Abstract
Hepatocellular carcinoma (HCC) accounts for 6% of all cancers worldwide. In the United States, the incidence is expected to increase due to the increased rate of hepatitis C viral infection affecting that region. Other factors that will influence higher incidence rates for HCC include the persistent presence of alcoholic cirrhosis and the recently recognized correlation between non-alcoholic steatohepatitis (NASH) and HCC. In most cases, cirrhosis is an integral part of the morbidity and mortality associated with HCC, and must be accounted for in order to manage patients with HCC properly. Historically, medical oncologists used the Child-Pugh scoring system of cirrhosis. However, Child-Pugh only categorizes the cirrhosis and does not address factors intrinsic to the cancer itself, which is recognized as a major limitation of that system. The idea of incorporating cancer-related parameters was developed by several research groups. The Cancer of the Liver Italian Program (CLIP) score and the Chinese University Prognostic Index (CUPI) are among many others that were developed and are of great use for patients with hepatitis C- and hepatitis B-associated HCC, respectively. Many chemotherapeutic agents have been tested in HCC, with reported response rates between 10% and 15% and no demonstrated survival advantage. Over the past decade, several molecular targets involved in the etiology of HCC have been identified. Recently, sorafenib, an antiangiogenic and Raf kinase inhibitor, has shown a survival advantage. The innovative therapeutic outcomes associated with novel targeted therapies illustrates the need for biologic and pharmacokinetic end points to define their optimal doses and therapeutic effects.
Hepatocellular carcinoma (HCC) accounts for 6% of all cancers worldwide. It is the fifth most common malignancy, with an estimated half million new cases diagnosed per year globally, and a mortality rate equivalent to its incidence.1 In the United States, an estimated 19,160 new cases of liver and intrahepatic biliary tumors are expected to have been diagnosed in 2007.2 This incidence is expected to increase due to the increasing rate of hepatitis C infection that has been seen over the past few decades in the United States.3 Other factors that contribute to higher incidence rates of HCC include persistent presence of alcoholic cirrhosis4 and the recently recognized correlation between nonalcoholic steatohepatitis (NASH) and HCC. NASH cases are expected to increase in frequency based on the correlation between NASH and obesity, a rising epidemic in the United States.5,6 Medical oncologists in North America will therefore need to become more familiar with the diagnosis, staging, and management of this lethal malignancy.
EPIDEMIOLOGY
Although the majority (~80%) of new cases of HCC are reported from the developing world,7 the incidence, as noted above, is increasing in economically developed countries.8 The primary risk factor for HCC is cirrhosis, particularly cirrhosis attributable to hepatitis B (HBV) and hepatitis C (HCV) infections.9,10 In the United States, a 3-fold increase in the age-adjusted rates for HCC associated with hepatitis C occurred—from 2.3 per 100,000 between 1993 and 1995 to 7.0 per 100,000 between 1996 and 1998.3 This likely reflects the increasing incidence of hepatitis C observed in North America during this time. HBV and HCV account for over 70% of HCC cases worldwide.10 Alcohol-induced cirrhosis is another important risk factor, especially in the western hemisphere, accounting for 32%–45% of HCC cases in Italy and the United States.4
The relative risk of death from HCC in morbidly obese patients is 1.68 times higher among women whose body mass index (BMI) equals or exceeds 35 and 4.52 times higher among men with similar BMI values compared to a reference group whose baseline BMI ranged between 18.5- and 24.9. This observation highlights NASH as an important risk factor.5 In addition, El-Serag demonstrated that diabetes mellitus increases the risk of developing HCC in patients with other risk factors, such as excessive alcohol consumption or hepatitis B or C.6 Morbid obesity and diabetes have thus been shown to exacerbate the risk of developing cirrhosis in patients with established NASH. The independent contribution of these risk factors to the increased risk of cirrhosis in patients without NASH, however, has not been demonstrated. Given the high prevalence of diabetes and obesity in United States, NASH may therefore be expected to become a risk factor of increasing importance.
TREATMENT
Although surgery (partial hepatectomy or total hepatectomy with orthotopic liver transplantation) can be curative for localized small liver tumors, therapeutic options for patients with advanced or metastatic HCC are limited, and survival in surgically incurable HCC patients has not increased significantly over the past 30 years.11 Even in patients eligible for conventional systemic chemotherapy, response rates are low (discussed in more detail below), and a survival advantage with cytotoxic chemotherapy has not been convincingly demonstrated. Poor outcomes in HCC may be attributed both to the molecular complexity of the cancer and the frequent coexistence of cirrhosis with associated liver dysfunction.
