Abstract
Exogenous neurotrophins (NTs) have been shown to rescue spiral ganglion neurons (SGNs) from degeneration following a sensorineural hearing loss (SNHL). Furthermore, chronic electrical stimulation (ES) has been shown to retard SGN degeneration in some studies but not others. Since there is evidence of even greater SGN rescue when NT administration is combined with ES, we examined whether chronic ES can maintain SGN survival long after cessation of NT delivery. Young adult guinea pigs were profoundly deafened using ototoxic drugs; five days later they were unilaterally implanted with an electrode array and drug delivery system. Brain derived neurotrophic factor (BDNF) was continuously delivered to the scala tympani over a four week period while the animal simultaneously received ES via bipolar electrodes in the basal turn (i.e. turn 1) scala tympani. One cohort (n=5) received ES for six weeks (i.e. including a two week period after the cessation of BDNF delivery; ES6); a second cohort (n=5) received ES for 10 weeks (i.e. a six week period following cessation of BDNF delivery; ES10). The cochleae were harvested for histology and SGN density determined for each cochlear turn for comparison with normal hearing controls (n=4). The withdrawal of BDNF resulted in a rapid loss of SGNs in turns 2–4 of the deafened/BDNF-treated cochleae; this was significant as early as two weeks following removal of the NT when compared with normal controls (p<0.05). Importantly, there was not a significant reduction in SGNs in turn 1 (i.e. adjacent to the electrode array) two and six weeks after NT removal, as compared with normal controls. This result suggests that chronic ES can prevent the rapid loss of SGNs that occurs after the withdrawal of exogenous NTs. Implications for the clinical delivery of NTs are discussed.
Keywords: neural degeneration, neurotrophin, deafness, electrical stimulation, cochlear implant, neural prostheses
Introduction
Deafness is one of the most common disabilities in society. In the majority of cases this occurs as a result of loss of sensory hair cells leading to a permanent sensorineural hearing loss (SNHL). Loss of hair cells sets in place a gradual but ongoing degeneration of spiral ganglion cells (SGNs), the primary afferent neurons of the cochlea (Gillespie et al., 2004; Hardie et al., 1999; Heid et al., 1998; Leake et al., 1988; Liberman et al., 1978; Mair, 1973; McGuinness et al., 2005; Ryugo et al., 1998; Shepherd et al., 1997; Shepherd et al., 2004; Shepherd et al., 2005; Steel et al., 1984; Takeno et al., 1998). SGNs are the target neurons for cochlear implants; the functional integrity of these neurons is therefore important for the success of these neural prostheses.
The removal of afferent input to SGNs removes both the neural activity and neurotrophin support that is normally supplied by hair cells, leading to cell death via apoptosis (Alam et al., 2007; Ladrech et al., 2004; Scarpidis et al., 2003). It is therefore not surprising that attempts to rescue SGNs centre on the use of electrical stimulation (ES) to induce neural activity in these neurons and/or the application of exogenous neurotrophins (NT).
In vitro studies have demonstrated that prolonged periods of depolarization of deafferented SGN cultures via elevated levels of extracellular potassium promotes neural survival via the activation of L-type voltage gated calcium channels (Hegarty et al., 1997; Miller et al., 2003). The resultant elevated intracellular calcium levels activate a number of down-stream pro-survival signalling pathways including cyclic AMP protein kinase, and calcium/calmodulin-dependent kinases II and IV (Bok et al., 2003; Hansen et al., 2001a; Roehm et al., 2005).
The pro-survival effects of chronic ES of SGNs remain less clear in vivo. Using a variety of animal models, a number of studies have demonstrated rescue effects associated with ES (Hartshorn et al., 1991; Kanzaki et al., 2002; Leake et al., 1999; Leake et al., 1991; Leake et al., 1992; Leake et al., 1995; Lousteau, 1987; Miller et al., 1995; Mitchell et al., 1997). In contrast, other studies report no trophic effects associated with ES per se (Araki et al., 1998; Coco et al., 2006; Li et al., 1999; Shepherd et al., 1994; Shepherd et al., 2005; Widijaja et al., 2006); these findings are consistent with a recent temporal bone study of 11 cochlear implant patients that showed no evidence of enhanced SGN survival in the implanted ear compared to the contralateral deafened control ear (Khan et al., 2005).
A number of growth factor families have been shown to play important roles in both the development and maintenance of SGNs (Fritzsch et al., 1999; Rubel et al., 2002). Both pre-synaptic hair cells and support cells within the organ of Corti, and post-synaptic neurons within the cochlear nucleus are necessary for SGN survival, reflecting complementary neurotrophic support from both sources (Hafidi, 1999; Lefebvre et al., 1992b; Lefebvre et al., 1994; Schecterson et al., 1994; Stankovic et al., 2004; Tan et al., 2006; Tierney et al., 2001; Ylikoski et al., 1993). These NTs include both brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3; (Lefebvre et al., 1992a; Schecterson et al., 1994; Tan et al., 2006; Ylikoski et al., 1993), with receptors for both of these NTs expressed on SGNs (Schecterson et al., 1994; Tan et al., 2006; Ylikoski et al., 1993).
The long-term delivery of exogenous NTs into the cochlea promotes a significant increase in SGN survival following SNHL. Unlike the in vivo ES studies described above, the trophic effects of NT delivery on SGNs have been highly significant and universally observed across studies. The majority of these studies delivered BDNF and/or NT-3 to deafened guinea pig cochleae over treatment periods of 2–8 weeks via an osmotic pump (Ernfors et al., 1996; Gillespie et al., 2004; Miller et al., 1997; Richardson et al., 2005; Staecker et al., 1996; Wise et al., 2005; Yamagata et al., 2004), although NT released from alginate beads placed on the round window have also been effective (Noushi et al., 2005). In addition, nerve growth factor (Gillespie et al., 2004; Schindler et al., 1995; Shah et al., 1995), glial cell line-derived neurotrophic factor (Kanzaki et al., 2002; Yagi et al., 2000; Ylikoski et al., 1998) and neurotrophin-4/5 (Gillespie et al., 2004) delivered alone; or ciliary-derived neurotrophic factor (Miller et al., 1997) or fibroblast growth factor (Miller et al., 2007) delivered in combination with other NTs, have also demonstrated SGN rescue in vivo. These robust findings have also been reported following NT delivery using viral vectors (Kanzaki et al., 2002; Staecker et al., 1998), and in species other than the guinea pig (McGuinness et al., 2005; Staecker et al., 1998). Pettingill et al., (2007) provides a detailed overview of these studies. Finally, in contrast to long-term NT delivery, a single infusion of NT directly into the scala tympani does not appear to promote long-term SGN survival (Richardson et al., 2005).
