Abstract
Background
The Intergroup 0116 trial demonstrated that adding postoperative chemoradiotherapy for gastric cancer resulted in a significant overall survival improvement of 9 months. The purpose of this study was to perform a cost-effectiveness analysis of adjuvant chemoradiotherapy for resected gastric cancer.
Methods
An economic model was constructed to examine the costs and quality-adjusted survival benefit of adjuvant chemoradiotherapy for gastric cancer. Medicare reimbursement rates were used for chemotherapy and radiotherapy costs. Costs of managing toxicities were also included. Patient utilities were derived from published literature. The analysis was performed from the third-party payer perspective, with results reported in 2007 US dollars. A lifetime time horizon and 3% discount rate were used. One-way and Monte Carlo probabilistic sensitivity analyses were performed.
Results
For the base case, the incremental cost of adding adjuvant chemoradiotherapy was $20,100. The net gain in quality-adjusted life years (QALYs) was 0.53. The incremental cost-effectiveness ratio (ICER) was $38,400/QALY. The probabilistic sensitivity analysis predicted a 67% likelihood that the ICER would be less than $50,000/QALY.
Conclusion
Our model suggests that the ICER of adjuvant chemoradiotherapy for resected gastric cancer compares favorably to other widely used cancer treatments.
Surgery remains the cornerstone of curative therapy for localized gastric adenocarcinoma. Survival, however, is still suboptimal, even after curative resection, with 5-year overall survival rates between 15% and 22%.1,2 In an effort to improve survival outcomes, adjuvant therapy regimens with chemotherapy and/or radiotherapy have been used.3–8 The most notable improvements in outcomes to date were demonstrated by the Intergroup 0116 trial (INT-0116/SWOG [Southwest Oncology Group] 9008), which studied the addition of adjuvant chemoradiotherapy after complete surgical resection,9,10 and the more recent MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial, which used a combination of neoadjuvant and adjuvant chemotherapy without radiation.11 Both trials showed an improvement in overall survival with the addition of adjuvant therapy, indicating that either perioperative chemotherapy alone or postoperative chemoradiotherapy is an acceptable adjuvant treatment regimen for gastric cancer.12
In an era of rising health care costs in the face of limited resources, there is increasing interest in evaluating the economic implications of cancer treatments,13–18 particularly from phase III randomized controlled clinical trials. Bruner19 lists a summary of 13 economic trials previously published on the cost-effectiveness of interventions from cooperative group clinical trials.
In the United States, the current National Comprehensive Cancer Network recommendations for the treatment of nonmetastatic gastric cancer of stage IB or higher is adjuvant chemoradiotherapy after resection. 20 The purpose of this study was to build an economic model to evaluate the cost-effectiveness of this regimen. Thus, we performed an economic analysis of an adjuvant chemoradiotherapy treatment regimen for completely resected gastric cancer predicated on the clinical results from the INT-0116/SWOG 9008 trial.9,10
METHODS
Clinical Trial
Intergroup 0116 was a large, randomized phase-III clinical trial that investigated the effect of surgery plus postoperative chemoradiotherapy on the survival of patients with resectable adenocarcinoma of the stomach or gastroesophageal junction. Patients with surgically resected stage IB to IV (M0) gastric adenocarcinoma were randomized to receive postoperative chemoradiotherapy or no additional treatment. The adjuvant treatment arm received five cycles of bolus intravenous 5-fluorouracil (5-FU)/leucovorin (LV) concurrently with 45 Gy of radiation. Adjuvant 5-FU was dosed at 425 mg/m2/day, and leucovorin at 20 mg/m2/day, each for 5 days for cycles 1, 4, and 5. Concurrent radiotherapy was given during chemotherapy cycles 2 and 3, consisting of 1.8 Gy per day for 25 days using either two- or four-field conventional techniques. Chemotherapy cycle 2 was given for the first 4 days of radiotherapy, and cycle 3 was given on the last 3 days of radiotherapy. The updated results of INT- 0116 showed that the adjuvant therapy arm had improved overall survival of 35 vs. 26 months for the surgery-alone arm.10
