Abstract
In the thalamus of the rat the reversal potential of GABA-induced anion currents is more negative in relay cells than in neurones of the reticular nucleus (nRt) due to different chloride extrusion mechanisms operating in these cells. The distribution of KCl cotransporter type 2 (KCC2), the major neuronal chloride transporter that may underlie this effect, is unknown in the thalamus. In this study the precise regional and ultrastructural localization of KCC2 was examined in the thalamus using immunocytochemical methods. The neuropil of all relay nuclei was found to display intense KCC2 immunostaining to varying degrees. In sharp contrast, the majority of the nRt was negative for KCC2. In the anterior and dorsal part of the nRt, however, KCC2 immunostaining was similar to relay nuclei and parvalbumin and calretinin were found to colocalize with KCC2. At the ultrastructural level, KCC2 immunoreactivity was mainly located in the extrasynaptic membranes of thick and thin dendrites and the somata of relay cells but was also found in close association with asymmetrical synapses formed by cortical afferents. Quantitative evaluation of KCC2 distribution at the electron microscopic level demonstrated that the density of KCC2 did not correlate with dendritic diameter or synaptic coverage but is 1.7 times higher on perisynaptic membrane surfaces than on extrasynaptic membranes. Our data demonstrate that the regional distribution of KCC2 is compatible with the difference in GABA-A reversal potential between relay and reticular nuclei. At the ultrastructural level, abundant extrasynaptic KCC2 expression will probably play a role in the regulation of extrasynaptic GABA-A receptor-mediated inhibition.
Keywords: GABA, KCl cotransporter, rat, reticular nucleus, thalamus
Introduction
The polarity of the response to the major inhibitory transmitter GABA in the central nervous system critically depends on the intracellular chloride concentration (Sivilotti & Nistri, 1991). Ligand-gated GABA-A receptors are chloride channels whose activation induces chloride flux through the plasma membrane. The direction of the chloride movement, however, depends on the chloride equilibrium potential (ECl). In most cell types this is kept slightly below the resting membrane potential, thus activation of GABA-A receptors results in a chloride influx from the extracellular space into the neurone inducing membrane hyperpolarization (Kaila, 1994). However, in a number of cell types or during ontogenesis ECl equals or is more depolarized than the resting membrane potential which results in shunting or depolarizing GABA-A responses (Rivera et al., 1999; Vardi et al., 2000; Gulacsi et al., 2003).
Inhibitory synaptic activity plays a pivotal role in shaping normal and pathophysiological rhythms in the thalamus. The firing mode of the two main cell types, excitatory thalamocortical relay cells and inhibitory reticular cells, is greatly influenced by the polarity of GABA-A responses (Bazhenov et al., 1999). Interestingly, different chloride transport mechanisms seem to operate in neurones of the relay and the reticular nuclei. Active chloride extrusion in relay cells sets the reversal potential of the GABA-A current 10 mV more hyperpolarized than in reticular cells, which results in hyperpolarizing and shunting GABA-A responses, respectively (Ulrich & Huguenard, 1997). Cation-chloride cotransporters are good candidates to account for the differential chloride distribution in relay and reticular cells as they participate in the active transport of chloride ions into or out of the cell (Payne et al., 2003). At present, there are seven types of cation-chloride cotransporters in mammals (Delpire, 2000), four of which are KCl cotransporters (KCCs) (Gillen et al., 1996). Of the four KCC isoforms, the neurone-specific KCC2 (Payne et al., 1996; Lu et al., 1999) seems to be essential for maintaining the transmembrane chloride gradient that underlies hyperpolarizing inhibitory postsynaptic potentials (Rivera et al., 1999). KCC2 expression has been shown to correlate with neuronal maturation and change in polarity of the GABA-A reversal potential (Rivera et al., 1999; Stein et al., 2004). Indeed, in early postnatal life, when the KCC2 expression level in the hippocampus is low, GABA-A responses are depolarizing (Ben-Ari et al., 1989) and, in KCC2 knockout mice, GABA is excitatory in spinal cord motoneurones (Hubner et al., 2001). Ion transporters also regulate the concentration of osmolytes in the intra- and extracellular compartments, implying a role in the control of cellular volume in the normal condition and during pathological states of extreme synaptic activity (Reid et al., 2001; Woo et al., 2002; Payne et al., 2003). In the hippocampus, KCC2 has been shown to be located preferentially in pyramidal cell spines in the proximity of excitatory synapses (Gulyas et al., 2001), suggesting a role in buffering the osmotic load accompanying excitatory synaptic activity. However, it was not clear whether increased KCC2 density in the spines was due to the small size of the compartment (i.e. relatively large osmolytic load) or to the proximity of the synapse. The distribution of KCC2 in the thalamus is unknown at present. In this study, using highly selective immunocytochemical methods, we first examined whether the distribution of KCC2 in the thalamus can explain the observed difference in chloride gradients in reticular and relay cells. Next, using quantitative immunogold methods, we also examined the precise localization of KCC2 in various dendritic compartments and in relation to excitatory synapses.
Materials and methods
Perfusion and preparation of tissue section
Eighteen male Wistar rats (Charles River, Hungary) were used for KCC2 immunocytochemistry. All experimental procedures were performed according to the ethical guidelines of the Institute of Experimental Medicine, Hungarian Academy of Sciences and approved by the Ethical Committee. Rats were deeply anaesthetized by Equithesin (chlornembutal, 0.3 mL/100 g), then perfused through the heart first with physiological saline for 2 min and then with either of the following two types of fixative. Fixative A (n = 10) contained 4% paraformaldehyde (TAAB, UK), 15% picric acid and 0.05% glutaraldehyde (TAAB) in phosphate buffer. In the case of fixative B (n = 8), the saline was followed first by 100 mL 2% paraformaldehyde and 3.6% acrolein (Sigma, St Louis, MO, USA) in phosphate buffer and then by 300 mL 2% paraformaldehyde. Following perfusions, coronal, horizontal or parasagittal sections (60 μm thick) were cut from the thalamus on a Vibratome, washed, cryoprotected in 30% sucrose in 0.1 m phosphate buffer overnight and freeze-thawed in an aluminium foil boat over liquid nitrogen.