Scoring and Staging
From a prognostic perspective, HCC is essentially two diseases in one, as cirrhosis is an integral part of the morbidity and mortality associated with HCC in most instances. Thus, in addition to staging the cancer using the pathologic Tumor, Node, Metastasis (TNM) classification system, the extent of cirrhosis must be defined to assess prognosis and treatment options properly. Historically, medical oncologists have used the Child-Pugh scoring system of cirrhosis (Table 1).12,13 A major limitation of the Child-Pugh system, however, is that it only categorizes the cirrhosis and does not address factors intrinsic to the cancer itself. Okuda and co-workers subsequently developed a scoring system that takes the bilirubin, albumin, and ascites parameters from Child-Pugh and adds it to an assessment of the tumor extent in the liver.14
Table 1.
Child-Pugh scoring of liver cirrhosis.
| Points
|
|||
|---|---|---|---|
| Parameter | 1 | 2 | 3 |
| Albumin (g/dL) | > 3.5 | 2.8–3.5 | < 2.8 |
| Bilirubin (mg/dL) | < 2 | 2–3 | > 3 |
| Ascites | Absent | Slight | Moderate |
| Encephalopathy | None | I–II | II–IV |
| PT (INR) | < 1.7 | 1.8–2.3 | > 2.3 |
| Score | A | B | C |
|---|---|---|---|
| Points | 5–6 | 7–9 | 10–15 |
Pugh RN, et al: Brit J Surg 60:646–649, 1973
The idea of incorporating cancer-related parameters was further developed by several other research groups. The Cancer of the Liver Italian Program (CLIP) score (Table 2)15,16 includes the Child-Pugh score parameters plus an assessment of tumor extent in the liver, the presence or absence of portal vein thrombosis, and the level of alpha-fetoprotein (AFP). Another scoring system was developed by Leung and colleagues from the Chinese University in Hong Kong. Known as the Chinese University Prognostic Index (CUPI), this system, like the CLIP score, identifies a number of potentially pertinent prognostic factors (Table 3).17 These include bilirubin, ascites, alphafetoprotein, alkaline phosphatase, tumor extent as defined by the TNM staging system, and the absence or presence of clinical symptoms on presentation.
Table 2.
The Cancer of the Liver Italian Program (CLIP) scoring system for HCC.
| Points
|
|||
|---|---|---|---|
| Parameter | 0 | 1 | 2 |
| Child-Pugh score | A | B | C |
| Tumor morphology | Uni-nodular & extension ≤50% | Multi-nodular & extension ≤50% | Massive or extension >50% |
| Portal vein thrombosis | No | Yes | |
| AFP (ng/dL) | <400 | ≥400 | |
| Score | 0 | 1 | 2 | 3 | 4–6 |
CLIP. Hepatology 28:751–755, 1998 & CLIP. Hepatology 31:840–845, 2000
Table 3.
Chinese University Prognostic Index (CUPI) risk groups in HCC.
| Parameter | Weight (CUPI Score) | |||||
|---|---|---|---|---|---|---|
| Bilirubin (mg/dL) | <1.9 | 0 | 1.9– 2.8 | 3 | > 2.9 | 4 |
| Ascites | Present | 3 | ||||
| Alkaline phosphatase | ≥ 200 IU/L | 3 | ||||
| TNM Stage | I & II | −3 | IIIa & IIIb | −1 | IVa & IVb | 0 |
| AFP (ng/mL) | ≥500 | 2 | ||||
| Disease symptoms on presentation | None | −4 | ||||
| Risk Group | Low | Intermediate | High |
|---|---|---|---|
| Score | −7 to 1 | 2 to 7 | 8 to 12 |
Leung T, et al: Cancer 94:1760–1769, 2002
The CLIP and CUPI scoring systems, however, were developed and assessed in two different populations. Hepatitis C patients were predominantly involved in the development of the CLIP score, whereas hepatitis B patients were primarily involved in the development of the CUPI index. An attempt by Leung from Hong Kong to retrofit the data from their predominantly Asian patient population (with hepatitis B-associated disease) to the CLIP scoring system was unsuccessful, leading to erroneous predictions of outcome.17 This finding suggests that different scoring systems might apply to specific patient populations.
Among other scoring systems are the Groupe d’Etude et de Traitement du Carcinoma Hepatocellulaire (GRETCH) staging system18 and the Japan Integrated Staging (JIS) Score,19 which is based on the TNM staging of the Liver Cancer Study Group of Japan (LCSGJ). Another system is the Barcelona Clinic Liver Cancer (BCLC) classification system,20 which was recently validated prospectively.21 While the BCLC staging system successfully separates advanced HCC patients from the rest, it fails to offer reliable prognostic information for this group, and so fails to help delineate outcomes and appropriateness of therapy.
A retrospective analysis of patients with advanced HCC seen by medical oncologists at Memorial Sloan-Kettering Cancer Center between 2001–2006 used two statistical tools, the likelihood ratio and Akaike Information Criterion,22 to compare the different scoring systems and help find out which one is most informative for survival outcome in this specific population of advanced HCC seen by medical oncologists. The GRETCH scoring system performed best and this was commensurate with the findings of Collete who came to the same conclusion independently.23 Nonetheless, this conclusion is limited by the retrospective nature of this analysis and needs to be further validated. Despite considerable concerted efforts, however, no uniformly accepted scoring system for the HCC/cirrhosis dyad exists, and, hence, no global consensus has been developed on the use of a standard staging system for HCC.