Significantly, in vitro studies have demonstrated that the trophic support of SGNs via depolarization appears to be additive with the actions of some NTs (Hansen et al., 2001a). This research has been extended to in vivo studies, demonstrating enhanced SGN survival in deafened cochleae treated with both exogenous NTs and ES (Kanzaki et al., 2002; Shepherd et al., 2005). Moreover, this work has also shown a functional advantage, in the form of significantly reduced electrically evoked auditory brainstem response (EABR) thresholds in ears treated with NTs (Shepherd et al., 2005; Shinohara et al., 2002), that may be associated with a NT mediated growth of SGN peripheral processes towards the scala tympani (Miller et al., 2007; Staecker et al., 1996; Wise et al., 2005) or reflect the increased diameter of NT treated SGNs (McGuinness et al., 2005; Shepherd et al., 2005), as threshold to ES decreases with increasing neuron size (Grill, 2004).
The duration of the exogenous NT supply is a key issue that impacts on the safe clinical delivery of these drugs. Although there is evidence that SGN survival is maintained well after cessation of NT administration (Miller et al., 2006), other research has demonstrated that the withdrawal of NT support leads to an accelerated loss of SGNs (Gillespie et al., 2003) - a finding that implies that NTs must be supplied continuously. Given the positive results of combining ES and NT delivery, we have examined whether chronic intracochlear ES can provide trophic support to SGNs long after removal of the source of exogenous NTs.
Material and Methods
Experimental subjects
Twelve healthy young pigmented guinea pigs weighing between 400–844 g (mean 569 ±133g) were used in the present study under approval of the Royal Victorian Eye and Ear Hospital’s Animal Research and Ethics Committee, and conformed to the guidelines of the National Health and Medical Research Council of Australia.
All animals had otoscopically normal tympanic membranes and normal hearing determined by click-evoked auditory brainstem response (ABR) thresholds of < 43 dB peak equivalent sound pressure level re 20 µPa (p.e. SPL; Hardie et al., 1999). Guinea pigs were systemically deafened using a single co-administration of kanamycin (400 mg/kg subcutaneously [sc]) and frusemide (100 mg/kg intravenously [iv]; Gillespie et al., 2003). Only animals exhibiting a severe-profound SNHL (i.e. ABR click thresholds >93 dB p.e. SPL in both ears) were used in this study. The animals were divided into two treatment groups. Both groups received BDNF over a four week period in combination with chronic ES delivered for either six (ES6; n=5) or 10 weeks (ES10; n=5; Table 1). The remaining animals served as normal hearing controls (n= 2; i.e. n=4 cochleae).
Table 1.
Summary of treatment groups
| Treatment group | Total implant duration (weeks) | Duration of BDNF treatment (weeks) | Duration of ES alone (weeks) |
|---|---|---|---|
| ES6 | 6 | 4 | 2 |
| ES10 | 10 | 4 | 6 |
Notes: 62.5 µg of BDNF/ml in 0.1% guinea pig albumin in 200 µl of Ringer’s solution.
Electrode array and delivery techniques
The electrode array consisted of three platinum (Pt) band electrodes on a 0.6 mm diameter silicone carrier. Each Pt electrode was connected to a stainless-steel leadwire system via a 25 µm diameter platinum/iridium (90/10) wire (Shepherd et al., 2002). A Pt marker was located 5 mm from the tip of the array as a guide to insertion depth. A 0.75 mm internal diameter (ID) polyvinyl chloride tube connected a mini-osmotic pump (Alzet 2004) to a 0.124 mm ID delivery-tube located within the central core of the electrode array. The contents of the osmotic pump were delivered to the scala tympani of the cochlea through a lumen in the delivery-tube at the tip of the array (Shepherd et al., 2002).
Preparation of the osmotic pump
The Alzet 2004 mini-osmotic pump has a reservoir capacity of 200 µl and a flow rate of 0.25 µl/hour, providing a continuous infusion period of 28 days. Under sterile conditions pumps were loaded with recombinant human BDNF (PeproTech) containing 0.1% guinea pig albumin in 200 µl of Ringer’s solution giving a BDNF concentration of 62.5 µg/ml. After allowing for 20% absorption by the pump and delivery cannula this effectively delivers 10 µg of BDNF per cochlea (Gillespie et al., 2003). The loaded pumps were incubated in sterile Ringer’s solution for 36–48 h at 37°C prior to surgery in accordance with the manufacturers’ specifications.
Surgical details
Five days following deafening each animal was anaesthetized with ketamine (60 mg/kg intramuscularly [im]) and xylazine (4 mg/kg im), and prepared for surgery. ABRs were recorded to confirm that the hearing loss was severe to profound. Surgery was performed under aseptic conditions. Supplemental doses of anaesthesia were administered during surgery at a level sufficient to maintain the animal in an areflexic state. Carprofen (4 mg/kg sc) and Baytril (10 mg/kg sc) were administered to provide long-term analgesia and broad-spectrum antibiotic cover respectively. A dorsal approach was used to expose the left cochlea of each animal. The round window membrane was incised and the electrode array was inserted ~4.5 mm into the scala tympani. The round window was sealed with muscle, the leadwire proximal to the electrode array was fixed using dental cement (Durelon), and the distal leadwire exited the skin via a small incision in the neck. The osmotic pump was connected to the delivery-tube and implanted into a subcutaneous pocket between the scapulae (Brown et al., 1993) and the wounds were sutured. Each animal was given 10 ml of Hartmann’s solution sc. During surgery the animal’s temperature was maintained at 37°C using a heating pad. No surgery was performed on the right cochlea of each animal; these cochleae served as deafened, untreated controls.
Electrically-evoked ABRs and chronic electrical stimulation
Immediately following implant surgery, EABRs were recorded in response to 100 µs/phase biphasic current pulses delivered to the bipolar electrode pair. Recording techniques were similar to those used to evoke ABRs with the addition of a sample- and- hold artefact suppressor designed to block the stimulus artefact (Shepherd et al., 2005). Threshold was defined as the smallest current level required to evoke a peak-trough response amplitude of >0.25 µV for wave III of the EABR, i.e. within a latency window of 1.5–2.5 ms following stimulus onset for both responses. During recording the animal’s temperature was maintained at 37°C using a heating pad.
Five days following implant surgery both groups commenced a chronic ES program using programmable current source stimulators. The output of the stimulator delivered 100 µs/phase charge balanced biphasic current pulses to a bipolar electrode pair at a stimulus rate of 1200 pulses per second (pps) and was amplitude-modulated to a depth of 50% at 30 Hertz. Electrode shorting and capacitive coupling were used to ensure complete charge recovery (Huang et al., 1999). The amplitude of the stimulus waveform was set so that the minimum current level equalled the post-operative EABR threshold (i.e. maximum stimulus intensity 6 dB above EABR threshold). EABR thresholds were monitored each month and if necessary stimulator thresholds were adjusted accordingly. All animals in the ES groups received 6 h of stimulation per day, five days per week for a period of six (ES6) or 10 weeks (ES10) following implantation beginning five days following implantation resulting in total stimulation times of 150 and 285 hours respectively.
