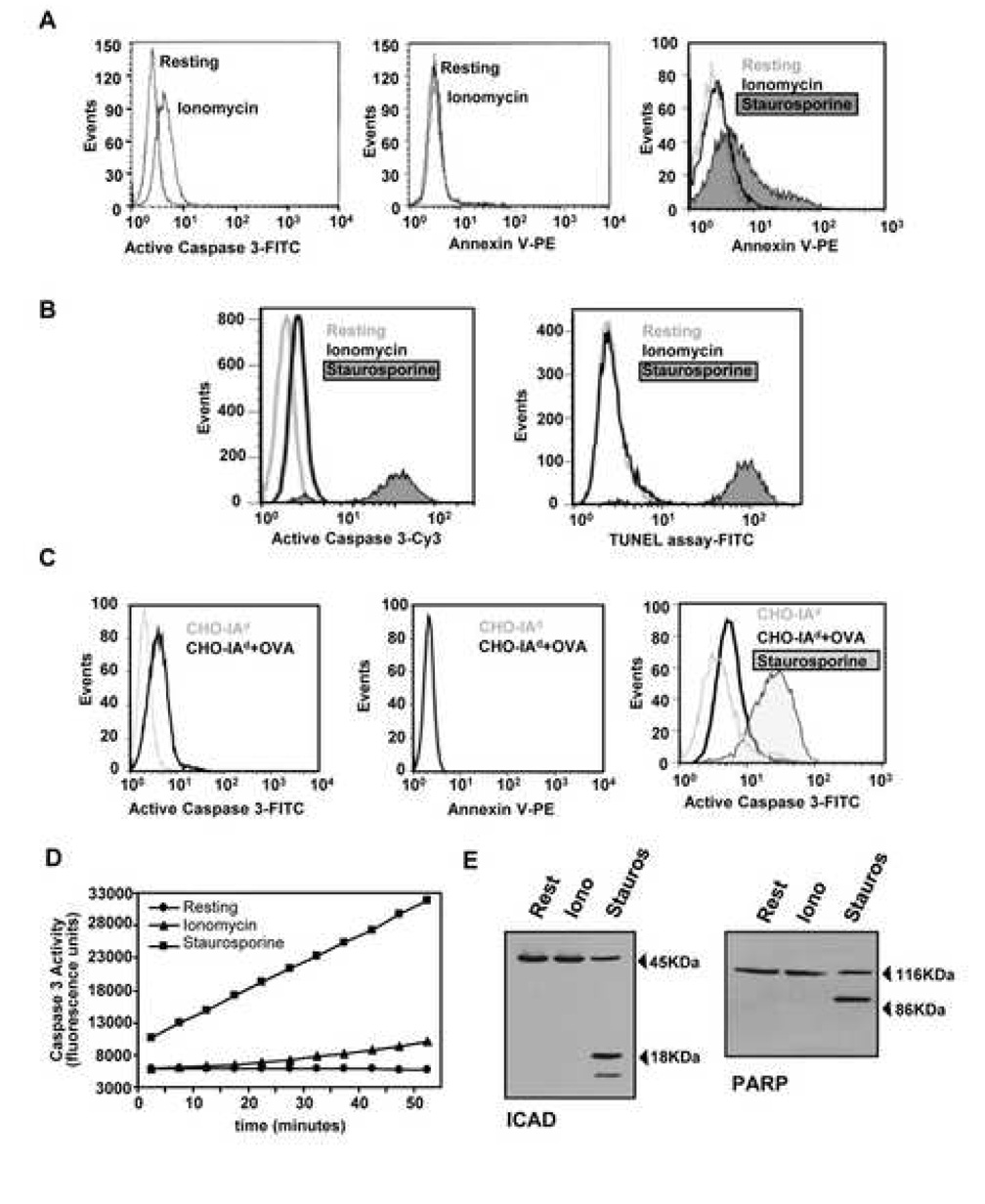
Caspase 3 activation in anergic T cells does not induce apoptosis
Given the key role that caspase 3 plays during apoptosis, we examined whether its increased activation in anergic T cells could be a consequence of the induction of apoptosis. We analyzed changes in the distribution of membrane phosphatidyl-serine, staining with phycoeritrin (PE)-conjugated Annexin V, and DNA fragmentation, using a TUNEL assay. Th1 cells were rendered unresponsive by ionomycin treatment and compared with untreated controls, while cells treated with staurosporine were used as apoptotic controls. Anergic cells showed a clear increase in the levels of active caspase 3, but no differences in Annexin V or TUNEL staining were observed when compared to control cells (Fig. 2A and B). Similar results were obtained when T cells were anergized in the presence of CHO-IAd and OVA and compared with T cells cultured with unloaded CHO-IAd cells (Fig 2C). To understand the differences in caspase 3 activation between anergic and apoptotic T cells, we measured the magnitude of caspase 3 activity in anergic cells and in apoptotic T cells using a caspase 3 activity assay (Fig. 2D). Results showed that, as suggested by our FACS analysis, the increase in caspase 3 activity in anergic cells was much lower than in apoptotic cells. We also compared the cleavage of two proapoptotic substrates processed by caspase 3 in apoptotic cells. While PARP and ICAD were clearly cleaved in apoptotic T cells, no processing of these substrates could be detected in anergic cells (Fig. 2E).
Figure 2. Activation of Caspase 3 does not induce apoptosis in anergic T cells.
A. DO11.10 Th1 cells treated with ionomycin or left resting for 12 hours. T cells were then recovered, washed, stained with an antibody against the active form of caspase 3 (left panel) or with Annexin V-PE (center panel) and analyzed by FACS. T cells treated with staurosporine (1µM, 4 hours) were used as positive control for Annexin V-PE staining (right panel). B. Levels of apoptosis were also detected by TUNEL assay in Th1 cells treated with 1µM ionomycin for 12 hours or with staurosporine for 4 hours. The same cells were also stained for active caspase 3 (left panel). C. Similar analyses were performed in T cells cultured for 12 hours with CHO-IAd cells loaded or not with OVA. D. Caspase 3 activity was measured using a fluorogenic caspase-3 substrate in cell lysates from Th1 cells left resting, anergized with ionomycin for 12 hours or treated with 1µM staurosporine for 4 hours. E. Th1 cells were left resting, incubated with 1µM ionomycin for 12 hours or treated with 1µM staurosporine for 4 hours. Total cell lysates were and analyzed by western blot using antibodies that recognized the proapoptotic caspase 3 substrates PARP and ICAD.