Cytotoxic Chemotherapy
At the time of this writing, no widely accepted standard approach to chemotherapy for HCC exists. Many chemotherapeutic agents have been tested in HCC, with response rates ranging between 10% and 15%, but no survival advantage has been demonstrated. The most studied chemotherapeutic agent is doxorubicin. An initial phase II trial in 1975 reported dramatic clinical activity with a 79% response rate;24 however, investigators have been uniformly unsuccessful in replicating those remarkable numbers in subsequent trials.25–35
One small randomized trial with no prespecified statistical hypothesis compared doxorubicin to no chemotherapy.36 A total of 106 patients with Karnofsky Performance Status ≥ 50% were randomized to receive doxorubicin 60 to 75 mg/m2 at 3-week intervals or to receive no anticancer therapy. Karnofsky performance status was well balanced between the two arms. In this trial, the median overall survival of the doxorubicin group over the control arm was approximately 3 weeks—10.6 weeks for doxorubicin vs. 7.5 weeks with no chemotherapy (P = .036). Although this 3-week median survival difference technically reached a level of statistical significance, the trial was fraught with serious methodologic flaws. Twenty-five percent of deaths in the doxorubicin group were considered treatment-related deaths due to toxicity, which makes the 3-week survival advantage questionable. Although it can be argued that management of marrow suppression and neutropenic septicemia has improved since 1988, the conclusion from that trial was that doxorubicin was not an ideal drug for the treatment of HCC, which further dampened any enthusiasm for this as a routine therapeutic approach.
Nevertheless, despite the substantial limitations of these trials, doxorubicin has become a default standard of treatment and serves as a control agent for several comparative trials evaluating other single agents or combination regimens.37 Objective response rates with single-agent doxorubicin in two recent phase III trials employing computed tomography (CT) scanning and modern response criteria were 10.5% and 4%.38,39 These more current trials can be considered a more accurate assessment of true activity, or lack thereof, of doxorubicin compared to the results of older trials.
Given the disappointing results of single-agent therapies, combination regimens have also been investigated. A regimen that initially demonstrated promising phase II activity in HCC is PIAF (cisplatin/interferon/doxorubicin [Adriamycin]/5-fluorouracil [5-FU]). This regimen yielded a response rate of 26% and a median survival of 9 months in a recently reported single-arm phase II trial.40 Of the 13 patients (26%) who had a partial response in that study, 9 underwent surgery, and 4 (9%) were found to have had a complete pathologic response to chemotherapy. These promising results led to one of the few large-scale randomized phase III studies conducted in patients with advanced HCC. Yeo and co-workers randomized 188 previously untreated patients with good hepatic function and good performance status to either PIAF or to singleagent doxorubicin, with overall survival (OS) as the primary end point.38 Unfortunately, the results of this study were negative in terms of the specified primary end point, with median survival of 8.6 months vs. 6.8 months for PIAF and doxorubicin, respectively (P = .83). The study did, however, show a doubling of response rate that trended strongly toward, but did not achieve, statistical significance; 20.9% for the PIAF arm vs. 10.5% for the doxorubicin arm (P = .058). The higher response rate in the PIAF arm was achieved at the expense of significantly increased toxicity, particularly related to myelosuppression and neutropenic fever.
Given the increase in serious toxicity, and lack of a demonstrable survival benefit, PIAF should not be routinely administered to HCC patients in standard practice. PIAF may be considered for carefully selected, medically fit patients with good liver function in whom cytoreduction is necessary to permit resectability. These conditions would justify the risk of the high toxicity of the regimen as an acceptable trade-off for potentially curative surgery.
Finally, a recently reported phase III randomized control trial tested the investigational agent nolatrexed as a single drug vs. doxorubicin in patients with unresectable HCC.39 The study was an extension of two phase II studies of nolatrexed in HCC.41,42 Results showed a median OS of 5.2 months for nolatrexed vs. 7.8 months for doxorubicin (P = .0055). It is unknown whether or not the nolatrexed conferred a negative survival effect, or was simply inactive.
In summary, cytotoxic chemotherapy has, at best, modest activity in patients with HCC, and has the potential for inducing significant toxicity. No treatment has convincingly demonstrated a survival advantage, even in those medically fit patients selected for investigational trials. Clearly, new agents for the treatment of HCC need to be identified.
Targeted Therapies in HCC
Over the past decade, several molecular targets involved in the etiology of HCC have been identified. These include growth factors, growth factor receptors, and components of intracellular signaling pathways that regulate the cell cycle, cellular survival, angiogenesis, invasion, and metastasis.43 Targeted agents that modulate growth factors, growth factor receptors, or kinases involved in the above pathways may have therapeutic potential in HCC. Therapies directed against these targets have shown benefit in other neoplasms.44 Inhibitors of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) are the two most studied classes of targeted therapeutics in HCC.