Cochlear histology
Each animal was euthanized with an overdose of anaesthetic (150 mg/kg sodium pentobarbital; intraperitoneal) and systemically perfused with heparinized normal saline at 37°C followed by phosphate buffered 4% paraformaldehyde at 4°C. The insertion depth of the electrode array was determined, the drug delivery system was checked for its patency, the presence of leaks and an intact connection to the osmotic pump, and the pump was examined for evidence of residual fluid (Shepherd et al., 2005).
Cochleae were decalcified, dehydrated, embedded in resin and serially sectioned at 2 µm. Sections every 126 µm were stained with haematoxylin and eosin. SGN densities for each of the four cochlear turns (basal turn T1; T2; T3; and apical turn T4) were measured from five representative mid-modiolar sections. Each cochlear turn was identified and the cross-sectional area of Rosenthal’s canal within each turn was measured using NIH Image. All neurons with a clear nucleus were then counted in each turn and SGN density (cells/mm2) calculated. The SGN soma area measurements were recorded from the same five sections. In this case the soma area of cells clearly exhibiting a nucleolus was measured using NIH Image. SGN density and soma area measurements were made from the treated (left) and the deafened untreated (right) cochlea of each animal, as well as the cochleae obtained from the two normal control animals. A single observer performed all measurements blind. Results were expressed as mean ± standard error of the mean (SEM) and statistical analysis was performed using a Kruskal- Wallis one-way ANOVA followed by a Dunn’s pairwise multiple comparison test. A difference was considered statistically significant at p<0.05.
Results
Status of the electrode arrays and drug delivery system
At post mortem examination all 10 chronically implanted electrode arrays were confirmed to be located within the scala tympani, the drug delivery system was functioning normally and there was no residual fluid detected in any osmotic pump.
Chronic depolarization prolongs the trophic effects on SGNs following the withdrawal of BDNF
Low power micrographs of the first three turns of both an ES6 and ES10 treated cochleae compared with their deafened untreated contralateral controls (ES6c and ES10c, respectively) are illustrated in Figure 1. A normal hearing control cochlea (control) is included for comparison. There are a number of histological features of note. First, with one important exception, there was extensive loss of SGNs throughout all three cochlear turns of both the BDNF/ES treated cochleae and the unimplanted, deafened controls. The exception to this trend was the increased survival evident in turn 1 of both the ES6 and ES10 cohorts as well as turn 2 of the ES6 cohort. This increase in survival, which was observed close to the stimulating electrode array located in the scala tympani, is illustrated at higher magnification in Fig. 2. A fine fibrous tissue capsule was evident in turn 1 of the treated cochlea, illustrating the location of the electrode array (*; Fig. 1).
Figure 1.
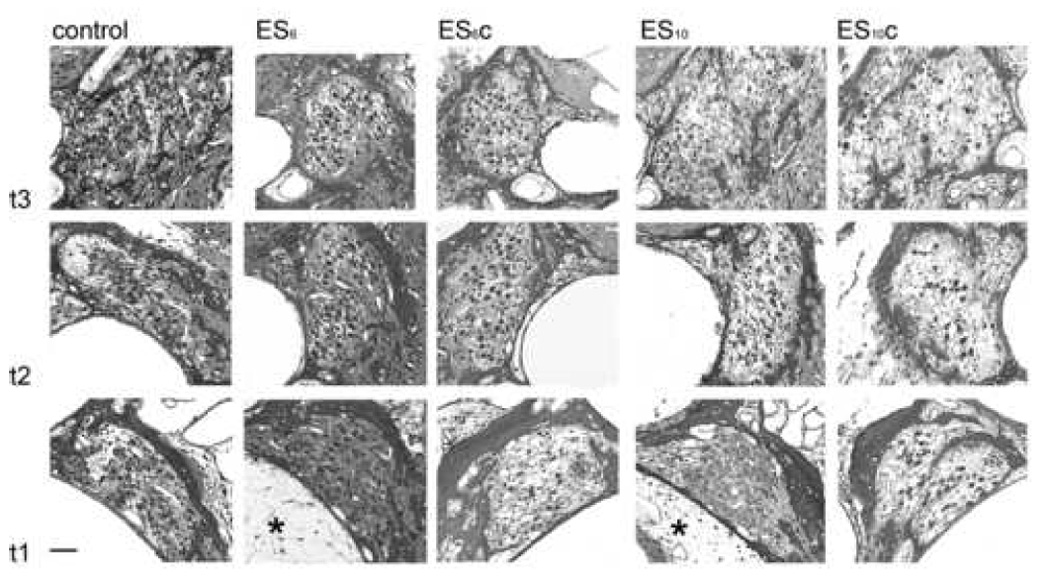
Representative photomicrographs of turns 1, 2 and 3 of a normal control, an ES6 cochlea and its contralateral control (ES6c), and an ES10 cochleae and its contralateral control (ES10c), showing the localized trophic effects of continued ES in turn 1 of the stimulated side (*), compared to the more severe loss of SGNs in higher turns. Scale bar = 40µm.
Figure 2.