Immunocytochemistry
After extensive washes in Tris-buffered saline (TBS, pH 7.4), sections were incubated in 3% bovine serum albumin (Sigma) for 45 min and then with rabbit anti-KCC2 antibody (1 : 300-500; Williams et al., 1999) for 2 days. For 3,3′-diaminobenzidine (DAB) immunostaining, sections were incubated in goat anti-rabbit antiserum (1 : 300; Vector Laboratories, Burlingame, CA, USA) for 2 h followed by avidin-biotinylated-horseradish peroxidase complex (ABC, 1 : 300; Vector Laboratories) for 2 h. Between each step sections were washed in TBS three times for 10 min. The specificity of the antibodies was studied extensively by the laboratories of origin. The immunoperoxidase reaction was developed with DAB as a chromogen. For pre-embedding immunogold staining following the primary antibody, the sections were incubated in 1 nm gold-conjugated goat anti-rabbit antiserum (1 : 60; Amersham, UK) dissolved in TBS containing 0.8% bovine serum albumin and 0.1% gelatin overnight, postfixed in 1% glutaraldehyde in TBS and then silver intensified with an R-Gent intensification kit (Aurion, Wageningen, the Netherlands). All sections were treated with OsO4 (1% for 45 min for DAB staining and 0.5% for 30 min at 4 °C for immunogold staining in 0.1 m phosphate buffer), dehydrated in ethanol and propylene oxide and embedded in Durcupan (ACM, Fluka, Buchs, Switzerland). During dehydration, the sections were treated with 1% uranyl acetate in 70% ethanol for 40 min. Selected blocks containing identified thalamic nuclei were re-embedded and 65-nm-thick ultrathin sections were cut with an Ultramicrotome (Reichert), mounted on grids and examined with an electron microscope (7100; Hitachi). Digital images were collected using a GATAN camera. For fluorescent double immunostainings, the combinations of rabbit anti-KCC2 antiserum and mouse anti-parvalbumin (1 : 500; Swant, Bellinzona, Switzerland) or mouse anti-calretinin (1 : 1500; Swant) were used. The second layer was Alexa 594-conjugated donkey anti-rabbit (1 : 200; Molecular Probes) and fluoroscein isothiocyanate-conjugated donkey anti-mouse (1 : 50; Jackson). Following washes in TBS the sections were mounted and covered by Vectashield. The sections were evaluated using a fluorescent microscope (Axioscope; Zeiss) using the following filter sets: fluoroscein isothiocyanate, 450-490/512-542 and Alexa 594, 546 ± 12/590 LP (absorption and emission in nm). Light microscopic images were scanned with a digital camera (DP 70; Olympus). Brightness and contrast were adjusted, if necessary, using Adobe Photoshop 7.0.
Quantitative electron microscopic analysis
The relative KCC2 density across different subcellular compartments was estimated in 151 dendrites and 12 somata randomly selected from the electron micrographs. The dendrites were subdivided into five categories according to their minor diameter (< 0.4, 0.4-0.6, 0.6-0.8, 0.8-1.0 and > 1.0 μm smaller diameter). The perimeter and area were measured with an NIH Image Analyzer and the number of gold particles was counted for each profile. For the calculation of density, the perimeter and surface area data were pooled within given dendritic categories and were divided by the total number of gold particles belonging to this category (for this reason we have not put error bars on the figures). Pooling was preferred to taking the gold particle: perimeter ratio for each profile and averaging because the number of profiles containing zero gold particles was higher in the thin than in the thick dendrites and this would have biased the grand average.
In the electron micrographs, the synaptic area was calculated using a polygon made up of a series of trapezoids, each 0.065 μm high (the thickness of the electron microscopic section), using the following formula
where lfirst and lsecond ... llast refer to the length of the synapse in the first and second ... last section of the series containing the given synapse.
Perisynaptic areas were defined as an annulus surrounding a synapse by 200 nm, composed of two rectangles on the top and bottom of the synapse, four parallelograms on the sides of the synapse and four quarters of a cycle connecting the rectangles and the parallelograms. For qualitative purposes this area was approximated using the following equation
The first two parts of the equation refer to the area of the rectangle, the second part refers to the parallelograms, where n is the number of sections containing the synapse, and the last part is the sum of the four quarters of the circle. If the synapses continued outside or ended closer than 200 nm to the end of the reconstructed sample, the outlying area was not considered. No overlapping of perisynaptic areas occurred in our sample and thus no gold particle was assigned to two synapses. The parameters of the dendrites were measured using NIH Image Analyzer. After calculation of peri- and extrasynaptic areas, the number of gold particles in each area was calculated, the data pooled over all dendrites and the ratio taken.
Results
Regional distribution
At the light microscopic level dense to moderate KCC2 immunostaining was seen in all thalamic nuclei except the reticular nucleus (nRt) (Fig. 1). No qualitative difference was observed between the different fixatives but, in general, animals perfused with fixative B (containing acrolein, see Materials and methods) showed stronger immunostaining.
Fig. 1.