Inhibition of caspase activity compromises anergy induction
To determine the role of caspase 3 in the establishment of T cell anergy, we studied the effect of inhibiting its activation during the induction of anergy. For this purpose, we added the caspase inhibitor Z-VAD to T cells 30 minutes before being anergized with OVA-loaded CHO-IAd cells. Inhibition of caspase 3 activation was confirmed in a fraction of the treated cells (Fig. 3A). Following the anergizing treatment, the caspase inhibitor was washed out, and cells were rested for 3 days before being re-stimulated with antiCD3 and antiCD28. We observed a marked impairment in the induction of anergy in Th1 cells anergized in the presence of Z-VAD. While anergic cells show very poor proliferative responses to stimulation, cells anergized in the presence of Z-VAD proliferated upon re-stimulation almost as well as untreated or previously stimulated control T cells (Fig. 3B). To confirm these results, cells were also anergized using irradiated splenocytes loaded with OVA and CTLA-4-Ig to block B7 molecules. Using this protocol, cells anergized in the presence of Z-VAD produced, following re-stimulation, several fold more IL-2 than cells anergized without Z-VAD (Fig. 3C and D).
Figure 3. Inhibition of caspase activity compromises anergy induction in T cells.
A and B. DO11.10 Th1 cells were anergized by coculture for 12 hours with OVA-loaded CHO-IAd cells. Z-VAD or control vehicle (DMSO) were added where indicated. Th1 cells cocultured with CHO-IAd cells or with OVA-loaded CHO-IAd-B7 cells were used as controls. (A) Z-VAD inhibition of caspase 3 activation was determined by intracellular staining for active caspase 3 or western blot. (B) Level of anergy following treatments was determined by measuring BrdU incorporation after antiCD3+antiCD28 stimulation. C and D. DO11.10 Th1 cells were anergized with irradiated splenocytes preincubated with OVA and CTLA-4-Ig. Z-VAD was added where indicated. After 12h, T cells were recovered, washed and rested for 3 days before re-stimulation with antiCD3+antiCD28. IL-2 production was measured by (C) RNase protection assay or (D) ELISA. Untreated control cells were also analyzed. S: stimulated. R: resting. Values are mean+SEM of at least 3 different experiments.
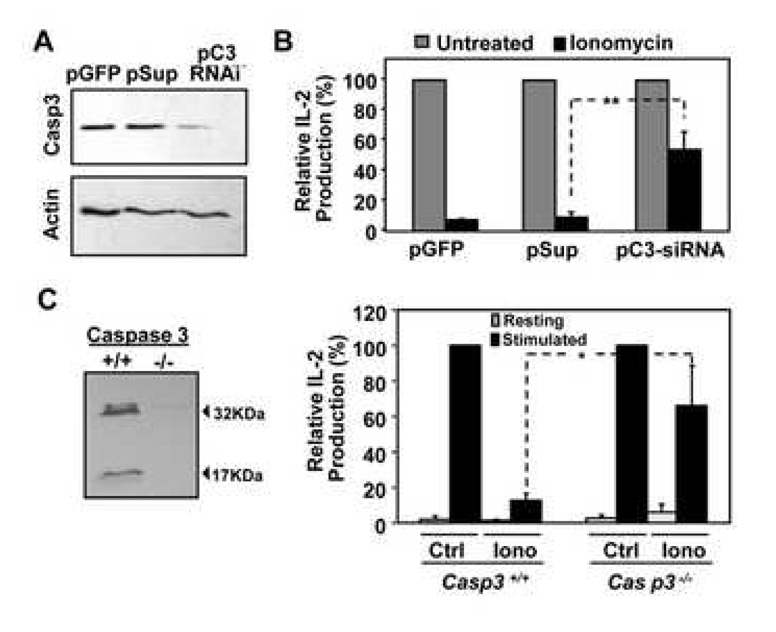
Caspase 3 is necessary to induce anergy in T cells
We next addressed if specifically caspase 3 activity was required to induce T cell hyporesponsiveness. Th1 cells were transfected with a plasmid expressing a shRNA specific for caspase 3 mRNA, which led to a marked reduction of caspase 3 expression (approx. 80%), and almost no detectable caspase 3 activation after anergy induction (Fig. 4A and data not shown). Inhibition of IL-2 expression following anergizing treatment with ionomycin was significantly impaired in caspase 3 knocked-down cells compared to control cells (1.8 vs. 7.1 fold reduction respectively) (Fig. 4B). We confirmed these results using Th1 cells from Casp3−/− mice. Cells were anergized using ionomycin for 12 hours and IL-2 production was measured following re-stimulation with antiCD3 and antiCD28. As previously seen in knocked-down cells, Casp3−/− T cells were significantly less susceptible to become anergic than Casp3+/+ T cells (1.5 vs. 7.7 fold reduction, respectively) (Fig. 4C). Under these conditions, we could not detect significant differences in the levels of apoptosis induced in anergic wild type or Casp3−/− T cells following stimulation with antiCD3 and antiCD28 (Suppl. Fig. 3).
Figure 4. Blocking caspase 3 expression interferes with the induction of anergy.