Epidermal Growth Factor Receptor Inhibitors
The importance of the EGFR pathway in HCC remains controversial.45,46 Nonetheless, as in many other solid tumors, erlotinib, an EGFR small-molecule kinase inhibitor, has been tested in a phase II trial of 38 patients with advanced HCC.47 Prior chemotherapy was permitted, and 47% of patients in this study had received prior treatment. Seventy-one percent of patients were classified Child-Pugh A (C-P A). Three partial responses (8%), as defined by RECIST (Response Evaluation Criteria in Solid Tumors) criteria, were observed. A 6-month RECIST progression-free survival (PFS) end point was achieved in 12 of 38 patients (32%), with a median PFS of 3.8 months. Median overall survival was 13 months. As with other tumors, immunohistochemical labeling of EGFR expression was not associated with outcome. The most frequent grade 3/4 toxicities were skin rash (13%), diarrhea (8%), and fatigue (8%). Because of the limited number of responses and short progression- free survival, despite the high number of C-P A patients who are likely to have better outcomes, the results of this trial do not provide sufficient support to recommend the use of single-agent erlotinib in the routine management of HCC. Cetuximab, another EGFR inhibitor, has shown preclinical activity in HCC;48 however, no clinical trials of cetuximab in HCC have been reported to date, so routine use cannot be recommended.
Lapatinib, a dual inhibitor of EGFR tyrosine kinase 1 and 2 (Her2/Neu), has also been studied in HCC.49 In a small phase II trial, 17 patients with advanced HCC were treated with oral lapatinib at a starting dose of 1,500 mg/day. Two confirmed partial responses (12%) were observed, and 8 patients exhibited some degree of stable disease. The median PFS was only 1.8 months, which falls short of other reported data in the literature.
Inhibitors of the Proangiogenic Pathway
HCC is a highly vascularized solid tumor, and VEGF augments HCC development and metastasis in preclinical models.50 Overexpression of the proangiogenic platelet-derived growth factor receptorbeta (PDGFR-β) in vascular endothelial cells of HCC tumors is associated with a highly metastatic phenotype, and activation of either the VEGF or PDGF-β pathway also activates downstream Raf-1.51 Sorafenib is one of the new molecular targeted agents that inhibits both proangiogenic (VEGFR-1, -2, -3; PDGFR-β) and tumorigenic (RET, Flt-3, c-Kit) receptor tyrosine kinases (RTKs); it also inhibits the serine/threonine kinase Raf-1 in vitro.52–54 Sorafenib has been tested extensively in HCC. In a phase I trial of sorafenib, a confirmed partial response was observed in a patient with metastatic HCC.55
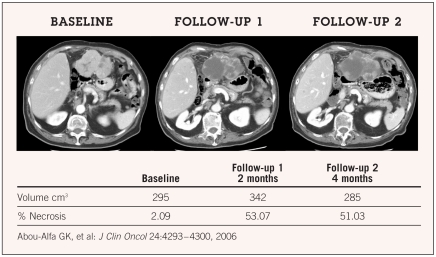
A phase II trial of sorafenib in patients with advanced HCC demonstrated stabilization of disease (≥ 16 weeks) in 33.6% of patients and a partial response in 2% of patients.56 Median time to progression (TTP) was 4.2 months and median OS was 9.2 months. Grade 3/4 toxicities potentially related to treatment included fatigue (9.5%), diarrhea (8%), and hand-foot skin reaction (5.1%). Interestingly, central tumor necrosis was observed in many patients in the study (Figure 1); however, the clinical implications of this observation have not yet been determined. Another finding from this study worth noting is the significant difference in TTP in 18 of 33 patients with higher (2 to 4+) pERK staining vs. those with lower (0 to 1+) intensity (P = .00034), suggesting the importance of raf inhibition as one of the mechanisms of action of sorafenib.
Figure 1.
Stable disease and “tumor necrosis.”
In the case of hepatitis C, data have shown that HCV-1 core protein may result in high basal activity of Raf-1, increasing the possibility of oncogenesis.57 In the above-mentioned study, it was noted retrospectively that patients with evident hepatitis C as a risk factor have an improved median TTP of 6.5 months vs. 4 months in their counterpart hepatitis B patients (P = .05).58 These particular findings are of limited value, considering the retrospective analysis, small sample size, and subjective assessment used in immunostaining. Thus, the actual contribution of this pathway to outcomes remains to be determined.