Economic Model
To determine the cost-effectiveness of the INT-0116 protocol, we constructed an economic model to evaluate the costs and utilities for patients receiving this intervention. The reference treatment strategy for our analysis was the control arm of INT-0116 (surgery alone). Since cost and utility information were not available from the actual patients enrolled in this trial, these data were extrapolated from other sources as described below. A third-party payer’s economic perspective was used. A 3% annual discount rate for costs and utilities was assumed,21 and a lifetime time horizon was used. All costs were converted to 2007 US dollars using historical US Gross Domestic Product deflator indices.22
Cost Data
Cost data for radiotherapy and chemotherapy were based on median Medicare-allowed charges from the 2007 Medicare Physician Fee Schedule.23 We assumed that all chemoradiotherapy was given in accordance with the INT-0116 trial protocol. Radiotherapy was planned using computed tomography (CT)-based simulation and was delivered in 25 daily fractions with a 2- or 4-field conventional setup. For radiotherapy, the model included costs for the initial physician consultation,24 simulation and treatment planning, dosimetry, blocks, weekly on-treatment visits, weekly laboratory tests,25 and daily radiotherapy delivery (Table 1). For chemotherapy, the model included the costs of the initial physician consult and management visits,24 chemotherapy drugs, chemotherapy administration, and laboratory analyses25 performed according to the SWOG 9008 protocol schedule (Table 1).
Table 1.
Cost data* for radiotherapy, chemotherapy, and toxicity management.
| Sensitivity analysis range
|
|||||||
|---|---|---|---|---|---|---|---|
| Description | HCPCS | Unit cost | Quantity or % | Base-case total cost | Low | High | Reference |
| Radiotherapy | |||||||
| Initial consultation | 99245 | $213.93 | 1 | $213.93 | $149.75 | $342.29 | 24 |
| Complex treatment plan | 77263 | $153.97 | 1 | $153.97 | $137.57 | $183.67 | 23 |
| Complex simulation | 77290 | $388.66 | 2 | $777.32 | $571.72 | $1,144.60 | 23 |
| 3D simulation | 77295 | $1,114.21 | 1 | $1,114.21 | $805.68 | $1,607.64 | 23 |
| Blocks/moulds | 77334 | $179.60 | 5 | $898.00 | $677.75 | $1,258.65 | 23 |
| Basic dosimetry calculation | 77300 | $79.32 | 4 | $317.28 | $242.44 | $440.12 | 23 |
| Continuing medical physics consults | 77336 | $99.71 | 5 | $498.55 | $341.55 | $757.65 | 23 |
| Radiation treatment management | 77427 | $175.52 | 5 | $877.60 | $791.60 | $1,038.75 | 23 |
| Concurrent chemotherapy | 77470 | $447.16 | 1 | $447.16 | $325.75 | $640.31 | 23 |
| Weekly CBC count | 85025 | $14.68 | 5 | $73.40 | $51.38 | $117.44 | 25 |
| Weekly port films | 77417 | $21.26 | 5 | $106.30 | $72.15 | $160.10 | 23 |
| Radiation treatment delivery | 77414 | $140.84 | 25 | $3,521.00 | $2,468.75 | $5,469.00 | 23 |
| Total radiotherapy costs: | $8,998.72 | $6,636.09 | $13,160.22 | ||||
| Chemotherapy | |||||||
| Initial consultation | 99245 | $231.10 | 1 | $231.10 | $161.77 | $369.75 | 24 |
| Management office visits | 99213 | $53.09 | 5 | $265.46 | $185.82 | $424.73 | 24 |
| 5-Fluorouracil 500-mg vial (x2) | J9190 | $1.66 | 44 | $73.04 | $51.13 | $116.86 | 28 |
| Initial CTX administration | 96409 | $117.12 | 22 | $2,576.64 | $1,874.62 | $3,938.66 | 23 |
| Leucovorin 50-mg vial | J0640 | $1.03 | 22 | $22.66 | $15.86 | $36.26 | 28 |
| Additional CTX administration | 96411 | $67.48 | 22 | $1,484.56 | $1,083.72 | $2,227.72 | 23 |
| CBC count | 85025 | $14.68 | 17 | $249.56 | $174.69 | $399.30 | 25 |
| Complete metabolic panel | 80053 | $19.96 | 2 | $39.92 | $27.94 | $63.87 | 25 |
| Total chemotherapy costs: | $4,942.94 | $3,460.05 | $7,908.70 | ||||
| Toxicity management | |||||||
| Neutropenia | |||||||
| Neutropenic hospitalizations | $13,224.12 | 2.98% | $394.08 | $197.04 | $1,182.24 | 26 | |
| Outpatient neutropenia management | $1,544.01 | 51% | $787.75 | $393.88 | $2,363.26 | 27 | |
| Filgrastim | $3,978.87 | 51% | $2,030.02 | $1,015.01 | $6,090.06 | 28 | |
| Nausea/vomiting | |||||||
| Management of CINV × 5 cycles | $2,976.09 | 100 | $2,976.09 | $1,488.04 | $8,928.27 | 29 | |
| Total toxicity costs: | $6,187.94 | $3,094.00 | $18,564.00 | ||||
All costs converted to 2007 US dollars.22
Abbreviations: HCPCS = Healthcare Common Procedure Coding System; CBC = complete blood cell; CTX = chemotherapy; CINV = chemotherapy-induced nausea & vomiting.