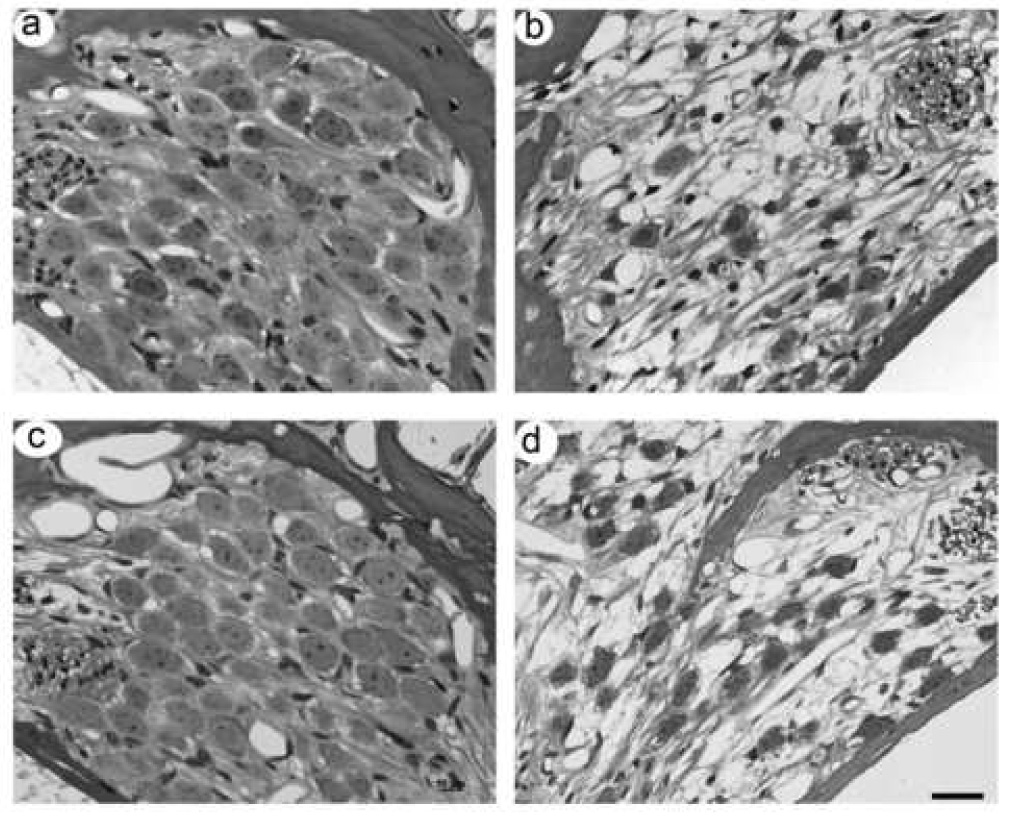
High power photomicrographs of turn 1 Rosenthal’s canal from the stimulated and deafened control cochleae of an animal from the ES6 cohort (a and b, respectively) and the ES10 cohort (c and d, respectively). SGNs in turn 1 of the stimulated cochleae (a and c) were adjacent to the scala tympani electrode array. SGN density in turn 1 of the treated cochleae were at or near normal levels and their mean soma area slightly greater than normal despite the cessation of BDNF two (a) or six (b) weeks prior to sacrifice. In contrast, there was a significant reduction in the number of surviving SGNs in the untreated deafened controls (c and d). Surviving SGNs in these cochleae exhibited significant shrinkage. Scale bar = 20 µm.
Figure 3 illustrates the mean SGN density for the ES6 and ES10 cohorts and their respective deafened controls expressed as a percentage of normal cochleae. For comparison, we have also included our previously published data illustrating SGN densities in deafened cochleae following four weeks of concurrent BDNF and ES treatment (Fig 3; Shepherd et al., 2005). The withdrawal of BDNF after four weeks resulted in a loss of SGNs in all turns of the treated cochleae. Significantly, the rate of SGN loss appeared greater in turns 2–4 than the natural rate of SGN degeneration observed following a SNHL (compare gradients of graphs for treated and deafened control data; Fig. 3). Moreover, the rate of SGN degeneration following cessation of BDNF increased with increasing distance from turn 1. While there was a significant difference in SGN density between the treated and deafened control cochleae at both six and 10 weeks (i.e. two and six weeks following cessation of BDNF delivery) in turn 1 and – to a lesser extent - turn 2, the rapid loss of SGNs in turns 3 and 4 resulted in no statistically significant difference between treated and deafened control cochleae as early as two weeks following completion of the BDNF delivery in these turns (Table 2).
Figure 3.
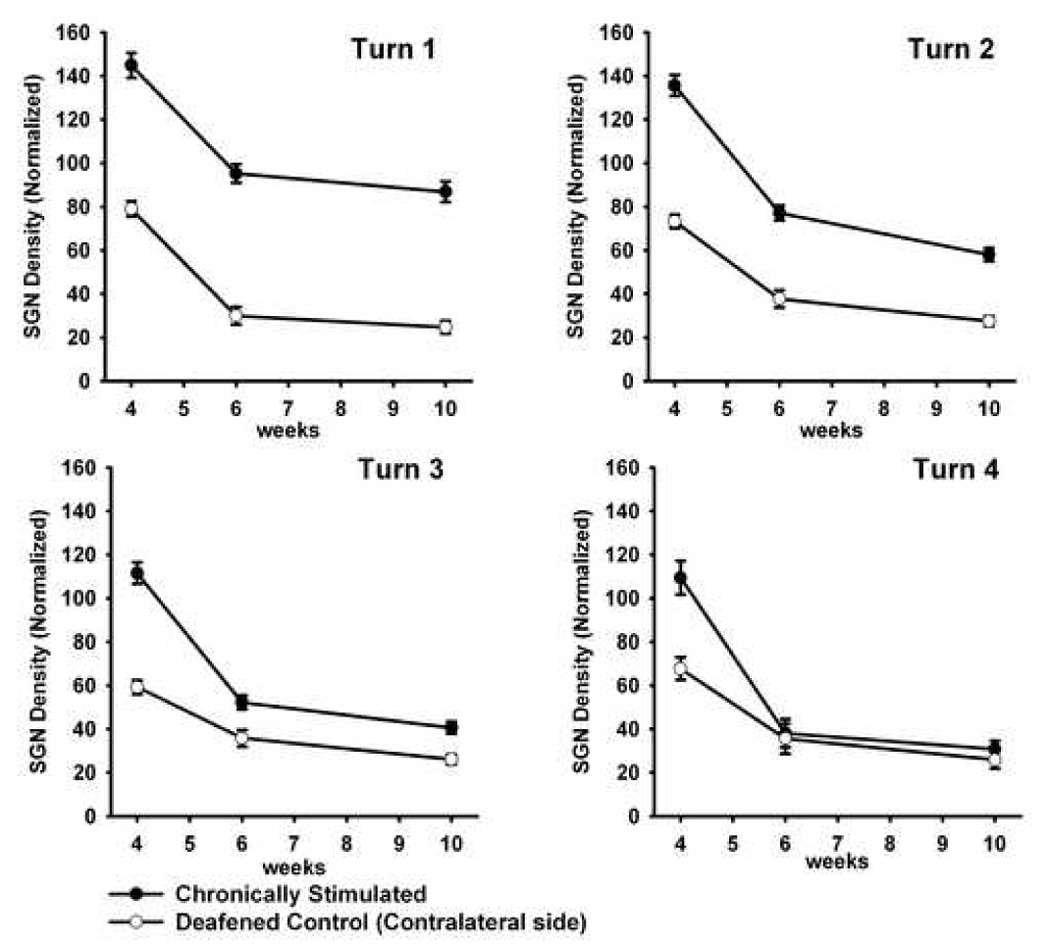
Mean SGN density (± SEM) of ES6 and ES10. These data are illustrated from turn 1 to turn 4 and show a reduction in SGN density in all turns following the cessation of exogenous BDNF (ie. from weeks four to six). Data are normalized to normal hearing controls. For comparison, these data are also compared with our previously published data illustrating SGN densities in deafened cochleae following four weeks of concurrent BDNF and ES treatment (Shepherd et al., 2005).