Differential distribution of KCl cotransporter type 2 (KCC2) in relay and reticular thalamic nuclei. Heterogeneity of staining pattern within the thalamic reticular nucleus (nRt). (A and B) Low power light micrographs showing the distribution of KCC2 on coronal thalamic sections at two antero-posterior levels. Note in A that relay nuclei are immunopositive but the majority of the nRt lacks the protein. In the dorsal portion of the nRt (arrows), however, KCC2-immunoreactivity is similar to the adjacent relay nuclei. D, dorsal; L, lateral. (C) In a horizontal thalamic section only the rostral portion of the nRt is positive for KCC2. A, anterior. Boxed areas are enlarged in D and F. The corresponding parts of the adjacent sections are shown (E-G) immunostained for parvalbumin (PV) that precisely outlines the nRt. Note that the sharp boundary of KCC2 immunostaining (arrowheads in D) present at the border of the nRt and ventral posterolateral nucleus (VPL) in the posterior part of the thalamus is hardly visible (arrowheads in F) between the anterior nRt and ventrolateral nucleus (VL). Small arrows label landmark capillaries. Scale bars, 500 μm (A-C); 100 μm (D-G). APT, anterior pretectal nucleus; CL, centrolateral nucleus; DLG; dorsal lateral geniculate nucleus; fr, fasciculus retroflexus; LD, laterodorsal nucleus; LP, lateral posterior nucleus; MD, mediodorsal nucleus; ml, medial lemniscus; Po, posterior nucleus; PC, paracentral nucleus; Pf, parafascicular nucleus; Re, reuniens nucleus; Rh, rhomboid nucleus; VLG, ventral lateral geniculate nucleus; VM, ventromedial nucleus; VPM, ventral posteromedial nucleus; ZI, zona incerta.
The KCC2 immunoreactivity was prominent in the primary sensory relay nuclei (first order nuclei), being strongest in the ventral posterolateral and ventral posteromedial nuclei (Fig. 1A). Higher order (associational) nuclei (Guillery & Sherman, 2002) of the same sensory modalities always expressed slightly less dense immunoreactivity than their first order counterpart, i.e. immunostaining in the somatosensory ventral posterolateral and ventral posteromedial nuclei was more dense than in the nucleus posterior (Fig. 1A). The visual, dorsal lateral geniculate nucleus displayed stronger KCC2 immunoreactivity than the higher order lateral posterior nucleus (Fig. 1B) and KCC2 expression in the ventral part of the auditory medial geniculate nucleus was stronger than in the dorsal part of the medial geniculate. KCC2 immunoreactivity in the ventral motor nuclei (including ventromedial, ventrolateral and ventral anterior) was similar to first order sensory nuclei. The intralaminar and the midline nuclear group showed stronger immunostaining than the adjacent mediodorsal nucleus and posterior thalamic nucleus. The most prominent expression of KCC2 among the midline nuclei was found in the reuniens and rhomboid nuclei (Fig. 1A). The anterior nuclear group showed weak immunostaining.
Other diencephalic sites, including zona incerta, ventral lateral geniculate nucleus and anterior pretectal nucleus, expressed prominent KCC2 immunostaining that was similar or even stronger than the densest thalamic immunoreactivity observed in the ventral posterolateral nucleus and ventral posteromedial nucleus (Fig. 1B).
At higher power KCC2 immunoreactivity showed homogeneous neuropil staining in all nuclei. The staining had a punctate appearance in both immunogold- and DAB-reacted material. In good quality DAB immunostainings individual somata and proximal dendrites could be distinguished. In these cases the labelling was clearly localized to the plasma membranes.
Reticular nucleus
The most conspicuous aspect of KCC2 immunostaining in the thalamus was the lack of immunoreactivity in the majority of the nRt. In contrast, the dorsal ‘head’ region and the anterior part of the nRt displayed KCC2 immunoreactivity similar to the adjacent relay nuclei (Figs 1 and 3).
Fig. 3.

Colocalization of KCl cotransporter type 2 (KCC2) and calretinin (CR) in the dorsal thalamic reticular nucleus (nRt). (A-C) Low power fluorescent images of coronal thalamic sections double immunostained for KCC2 and CR demonstrate the overlap of the two markers in the dorsal nRt. (D-G) In higher power images both CR-positive (arrowheads) and CR-negative (small arrows) KCC2-containing dendrites can be observed in the dorsal nRt. The CR-positive soma (s) in G is negative for KCC2. Scale bars, 200 μm (A-C); 5 μm (D-G). VPL, ventral posterolateral nucleus; VPM, ventral posteromedial nucleus. D, dorsal; L, lateral.
Examination of thalamic sections cut in coronal (Fig. 1A), horizontal (Fig. 1C) or parasagittal planes revealed that the KCC2-positive part of the nRt comprised the dorsal part of the nucleus throughout its anteroposterior extent. The anterior nRt, which envelops the anterior surface of the dorsal thalamus, was positive for KCC2 throughout its dorsoventral extent. In addition, weak KCC2 staining was also present in the caudal-most part of the nRt. The rest of the nucleus was free of labelling.
To verify the presence of KCC2 within the dorsal and anterior part of the nucleus, adjacent sections were immunoreacted for parvalbumin (Fig. 1D-G), a well-known marker of nRt neurones, and fluorescent double immunostainings were performed for KCC2 and parvalbumin (Fig. 2). Low power images of the double-immunostained material clearly demonstrated KCC2-immunoreactive structures in the dorsal part of the nRt, whereas the ventral part was free of labelling. Examination of individual dendrites in the head of the nRt with a high power fluorescent microscope demonstrated the colocalization of parvalbumin and KCC2 in the same dendritic profiles (Fig. 2). It should be noted that the somatic membranes of nRt cells were either not or only weakly labelled for KCC2.
Fig. 2.

Colocalization of KCl cotransporter type 2 (KCC2) and parvalbumin (PV) in the dorsal thalamic reticular nucleus (nRt). (A and B) High power fluorescent images of nRt neurones double immunostained for KCC2 and PV. Note the colocalization of the two markers in the dendrites (arrowheads) but the scarcity of the KCC2 signal in the PV-positive soma (s). Scale bars, 10 μm.