Chemoradiotherapy toxicity management costs (Table 1) were based on the type, frequency, and grade of the most common toxicities reported in the Intergroup trial.9 We included costs for toxicity prophylaxis and for the management of acute toxicities, including downstream hospitalization costs for patients experiencing the most severe toxicities. The most common toxicity reported in the trial was hematologic, primarily neutropenia (54%).
We contacted the SWOG Statistical Center (www.swogstat.org) to determine if actual hospitalization rates were recorded for patients in the SWOG 9008 trial experiencing toxicities, but found that this information was not recorded. Because the actual hospitalization rate was not recorded, we used data from Caggiano,26 who calculated the rate (2.98%) and cost ($10,900 in 1999 US dollars) of hospitalization for neutropenic patients who receive chemotherapy for stomach cancer, based on actual hospital discharge databases from seven states. We assumed that the remainder of the patients experiencing grade 3 or 4 neutropenia could be treated as outpatients and used data from Bennett,27 who found that the cost of outpatient treatment of neutropenia for several cancer types was $1,329 (in 2001 US dollars). We also included the costs of filgrastim,28 which was assumed to have been given to all patients with neutropenia.
Cost estimates for managing chemotherapy-induced nausea and vomiting (CINV) are based on a study by Stewart,29 who calculated the total costs (outpatient and hospitalization) for prevention and management of CINV after the introduction of ondansetron. When converted to 2007 US dollars,22 this was calculated to be $2,976 per patient (Table 1). The 2004 update of INT-0116 confirmed that there were no significant late toxicities in the chemoradiotherapy arm.10
Costs for surgery were assumed equivalent in both groups and were not tabulated in this analysis, as all patients in the series underwent surgery prior to trial enrollment.
Patient Utilities
Patient health state utility values were used to adjust survival for the decrease in health-related quality of life from the various treatment interventions. Utilities were estimated based on published literature from patients who had similar health states to those enrolled in INT-0116. Patients in both arms of the study were given a baseline utility of the postgastrectomy state, while those in the intervention group had a further reduction of their utility during chemoradiotherapy (Table 2). The utility for the postgastrectomy state was estimated to be 0.81, based on a study by Gockel,30 who evaluated patients after subtotal resection and gastrectomy with a gastrointestinal quality-of-life index. This utility was estimated by taking the percentage of the gastrointestinal quality-of- life index score for postgastrectomy patients (116/144). The utility for nausea/vomiting was estimated to be 0.27, based on a study by Grunberg,31 who evaluated quality of life of patients experiencing chemotherapy-induced nausea and vomiting. The utility for leukopenia was estimated to be 0.71, based on a study by Fortner,32 who evaluated the effects of neutropenia on quality of life.
Table 2.
Patient health state utility values.*
| Utility | Range | Reference | |
|---|---|---|---|
| Postgastrectomy | 0.81 | 0.4–1.0 | 27 |
| Nausea/vomiting | 0.27 | 0.14–1.0 | 28 |
| Leukopenia | 0.70 | 0.35–1.0 | 29 |
| Net utility during chemoradiation† | 0.59 |
Utilities were estimated based on quality-of-life data in the indicated references.