A. Th1 cells were transfected with a GFP expressing vector only or together with the pC3RNAi, which expresses a specific caspase 3 shRNA, or control pSup vector. Cells were then sorted for GFP expression, left resting or treated with 1µM ionomycin for 12 hours, and caspase 3 and actin were detected by western blot. B. Control and ionomycin-treated caspase 3 knocked-down cells were stimulated with antiCD3+antiCD28 and IL-2 production measured by ELISA. Bars show percentage of IL-2 expression relative to control stimulated cells, and are mean+SEM of 3 experiments. **=p<0.01. C. CD4+ T cells were isolated from casp3−/− mice and wild-type littermates. Absence of caspase 3 expression in Casp3−/− cells was confirmed by western blot. T cells were differentiated under Th1 skewing conditions and then left resting or treated with 1µM ionomycin for 12 hours. Cells were then washed and left resting for 6–8 hours before re-stimulation with antiCD3+antiCD28. Inhibition of IL-2 production in Casp3−/− T cells was compared with that of wild type anergized T cells. Bars show percentage of IL-2 expression in anergic cells compared with control stimulated cells, and are mean+SEM of 6 mice. *=p<0.05.
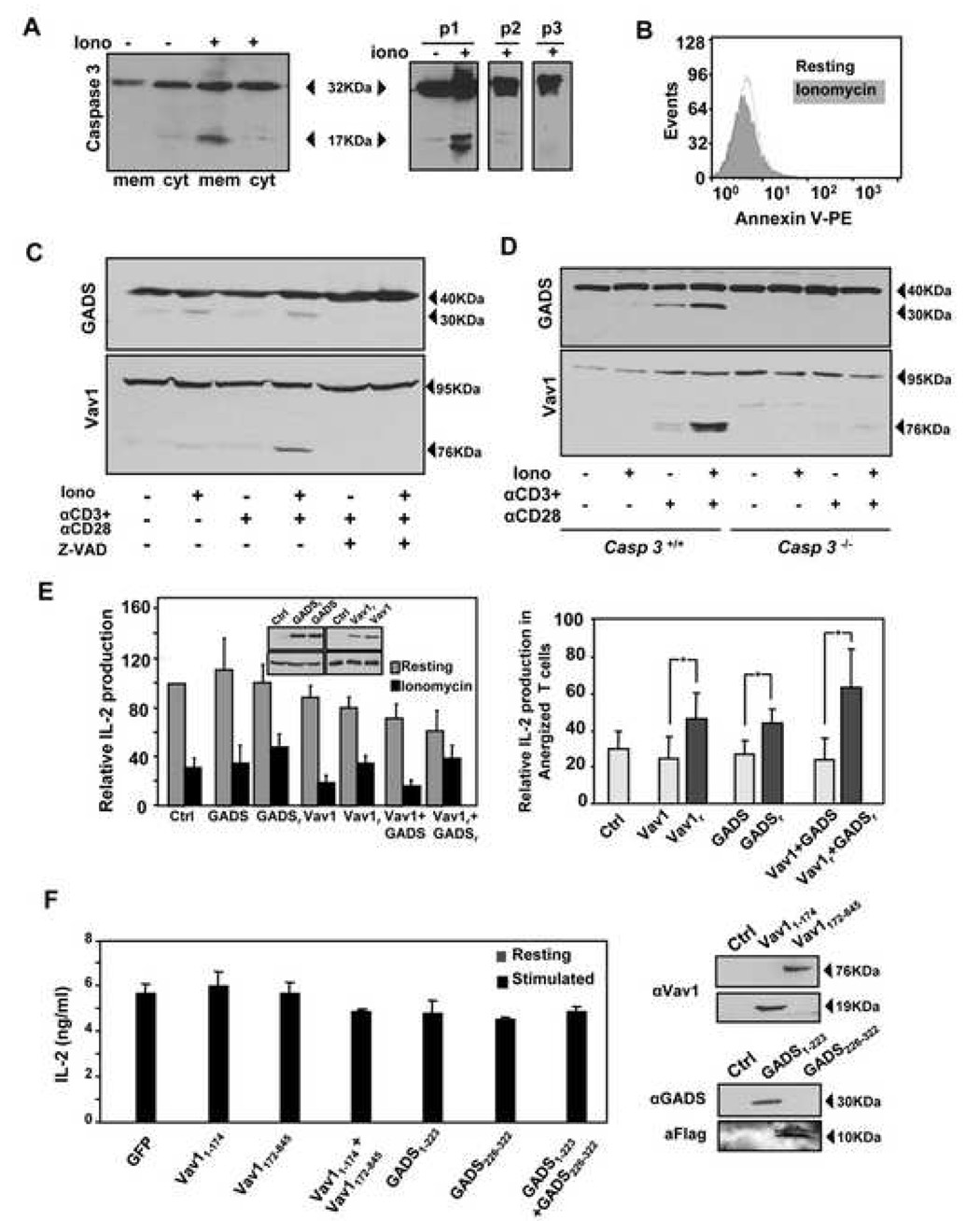
Caspase 3 cleaves GADS and Vav1 in anergic T cells
Several TCR signaling components have been reported as caspase substrates during apoptosis or following CD95-induced inhibition of antigen receptor signaling, including the Grb2-related adaptor downstream of shc (GADS) (Berry et al., 2001), the guanine-nucleotide exchange factor Vav1 (Hofmann et al., 2000), the TCRζ chain (Gastman et al., 1999) and PLCγ (Bae et al., 2000). Analyzing total cell extracts from anergic T cells we were able to detect degradation of GADS (Fig. 5A). No detectable cleavage was found in other substrates (data not shown). This cleavage was caspase 3-dependent as it did not occur in Casp3−/− Th1 cells (Fig. 5B). Similar results were obtained when T cells from DO11.10 mice were tolerized in vivo by oral feeding with ovalbumin (Fig. 5C left panel). Orally tolerized CD4+ T cells activated caspase 3 and showed cleavage of GADS and Vav1 (Fig. 5C right panels)
Figure 5. Caspase 3 cleaves GADS and Vav1 in anergic T cells.