Table 2.
Summary of statistical comparison between treated cochleae and deafened controls using Dunn’s pairwise comparison: SGN density
| Cochlear turn | 6 weeks: | 10 weeks: | ||
|---|---|---|---|---|
| Q | P | Q | P | |
| T1 | 14.9 | <0.05 | 9.7 | <0.05 |
| T2 | 9.0 | <0.05 | 7.0 | <0.05 |
| T3 | 2.1 | ns | 1.2 | ns |
| T4 | 0.4 | ns | 0.8 | ns |
The far more gradual rate of SGN degeneration evident in turn 1, compared with more apical regions of the treated cochleae, are further emphasized when they are compared with normal hearing controls (Table 3). While there was a reduction in SGN density in turn 1 (i.e. adjacent to the electrode array) after NT removal, this reduction was not significant compared with normal control cochleae for both ES6 and ES10 cohorts. Although the reduction in SGNs in turn 2 of the ES6 cohort was also not significant, turn 2 of the ES10 cohort and turns 3 and 4 of both cohorts exhibited significant reductions in SGN density.
Table 3.
Summary of statistical comparison between treated cochleae and normal hearing controls using Dunn’s pairwise comparison: SGN density
| Cochlear turn | 6 weeks: | 10 weeks: | ||
|---|---|---|---|---|
| Q | P | Q | P | |
| T1 | 1.2 | ns | 1.2 | ns |
| T2 | 2.7 | ns | 5.1 | <0.05 |
| T3 | 4.5 | <0.05 | 6.4 | <0.05 |
| T4 | 3.7 | <0.05 | 4.2 | <0.05 |
Figure 4 illustrates the mean soma area for the ES6 and ES10 cohorts and their respective deafened controls, expressed as a percentage of normal cochleae. These data are also compared with our previously published data illustrating soma area of SGNs in deafened cochleae following four weeks of concurrent BDNF and ES treatment (Fig 4; Shepherd et al., 2005). The cessation of BDNF delivery after four weeks resulted in a dramatic reduction in soma area of the SGNs in all turns, however soma area remained greater than normal in turn 1 (p<0.05; Fig. 4). Again in turns 2–4, the rate of SGN shrinkage appeared greater than the natural rate observed following a SNHL (compare gradients of graphs for treated and deafened control data; Fig. 4). While there was a significant difference in SGN soma area between treated and deafened control cochleae at both six and 10 weeks (i.e. two and six weeks following cessation of BDNF delivery) in turn 1 and 2, the rapid reduction in SGN soma area in turns 3 and 4 resulted in no statistically significant difference between treated and control cochleae as early as two weeks following cessation of BDNF delivery in these turns (Table 4).
Figure 4.
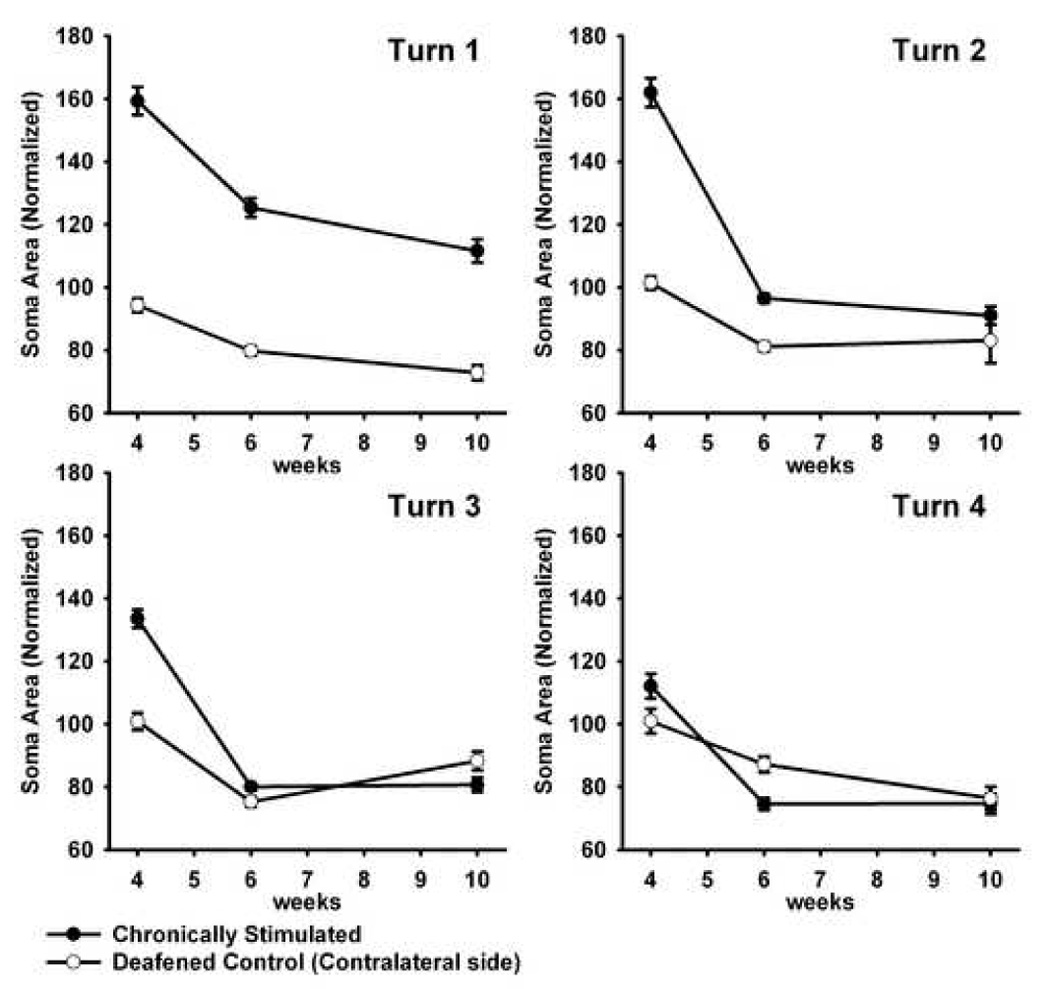
Mean SGN soma area (± SEM) of ES6 and ES10. These data are illustrated from turn 1 to turn 4 and illustrate a reduced soma area of the SGNs in all turns following the cessation of exogenous BDNF (ie. from weeks four to six). In turn 1 the soma area remained greater than normal. Data are normalized to normal hearing controls. For comparison, these data are also compared with our previously published data illustrating SGN soma areas following four weeks of concurrent BDNF and ES treatment (Shepherd et al., 2005).
Table 4.