Calculated as a weighted average based on percentage of patients experiencing these toxicities in INT-0116.
The overall net utility during chemoradiotherapy administration was estimated to be 0.59, calculated as a weighted average of the fraction of patients experiencing the specified toxicities in INT-0116.9 Quality-adjusted life-years (QALYs) were calculated by multiplying the utilities by the time the patient spent in that state.
Base-Case and Sensitivity Analyses
Baseline values for the costs, utilities, and expected survival benefit from adjuvant chemoradiotherapy were used for the base-case analysis. The primary outcome measure for this analysis was the incremental cost-effectiveness ratio (ICER), defined as the incremental cost divided by the number of QALYs saved. The incremental cost was the difference in cost between the chemoradiotherapy arm and the control arm.
One-way and probabilistic sensitivity analyses were performed. For the one-way sensitivity analyses, the upper and lower bounds for chemotherapy and radiotherapy costs were derived from the range in Medicare geographic practice cost indices, which ranged from 70% to 160% of the median. Because of the greater uncertainty regarding toxicity costs, a wider range of 50% to 300% of the base-case estimate was assigned. The upper bound for hospitalization rate was set at 29%, to reflect the percentage of patients (79/273) experiencing grade 4 hematopoietic toxicities in the INT-0116 trial. For patient utilities, we allowed values to vary from 50% of baseline to a maximum value of unity (representing no decrease in quality of life). We also allowed the expected survival gain to vary from 2.8 to 16.8 months, which corresponds to the 95% confidence interval for the hazard ratio reported in INT-0116.
The probabilistic sensitivity analysis was performed using a second-order Monte Carlo simulation using TreeAge Pro software.33 For this analysis, 1,000 simulated trials were run, where each input was sampled at random from probability distribution functions assigned to each variable. The cost input variables were modeled with normal distributions. A standard deviation (SD) of 25% of the baseline value was used for the chemotherapy and radiotherapy costs since this most closely corresponded to the Medicare geographic practice cost index range. For toxicity costs, a larger SD of 50% was used to reflect greater uncertainty in these estimates. Utilities were modeled using beta distributions with SD = 0.1. Hospitalization rate was modeled with a log-normal distribution (mode 3%) with a large right-sided tail to reflect the potential for higher hospitalization rates. The expected survival benefit was also modeled with a normal distribution with SD = 3.5 months to approximate the 95% confidence intervals for the hazard ratio in the Intergroup trial.10
RESULTS
When the baseline survival for patients in the adjuvant chemoradiotherapy arm (35 months) was adjusted for quality of life using the patient utilities, this resulted in a quality-adjusted survival of 27.01 months, or 2.25 QALYs. Similarly, for patients in the surgery alone arm, the baseline survival of 26 months was adjusted to 20.68 months, or 1.72 QALYs (Table 3). The 9-month net survival gain with chemoradiotherapy was adjusted to 6.32 months (0.53 QALYs). For the base-case analysis, the incremental cost of adjuvant chemoradiotherapy was $20,100 (Table 4). This resulted in an ICER of $38,400 per QALY.
Table 3.
Quality-adjusted life-years.
| Survival (mo) | QALYs | |
|---|---|---|
| Chemoradiation | 35 | 2.25 |
| Surgery alone | 26 | 1.72 |
| Net survival gain | 9 | 0.53 |
Abbreviation: QALY = quality-adjusted life-year.
Table 4.
Base case results.
| Incremental cost of chemoradiation | $20,100 |
| QALYs gained | 0.53 |
| Incremental cost-effectiveness ratio | $38,400/QALY |
Abbreviation: QALY = quality-adjusted life-year.