A. DO11.10 Th1 cells were cocultured for 12 hours with CHO-IAd cells loaded or not with OVA, or with OVA-loaded CHO-IAd-B7 cells. T cells were then recovered and caspase 3 and GADS processing determined. B. Casp3−/− and wild-type littermates Th1 cells were treated for 12 hours with ionomycin and processing of caspase 3 and GADS detected by western blot. C. DO11.10 mice were orally tolerized with ovalbumin. Seven days later, CD4+ cells were isolated and stimulated with antiCD3+antiCD28. IL-2 production was measured by ELISA. Proteolytic cleavage of caspase 3, GADS and Vav1 was determined by western blot. Results are from two different mice from each group (mean+SEM).
Levels of GADS and Vav1 cleavage detected were moderate, suggesting that only a reduced fraction of the total cell content of those proteins was being processed by caspase 3. One possible explanation could be that only the fraction of GADS and Vav1 recruited to the TCR-associated signaling complex could become target of caspase 3 activity. To test this hypothesis, we first analyzed the subcellular localization of active caspase 3 in anergic T cells. The active form of caspase 3 was only detected in the pellet but not in the soluble fraction of a S100 lysate, suggesting membrane localization (Fig. 6A, left panel). Further subfractionation assays showed that active caspase 3 appeared clearly associated with the plasma membrane fraction, but not with those containing other organelles or with nuclear fractions (Fig 6A, right panel). Purity of the fractions was assessed using antibodies against connexin43, GM130 and Bcl-2 (data not shown). This specific subcellular localization would provide a way to restrict caspase 3 activity to signaling molecules recruited to the membrane during T cell activation. In order to test this hypothesis, we analyzed the cleavage of GADS and Vav1 in anergic cells following re-stimulation. Th1 cells derived from Casp3+/+ or Casp3−/− mice were treated with ionomycin in the presence or absence of the caspase inhibitor Z-VAD for 12 hours to induce anergy. Cells were then washed and left resting for several hours before re-stimulation. No differences in the levels of Annexin V staining could be detected between stimulated anergic and non-anergic cells, ruling out the possibility that increased caspase 3-mediated processing might result from increased apoptosis in anergic T cells (Fig 6B). As hypothesized, stimulation of T cells led to increased GADS and Vav1 cleavage in anergic cells (Fig. 6C and D). Very low levels of processing could also be observed in non-anergic cells, which could result from a fraction of cells that might have been only suboptimally stimulated in our assay, or from some small level of apoptosis induced during stimulation. As shown before, this cleavage was caspase 3 dependent, as it was blocked by a caspase inhibitor (Fig. 6C) and was absent in Casp3−/− T cells (Fig. 6D).
Figure 6. Active caspase 3 associates to the plasma membrane in anergic T cells where it cleaves GADS and Vav1 following TCR stimulation.
A. S100 fractions were prepared from resting Th1 cells or cells treated with 1µM ionomycin for 12 hours (left panel). Lysates from those cells were also fractionated by differential centrifugation in sucrose gradients into fractions containing the plasma membrane (P1), mitochondria, lysosomes, endosomes and peroxisomes (P2) and the Golgi and ER (P3) (right panel). The presence of active caspase 3 in all fractions was detected by western blot. B and C. Th1 cells from Casp3−/− or wild type mice were treated with 1µM ionomycin for 12 hours, washed and rested for 4 hours before stimulation with antiCD3+antiCD28 for 6 hours. Annexin V staining was detected by FACS in stimulated cells (B) and proteolytic processing of GADS and Vav1 determined in lysates from these cells (C). D. Th1 cells were left untreated or treated with 1µM ionomycin for 16 hours, washed and left resting for 4 hours before re-stimulation, in the presence or absence of Z-VAD. After 6 hours, cells were collected and proteolytic cleavage of GADS and Vav1 detected by western blot. E. Th1 cells from C57BL6/J mice were transfected with plasmids expressing wild-type or caspase-resistant (GADSr and Vav1r) murine GADS and/or Vav1. Amounts of DNA used were adjusted to obtain similar levels of expression (inset). Control cells were transfected with pGFP. 36 hours post-transfection, cells were rested or treated with ionomycin 1µM for 12 hours. IL-2 was measured by ELISA after stimulation with antiCD3+antiCD28. Left panel shows normalized (to control untreated cells) IL-2 values, whereas right panel shows percentage of IL-2 expression in anergic cells compared with control cells in each experimental condition. Bars are mean+SEM of 4 experiments. *p<0.05. F. Th1 cells were transduced with retrovirus expressing the N-terminal (Vav11–174, GADS1–223) and/or the C-terminal (Vav1172–845, GADS226–322) fragments of Vav1 and GADS. Expression of these peptides was verified by western blot in infected NIH3T3 cells. IL-2 production was measured in sorted T cells after stimulation with antiCD3+antiCD28. Bars are mean+SEM of at least 2 experiments.
If the blockade of TCR signaling was a consequence of the caspase-dependent cleavage of Vav1 and GADS, we should be able to reverse this effect by using caspase-resistant forms of these proteins. Th1 cells were transfected with plasmids expressing either wild type or caspase-resistant forms of GADS and Vav1 bearing mutations in the caspase recognition site that rendered them resistant to caspase 3-mediated cleavage (Berry et al., 2001; Hofmann et al., 2000). Ionomycin-induced hyporesponsiveness, measured as decreased IL-2 production upon re-stimulation, was similar in control cells and in cells transfected with wild type proteins, but was significantly reduced in cells expressing the caspase-resistant GADS or Vav1. This effect was even more pronounced when both proteins were co-expressed together (Fig. 6 E).
To exclude the possibility that the cleaved fragments might have an effect on T cell activation, we transduced primary T cells with retrovirus expressing the different fragments that would result from the caspase 3-mediated cleavage of Vav1 or GADS. Cells expressing either of the two different fragments or both fragments at the same time were then stimulated with antiCD3 and antiCD28. The expression of those fragments did not cause any effect on IL-2 production, suggesting that the loss of function seen in anergic T cells was likely a consequence of the cleavage of these proteins and not of alterations in signaling pathways caused by the cleaved fragments (Fig. 6F).