Summary of statistical comparison between treated and deafened controls using Dunn’s pairwise comparison: SGN soma area.
| Cochlear turn | 6 weeks: | 10 weeks: | ||
|---|---|---|---|---|
| Q | P | Q | P | |
| T1 | 14.9 | <0.05 | 9.7 | <0.05 |
| T2 | 9.0 | <0.05 | 7.0 | <0.05 |
| T3 | 2.1 | ns | 1.2 | ns |
| T4 | 4.1 | <0.05 | 1.8 | ns |
Discussion
The present results demonstrate that the withdrawal of exogenous NT support to SGNs in deafened cochleae results in a rapid loss of SGNs; a finding consistent with a previous study from our laboratory (Gillespie et al., 2003). It must be noted, however, that these effects of neurotrophin withdrawal have not been consistent across laboratories (e.g. Miller et al., 2006). Importantly, chronic ES - continuing after cessation of the NT delivery – significantly reduced the rate of SGN loss although this effect was localized to the region of the cochleae proximal to the electrode array (i.e. T1). Although SGN density in T2 of the ES6 cohort (i.e. 2 weeks after cessation of BDNF) had not reduced to levels significantly less than normal control cochleae, the ES10 cohort (6 weeks after cessation of BDNF) exhibited a significant SGN loss in T2 compared with normal controls. Importantly, ongoing ES also maintained SGN soma area at near normal levels for SGNs adjacent to the electrode array, in contrast to more distal SGNs that exhibited rapid neuronal shrinkage following the withdrawal of NT support. These novel findings have important implications for the application of NTs in a clinical setting.
The most marked evidence of a trophic effect of electrical stimulation on SGNs following the withdrawal of NT support occurred in the basal turn of the cochlea, adjacent to the bipolar electrode array. Given the relatively localized current distribution for this electrode geometry (Black et al., 1980; Brown et al., 1992; Snyder et al., 2004; van den Honert et al., 1987), this finding implies that the observed changes were a result of the depolarization of SGNs rather than a more widespread effect of ES on the neural population that has been reported by others (e.g. Mitchell et al., 1997). While we have not observed evidence of SGN rescue following chronic ES alone (Araki et al., 1998; Coco et al., 2007; Shepherd et al., 1994; Shepherd et al., 2005), ES has been shown to retard the degeneration of SGNs in other studies (Hartshorn et al., 1991; Kanzaki et al., 2002; Leake et al., 1999; Leake et al., 1991; Leake et al., 1992; Leake et al., 1995; Lousteau, 1987; Miller et al., 1995; Mitchell et al., 1997). Preliminary studies from our laboratory have shown increased expression levels of the NT receptor Trk B in the lower basal turn of SGNs from deafened cochleae subject to chronic ES compared with deafened controls (Widijaja et al., 2006), a finding that has also been observed in the central nervous system (Du et al., 2000; Meyer-Franke et al., 1998). An ES induced increase in TrkB expression in SGNs would support the observed enhancement of SGNs in NT treated cochleae subject to ES and may contribute to the ongoing maintenance of SGNs following the NT withdrawal. Interestingly, the systemic application of the GM1 ganglioside - which is known to up-regulate TrkB signalling (Rabin et al., 2002) - has also been shown to rescue SGNs following SNHL (Leake et al., 2007). Moreover, this study demonstrated that GM1 and ES resulted in an additive rescue effect on SGNs, implying that two complementary mechanisms were associated with this response. Of particular relevance to the present study, ES was shown to maintain a modest trophic effect on SGNs following the withdrawal of the ganglioside (Leake et al., 2007).
There are a number of potential sources of endogenous NT within the deafened cochlea (i.e. cochleae devoid of an organ of Corti) following the cessation of exogenous sources. First, a number of NTs, including BDNF and NT-3, are known to be widely distributed in the cochlear nucleus (Hafidi, 1999; Lefebvre et al., 1994; Tierney et al., 2001) and are thought to exert a trophic influence on SGNs (Lefebvre et al., 1994). Second, neural activity – including patterned ES - is known to up-regulate the secretion of endogenous BDNF in various neurons by regulating the transcription of the BDNF gene (Balkowiec et al., 2000; Balkowiec et al., 2002; Gartner et al., 2002; Hansen et al., 2001a; Lever et al., 2001; Lu, 2003; Nanda et al., 2000; Rocamora et al., 1996; Tan et al., In Press). Thus chronic intracochlear ES could be expected to increase endogenous levels of BDNF in SGNs; this mechanism may contribute to the reduced SGN degeneration following ES reported in some studies. Finally, Schwann cells are known to produce NTs (Hansen et al., 2001b; Mirsky et al., 1999), prompting a number of authors to suggest the possibility of reciprocal neuron-glial interactions in the auditory nerve resulting in SGN rescue (Hansen et al., 2001b; Lefebvre et al., 1992b). Both in vitro and in vivo studies have provided evidence that Schwann cells can promote SGN survival (Andrew, 2003; Whitlon et al., 2003). Importantly, the ability of Schwann cells to respond to neurotrophins (Mirsky et al., 1999), their long-term survival following SNHL (Hurley et al., 2007), and apparent proliferation following exogenous neurotrophin infusion (Wise et al., 2005), suggest that Schwann cells may be well placed to stimulate and / or enhance any reparative response within the deafened cochlea following NT administration and chronic ES.
The accelerated loss of SGNs following cessation of NT delivery observed in turns 3–4 of the ES6 cohort and turns 2–4 of the ES10 cohort were consistent with the findings of Gillespie et al., (2003) who demonstrated SGN losses of ~60% over a 2 week period. Although there is evidence that SGN survival is maintained well after cessation of NT administration (Miller et al., 2006), reports of accelerated neural loss occurring following NT withdrawal has also been described in other neural systems. For example, short-term administration of nerve growth factor is not sufficient to permanently rescue cholinergic neurons following a lesion of the septohippocampal pathway (Montero et al., 1988). In addition, although BDNF treatment supported the survival of retinal ganglion cells following the transection of the optic nerve, most of these ganglion cells degenerated soon after BDNF administration ceased (Mansour-Robaey et al., 1994).
Shrinkage of SGN soma following SNHL observed in the present study, has also previously been reported in other deafness models (Araki et al., 1998; Elverland et al., 1980; Leake et al., 1988; Leake et al., 1999; Shepherd et al., 1997). Neuronal shrinkage presumably reflects a down regulation in biosynthetic activity of the SGNs following a SNHL. For example, the activity driven metabolic needs of these cells is related to the re-establishment of the resting membrane potential following action potential propagation (Kadekaro et al., 1985). Such high metabolic requirements would be greatly diminished as a result of the significant reduction in both driven and spontaneous activity that occurs following deafness (Hartmann et al., 1984; Liberman et al., 1978; Shepherd et al., 1997). To this end, studies of chronic ES of the SGNs in deafened subjects have reported small but significant increases in SGN soma area in the stimulated cochlea (Araki et al., 1998; Coco et al., 2007; Leake et al., 1999). Our results, illustrating the maintenance of SGN soma area localized to neurons close to the stimulating electrode array, are consistent with these results. Finally, in contrast to deafened control cochleae, the soma area of SGNs treated for four weeks with exogenous BDNF were significantly greater than those of normal controls (Fig 4; Shepherd et al., 2005). The increases in soma area associated with NT administration are much greater than the increases observed following chronic ES. Although the mechanisms underlying an increased soma area following NT delivery remain unclear, this is a common neural response (Gratto et al., 2003; McGuinness et al., 2005; Perez-Navarro et al., 1999) that may be associated with elevated concentrations of the exogenous NT compared with normal endogenous levels.