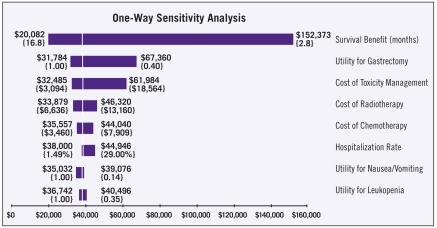
For the one-way sensitivity analysis, the inputs to the model were allowed to vary across their specified ranges (Table 5). The results of the one-way sensitivity analysis are shown in the tornado diagram in Figure 1, which depicts graphically how variations in each input affect the outcome. The tornado diagram is stacked in order of decreasing width, indicating that variations in inputs near the top (expected survival benefit) have the greatest effect on the outcome, while variations in inputs near the bottom (hospitalization rate and utilities) have relatively small effects on the outcome.
Table 5.
Inputs for one-way sensitivity analysis.
| Input | Base case | Low | High |
|---|---|---|---|
| Cost of radiotherapy | $9,000 | $6,600 | $13,200 |
| Cost of chemotherapy | $5,000 | $3,500 | $7,900 |
| Cost of toxicity management | $6,200 | $3,100 | $18,600 |
| Utility for postgastrectomy | 0.81 | 0.40 | 1.00 |
| Utility for nausea/vomiting | 0.27 | 0.14 | 1.00 |
| Utility for leukopenia | 0.70 | 0.35 | 1.00 |
| Hospitalization rate | 2.98% | 1.5% | 29% |
| Survival benefit (months) | 9 | 2.8 | 16.8 |
Figure 1.
Tornado diagram of the one-way sensitivity analysis indicates that the outcome is most sensitive to variation in the expected survival benefit, and least sensitive to variation in the utilities for nausea/vomiting and leukopenia. Dollar amounts indicate the incremental cost per quality-adjusted life year; brackets contain the upper and lower values for each input.
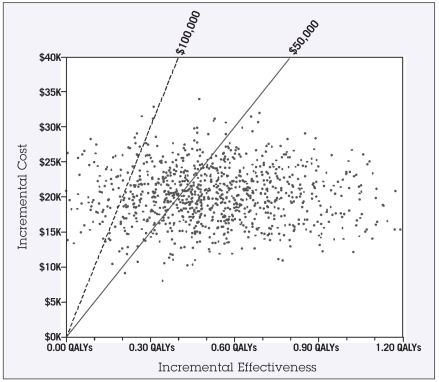
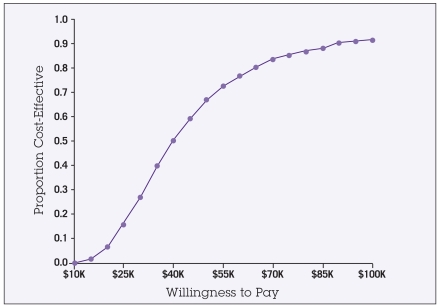
For the probabilistic sensitivity analysis, the results of the second-order Monte Carlo simulations are shown in the scatter plot in Figure 2. Each point represents one of the 1,000 trials run where each input was assigned a random value according to its probability density function. The solid and dashed diagonal lines indicate the $50,000 and $100,000 thresholds. Trial points that fall to the right and below these diagonal lines indicate a cost-effectiveness below the given threshold level. This analysis indicates a 67% probability that the ICER would be less than $50,000/QALY, and a 91% probability of the ICER being less than $100,000/QALY. The acceptability curve in Figure 3 can be used to interpret the cost-effectiveness of this intervention for any given threshold level. For example, at a threshold level (“willingness to pay”) of $30,000, there would be a 25% likelihood that this intervention would be considered cost-effective. However, at a threshold of $100,000, there would be a 91% probability that the intervention would be considered cost-effective.
Figure 2.
Scatter plot showing results of Monte Carlo probabilistic sensitivity analysis. Each point represents the outcome of 1,000 simulated trials in which each input was assigned a random value according to its probability density function. The solid and dashed diagonal lines indicate the $50,000 and $100,000 thresholds, respectively. The percentage of points that fall to the right of these lines (67% and 91%) indicate the likelihood that this intervention would be cost-effective at that threshold level.
Abbreviation: QALY = quality-adjusted life-year.
Figure 3.
Acceptability curve depicts the likelihood that the intervention would be interpreted as cost-effective at any given societal threshold level (“willingness to pay”). For example, the model predicts a 67% probability that the incremental cost-effectiveness of this intervention would be < $50,000 per QALY (quality-adjusted life-year), and a 91% probability that this intervention would be < $100,000 per QALY.
