CASE REPORT
A 71-year-old man, who had been hepatitis B positive for over 20 years, underwent left hepatectomy in October 2004 after a 5.0 cm lesion was found in the left lobe of his liver. Two years later, in October of 2006, at least 3 large lesions were found in his right lobe. Alpha fetoprotein (AFP) level was 520 ng/mL. This patient was not a candidate for liver transplantation. He underwent transarterial chemoembolization (TACE) followed by radiofrequency ablation (RFA). In January of 2007 magnetic resonance imaging (MRI) revealed that the liver lesions were well controlled, and his AFP had decreased to 68 ng/mL [Figure 1]. Two months later, in March of 2007, imaging studies showed stable post treatment changes in the liver [Figure 2], but lesions were present in the lung and hilum [Figure 3]. Biopsy was performed to determine whether the lesions represented newly developed lung cancer or metastases from liver disease. Results of the biopsy confirmed metastatic hepatocellular carcinoma (HCC)
Figure 1.
MRI images obtained in October 2006 (top) and in January 2007, (below) 1 year after transarterial chemoablation and radiofrequency ablation.
Figure 2.
Images of the liver obtained in March 2007 show stable post treatment changes.
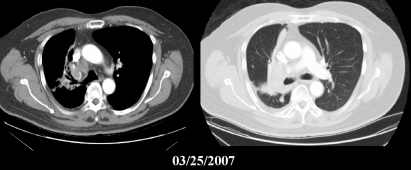
Figure 3.
Images of the lung obtained in March 2007 reveal hilar and lung lesions.
Laboratory studies indicated Child-Pugh stage A disease; total bilirubin 1.0 mg/dL, albumin 3.9 g/dL, INR 0.9, aspartate aminotransferase (AST) 73 U/L, alanine aminotransferase (ALT) 48 U/L, platelets 190 × 109/L, creatinine 0.9 mg/dL, AFP 430 ng/mL. Figures 2 and 3 show post RFA images of the liver and lung, respectively.
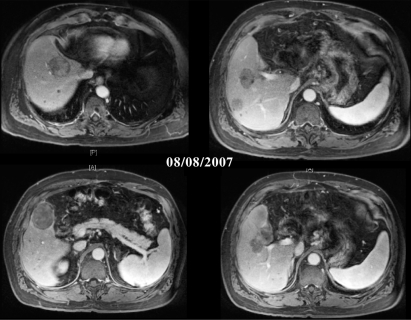
Treatment with sorafenib was not an option at the time, so the patient entered a phase II clinical trial of bevacizumab 5 mg/kg, IV over 90 minutes for the first treatment (then 60 minutes in later cycles) on day 1, oxaliplatin 130 mg/m2 IV on day 1, capecitabine 825 mg/m2 twice a day administered orally on days 1 to 14. Treatments were repeated every 21 days as one cycle. Post treatment evaluation of this patient in August 2007 revealed stable disease [Figure 4]. Some of the smaller liver lesions were no longer visible on MRI; no changes were seen in large lesions, but decreased vascularization was noted. Lung scan revealed stable disease, with a slight decrease in tumor size. Laboratory values were as follows: total bilirubin 1.3 mg/dL, albumin 3.8 g/dL, AST 72 U/L, ALT 52 U/L, platelets 130 × 109/L, creatinine 1.2 mg/dL, INR 0.9, AFP 34 ng/mL.
Figure 4.
Post treatment scans show stable disease.
DISCUSSION
On November 16, 2007, the US Food and Drug Administration (FDA) approved sorafenib for the treatment of patients with unresectable HCC. Approval was based on the results of the SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) study, an international, multicenter, randomized, double-blind, placebo-controlled trial in 602 patients with unresectable, biopsy-proven hepatocellular carcinoma.1 The trial was stopped after a prespecified second interim analysis for survival revealed that sorafenib extended overall survival by 44% in patients with HCC (HR=0.69; P = .0006) vs. placebo. Separate analysis demonstrated a statistically significant improvement in time to tumor progression in the sorafenib arm (median 5.5 vs. 2.8 months; HR=0.58, P = .000007). The availability of sorafenib has, essentially, changed the treatment paradigm for HCC. The question now is where do we go from here?
Data from other studies with biologic and cytotoxic agents in HCC are available. The patient in this report, for example entered a study including bevacizumab, and indeed, a number of ongoing studies are evaluating bevacizumab as both a single-agent, and in combination with other agents, such as gemcitabine, oxaliplatin, capecitabine, and others. In addition, other studies are looking at epidermal growth factor receptor (EGFR) inhibitors alone and in combination regimens. Data thus far, suggest that other biologic agents, besides sorafenib, will prove effective. So, what would be the next step?
A number of questions need to be addressed pertinent to continued research in HCC. Will future phase III studies be sorafenib based or based on other biologic agents currently under evaluation? Will phase II studies reveal other biologic agents with potential superioriority to sorafenib? What role will cytotoxic drugs play in HCC (eg, combined with sorafenib)? What are the most important primary end points to consider in study design: progression-free survival vs. overall survival vs. time to tumor progression? The importance of quality-of-life measures is increasingly recognized. And how should we be measuring response of the disease to treatment? RECIST criteria are not appropriate in assessing treatment response of HCC. What criteria, then, should we be using? Can we use dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) studies to improve disease assessment?
In an effort to address these and other questions, a special working group was created under the auspices of the Gastrointestinal Steering Committee of the National Cancer Institutes. The HCC task force was created in response to initiatives of the Cancer Trials Working Group (CTWG) in an effort to refine concepts from cooperative groups, define gaps and opportunities, facilitate the development of phase III studies, avoid redundancy in research, and optimize collaborations. Current members of the HCC task force include 10 major cooperative groups. Representatives of these groups will pool their resources in a joint effort to develop a more standardized approach to studying HCC, so that we might understand the disease better and foster multidisciplinary research initiatives in a timely, efficient manner.
Footnotes
Disclosures of Potential Conflicts of Interest
The author indicated no potential conflicts of interest.
REFERENCES
- 1.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): Results of a Phase III randomized placebo- controlled trial (SHARP trial). 2007 ASCO Annual Meeting. J Clin Oncol. 2007;25(18S) (abstr LBA1) [Google Scholar]