Clinically viable neurotrophin delivery techniques
While the present results are promising, from a clinical perspective further research is necessary before NTs can be combined with cochlear implants. First, the long-term safety and efficacy of NT delivery to the cochlea must be examined. This is particularly important when applying drugs to the inner ear as the scala tympani is patent with cerebrospinal fluid and the brain via the cochlear aqueduct (Gopen et al., 1997). Second, an appropriate strategy for long-term delivery of NTs must be established. The delivery of a neurotrophin protein in solution via a pump is not clinically acceptable in the long-term because of increased risks of infection. There are several alternative options for neurotrophin delivery within a clinical setting including NT capture in a polymer, or via overexpression using cell- or gene- based therapies. While these techniques are capable of maintaining neurotrophin levels during the few weeks after the implant is inserted and before electrical stimulation begins, their application must be considered as part of a safe implant surgical protocol; i.e. their administration must not significantly prolong the surgery, increase the risks of trauma to cochlear structures, or predispose the cochlea to an extensive inflammatory response or infection. Although no technique has been used clinically, several are currently under experimental evaluation. Hendricks et al., (in press) contain a detailed discussion on available methods.
While increased SGN survival could be expected to result in improved clinical performance, it should be noted that human temporal bone studies to date have not demonstrated a correlation between total SGNs and word recognition scores in cochlear implant subjects (Fayad et al., 2006; Nadol et al., 2006; Nadol et al., 2001), emphasising the importance of central processing and brain plasticity in clinical performance.
Conclusion
The present results suggest that SGNs can maintain a trophic advantage through ES alone, following initial treatment with both NT and ES. From a clinical perspective this work implies that while delivery of exogenous NTs is necessary to promote SGN rescue, when performed in concert with ES, NTs may not need to be delivered continuously to promote SGN survival. Moreover, the results support and extend our knowledge of the response of the deafened cochlea to NT delivery by demonstrating an accelerated loss of SGNs following the removal of the exogenous NT in regions of the cochlea not subject to ES.
Acknowledgements
We are grateful to Dr James Fallon for software development, Dr Jin Xu and Ms Helen Feng for electrode manufacture, Mr. Rodney Millard for Engineering support, Ms. Jacqueline Andrew for Research Assistance, Ms Maria Clarke for histology, Dr Sue Pierce and Ms Elisa Borg for veterinary advice and animal husbandry, and Dr. Lisa Pettingill, Dr. James Fallon and Prof. Bryan Pfingst for comments on earlier versions of this manuscript.
Supporting grants: This work was funded by the NIDCD (NO1-DC-3-1005 and HHS-N-263-2007-00053-C).
Abbreviations
- ABR
Auditory Brainstem Response
- ANOVA
Analysis of Variance
- BDNT
Brain Derived Neurotrophin Factor
- dB
Decibel
- EABR
Electrically-evoked Auditory Brainstem Response
- ES
Electrical Stimulation
- ID
Internal Diameter
- IM
Intramuscular
- IV
Intravenous
- NT
Neurotrophin
- NT-3
Neurotrophin 3
- Pa
Pascal
- Pt
Platinum
- SC
Subcutaneous
- SEM
Standard Error of Mean
- SGN
Spiral Ganglion Neuron
- SNHL
Sensorineural Hearing Loss
- SPL
Standard Pressure Level
- T1
Cochlear turn 1 (base)
- T2
Cochlear turn 2
- T3
Cochlear turn 3
- T4
Cochlear turn 4 (apex)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Andrew J. Honours Thesis. Melbourne: University of Melbourne; 2003. Rehabilitation of the deafened auditory nerve with Schwann cell transplantation. [Google Scholar]
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. Journal of Neuroscience. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RC, Clark GM. Differential electrical excitation of the auditory nerve. J Acoust Soc Am. 1980;67:868–874. doi: 10.1121/1.383966. [DOI] [PubMed] [Google Scholar]
- Bok J, Zha XM, Cho YS, Green SH. An extranuclear locus of cAMP-dependent protein kinase action is necessary and sufficient for promotion of spiral ganglion neuronal survival by cAMP. J Neurosci. 2003;23:777–787. doi: 10.1523/JNEUROSCI.23-03-00777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Altschuler RA, Nuttall AL. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- Brown M, Shepherd RK, Webster WR, Martin RL, Clark GM. Cochleotopic selectivity of a multichannel scala tympani electrode array using the 2-deoxyglucose technique. Hear Res. 1992;59:224–240. doi: 10.1016/0378-5955(92)90119-8. [DOI] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2006;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150:1423–1434. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elverland HH, Mair IW. Hereditary deafness in the cat. An electron microscopic study of the spiral ganglion. Acta Otolaryngol. 1980;90:360–369. doi: 10.3109/00016488009131737. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell & Tissue Research. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci U S A. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to an accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Gopen Q, Rosowski JJ, Merchant SN. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Gratto KA, Verge VM. Neurotrophin-3 down-regulates trkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J Neurosci. 2003;18:1535–1548. doi: 10.1046/j.1460-9568.2003.02881.x. [DOI] [PubMed] [Google Scholar]
- Grill WM. Electrical stimulation of the peripheral nervous system: Biophysics and excitation properties. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Vol. 2. Singapore: World Scientific Publishing Co.; 2004. pp. 319–341. [Google Scholar]
- Hafidi A. Distribution of BDNF, NT-3 and NT-4 in the developing auditory brainstem. Int J Dev Neurosci. 1999;17:285–294. doi: 10.1016/s0736-5748(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neurosci. 2001a;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear. Res. 2001b;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Archives of Otolaryngology - Head and Neck Surgery. 1991;104:311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid S, Hartmann R, Klinke R. A model for prelingual deafness, the congenitally deaf white cat--population statistics and degenerative changes. Hear Res. 1998;115:101–112. doi: 10.1016/s0378-5955(97)00182-2. [DOI] [PubMed] [Google Scholar]
- Hendricks JL, Chikar JA, Raphael Y, Martin DC. Methods and materials for controlled drug delivery in the cochlea. Hear Res. (in press). (this issue) [Google Scholar]