DISCUSSION
As health care costs inexorably rise, interest in assessing the economic burden of cancer treatments continues to increase; this is particularly true when it comes to the treatment of aggressive malignancies, where incremental progress in therapeutic advances is often measured by survival improvement in months rather than years. While prospective collection of cost and utility information concurrently during a clinical trial would be ideal,17 this goal is still not commonly achieved because of time and resource constraints. When actual cost data from trial participants are either not collected or not available, an economic modeling approach34 based on costs and utilities estimates from other sources can be used to simulate the cost-effectiveness of phase III clinical trials.35–37 Our study is an example of an economic model that can be used to perform a cost-effectiveness analysis of a recommended treatment regimen based on a major phase III clinical trial.
The recently published MAGIC trial11 employed an alternative adjuvant treatment regimen for gastric cancer. The results of this trial demonstrated that a perioperative chemotherapy regimen (without radiotherapy), consisting of three preoperative and three postoperative cycles of epirubicin, cisplatin, and 5-FU, also resulted in an improvement in overall and progression-free survival. To determine how the cost-effectiveness of the MAGIC regimen compares with INT-0116, a complete economic analysis of the MAGIC regimen would need to be performed. However, a few preliminary observations can be made. The costs of the MAGIC chemotherapy regimen would likely be higher than the Intergroup 5-FU/LV regimen, because of the higher cost of epirubicin and continuous infusion 5-FU. On the other hand, total expenses for the MAGIC regimen might be lower, because no costs were incurred for radiotherapy. Both regimens demonstrated an improvement in overall survival, but a full analysis would be needed to determine how the improvement in QALYs in the MAGIC trial compares to that in INT-0116. Future adjuvant treatment for gastric cancer may involve some combination of both regimens, with both preoperative chemotherapy and postoperative chemoradiotherapy.12
Markov modeling is often used in other economic analyses, as this technique effectively models disease processes that involve ongoing risk over extended periods. Markov modeling is useful when attempting to extrapolate results beyond the follow-up period of a clinical trial. Since the median survival in both arms was well within the follow-up period of the trial (> 6 years), we used the direct assessment of median survival from the clinical trial data as an approximation for mean survival time, allowing us to forgo Markov modeling for this analysis.
This study has several limitations. As discussed above, since we did not have access to actual cost data from patients enrolled in this Intergroup trial, chemoradiotherapy costs were obtained from Medicare fee schedules. While other insurers may reimburse at greater or less than Medicare rates, Medicare—as the largest single domestic payer—represents a reasonable approximation of national norms.
Since actual hospitalization rates for patients experiencing acute toxicities from adjuvant chemoradiotherapy were not recorded in INT-0116, we used data from a study by Caggiano.26 This study measured the rates and costs of hospitalization for neutropenic patients with gastric cancer using data drawn from hospital discharge databases in seven states. Interestingly, while the costs per hospitalization for gastric cancer patients were among the highest among all solid tumors ($10,900 in 1999 US dollars), the rates of hospitalization (2.98%) were rather modest when compared to other solid tumor types, such as pancreatic cancer (11.1%) and lung cancer (5.2%). We speculate that this may be due to a combination of factors, including the tumor site and the chemotherapy regimens used. Nevertheless, if the actual hospitalization rates turn out to be higher than in the Caggiano study, the total costs of this adjuvant treatment regimen would increase.
In this study, we assumed that patients experiencing CINV would be managed with ondansetron, with costs based on a study by Stewart.29 With the recent introduction of more expensive antiemetic agents, such as granisetron, dolasetron, palonosetron, and aprepitant, it is possible that the costs of management of CINV may increase further. The overall effect on costs associated with these more expensive antiemetics is unclear, however, since Stewart29 showed that better management of CINV after the introduction of a more effective antiemetic agent (ondansetron) actually resulted in an overall decrease in total costs, despite the higher price of the antiemetic agent itself.