Calretinin-immunoreactive cells have been reported to be present selectively in the dorsal and anterior part of the nRt (Lizier et al., 1997). The similar distribution of calretinin and KCC2 prompted us to examine the codistribution of the two markers. In our fluorescent double-immunostained material, calretinin-positive cells formed a rim at the periphery of the nRt head as described previously (Lizier et al., 1997). In this part of the nRt, calretinin and KCC2 showed an overlapping distribution but KCC2 immunostaining was not confined to the periphery of the nucleus and was present in the entire width of the nRt (Fig. 3A-C). At the caudal part of the nRt, where calretinin-positive cells are scattered throughout the nucleus, the nRt also showed weak KCC2 immunostaining. Examination of individual dendrites at high power demonstrated the colocalization of KCC2 and calretinin in nRt dendrites at both the rostral and caudal levels (Fig. 3D-G). However, not all KCC2-positive nRt dendrites expressed calretinin.
Ultrastructural distribution
The KCC2 immunoreactivity was examined at the ultrastructural level in the ventral posteromedial nucleus and in the nRt using two chromogens. DAB was used to examine the general distribution of KCC2 and silver-intensified immunogold labelling was applied to examine the precise ultrastructural localization and the quantitative distribution of KCC2 protein.
For the identification of axon terminals in the ventral posteromedial nucleus, Guillery’s classification was used (Guillery, 1969a,b). Terminals establishing excitatory synapses belonged to either small (RS type) terminals, which are known to be mainly of cortical origin (Jones & Powell, 1969; Wong-Riley, 1972), or large (RL type) terminals. RL terminals may have a peripheral origin (Szentagothai, 1963; Colonnier & Guillery, 1964; (Ralston & Herman, 1969) or, in the case of higher order nuclei, may also originate in cortical layer V (Mathers, 1972; Hoogland et al., 1991). It should be noted that axons of brainstem cholinergic neurones are also known to have RS morphology in the thalamus (Erisir et al., 1997).
In the DAB-stained material of the ventral posterior nucleus KCC2 immunoreactivity was present exclusively postsynaptically (data not shown). Nearly all dendritic profiles displayed KCC2 immunoreactivity. Thin dendrites were entirely filled by the DAB precipitate whereas in the somata and thick dendrites of relay cells the immunoprecipitate was localized along the plasma membrane. In our material no KCC2 immunostaining was seen in either RL, RS or reticular (F) axon terminals.
The KCC2 immunogold staining demonstrated a highly specific staining pattern and replicated the results of the DAB material. Most gold particles were associated with the cytoplasmic side of plasma membranes of dendrites and somata, with the exception of a few which could be localized to inner membrane structures and may indicate proteins transported in the dendrites (Figs 4 and 5). Gold particles were often seen adjacent to asymmetrical synapses (Fig. 4C-D). Accumulation of gold particles near symmetrical synapses or synapses formed by primary sensory afferent terminals was not readily observed in the cases examined (Fig. 4A and B).
Fig. 4.

Ultrastructural localization of KCl cotransporter type 2 (KCC2) molecules in the ventrobasal complex. (A and B) High power electron micrographs showing thick proximal dendrites (d1) of a relay cell receiving inputs from primary afferents (RL) and from terminals showing the characteristics of the thalamic reticular nucleus input (F). Small calibre distal dendrites (d2-4) are contacted by corticothalamic terminals (asterisks). Silver-intensified gold particles (some of them labelled by arrowheads) on the intracellular surface of the membranes also indicate the location of KCC2 molecules in thick and thin dendrites. In these cases the distribution of the silver signal is rather homogeneous along the membranes. There is no apparent accumulation of gold particles in the vicinity of excitatory or inhibitory synapses. (C and D) In these examples of thin relay cell dendrites (d1-4) almost all asymmetrical synapses established by RS-type terminals (asterisks) are accompanied by silver deposits (small arrows) in a perisynaptic position. Arrowheads indicate extrasynaptic labelling. Scale bars, 1 μm (A-C); 0.5 μm (D).
Fig. 5.
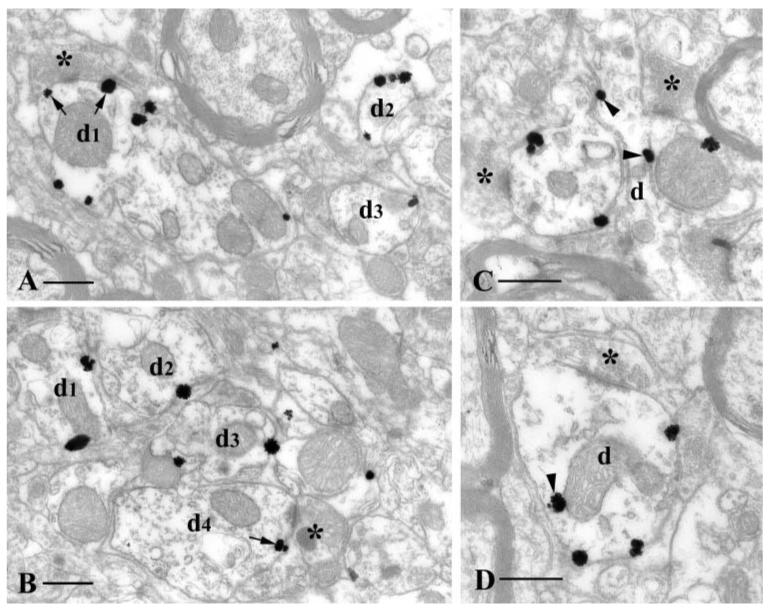
High power electron micrographs showing KCl cotransporter type 2 (KCC2) immunoreactivity in the dorsal thalamic reticular nucleus (nRt). (A-D) Silver-intensified gold particles demonstrate the membrane localization of the transporter on nRt dendrites (d1-d4). Some of the particles (small arrows in A and B) are localized near the asymmetrical synapses established by terminals of presumably cortical (asterisk in A, C and D) or thalamic (asterisk in B) origin. Arrowheads in C and D indicate KCC2 protein associated with intracellular membrane organelles. Scale bars, 0.5 μm (A-D).