- Huang CQ, Shepherd RK, Carter PM, Seligman PM, Tabor B. Electrical stimulation of the auditory nerve: direct current measurement in vivo. IEEE Trans Biomed Eng. 1999;46:461–470. doi: 10.1109/10.752943. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur J Neurosci. 2007;26:1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985;82:6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Damian D, Eddington DK, Nadol JB., Jr Effect of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann Otol Rhinol Laryngol. 2005;114:381–385. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- Ladrech S, Guitton M, Saido T, Lenoir M. Calpain activity in the amikacin-damaged rat cochlea. J Comp Neurol. 2004;477:149–160. doi: 10.1002/cne.20252. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res. 1991;54:251–271. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear electrical stimulation in neonatally deafened cats: effects of intensity and stimulating electrode location. Hear. Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Consequences of chronic extracochlear electrical stimulation in neonatally deafened cats. Hear. Res. 1995;82:65–80. doi: 10.1016/0378-5955(94)00167-o. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Vollmer M, Rebscher SJ. Neurotrophic effects of GM1 ganglioside and electrical stimulation on cochlear spiral ganglion GM1 ganglioside and electrical stimulation on cochlear spiral ganglion neurons in cats deafened as neonates. J Comp Neurol. 2007;501:837–853. doi: 10.1002/cne.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre PP, Weber T, Rigo J-M, Staecker H, Moonen G, Van De Water TR. Peripheral and central target-derived trophic factor(s) effects on auditory neurons. Hear. Res. 1992a;58:185–192. doi: 10.1016/0378-5955(92)90127-9. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Weber T, Rigo J-M, Staecker H, Moonen G, Van De Water TR. Peripheral and central target-derived trophic factor(s) effects on auditory neurons. Hear Res. 1992b;58:185–192. doi: 10.1016/0378-5955(92)90127-9. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5:865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res. 1999;133:27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–842. [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair IW. Hereditary deafness in the white cat. Acta Otolaryngol. Suppl. 1973;314:1–48. [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Prieskorn DM, Altschuler RA, Miller JM. Mechanism of electrical stimulation-induced neuroprotection: effects of verapamil on protection of primary auditory afferents. Brain Res. 2003;966:218–230. doi: 10.1016/s0006-8993(02)04170-7. [DOI] [PubMed] [Google Scholar]
- Miller CA, Woodruff KE, Pfingst BE. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear Res. 1995;92:85–99. doi: 10.1016/0378-5955(95)00204-9. [DOI] [PubMed] [Google Scholar]
- Miller J, Prieskorn DM, Lee SD, Kim J-S, Mitchell A, Altschuld RA. Auditory nerve survival remains enhanced four weeks following cessation of treatment with glial cell-line derived neurotrophic factor; ARO Midwinter meeting; Baltimore, MD: 2006. pp. Abstract 891. [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: Effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007 doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9:293–311. doi: 10.1111/j.1750-3639.1999.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Montero CN, Hefti F. Rescue of lesioned septal cholinergic neurons by nerve growth factor: specificity and requirement for chronic treatment. J Neurosci. 1988;8:2986–2999. doi: 10.1523/JNEUROSCI.08-08-02986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol. 2006;64:31–49. doi: 10.1159/000094643. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res Mol Brain Res. 2000;78:1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Noushi F, Richardson RT, Hardman J, Clark G, O'Leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- Perez-Navarro E, Alberch J, Neveu I, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons after excitotoxicity in vivo. Neuroscience. 1999;91:1257–1264. doi: 10.1016/s0306-4522(98)00723-4. [DOI] [PubMed] [Google Scholar]
- Pettingill LN, Richardson RT, Wise AK, O'Leary SJ, Shepherd RK. Neurotrophic factors and neural prostheses: Potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1138–1148. doi: 10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin SJ, Bachis A, Mocchetti I. Gangliosides activate Trk receptors by inducing the release of neurotrophins. J Biol Chem. 2002;277:49466–49472. doi: 10.1074/jbc.M203240200. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O'Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Rosenbaum BT, Kim PJ, Niparko JK, Saada AA. Single unit recordings in the auditory nerve of congenitally deaf white cats: morphological correlates in the cochlea and cochlear nucleus. J Comp Neurol. 1998;397:532–548. doi: 10.1002/(sici)1096-9861(19980810)397:4<532::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scarpidis U, Madnani D, Shoemaker C, Fletcher CH, Kojima K, Eshraghi AA, Staecker H, Lefebvre P, Malgrange B, Balkany TJ, Van De Water TR. Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otol Neurotol. 2003;24:409–417. doi: 10.1097/00129492-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Schindler RA, Gladstone HB, Scott N, Hradek GT, Williams H, Shah SB. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factors. American Journal of Otology. 1995;16:304–309. [PubMed] [Google Scholar]
- Shah SB, Gladstone HB, Williams H, Hradek GT, Schindler RA. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol. 1995;16:310–314. [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear. Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Xu J. A multichannel scala tympani electrode array incorporating a drug delivery system for chronic intracochlear infusion. Hear Res. 2002;172:92–98. doi: 10.1016/s0378-5955(02)00517-8. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci. 2004;20:3131–3140. doi: 10.1111/j.1460-9568.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II Deafened kittens. Hear. Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–322. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H, Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel KP, Bock GR. Electrically-evoked responses in animals with progressive spiral ganglion degeneration. Hear Res. 1984;15:59–67. doi: 10.1016/0378-5955(84)90225-9. [DOI] [PubMed] [Google Scholar]
- Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol Neurootol. 1998;3:281–290. doi: 10.1159/000013800. [DOI] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–543. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Widijaja S, Xu J, Shepherd RK. Cochlear implants stimulate activity dependent CREB pathway in the deaf auditory cortex: implications for molecular plasticity induced by prosthetic devices. Cereb Cortex. doi: 10.1093/cercor/bhm206. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney TS, T PD, Xia G, Moore DR. Development of brain-derived neurotrophic factor and neurotrophin-3 immunoreactivity in the lower auditory brainstem of the postnatal gerbil. Eur J Neurosci. 2001;14:785–793. doi: 10.1046/j.0953-816x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hear. Res. 1987;29:195–206. doi: 10.1016/0378-5955(87)90167-5. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Ketels K. Co-culture of dissociated spiral ganglion with adjacent non-neuronal cells improves neuronal survival., Association for Research in Otolaryngology. Vol. 26. Daytona Beech, FL: 2003. p. 467. [Google Scholar]
- Widijaja S, Tan J, Xu J, Shepherd RK. Chronic electrical stimulation in deafened rats: effects on spiral ganglion neuron survival, Bionics and Regeneration of the Ear. Melbourne, Victoria, Australia: 2006. [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear. Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]