Patient utilities used in this study were estimated from the literature, rather than from the actual INT-0116 participants. Data are available in the literature regarding health-related quality of life for patients in comparable health states,30–32 but these data are not always expressed in a form that can be directly used in cost-effectiveness analyses. Thus, we estimated patient utilities based on the available data in these studies. There are obvious limitations to the accuracy of this approach, because these quality-of-life studies were from different settings. However, our sensitivity analyses demonstrated that the results were not particularly sensitive to utility variation, except for extremely pessimistic estimates of the postgastrectomy utility; hence it may be surmised that more accurate utility data would not have had an appreciable effect on outcomes.
We assumed that conventional radiotherapy would be used as specified in INT-0116. The implementation of newer, more expensive radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT), would significantly increase the total cost of radiotherapy and make this intervention appear less cost-effective if there were no corresponding gain in QALYs from the use of IMRT.
Cost-effectiveness analysis is a useful metric that simultaneously incorporates clinical and economic data for evaluating the economic implications of cancer treatments, an increasingly important consideration in an age when novel agents and technical advances may portend a modicum of benefit at substantial costs. These studies can help detect potential misalignment of health care resources and enable more consistent resource allocation. Given that the health care budget in the United States cannot continue to rise indefinitely, cost-effectiveness analyses will play an increasingly important role in the future in determining how health care dollars are allocated.
This study demonstrates an example of an economic modeling approach that was successfully used to perform a cost-effectiveness analysis of a recommended adjuvant therapy based on a phase III clinical trial. Our analysis suggests that the ICER of administering adjuvant chemoradiotherapy after gastric cancer resection is comparable to those of other widely accepted cancer treatments.
Acknowledgments
The authors would like to thank Todd J. Scarbrough, MD, and Lisa A. Kachnic, MD, for their assistance in obtaining cost and billing data.
Footnotes
Portions of this work were published as an abstract in the 2005 Proceedings of the 47th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, in Denver, CO.
Disclosures of Potential Conflicts of Interest
Dr. Wang received support from a Resident Research Grant from the Radiological Society of North America Research & Education Foundation to conduct this study.
REFERENCES
- 1.Blanke CD, Coia LR, Schwarz RE, et al. Gastric cancer. In: Pazdur R, Coia LR, Hoskins WJ, et al., editors. Cancer Management: A Multidisciplinary Approach. 9. Manhasset, NY: CMP Healthcare Media; 2005. pp. 279–292. [Google Scholar]
- 2.Pisters PWT, Kelsen DP, Powell SM, et al. Cancer of the stomach. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer. 7. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 3.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–1064. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 4.Hermans J, Bonenkamp JJ, Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 5.Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597–608. doi: 10.1080/11024150201680005. [DOI] [PubMed] [Google Scholar]
- 6.Mari E, Floriani I, Tinazzi A, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)—report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 8.Hallissey MT, Dunn JA, Ward LC, et al. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309–1312. doi: 10.1016/s0140-6736(94)92464-3. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald JS, Smalley SR, Benedetti J, et al. Postoperative combined radiation and chemotherapy improves disease-free survival (DFS) and overall survival (OS) in resected adenocarcinoma of the stomach and gastroesophageal junction: Update of the results of Intergroup Study INT-0116 (SWOG 9008). American Society of Clinical Oncology 2004 Gastrointestinal Cancers Symposium; 22-24 January 2004; San Francisco. 2004. (abstr 6) [Google Scholar]
- 11.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol. 2005;90:166–170. doi: 10.1002/jso.20223. [DOI] [PubMed] [Google Scholar]
- 13.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 14.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 15.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 16.Hayman J, Weeks J, Mauch P. Economic analyses in health care: an introduction to the methodology with an emphasis on radiation therapy. Int J Radiat Oncol Biol Phys. 1996;35:827– 841. doi: 10.1016/0360-3016(96)00172-1. [DOI] [PubMed] [Google Scholar]
- 17.Konski A, Watkins-Bruner D. The RTOG Outcomes Model: economic end points and measures. Expert Opin Pharmacother. 2004;5:513–519. doi: 10.1517/14656566.5.3.513. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien BJ. Cost-effectiveness analysis in cancer: toward an interative framework for integration of evidence from trials to models. In: Lipscomb J, Gotay CC, Snyder C, editors. Outcomes Assessment in Cancer: Measures, Methods and Applications. Cambridge, UK: Cambridge University Press; 2005. pp. 503–521. [Google Scholar]
- 19.Bruner DW, Movsas B, Konski A, et al. Outcomes research in cancer clinical trial cooperative groups: the RTOG model. Qual Life Res. 2004;13:1025–1041. doi: 10.1023/B:QURE.0000031335.02254.3b. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Practice Guidelines in Oncology. Available at: www.nccn.org.