Caspase 3 cleavage of Vav1 prevents its recruitment to the plasma membrane
Vav1 is recruited to the membrane upon engagement of the TCR. To determine if cleavage by caspase 3 could prevent this recruitment, we analyzed Vav1 subcellular localization following TCR engagement in control and anergic T cells. As expected, in resting cells Vav1 resided mostly in the cytosol, but was readily recruited to the membrane upon stimulation. This effect was blocked in anergic cells, which showed markedly reduced Vav1 recruitment to the plasma membrane following stimulation with antiCD3 and antiCD28 (Fig. 7A). To corroborate the effect that cleavage of Vav1 had on its subcellular localization, we analyzed membrane recruitment in anergized cells previously transduced with retroviruses that expressed either wild type or a caspase 3-resistant Vav1. In control cells transduced with a virus expressing GFP, recruitment of Vav1 to the plasma membrane upon activation was greatly reduced in anergic T cells. Overexpression of a wild type Vav1 resulted in a small increase in membrane recruitment that was not significant and likely due to the overexpression of this protein. However, the expression of the caspase 3-resistant Vav1 completely restored recruitment of Vav1 to the membrane in anergic cells to levels similar to those found in non-anergic control T cells (Fig. 7B).
Figure 7. Caspase 3–mediated cleavage prevents activation-dependent recruitment of Vav1 to the membrane in anergic T cells.
A.Vav1 membrane recruitment was determined by western blot of pellet (mem) and supernatant (cyt) S100 fractions of control and anergic Th1 cells stimulated with antiCD3+antiCD28. Detection of flotillin1 was used as a membrane marker. B. Th1 cells, transduced with virus expressing wild-type or caspase-resistant (Vav1r) Vav1, were sorted and the translocation of Vav1 to the membrane in control and anergized cells determined after stimulation with antiCD3+antiCD28. Densitometric quantification of six experiments is shown. **p<0.01, *p<0.05.
DISCUSSION
It is becoming increasingly evident that caspases are versatile proteases that control many cellular functions other than apoptosis. Here we show that caspase 3 negatively regulates TCR signaling in anergic T cells. Anergy is induced in T cells by suboptimal stimulation that causes sustained calcium signaling and the consequent activation of the calcineurin/NFAT axis (Bandyopadhyay et al., 2007; Borde et al., 2006; Heissmeyer et al., 2005). We have shown that under these conditions the expression of a specific set of genes is upregulated (Macian et al., 2002), which encode proteins responsible for the inhibition of T cell activation (Anandasabapathy et al., 2003; Heissmeyer et al., 2004; Jeon et al., 2004; Olenchock et al., 2006; Su et al., 2006; Zha et al., 2006). Caspase 3 is also expressed in anergic T cells in a calcium/NFAT-dependent manner (Macian et al., 2002; Kaji et al., 2003). Our results show now that caspase 3 is activated in non-apoptotic anergic T cells, and that the activity of this protease is necessary to maintain anergic T cells hyporesponsive.
Originally described as a crucial regulator of apoptosis, caspase 3 has also been shown to regulate other processes including skeletal muscle differentiation, and the maturation of erythroid precursors, monocytes, and dendritic cells (De Maria et al., 1999; Fernando et al., 2002; Santambrogio et al., 2005; Sordet et al., 2002; Wong et al., 2004). In B cells, caspase 3 regulates entry into the cell cycle by cleaving p21 and preventing the formation of active cyclin/CDK complexes (Woo et al., 2003). In T cells, caspase 8 is activated upon stimulation and controls T cell proliferation (Alam et al., 1999; Kennedy et al., 1999). Blocking caspase activity during T cell stimulation leads to decreased activation-induced proliferation and cytokine production, which is also evident in caspase 8 deficient T cells (Alam et al., 1999; Falk et al., 2004; Kennedy et al., 1999; Salmena and Hakem, 2005; Salmena et al., 2003). The mechanisms responsible for this effect may reside in the ability of active caspase 8 to induce NF-κB activation and to regulate cell cycle in T cells (Falk et al., 2004; Su et al., 2005). Here we report that another member of the caspase family of proteases, caspase 3, has a different role in T cell physiology and negatively regulates TCR signaling in anergic T cells. Although we cannot rule out that other mechanisms may also contribute to the activation of caspase 3 in response to tolerogenic stimuli, our results indicate that calcium signaling induces the calpain-mediated activation of this protease in anergic T cells. Caspase 3 activation has also been reported in fully stimulated T cells, but with delayed kinetics compared to its activation in anergic cells (Alam et al., 1999; Misra et al., 2005). It is possible, thus, that caspase 3 may contribute to terminate T cell activation through a mechanism similar to the one that operates in anergic T cells.
Ubiquitin ligases, such as Itch and Cbl-b, have been implicated in downregulating TCR signaling by targeting components of the TCR signalosome to the endocytic pathway, leading to their functional inactivation or degradation (Heissmeyer et al., 2004). We propose that caspase 3-mediated cleavage of proteins involved in TCR signal transduction, such as GADS and Vav1, is also responsible for maintaining a status of hyporesponsiveness in anergic T cells. GADS is an adaptor protein that interacts with SLP-76 and LAT (Boerth et al., 2000; Yankee et al., 2004). GADS-deficient T cells have profound defects in TCR signaling that severely affect thymocyte development (Yankee et al., 2004). Induction of apoptosis or CD95 engagement lead to the caspase-dependent cleavage of GADS, which blocks productive TCR signaling (Berry et al., 2001; Yankee et al., 2001). Vav1, a nucleotide exchange factor for the RhoA, Rac1 and cdc42 GTPases (Fujikawa et al., 2003; Tybulewicz et al., 2003), is also cleaved by caspase 3 in apoptotic T cells (Hofmann et al., 2000). The resulting fragment fails to induce p38 activation and cannot support transcription from NFAT, AP-1 or NF-κB reporter plasmids (Hofmann et al., 2000). We report here that cleavage of these two proteins occurs also in anergic T cells. Whereas in apoptotic cells inhibition of these signaling pathways may prevent aberrant cytokine synthesis in a cell destined to die, in anergic T cells this same mechanism would engage to block T cell activation. We have confirmed that the cleavage of GADS and Vav1 in anergic T cells is mediated by caspase 3. Blocking caspase 3 expression with siRNA or using T cells from caspase 3-deficient mice leads to defective anergy induction, which correlates with lack of GADS and Vav1 cleavage. Furthermore, cells that express caspase-resistant forms of these proteins are also resistant to anergy induction. Nevertheless, some degree of hyporesponsiveness is still induced in these cells, which might be due to the activation of the other mechanisms that also dampen TCR signaling in anergic T cells. Although the results from the experiments performed using caspase-resistant Vav1 and GADS strongly suggest a critical role for these two proteins in controlling TCR signaling in anergic T cells, we cannot rule out that other targets of caspase 3 exist in anergic T cells and that their proteolytic processing also contributes to keep anergic cells hyporesponsive.