In the KCC2-positive dorsal sector of the nRt the staining pattern was similar to that of the ventral posteromedial nucleus (Fig. 5). Labelling was seen exclusively on postsynaptic structures and never on axon terminals. Other parts of the nRt, in contrast, were almost entirely devoid of immunostained profiles.
Distribution of KCC2 in different soma-dendritic compartments
Relay cells are highly polarized. Somata, proximal and distal dendrites receive distinct afferents and have different surface : volume ratios (Steriade et al., 1997). In addition, proteins responsible for ion transport can be differentially distributed along the plasma membranes (Budde et al., 1998; Destexhe et al., 1998; Lorincz et al., 2002). This prompted us to examine the density of gold particles indicating KCC2 molecules in the somata and in five dendritic categories of relay cells: dendrites with minor diameter above 1 μm receiving mainly large excitatory terminals of primary sensory afferents and dendrites with minor diameter between 1 and 0.8, 0.8 and 0.6, 0.6 and 0.4 and below 0.4 μm receiving small excitatory terminals of RS type. In total 157 profiles were measured in the ventral posterior nucleus.
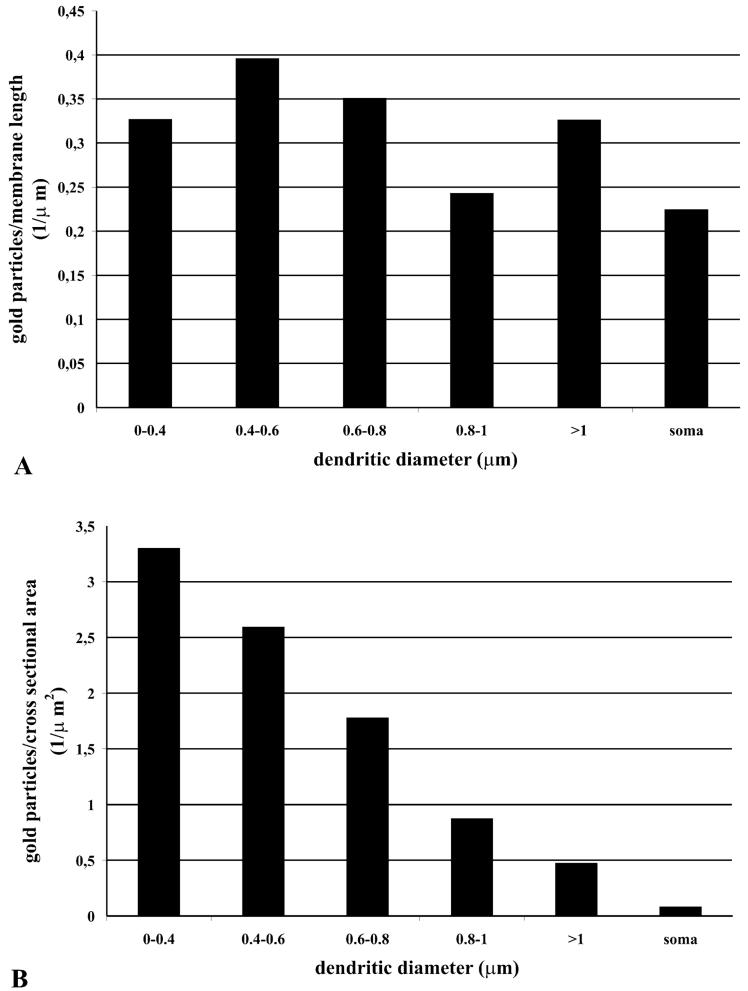
The density of KCC2 immunoreactivity was relatively even in the various soma-dendritic compartments. No clear preference or gradient of KCC2 molecules to any of these subcellular compartments was revealed (Fig. 6A). KCC2 density, as measured by gold particle/membrane length (see Materials and methods), was similar for the somata and the five dendritic categories. However, due to the different surface : volume ratio of thin dendrites, this results in a higher density of KCC2 in thinner dendrites if the same data are illustrated as gold particles/cross-sectional area, which reflects the volume of the postsynaptic elements (Fig. 6B).
Fig. 6.
Relationship between gold particle density indicating KCl cotransporter type 2 immunoreactivity and dendritic diameter. Bar graphs demonstrate the density of gold particles on different dendritic categories and on the somata of relay cells measured in single ultrathin sections in the ventrobasal complex. The density of gold particles relative to membrane area (A) is similar across all compartments. The density of gold particles relative to volume (B), however, shows a marked decrease from thin dendrites to soma. For the lack of error bars see Materials and methods.
Distribution of KCC2 in perisynaptic membrane surfaces
A recent study described strong KCC2 immunoreactivity in the spines and thorny excrescences of hippocampal pyramidal cells adjacent to asymmetrical synapses (Gulyas et al., 2001). In order to determine whether KCC2 has a preferential association to excitatory synapses in the thalamus, the gold particle density was measured in peri- and extrasynaptic areas of 1-μm-long distal dendritic segments of relay cells (n = 22) reconstructed from serial ultrathin sections in the ventral posterior nucleus. The perisynaptic area was defined as a 200-nm annulus around the synapse. The perisynaptic area defined in this way accounted for 15% of the total dendritic surface. Fifty-five synapses were encountered in our sample.
We included only RS type terminals in our analysis. Synapses formed by primary afferent terminals (RL) were not analysed because of the convoluted shape of the postsynaptic target, their low density and the apparent lack of accumulation of gold particles around these synapses. The number of RS type synapses ranged from one to six per dendritic segment in our sample. The summed gold particle density was 4.34 gold particles/μm2 in perisynaptic areas, whereas it was only 2.49 gold particles/μm2 in extrasynaptic membranes (Fig. 7). The distribution of gold particles in peri- and extrasynaptic areas differed significantly from a homogeneous distribution (Chi-square probe P < 0.01). Using our definition of perisynaptic area (see Materials and methods) the ratio of the total number of extrasynaptically vs. perisynaptically located gold particles was 3 : 1. Our conclusion is that the KCC2 protein has a certain preference for perisynaptic localization but a significant proportion is located in the extrasynaptic position.