- 21.Lipscomb J, Weinstein MC, Torrance GW. Time Preference. In: Gold M, Siegel JE, Russell LB, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. 214–235. [Google Scholar]
- 22.Budget of the United States Government Historical Tables Fiscal Year 2008. Gross Domestic Product and Implicit Outlay Deflators. Available at: http://www.gpoaccess.gov/usbudget/fy08/hist.html.
- 23.Centers forMedicare andMedicaid Services. Medicare Physician Fee Schedule Lookup. Available at: http://www.cms.hhs.gov/apps/pfslookup/
- 24.Centers for Medicare & Medicaid Services. Evaluation & Management Codes by Specialty. Available at: http://www.cms.hhs.gov/MedicareFeeforSvcPartsAB.
- 25.Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule. Available at: http://www.cms.hhs.gov/ClinicalLabFeeSched/
- 26.Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 27.Bennett CL, Calhoun EA. Evaluating the total costs of chemotherapy-induced febrile neutropenia: results from a pilot study with community oncology cancer patients. Oncologist. 2007;12:478–483. doi: 10.1634/theoncologist.12-4-478. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Medicare & Medicaid Services. Medicare Part B Drug Average Sales Price 2007 2nd Qtr. Available at: http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice.
- 29.Stewart DJ, Dahrouge S, Coyle D, et al. Costs of treating and preventing nausea and vomiting in patients receiving chemotherapy. J Clin Oncol. 1999;17:344–351. doi: 10.1200/JCO.1999.17.1.344. [DOI] [PubMed] [Google Scholar]
- 30.Gockel I, Pietzka S, Junginger T. [Quality of life after subtotal resection and gastrectomy for gastric cancer] Chirurg. 2005;76:250–257. doi: 10.1007/s00104-004-0950-5. [DOI] [PubMed] [Google Scholar]
- 31.Grunberg SM, Boutin N, Ireland A, et al. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer. 1996;4:435–439. doi: 10.1007/BF01880641. [DOI] [PubMed] [Google Scholar]
- 32.Fortner BV, Schwartzberg L, Tauer K, et al. Impact of chemotherapy-induced neutropenia on quality of life: a prospective pilot investigation. Support Care Cancer. 2005;13:522–528. doi: 10.1007/s00520-004-0757-4. [DOI] [PubMed] [Google Scholar]
- 33.TreeAge Software. TreeAge Pro 2006. Available at: www.treeage.com.
- 34.Owen JB, Grigsby PW, Caldwell TM, et al. Can costs be measured and predicted by modeling within a cooperative clinical trials group? Economic methodologic pilot studies of the radiation therapy oncology group (RTOG) studies 90-03 and 91-04. Int J Radiat Oncol Biol Phys. 2001;49:633–639. doi: 10.1016/s0360-3016(00)00770-7. [DOI] [PubMed] [Google Scholar]
- 35.Konski A, Bracy P, Weiss S, et al. Cost-utility analysis of a malignant glioma protocol. Int J Radiat Oncol Biol Phys. 1997;39:575–578. doi: 10.1016/s0360-3016(97)00373-8. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey SD, Moinpour CM, Lovato LC, et al. Economic analysis of vinorelbine plus cisplatin versus paclitaxel plus carboplatin for advanced non-small-cell lung cancer. J Natl Cancer Inst. 2002;94:291–297. doi: 10.1093/jnci/94.4.291. [DOI] [PubMed] [Google Scholar]
- 37.Konski A, Sherman E, Krahn M, et al. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86-10) Int J Radiat Oncol Biol Phys. 2005;63:788–794. doi: 10.1016/j.ijrobp.2005.03.010. [DOI] [PubMed] [Google Scholar]