Single-agent sorafenib has also been evaluated in a randomized, double-blinded, placebo-controlled phase III trial in C-P A patients with advanced HCC.59 The SHARP (Sorafenib HCC Assessment Randomized Protocol) trial, was powered for two primary end points; overall survival and time to symptomatic progression (TTSP). The latter end point was assessed using the Functional Assessment of Cancer Therapy (FACT) Hepatobiliary Symptom Index 8 (FHSI8) scale, an eight-question subset of FACT-Hepatobiliary. Results demonstrated a statistically significant (P = .00058) improvement in survival of 44% in favor of sorafenib (10.7 months) vs. placebo (7.9 months). The study did not, however, show any difference in TTSP (P = .77), which may be related to the relatively good performance status of the patients on study—the majority of patients had previously undergone surgical resection (20%) or transarterial chemoembolization (TACE)—or the failure of the FHSI8-TSP instrument to detect any such difference.
Sorafenib was well tolerated in the SHARP study, with adverse effects reported at 52% for sorafenib vs. 54% for placebo. Grade 3/4 toxicities in the sorafenib vs. placebo groups included diarrhea (11% vs. 2%), fatigue (10% vs. 15%), hand-foot reaction (8% vs. 1%), and bleeding was less than 1% in both arms. The positive outcome of the study applied to C-P A patients with good performance status. This population represents a large proportion of patients seen by oncologists. In a retrospective analysis of 187 patients seen by medical oncologists at Memorial Sloan-Kettering Cancer Center for advanced or systemic hepatocellular carcinoma, 80% had an ECOG performance status of 0–1, and 67% had a Child-Pugh A score.22
Using sorafenib for patients classified as Child-Pugh B (C-P B) has not been fully addressed. In the phase II study previously mentioned,56 28% of patients were classified C-P B. In 22 patients from whom blood samples were drawn for pharmacokinetic analysis, area under the concentration vs. time curve over 8 hours (AUC0-8) was comparable between C-P A (25.4 mg·h/L) and C-P B (30.3 mg·h/L) patients. Cmax values were 4.9 mg/L and 6.0 mg/L in C-P A and C-P B patients, respectively. Common adverse events associated with sorafenib were similar between C-P A and C-P B patients. However, cirrhosis worsened more frequently in C-P B patients. It remains unclear whether this is a drug-related effect or disease progression.
A Cancer and Leukemia Group B (CALGB) study evaluating sorafenib in patients with solid tumors and hematologic malignancies and liver or renal dysfunction reported elevated total bilirubin as a drug-limiting toxicity in several liver dysfunction cohorts.60 It is unclear whether this total bilirubin elevation is due to worsening liver function caused by sorafenib or simply due to the inhibitory effect of UGT1A1, to which sorafenib is a substrate.61 The question will be answered best in a prospective study that evaluates sorafenib in patients with advanced HCC and different degrees of liver dysfunction, looking at both toxicity and outcome.
A phase I study evaluated sorafenib in combination with doxorubicin in 34 patients with advanced, unresectable solid tumors, including HCC.62 The combination was adequately tolerated, without clear evidence of synergistic toxicity, and with the full planned doses of each agent being administered without encountering dose-limiting toxicity. Interestingly, all four patients with advanced, progressive HCC who received the sorafenib/doxorubicin combination experienced prolonged stable disease, remaining on treatment formore than 1 year.
The results of a randomized phase II, double-blind trial of sorafenib plus doxorubicin vs. placebo plus doxorubicin in chemotherapy- naïve HCC patients was recently reported.63 The primary end point, median TTP, was superior in the sorafenib/doxorubicin arm (9 months vs. 5 months), and toxicity profiles were similar for both groups. As a result, patients on the placebo/doxorubicin arm crossed over to the sorafenib combination arm. In this preliminary analysis, TTP and OS in the sorafenib/doxorubicin arm appear encouraging. This trial supports the growing body of evidence of the activity of sorafenib in HCC. However, any synergistic role between sorafenib plus doxorubicin in HCC still needs to be further defined.
The anti-VEGF monoclonal antibody bevacizumab has also been studied in patients with advanced HCC. Bevacizumab was initially studied as a single agent in HCC at 5 mg/kg and 10 mg/kg doses.64 Of 28 patients treated, 2 patients had a partial response and 18 had stable disease. As per this last report, 6 patients with stable disease were currently receiving treatment at 3.7+ to 23.3+ months on study. Median TTP was 6.5 months. Treatment was discontinued in 4 of the 28 patients due to serious adverse events, including one transient ischemic attack, and 3 patients developed serious esophageal bleeding, which led to a modification of the protocol to identify and treat esophageal varices prior to enrollment.65
Bevacizumab was combined with gemcitabine and oxaliplatin in a phase II trial in previously treated HCC patients with good performance status.66 In 30 patients with advanced HCC, the objective response rate was 20%, and 27% of patients had stable disease. Median PFS was 5.3 months and median OS was 9.6 months. Leukopenia/neutropenia, elevation of transaminases, hypertension, and fatigue were the most common grade 3/4 toxicities. Another phase II study evaluated the feasibility and efficacy of intravenous bevacizumab, oxaliplatin, and capecitabine in 17 patients with advanced HCC.67 The combination appeared tolerable, yet no data have yet been made available regarding efficacy.