Fig. 7.
Average gold particle density in perisynaptic areas (a 200-nm annulus around synaptic specializations) and extrasynaptic areas on the surface of reconstructed dendritic segments. Synapses in this analysis included asymmetrical membrane specializations established by RS terminals on distal relay cell dendrites. Perisynaptic membranes contain significantly more KCl cotransporter type 2 than extrasynaptic membranes. For the lack of error bars see Materials and methods.
KCC2 density does not correlate with synaptic coverage
In order to determine whether the KCC2 density on a given dendrite is correlated with the amount of synaptic input of the same dendrite, we reconstructed 47 dendritic segments from 15 ultrathin sections and examined the correlation of the synaptic coverage (summed synaptic area of each dendrite divided by the total dendritic surface) and gold particle density of the total dendritic surface. Synapses covered 1-8% of the total dendritic surface. Again our sample included small calibre dendrites where RS type terminals predominate. We found an eightfold difference in synaptic coverage and a fourfold difference in gold particle density among individual dendrites but no significant correlation was found between the two variables (Pearson R2 = 0.0095) (Fig. 8).
Fig. 8.
Scatter plots demonstrating the gold particle density as a function of synaptic coverage (synaptic areas/dendritic surface). Our sample included excitatory terminals established by small terminals. Each individual plot represents a relay cell dendritic segment reconstructed from 15 65-nm-thick ultrathin section in the ventrobasal complex. Both the synaptic coverage and the density of gold particles show high variability but there is no correlation between the two parameters.
Discussion
In the present study we found that all relay nuclei of the thalamus expressed KCC2 immunoreactivity, whereas the majority of the nRt lacked this chloride extrusion protein. However, unlike the rest of the nucleus, the rostral and dorsal parts of the nRt were positive for KCC2. Immunogold staining demonstrated that KCC2 is homogeneously distributed in different calibre somato-dendritic domains. KCC2 was more concentrated in the vicinity of excitatory synapses established by RS terminals, which are mainly of cortical layer VI origin. No correlation was found between the synaptic coverage and the density of KCC2 staining on individual dendrites.
Differential expression of KCC2 in relay and reticular neurones
Our results show that, while all relay nuclei express KCC2, the nRt is entirely devoid of this transporter, except for the rostral and dorsal part of the nucleus. Earlier work suggested that the expression of KCC2 protein has a strong influence on the GABA-A reversal potential. During development, an increasing KCC2 level has an important role in changing the originally depolarizing GABA-A response to a hyperpolarizing response (Rivera et al., 1999). Our anatomical data imply different responses to GABA in relay and reticular neurones. Indeed, it has been shown that the EGABA in relay cells is set more negative (−81 mV) than in reticular neurones (−71 mV) (Ulrich & Huguenard, 1997) via a furosemide-sensitive active chloride extrusion mechanism. Our data showing a lack of KCC2 in the majority of the nRt and strong expression of the cotransporter in relay cells are entirely consistent with these results and suggest that KCC2 is one of the major factors that establish the observed difference in GABA-A reversal potential between relay and reticular nuclei. This conclusion is also supported by the fact that KCC2 has been shown to be furosemide sensitive (Payne, 1997). A similar situation was observed in the retina where KCC2 is expressed in cells with EGABA-A more negative than the resting membrane potential, whereas another cationchloride cotransporter NKCC is expressed in those cells where EGABA-A is more positive than the resting membrane potential (Vardi et al., 2000). More recently, in the substantia nigra, a similar difference was observed between neurones of the pars reticulata and pars compacta (Gulacsi et al., 2003). KCC2-negative dopaminergic neurones in the pars compacta received significantly less negative GABA-A inhibitory postsynaptic potential than the KCC2-positive GABAergic cells in the pars reticulata. Other chloride transport mechanisms present in the dopaminergic neurones were not sufficient to reduce this difference, which further suggests that, regardless of the neuronal system studied, KCC2 has a major role in setting the GABA-A reversal potential.
The expression of KCC2 not only differed between reticular and relay nuclei but also various relay nuclei expressed different levels of KCC2. ECl measurements, however, have been performed only in first order somatosensory nuclei and thus the reason for this difference and its functional consequence are less clear. The more weakly stained higher order sensory nuclei receive different types of excitatory and inhibitory input from their first order counterparts (Sherman & Guillery, 1996; Bartho et al., 2002) and the ratio of various excitatory inputs is also dissimilar (Wang et al., 2003). This may result in different synaptic coverage and a difference in synapse-associated KCC2 expression.
Recently, besides the well-known nRt afferents, higher order thalamic nuclei have been shown to receive selective GABAergic input from the zona incerta (Bartho et al., 2002). Here we show that, unlike the nRt, the zona incerta shows very strong KCC2 immunostaining. These data suggest that a major difference is expected in local GABA-A-mediated events between the two nuclei supplying higher order nuclei with GABAergic inputs.
Heterogeneous KCC2 expression in the reticular nucleus
The rostral and dorsal parts of the nRt expressed KCC2 at a similar level to the relay nuclei but in sharp contrast to the rest of the nucleus. Based on these data we predict that the KCC2-positive rostrodorsal nRt will have an EGABA-A similar to relay cells and therefore GABA might have a direct hyperpolarizing effect instead of shunting. The morphological basis of inhibition within the nRt has been described previously (Yen et al., 1985; Pare & Steriade, 1993; Pinault et al., 1997; Landisman et al., 2002) and, in many experimental conditions, it seems to play a major role in shaping the synchrony of nRt activity. Reducing intra-nRt inhibition leads to prolonged nRt bursts (Bal & McCormick, 1996; von Krosigk et al., 1993; Huntsman et al., 1999) or to the increase of nRt synchrony (Sohal & Huguenard, 2003) which consequently elicits larger rebound bursts in relay cells. These events cause spike and wave discharges similar to absence epilepsy (DeLorey et al., 1998).