The role of caspase 3 as a tolerance-inducing factor does not seem to be restricted to the induction of clonal anergy in Th1 cells, as we can also detect caspase 3 activation and cleavage of GADS and Vav1 in T cells from orally tolerized mice. These findings support that activation of the calcium/NFAT dependent expression of caspase 3, and likely several other genes, may constitute a basic mechanism for the induction of T cell tolerance.
Substrate specificity may underlie the different roles of caspase 3 in different tissues and physiological conditions and explain why its activation does not cause apoptosis in anergic T cells. It is still not clear how this specificity might be achieved. We could not detect differences in the expression of several negative regulators of caspase activation (Suppl. Fig. 4); however, levels of caspase 3 activity in anergic cells were much lower than those detected in apoptotic cells. More importantly, active caspase 3 seems to be confined to the plasma membrane, where accessibility to substrates would be restricted. Supporting this idea, whereas we could not detect significant changes in the levels of total Vav1 in anergic T cells, the presence of this protein in the plasma membrane was drastically reduced in anergic cells, but could be restored by expressing a caspase-resistant form of Vav1. Hence, both quantitative and qualitative differences in caspase 3 activity between anergic and apoptotic T cells, may explain the different outcomes of the activation of this protease.
Maintenance of T cell tolerance may represent just a part of a global role of caspases as immunomodulators. Apart from its involvement in AICD and anergy, caspase 3 has also been shown to negatively regulate B cell progression into the cell cycle (Woo et al., 2003). Furthermore, basal caspase 3 activity is required to maintain dendritic cells in an immature state, and its inhibition by activating stimuli induces dendritic cell maturation and productive presentation of antigens (Santambrogio et al., 2005; Wong et al., 2004).
Caspase 3 plays, thus, a crucial role in the establishment and maintenance of T cell tolerance. Calcium signals engaged in response to tolerogenic stimuli are responsible for the activation of caspase 3, which inhibits T cell activation by inactivating key proteins involved in transducing signals from the TCR.
EXPERIMENTAL PROCEDURES
Mice
Mice were maintained in pathogen-free conditions. All animal work was performed according to the guidelines set by the Institutional Animal Care Committee of the Albert Einstein College of Medicine. Four to six-week-old C57BL6/J, BALB/c and DO11.10 mice were purchased from Jackson Laboratories. Casp3−/− mice (Woo et al., 2003) were kindly provided by Dr. T.W. Mak.
Cell Culture
Primary CD4+ T cells were isolated from lymph nodes and spleens using magnetic beads (Invitrogen), stimulated with 0.5µg/ml plate bound antiCD3 and 0.5µg/ml antiCD28 (BD) and differentiated for 7 days with 10ng/ml IL-12 (Cell Science), 10µg/ml anti-IL-4 and 10U/ml of recombinant human IL-2. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2mM L-glutamine, nonessential amino acids, essential vitamins (Cambrex), and 50µM 2-mercaptoethanol. Chinese hamster ovary (CHO) cells expressing IAd with or without B7.2 were kindly provided by S. Bhatia.
Induction of T cell tolerance
Four different systems were used to induce T cell tolerance: 1. Mitomycin C (Sigma) pretreated CHO-IAd cells loaded with 10µM OVA peptide were cocultured with DO11.10-derived Th1 cells for 12 hours. Th1 cells were recovered, rested for 3 days, before being analyzed. 2. Th1 cells were treated with 1µM ionomycin (EMD) for 12 hours, washed, and rested for 4 hours before being re-stimulated. 3. DO11.10 Th1 cells were cultured for 12 hours with irradiated BALB/c mice splenocytes preincubated with 10µM OVA and 10µg/ml CTLA-4-Ig (Chimerigen). Th1 cells were recovered, washed and rested for 3 days before being analyzed. In all procedures, the caspase inhibitor Z-VAD (EMD) was added at a final concentration of 100µM where indicated during anergy induction and then washed out. 4. Oral tolerance was induced by the administration of a single intragastric dose of 25mg of ovalbumin (Sigma) to DO11.10 mice. On day 7 CD4+ T cells were isolated and analyzed.
ELISA
2.5 or 5×104 Th1 cells were stimulated in 96 well plates. Supernatants were collected 24 hours after stimulation and IL-2 levels were measured in a sandwich ELISA (BD).
Proliferation Assay
25×103 Th1 cells were stimulated in 96 well plates. Sixty hours later 5-Bromo-2’-deoxy-uridine (BrdU) was added for 12 hours. Incorporation of BrdU was measured by ELISA following manufacturer’s instructions (Roche).
Immunoblotting
Whole cell extracts were prepared by boiling cell pellets directly in SDS to prevent proteolysis during cell lysis. Antibodies against caspase 3 and 9 and cleaved caspase 3 (Cell Signaling), GADS (Upstate Biotech), Vav1 (Santa Cruz Biotech), FLIP (EMD) and Caspase 8, XIAP, Flotillin1, Connexin-43, GM130 and Bcl-2 (BD) were used. Where indicated, the calpain inhibitor calpeptin (EMD) or the caspase inhibitor Z-VAD were added to cells 30 minutes before adding 1µM ionomycin for 6 to 12 hours. Densitometric quantification of the immunoblotted membranes and stained gels was done with ImageJ (National Institutes of Health, http://rsb.info.nih.gov/ij).