Recently, bevacizumab has been tested in combination with erlotinib in patients with HCC and CLIP ≥ 3.68 Based on an intent-to-treat analysis, 7 of 34 patients responded, and 27 (79.4%) had stable disease for up to 8 weeks. Median PFS was 9 months, and median OS was 19 months. Grade 3/4 fatigue and hypertension were each reported in 15% of patients, and similar grade gastrointestinal bleeds were reported in 9%. The outcome of this particular study is worth exploring further in a randomized phase III study to determine the existence of an additive or synergistic effect of antiangiogenic therapy and a tyrosine kinase inhibitor in HCC.
Sunitinib, another antiangiogenic agent, has also been studied in HCC.69 Of 26 patients treated, 10 (38.5%) showed stable disease, with a median PFS of 4.1 months. Another study showed similarly promising results at a dose of 50 mg, with median TTP of 21 weeks and median OS of 45 weeks.70
In view of the positive results of the SHARP study, which showed a 44% improvement in survival, targeting the VEGF pathway appears to be a potentially useful strategy in treating advanced HCC. Considering the vascularity of this tumor, however, a greater degree of antitumor activity might have been expected.51 Still, the possibility exists that antitumor activity may be reflected more in tumor necrosis rather than actual tumor shrinkage, as noted in the previously mentioned phase II study of sorafenib in HCC.56 This phenomenon, while already reported in the radiology literature, 71 would still require validation as a measure of response in HCC.
CONCLUSION
The steady rise in incidence of hepatitis C, the rise in diabetes and morbid obesity-associated NASH, and the continued substantial levels of alcohol abuse, comprise the major risk factors for the increasing incidence of HCC in the United States. Understanding the two components of the disease—the cirrhosis and the cancer itself—are essential to evaluating and managing patients with HCC properly. Currently available cytotoxic chemotherapy has little proven therapeutic benefit in HCC. A number of novel agents are under evaluation. Sorafenib is the first systemic treatment to demonstrate a survival advantage in the setting of a large, randomized, controlled phase III study.59 Data from ongoing trials and future research are required in order to gain a better understanding of the role this agent and others will play in combination therapies. The improved outcomes seen with bevacizumab in combination with erlotinib may be due to a synergistic effect and suggests that exploring sorafenib combined with erlotinib may be a worthy endeavor.
We continue to face many of the logistical challenges that have plagued the development of therapies for HCC. As stated above, assessment of response may be difficult in HCC, due to the frequent presence of hepatic fibrosis, cirrhosis, and scarring. No marker, such as alpha-feto-protein, has been adequately validated as a surrogate for response. The central necrosis reported in the phase II study of sorafenib in HCC also remains an interesting observation, 56 but in the absence of validation as a correlate for clinical benefit, the usefulness of this observation remains unclear. What is clear is that whatever benefits have been seen with sorafenib or other targeted agents have been modest at best. HCC remains a highly lethal disease, and efforts to develop newer, more effective treatments must continue to receive high priority.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Abou-Alfa has received research support from Bayer.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 7.McGlynn KA, Tsao L, Hsing AW, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–296. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 8.Stuver SO. Towards global control of liver cancer? Semin Cancer Biol. 1998;8:299–306. doi: 10.1006/scbi.1998.0079. [DOI] [PubMed] [Google Scholar]
- 9.Nissen NN, Martin P. Hepatocellular carcinoma: the high-risk patient. J Clin Gastroenterol. 2002;35:S79–S85. doi: 10.1097/00004836-200211002-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol. 2005;34(suppl 1):S75–S78. doi: 10.1016/s1386-6532(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 11.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391– 7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Child CG. The Liver and Portal Hypertension. Saunders; Philadelphia: 1964. p. 50. [Google Scholar]
- 14.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.the Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 16.The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 17.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 18.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 19.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–328. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 21.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Huitzil FD, Capanu M, O’Reilly E, et al. Ranking and improvement of staging systems (SS) in advanced hepatocellular carcinoma (AHCC). Presented at the 2008 Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. (abstr 210) [Google Scholar]