Unfortunately, no systematic studies have been performed within the nRt to discriminate the role of different nRt sectors in these events. Our data, however, strongly suggest that the polarity and magnitude of GABA-A responses can be significantly different in the KCC2-positive and -negative parts of the nucleus and will probably result in differences in burst pattern and synchrony. Indeed, recent data indicate that the polarity of evoked inhibitory postsynaptic potentials in nRt cells can change during different phases of cortical oscillation, demonstrating the importance of the GABA-A reversal potential in the nRt in vivo. Hyperpolarizing inhibitory postsynaptic potentials evoked during the depolarizing phase of the cortical slow oscillations are reversed during the opposite EEG phase and can trigger full-blown high frequency bursts in nRt cells (Bazhenov et al., 1999).
The KCC2-positive part of the nRt comprises the ‘limbic’ and ‘visual’ (Coleman & Mitrofanis, 1996) sectors of the nucleus. Many of the cells in this region are positive for the calcium-binding protein calretinin. In the dorsal part of the nRt calretinin-positive and -negative cells have been separated according to their projection pattern (Lizier et al., 1997). The colocalization of KCC2 and calretinin suggests that nRt neurones projecting to ipsilateral medial thalamic nuclei are among those in which the intracellular chloride concentration is lower than in the rest of the nucleus. The limbic sector of the nRt has been shown to project to anterior, intralaminar, midline, mediodorsal and certain higher order sensory nuclei (Steriade et al., 1984; Gonzalo-Ruiz & Lieberman, 1995; Lozsadi, 1995; Lizier et al., 1997) and also to contralateral relay and reticular nuclei (Raos & Bentivoglio, 1993). These nuclei are not directly involved in the classical relay of peripheral information but support cognitive thalamocortical functions including selective attention, memory formation or consciousness. Thus, if the presence of KCC2 induces different firing patterns in the rostral and dorsal nRt, it is expected to control relay cell activity involved in higher order cortical function.
KCC2 is distributed evenly in different subcellular compartments
If KCC2 only has a role in setting the chloride equilibrium, it is expected to concentrate in subcellular compartments where there is a strong chloride load, i.e. GABA-A receptor-mediated inhibition. This is not the case in the hippocampus, where KCC2 was found to accumulate near glutamatergic synapses on the dendritic spines of CA1 pyramidal cells and on the thorny excrescences of CA3 pyramidal cells (Gulyas et al., 2001). These sites are the loci of intense excitation. In addition, KCC2 was more strongly expressed in interneurone types receiving stronger excitatory input (Gulyas et al., 2001). These data were implicated to support the role of KCC2 in controlling the volume changes caused by the osmolyte load accompanying excitatory synaptic transmission. We reasoned that, if KCC2 has a role in volume regulation, it would display a higher density in small calibre dendrites where similar osmolyte entry would cause a proportionally larger effect. Our data, however, did not support this hypothesis. There was no correlation between synaptic coverage and KCC2 density and KCC2 was evenly distributed along the membranes of somatic and dendritic compartments with widely different diameters. Although, because of the different surface : volume ratio of small and large diameter compartments, this causes a larger KCC2/volume density in thin dendrites, we conclude that relay cells do not have specific mechanisms to accumulate KCC2 in small diameter compartments. We have also observed a higher KCC2 density in perisynaptic areas near excitatory synapses but this was not sufficient to produce a significant correlation between synaptic coverage and immunogold particle density, probably due to the abundant extrasynaptic KCC2 expression.
In summary, the accumulation of gold particles near excitatory synapses is less pronounced in the thalamus than in the hippocampus. Unlike in the hippocampus, where excitatory synapses are established exclusively on spines with restricted volumes, in the thalamus dendritic shafts are the prime target of excitatory corticothalamic terminals. Thus, our data do not directly rule out the role of KCC2 in osmolyte homeostasis coupled to excitatory synaptic transmission but suggest that the osmotic load may be better buffered in the larger compartments of dendritic shafts than in spines.
The only synapse type in relay cells that appears to be homogeneously distributed along proximal and distal dendrites is the GABAergic input from the nRt (Liu et al., 1995). Furthermore, the thalamus is enriched in GABA-A receptor subunits (delta and alpha4) which are characterized by high GABA affinity, slow desensitization and extrasynaptic localization (Pirker et al., 2000). In the cerebellum and the hippocampus these subunits have been shown to be activated by spillover mechanisms and apparently induce a significant tonic background GABA-A conductance in principal cells (Brickley et al., 1996; Nusser et al., 1998; Wei et al., 2003). Based on the receptor distribution, similar spillover mechanisms may operate in the thalamus, as also suggested by the abundant extrasynaptic expression of high affinity GABA-B receptors in relay cells (Kulik et al., 2002). Thus, the homogeneous distribution and abundant extrasynaptic KCC2 expression suggests that one of the main roles of KCC2 may be to lower chloride concentration along the entire somato-dendritic domain of relay cells to increase the driving force of hyperpolarizing GABA-A currents through both extrasynaptically and synaptically located GABA-A receptors.
Acknowledgements
We thank Katalin Lengyel, Erzsebet Oszwald and Gyõzõ Goda for technical assistance. This work was supported by the Hungarian Scientific Research Fund (OTKA F32327), Wellcome Trust and NIH (NS36296).