Detection of active caspases
Total cell extracts were prepared by lysing 5×106 cells in 1ml of RIPA buffer with a cocktail of protease inhibitor (Roche). 100µM of biotin-VAD-fmk (EMD) were added for 4hours at 4°C, followed by 50µl of streptavidin agarose beads (Invitrogen) and incubated for 4 hours at 4°C. Agarose beads were washed 3 times with RIPA buffer and boiled directly in SDS.
Apoptosis detection
Apoptosis was determined using Annexin V-PE apoptosis detection kit (BD), or by TUNEL assay using the In situ Cell Death Detection kit (Roche). Stained cells were analyzed on a FACSCAN (Beckton-Dickinson).
Intracellular staining
To detect caspase 3 activation, T cells were fixed in 4% paraformaldehyde, permeabilized in PBS/1% BSA/0.5% saponin and incubated with an antibody against the active fragment of caspase-3. A secondary FITC or Cy3-conjugated antibody was used. Cells were analyzed on a FACSCAN.
Caspase 3 activity
T cells were plated in 96-well pates at 5×104 cells/well. Fluorogenic caspase 3 substrate IX (EMD) was added at a final concentration of 50µM. Fluorescence was measured using a plate reader fluorimeter (BMG) every 5 minutes for 1 hour at 37°C.
RNase Protection Assay
Total RNA was obtained from T cells with Trizol Reagent (Invitrogen) and analyzed using the RiboQuant multiprobe RNase protection kit with the mCK-1b or the mAPO-1 probes (BD).
Quantitative RT-PCR (qPCR)
Total RNA was prepared from T cells with Trizol Reagent and used to synthesized cDNA using a Superscript kit (Invitrogen). qPCR was performed using a SYBR green master mix (Stratagene) as described before (Macian et al., 2002) using specific primers to amplify a fragment from the caspase 3 cDNA (5’-ACGCGCACAAGCTAGAATTT, 5’-CTTTGCGTGGAAAGTGGAGT).
Plasmids and T cell transfection
Plasmids expressing the wild-type murine GADS and a caspase resistant mutant form (D235A) were kindly provided by C.J. McGlade (Berry et al., 2001; Liu and McGlade, 1998). A wild-type Vav-1 expressing construct was kindly provided by X.R. Bustelo. Vav1 protein resistant to caspase cleavage (D150A/D161A) was generated using PCR-based site-directed mutagenesis. 5 to 20µg of DNA were introduced in T cells using a nucleofector device (AMAXA) following the manufacturer’s protocol. Cells were anergized 36 hours post-transfection.
Retroviral infection of primary Th1 cells
GADS, GADS1–223, flag-GADS226–322, Vav1, Vav11–174 and Vav1172–845 cDNAs were subcloned in the RV-IRES-GFP vector (Macian et al., 2002). Phoenix ecotropic packaging cells were transfected using a calcium/phosphate method with the different retroviral vectors. 48 hours after transfection, retroviral supernatants were collected, supplemented with polybrene (8 µg/ml), and used to infect T cells 24 hours after stimulation. Infected cells were sorted for GFP expression.
RNA Interference
A murine caspase 3 specific shRNA (5’- GTAAAGACCATACATGGGA-3’) was introduced into the pSUPER (Oligoengine). 20 µg of DNA (1:4 ratio pMaxGFP and pC3RNAi) were transfected into 5×106 T cells. GFP-positive cells were sorted 36 hours post-transfection and analyzed.
Subcellular fractionation
Cells were resuspended in ice-cold fractionation buffer (10mM Tris- HCl, pH 7.5, 5mM MgCl2, 1mM EDTA, and 1mM DTT with protease inhibitors) and disrupted by sonication. Nuclei were pelleted by centrifugation (700xg, 10 min, 4°C) and supernatants centrifuged at 100,000g for 1h at 4°C to obtain the membrane (P100) and the cytosolic (S100) fractions. Alternatively, cells were resuspended in ice-cold 0.25M sucrose and disrupted by sonication. Nuclei were pelleted and supernatants centrifuged at 1450g for 10 min at 4°C to obtain the membrane fraction (P1). Centrifugation at 17000g, 10 min, at 4°C was performed to obtain the organelles fraction (mitochondria, lysosomes, endosomes and peroxisomes) (P2). A final centrifugation at 100,000xg (1h at 4°C) was performed to separate endoplasmic reticulum and Golgi (P3).
Statistical analysis
Differences in the inhibition of IL-2 production in anergic T cells were analyzed with a Student's t-test (paired, two-tails).