- 23.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of hepatocellular carcinoma (HCC): comparison of four staging systems in two French clinical trials. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 4589) [Google Scholar]
- 24.Olweny CL, Med M, Toya T, et al. Treatment of hepatocellular carcinoma with Adriamycin. Preliminary communication. Cancer. 1975;36:1250–1257. doi: 10.1002/1097-0142(197510)36:4<1250::aid-cncr2820360410>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Ihde DC, Kane RC, Cohen MH, et al. Adriamycin therapy in American patients with hepatocellular carcinoma. Cancer Treat Rep. 1977;61:1385–1387. [PubMed] [Google Scholar]
- 26.Vogel CL, Bayley AC, Brooker RJ, et al. A phase II study of Adriamycin (NSC 123127) in patients with hepatocellular carcinoma from Zambia and the United States. Cancer. 1977;39:1923–1929. doi: 10.1002/1097-0142(197705)39:5<1923::aid-cncr2820390502>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Falkson G, Moertel CG, Lavin P, et al. Chemotherapy studies in primary liver cancer: a prospective randomized clinical trial. Cancer. 1978;42:2149–2156. doi: 10.1002/1097-0142(197811)42:5<2149::aid-cncr2820420510>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PJ, Williams R, Thomas H, et al. Induction of remission in hepatocellular carcinoma with doxorubicin. Lancet. 1978;1:1006–1009. doi: 10.1016/s0140-6736(78)90735-3. [DOI] [PubMed] [Google Scholar]
- 29.Olweny CL, Katongole-Mbidde E, Bahendeka S, et al. Further experience in treating patients with hepatocellular carcinoma in Uganda. Cancer. 1980;46:2717–2722. doi: 10.1002/1097-0142(19801215)46:12<2717::aid-cncr2820461230>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Melia WM, Johnson PJ, Williams R. Induction of remission in hepatocellular carcinoma. A comparison of VP 16 with Adriamycin. Cancer. 1983;51:206–210. doi: 10.1002/1097-0142(19830115)51:2<206::aid-cncr2820510206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Barbare JC, Ballet F, Petit J, et al. Hepatocellular carcinoma with cirrhosis: treatment with doxorubicin. Phase II evaluation. Bull Cancer. 1984;71:442–445. [PubMed] [Google Scholar]
- 32.Chlebowski RT, Brzechwa-Adjukiewicz A, Cowden A, et al. Doxorubicin (75 mg/m2) for hepatocellular carcinoma: clinical and pharmacokinetic results. Cancer Treat Rep. 1984;68:487–491. [PubMed] [Google Scholar]
- 33.Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma. Adria-mycin versus quadruple chemotherapy. Cancer. 1984;53:401–405. doi: 10.1002/1097-0142(19840201)53:3<401::aid-cncr2820530306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Falkson G, MacIntyre JM, Moertel CG, et al. Primary liver cancer. An Eastern Cooperative Oncology Group Trial. Cancer. 1984;54:970–977. doi: 10.1002/1097-0142(19840915)54:6<970::aid-cncr2820540604>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Sciarrino E, Simonetti RG, Le Moli S, et al. Adriamycin treatment for hepatocellular carcinoma. Experience with 109 patients. Cancer. 1985;56:2751–2755. doi: 10.1002/1097-0142(19851215)56:12<2751::aid-cncr2820561205>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Lai CL, Wu PC, Chan GCB, et al. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479–483. doi: 10.1002/1097-0142(19880801)62:3<479::aid-cncr2820620306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 37.Simonetti RG, Liberati A, Angiolini C, et al. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Annals of Oncology. 1997;8:117–136. doi: 10.1023/a:1008285123736. [DOI] [PubMed] [Google Scholar]
- 38.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 39.Porta C, Ruff R, Feld L, et al. ASCO 2006 Gastrointestinal Symposium Proceedings. Lippincott Williams & Wilkins; Baltimore: 2006. Results of a phase III randomized controlled study, the largest ever completed in hepatocellular carcinoma (HCC), comparing the survival of patients with unresectable HCC treated with nolatrexed (NOL) or doxorubicin (DOX) p. 133A. [Google Scholar]
- 40.Leung TW, Patt YZ, Lau WY, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clinical Cancer Research. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 41.Stuart K, Tessitore J, Rudy J, et al. A Phase II trial of nolatrexed dihydrochloride in patients with advanced hepatocellular carcinoma. Cancer. 1999;86:410–414. [PubMed] [Google Scholar]
- 42.Jhawer M, Rosen L, Dancey J, et al. Phase II trial of nolatrexed dihydrochloride [Thymitaq, AG 337] in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2007;25:85–94. doi: 10.1007/s10637-006-9003-x. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Alfa GK, Morse M. Novel therapies targeted at signal transduction in liver tumors. In: Clavien PA, editor. Malignant liver tumors: Current and emerging therapies. 2. Sudbury, MA: Jones and Bartlett; 2004. p. 307. [Google Scholar]
- 44.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 45.Kira S, Nakanishi T, Suemori S, et al. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17:177–182. doi: 10.1111/j.1600-0676.1997.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 46.Hamazaki K, Yunoki Y, Tagashira H, et al. Epidermal growth factor receptor in human hepatocellular carcinoma. Cancer Detect Prev. 1997;21:355–360. [PubMed] [Google Scholar]
- 47.Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657–6663. doi: 10.1200/JCO.2005.14.696. [DOI] [PubMed] [Google Scholar]