Abbreviations
- DAB
3,3′-diaminobenzidine
- KCC2
KCl cotransporter type 2
- nRt
thalamic reticular nucleus
- TBS
Tris-buffered saline
References
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur. J. Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Selfsustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat. Neurosci. 1999;2:168–174. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Munsch T, Pape HC. Distribution of L-type calcium channels in rat thalamic neurones. Eur. J. Neurosci. 1998;10:586–597. doi: 10.1046/j.1460-9568.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- Coleman KA, Mitrofanis J. Organization of the visual reticular thalamic nucleus of the rat. Eur. J. Neurosci. 1996;8:388–404. doi: 10.1111/j.1460-9568.1996.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Colonnier M, Guillery RW. Synaptic organization in the lateral geniculate nucleus of the monkey. Z. Zellforsch. Mikrosk. Anat. 1964;62:333–355. doi: 10.1007/BF00339284. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol. Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic lowthreshold calcium currents in thalamic relay cells. J. Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc. Natl Acad. Sci. U.S.A. 1997;94:1517–1520. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen CM, Brill S, Payne JA, Forbush B., 3rd Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996;271:16 237–16 244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Lieberman AR. GABAergic projections from the thalamic reticular nucleus to the anteroventral and anterodorsal thalamic nuclei of the rat. J. Chem. Neuroanat. 1995;9:165–174. doi: 10.1016/0891-0618(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Guillery RW. An abnormal retinogeniculate projection in Siamese cats. Brain Res. 1969a;14:739–741. doi: 10.1016/0006-8993(69)90213-3. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z. Zellforsch. Mikrosk. Anat. 1969b;96:1–38. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J. Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Sik A, Payne JA, Kaila K, Freund TF. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001;13:2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- Hoogland PV, Wouterlood FG, Welker E, Van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L) Exp. Brain Res. 1991;87:159–172. doi: 10.1007/BF00228517. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. An electron microscopic study of the mode of termination of cortico-thalamic fibres within the sensory relay nuclei of the thalamus. Proc. R. Soc. Lond. B Biol. Sci. 1969;172:173–185. doi: 10.1098/rspb.1969.0018. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur. J. Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Honda CN, Jones EG. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. J. Comp. Neurol. 1995;352:69–91. doi: 10.1002/cne.903520106. [DOI] [PubMed] [Google Scholar]
- Lizier C, Spreafico R, Battaglia G. Calretinin in the thalamic reticular nucleus of the rat: distribution and relationship with ipsilateral and contralateral efferents. J. Comp. Neurol. 1997;377:217–233. doi: 10.1002/(sici)1096-9861(19970113)377:2<217::aid-cne5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat. Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Lozsadi DA. Organization of connections between the thalamic reticular and the anterior thalamic nuclei in the rat. J. Comp. Neurol. 1995;358:233–246. doi: 10.1002/cne.903580206. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Mathers LH. The synaptic organization of the cortical projection to the pulvinar of the squirrel monkey. J. Comp. Neurol. 1972;146:43–60. doi: 10.1002/cne.901460104. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Steriade M. The reticular thalamic nucleus projects to the contralateral dorsal thalamus in macaque monkey. Neurosci. Lett. 1993;154:96–100. doi: 10.1016/0304-3940(93)90180-s. [DOI] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J. Biol. Chem. 1996;271:16 245–16 252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pinault D, Smith Y, Deschenes M. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J. Neurosci. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Ralston HJ, 3rd, Herman MM. The fine structure of neurons and synapses in ventrobasal thalamus of the cat. Brain Res. 1969;14:77–97. doi: 10.1016/0006-8993(69)90032-8. [DOI] [PubMed] [Google Scholar]
- Raos V, Bentivoglio M. Crosstalk between the two sides of the thalamus through the reticular nucleus: a retrograde and anterograde tracing study in the rat. J. Comp. Neurol. 1993;332:145–154. doi: 10.1002/cne.903320202. [DOI] [PubMed] [Google Scholar]
- Reid KH, Li GY, Payne RS, Schurr A, Cooper NG. The mRNA level of the potassium-chloride cotransporter KCC2 covaries with seizure susceptibility in inferior colliculus of the post-ischemic audiogenic seizure-prone rat. Neurosci. Lett. 2001;308:29–32. doi: 10.1016/s0304-3940(01)01973-5. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J. Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog. Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory interconnections control burst pattern and emergent network synchrony in reticular thalamus. J. Neurosci. 2003;23:8978–8988. doi: 10.1523/JNEUROSCI.23-26-08978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Steriade M, Parent A, Hada J. Thalamic projections of nucleus reticularis thalami of cat: a study using retrograde transport of horseradish peroxidase and fluorescent tracers. J. Comp. Neurol. 1984;229:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones E, McCormick D. Thalamus. Vol. 1. Elsevier Science; Oxford: 1997. [Google Scholar]
- Szentagothai J. The structure of the synapse in the lateral geniculate body. Acta Anat. (Basel) 1963;55:166–185. [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. Nucleus-specific chloride homeostasis in rat thalamus. J. Neurosci. 1997;17:2348–2354. doi: 10.1523/JNEUROSCI.17-07-02348.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J. Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Van Horn SC, Sherman SM. Synaptic distribution in the somatosensory thalamic nuclei of the cat. Soc. Neurosci. Abstr. 2003:699.20. Abstract viewer/Itinerary Planner. Washington, DC: Society for Neuroscience. [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10 650–10 661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J. Biol. Chem. 1999;274:12 656–12 664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT. Neuronal and synaptic organization of the normal dorsal lateral geniculate nucleus of the squirrel monkey, Saimiri sciureus. J. Comp. Neurol. 1972;144:25–59. doi: 10.1002/cne.901440103. [DOI] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB, Deutch AY, Lovinger DM, Delpire E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- Yen CT, Conley M, Hendry SH, Jones EG. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J. Neurosci. 1985;5:2254–2268. doi: 10.1523/JNEUROSCI.05-08-02254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]