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of our laboratories for helpful discussions and A.M. Cuervo and L. Santambrogio for critical reading of the manuscript. We thank T.W. Mak for the Casp3−/− mice, and S. Bathia for the CHO-IAd and CHO-IAd-B7 cell lines. We also thank C.J. McGlade for the GADS constructs and X.R. Bustelo for the Vav-1 expression plasmid. Human IL-2 and antibodies against murine IL-4 were obtained from the Biological Resources Branch preclinical repository. This work was supported by National Institutes of Health grants AI48213 (AR.) and AI059738 (FM.), and by the Irene Diamond Foundation (FM.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alam A, Cohen LY, Aouad S, Sekaly R-P. Early Activation of Caspases during T Lymphocyte Stimulation Results in Selective Substrate Cleavage in Nonapoptotic Cells. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis-independent functions of killer caspases. Current Opinion in Cell Biology. 2002;14:721–726. doi: 10.1016/s0955-0674(02)00384-8. [DOI] [PubMed] [Google Scholar]
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: An E3 Ubiquitin Ligase that Inhibits Cytokine Gene Transcription Is Expressed in Anergic CD4+ T Cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- Bae SS, Perry DK, Oh YS, Choi JH, Galadari SH, Ghayur T, Ryu SH, Hannun YA, Suh PG. Proteolytic cleavage of phospholipase C-gamma1 during apoptosis in Molt-4 cells. FASEB J. 2000;14:1083–1092. doi: 10.1096/fasebj.14.9.1083. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Soto-Nieves N, Macian F. Transcriptional regulation of T cell tolerance. Semin Immunol. 2007;19:180–187. doi: 10.1016/j.smim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DM, Benn SJ, Cheng AM, McGlade CJ. Caspase-dependent cleavage of the hematopoietic specific adaptor protein Gads alters signalling from the T cell receptor. Oncogene. 2001;20:1203–1211. doi: 10.1038/sj.onc.1204218. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Boerth NJ, Sadler JJ, Bauer DE, Clements JL, Gheith SM, Koretzky GA. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J Exp Med. 2000;192:1047–1058. doi: 10.1084/jem.192.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–119. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U, Peschle C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Denis F, Rheaume E, Aouad SM, Alam A, Sakaly R-P, Cohen LY. The role of caspases in T cell development and the control of immune responses. Cellular and Molecular Life Sciences (CMLS) 1998;V54:1005–1019. doi: 10.1007/s000180050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M, Ussat S, Reiling N, Wesch D, Kabelitz D, Adam-Klages S. Caspase inhibition blocks human T cell proliferation by suppressing appropriate regulation of IL-2, CD25, and cell cycle-associated proteins. J Immunol. 2004;173:5077–5085. doi: 10.4049/jimmunol.173.8.5077. [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K, Miletic AV, Alt FW, Faccio R, Brown T, Hoog J, Fredericks J, Nishi S, Mildiner S, Moores SL, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Caspase-mediated Degradation of T-Cell Receptor {{zeta}}-Chain. Cancer Res. 1999;59:1422–1427. [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Varma R, Im SH, Garcia-Cozar F, Horton HF, Byrne MC, Feske S, Venuprasad K, Gu H, et al. A molecular dissection of lymphocyte unresponsiveness induced by sustained calcium signalling. Novartis Found Symp. 2005;267:165–174. [PubMed] [Google Scholar]
- Hofmann TG, Hehner SP, Droge W, Schmitz ML. Caspase-dependent cleavage and inactivation of the Vav1 proto-oncogene product during apoptosis prevents IL-2 transcription. Oncogene. 2000;19:1153–1163. doi: 10.1038/sj.onc.1203406. [DOI] [PubMed] [Google Scholar]
- Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon M-S, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L. Essential Role of the E3 Ubiquitin Ligase Cbl-b in T Cell Anergy Induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kaji T, Hachimura S, Ise W, Kaminogawa S. Proteome Analysis Reveals Caspase Activation in Hyporesponsive CD4 T Lymphocytes Induced in Vivo. J Biol Chem. 2003;278:27836–27843. doi: 10.1074/jbc.M212820200. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase Activation Is Required for T Cell Proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbus A, Pilat S, Husak Z, Deiner EM, Stengl G, Beug H, Baccarini M. Raf-1 Antagonizes Erythroid Differentiation by Restraining Caspase Activation. J Exp Med. 2002;196:1347–1353. doi: 10.1084/jem.20020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Liu SK, McGlade CJ. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene. 1998;17:3073–3082. doi: 10.1038/sj.onc.1202337. [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- McCollum AT, Nasr P, Estus S. Calpain activates caspase-3 during UV-induced neuronal death but only calpain is necessary for death. J Neurochem. 2002;82:1208–1220. doi: 10.1046/j.1471-4159.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- Miossec C, Dutilleul V, Fassy F, Diu-Hercend A. Evidence for CPP32 Activation in the Absence of Apoptosis during T Lymphocyte Stimulation. J Biol Chem. 1997;272:13459–13462. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- Misra RS, Jelley-Gibbs DM, Russell JQ, Huston G, Swain SL, Budd RC. Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates. J Immunol. 2005;174:3999–4009. doi: 10.4049/jimmunol.174.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L, Potolicchio I, Fessler SP, Wong S-H, Raposo G, Strominger JL. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E, Dubrez-Daloz L. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- Su L, Lineberry N, Huh Y, Soares L, Fathman C. A Novel E3 Ubiquitin Ligase Substrate Screen Identifies Rho Guanine Dissociation Inhibitor as a Substrate of Gene Related to Anergy in Lymphocytes. J Immunol. 2006;177:7559–7566. doi: 10.4049/jimmunol.177.11.7559. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Vav1: a key signal transducer downstream of the TCR. Immunol Rev. 2003;192:42–52. doi: 10.1034/j.1600-065x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Wong SH, Santambrogio L, Strominger JL. Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc Natl Acad Sci U S A. 2004;101:17783–17788. doi: 10.1073/pnas.0408229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M, Hakem R, Furlonger C, Hakem A, Duncan GS, Sasaki T, Bouchard D, Lu L, Wu GE, Paige CJ, Mak TW. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol. 2003;4:1016–1022. doi: 10.1038/ni976. [DOI] [PubMed] [Google Scholar]
- Yankee TM, Draves KE, Ewings MK, Clark EA, Graves JD. CD95/Fas induces cleavage of the GrpL/Gads adaptor and desensitization of antigen receptor signaling. Proc Natl Acad Sci U S A. 2001;98:6789–6793. doi: 10.1073/pnas.111158598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankee TM, Yun TJ, Draves KE, Ganesh K, Bevan MJ, Murali-Krishna K, Clark EA. The Gads (GrpL) adaptor protein regulates T cell homeostasis. J Immunol. 2004;173:1711–1720. doi: 10.4049/jimmunol.173.3.1711. [DOI] [PubMed] [Google Scholar]
- Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Center DM, Wu DMH, Cruikshank WW, Yuan J, Andrews DW, Kornfeld H. Processing and Activation of Pro-Interleukin-16 by Caspase-3. J Biol Chem. 1998;273:1144–1149. doi: 10.1074/jbc.273.2.1144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.