- 48.Huether A, Hopfner M, Varadari V, et al. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular carcinoma. Biochemical Pharmacology. 2005;70:1568– 1578. doi: 10.1016/j.bcp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Ramanathan RK, Belani CP, Singh DA, et al. Phase II study of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase 1 and 2 (Her2/Neu) in patients (pts) with advanced biliary tree cancer (BTC) or hepatocellular cancer (HCC). A California Consortium (CCC-P) Trial. 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24:18S. (abstr 4010) [Google Scholar]
- 50.Yoshiji H, Kuriyama S, Yoshii J, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517–1524. doi: 10.1002/hep.20218. [DOI] [PubMed] [Google Scholar]
- 51.Zhang T, Sun HC, Xu Y, et al. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin Cancer Res. 2005;11:8557–8563. doi: 10.1158/1078-0432.CCR-05-0944. [DOI] [PubMed] [Google Scholar]
- 52.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral anti-tumor activity and targets the Raf/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 53.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 54.Levy AP, Pauloski N, Braun D, et al. Analysis of transcription and protein expression changes in the 786-O human renal cell carcinoma tumor xenograft model in response to treatment with the multi-kinase inhibitor sorafenib (BAY 43-9006) Proc Am Assoc Cancer Res. 2006;47:213. [Google Scholar]
- 55.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 56.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1– 8. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 57.Giambartolomei S, Covone F, Levrero M, et al. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene. 2001;20:2606–2610. doi: 10.1038/sj.onc.1204372. [DOI] [PubMed] [Google Scholar]
- 58.Huitzil FD, Saltz LS, Song J, et al. Retrospective analysis of outcome in hepatocellular carcinoma (HCC) patients (pts) with hepatitis C (C+) versus B (B+) treated with sorafenib (S). 2007 ASCO Gastrointestinal Cancers Symposium; Orlando, FL. January 19–21, 2007. [Google Scholar]
- 59.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): results of a phase III randomized placebo-controlled trial (SHARP trial). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr LBA1) [Google Scholar]
- 60.Miller AA, Murry DJ, Owzar K, et al. Pharmacokinetic (PK) and phase I study of sorafenib (S) for solid tumors and hematologic malignancies in patients with hepatic or renal dysfunction (HD or RD): CALGB 60301. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 3538) [Google Scholar]
- 61.Wayne NJ. Package insert. Nexavar (sorafenib) Bayer HealthCare Pharmaceuticals Inc; 2008 January [Google Scholar]
- 62.Richly H, Kupsch P, Passage K, et al. Results of a phase I trial of BAY 43-9006 in combination with doxorubicin in patients with primary hepatic cancer. Ann Oncol. 2004;15:iii 104h. doi: 10.5414/cpp42650. [DOI] [PubMed] [Google Scholar]
- 63.Abou-Alfa G, Johnson P, Knox J, et al. Preliminary results from a phase II, randomized, double- blind study of sorafenib plus doxorubicin versus placebo plus doxorubicin in patients with advanced hepatocellular carcinoma. 14th European Cancer Conference (ECCO); Barcelona, Spain. September 27–30, 2007; (abstr) [Google Scholar]
- 64.Schwartz JD, Schwartz M, Lehrer D, et al. Bevacizumab in unresectable hepatocellular carcinoma (HCC) for patients without metastasis and without invasion of the portal vein. 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24:18S. (abstr 4144) [Google Scholar]
- 65.Schwartz JD, Schwartz M, Goldman J, et al. Bevacizumab in hepatocellular carcinoma (HCC) for patients without metastasis and without invasion of the portal vein. 2005 ASCO Gastrointestinal Cancers Symposium; Hollywood, FL. January 27–29, 2005; (abstr 134) [Google Scholar]
- 66.Zhu AX, Blaskowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 67.Hwitt M, Sun W, Haller DG, et al. A phase II trial of combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): preliminary safety analysis. 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24:18S. (abstr 14098) [Google Scholar]
- 68.Thomas MB, Chadha R, Iwasaki M, et al. The combination of bevacizumab (B) and erlotinib (E) shows significant biological activity in patients with advanced hepatocellular carcinoma (HCC). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 4567) [Google Scholar]
- 69.Faivre SJ, Raymond E, Douillard J, et al. Assessment of safety and drug-induced tumor necrosis with sunitinib in patients (pts) with unresectable hepatocellular carcinoma (HCC). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 3546) [Google Scholar]
- 70.Faivre SJ, Raymond E, Douillard J, et al. Assessment of safety and drug-induced tumor necrosis with sunitinib in patients (pts) with unresectable hepatocellular carcinoma (HCC). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;2518S (abstr 3546) [Google Scholar]
- 71.Wang J, Chen LT, Tsang YM, et al. Dynamic Contrast-Enhanced MRI analysis of perfusion changes in advanced hepatocellular carcinoma treated with an antiangiogenic agent: A preliminary study. Am J Roentgenology. 2004;183:713–719. doi: 10.2214/ajr.183.3.1830713. [DOI] [PubMed] [Google Scholar]