Abstract
B. cereus, is a member of a genus of aerobic, gram-positive, spore-forming rod-like bacilli, which includes the deadly, B. anthracis. Preliminary experiments have shown that gC1qR binds to B.cereus spores that have been attached to microtiter plates. The present studies were therefore undertaken, to examine if cell surface gC1qR plays a role in B.cereus spore attachment and/or entry. Monolayers of human colon carcinoma (Caco-2) and lung cells were grown to confluency on 6 mm coverslips in shell vials with gentle swirling in a shaker incubator. Then, 2 μl of a suspension of strain SB460 B.cereus spores (3×108/ml, in sterile water), were added and incubated (1–4 h; 36° C) in the presence or absence of anti-gC1qR mAb-carbon nanoloops. Examination of these cells by EM revealed that: (1) When B. cereus endospores contacted the apical Caco-2 cell surface, or lung cells, gClqR was simultaneously detectable, indicating upregulation of the molecule. (2) In areas showing spore contact with the cell surface, gClqR expression was often adjacent to the spores in association with microvilli (Caco-2 cells) or cytoskeletal projections (lung cells). (3) Furthermore, the exosporia of the activated and germinating spores were often decorated with mAb-nanoloops. These observations were further corroborated by experiments in which B.cereus spores were readily taken up by monocytes and neutrophils, and this uptake was partially inhibited by mAb 60.11, which recognizes the C1q binding site on gC1qR. Taken together, the data suggest a role, for gC1qR at least in the initial stages of spore attachment and/or entry.
1 Introduction
Bacillus cereus is an opportunistic pathogen involved in several types of food poisoning and tissue destruction (Davey and Tauber 1987; Callegan, Engel, Hill, and O’Callaghan 1994; Beecher, Pulido, Barney and Wong, 1995; Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000; Day, Smith, Gregg, Turnbull, Head, Ives, and Ho 1981). It belongs to the genus of spore-forming aerobic bacteria, which includes B. thuringiensis, a bacterium which produces intracellular protein crystals toxic to a number of insect larvae —commonly used in many parts of the world as a biological pesticide in the protection of crops (Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000); and B.anthracis, the causative agent of anthrax and a potential bio-warfare agent (Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000; Whitney, Beatty, Taylor, Weyant, Sobel, Arduino, and Ashford). Traditionally, B. cereus has been regarded more as an opportunistic nuisance found in hospitals and in milk products causing mild gastroenteritis, rather than something more sinister. Toxin formation as well as formation of heat-resistant spores, which survive pasteurization, has been associated with food poisoning since many strains produce enterotoxins and emetic toxins that cause gastroenteritis, diarrhea, and vomiting (Davey and Tauber 1987; Callegan, Engel, Hill, and O’Callaghan 1994; Beecher, Pulido, Barney and Wong, 1995; Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000; Day, Smith, Gregg, Turnbull, Head, Ives, and Ho 1981; Whitney, Beatty, Taylor, Weyant, Sobel, Arduino, and Ashford; Gould and Dring, 1974). However, a plethora of experimental evidence has been rapidly accumulating showing that B.cereus infection is more serious than it had been previously thought. B.cereus is one of the most important causes of post-traumatic and metastatic bacterial endophthalmitis causing an average of 39% of trauma associated cases of endophthalmitis (Davey and Tauber 1987; Callegan, Engel, Hill, and O’Callaghan 1994; Beecher, Pulido, Barney and Wong, 1995) and is the most frequent cause of metastatic bacterial endophthalmitis in drug abusers (Davey and Tauber 1987; Callegan, Engel, Hill, and O’Callaghan 1994; Beecher, Pulido, Barney and Wong, 1995). Indeed, B.cereus is considered to be one of the most destructive organisms to affect the eye causing almost certain blindness even when aggressive and appropriate anti-microbial therapy is administered before the loss of visual acuity (Davey and Tauber 1987; Callegan, Engel, Hill, and O’Callaghan 1994; Beecher, Pulido, Barney and Wong, 1995). This is because highly potent toxins and virulence factors continue to damage the tissue and promote inflammation (Davey and Tauber 1987). Among these virulence factors are, cereolysin O, a thiol-activated cytolysin, which recognizes the cholesterol component of cell membranes prior to lysis (Shaney, Bernheimer, Grushoff, and Kim, 1974); cereolysin AB, another cytolytic unit unrelated to cereolysin O (Gilmore, Cruz-Rodz, Leimeister-Wachter, kreft and Goebel, 1989); hemolysin BL, a toxin with hemolytic, dermo-necrotic, and emetic activities (Beecher, Pulido, Barney and Wong, 1995); and collagenase, capable of degrading the collagen constituent of the vitreous and retinal architecture (Soderling and Pauino, 1981; Harrington, 1996 ).
Formed in response to starvation, bacterial spores such as those of B. cereus and its relative B.anthracis are among the most resilient cell types known (Harrington, 1996). Although they remain dormant for decades in a nutrient-deprived environment enduring a wide range of environmental stresses, they retain the capacity to germinate and give rise to bacterial colonies once the nutrient conditions return to a favorable state (Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000). Fundamental to spore resistance and germination is the coat, a tough proteinaceous, multilayered shield that provides mechanical integrity and excludes large toxic molecules, while allowing small nutrient molecules to access germination receptors beneath the coat (Driks 2002; Chada, Sanstad, Wang, and Driks 2003). For some spore species, such as B. anthracis, B. cereus and Clostridial species, an outer responsive membrane called the exosporium comprised of protein, carbohydrate, and lipid has been shown to function in spore attachment but is still poorly understood (Chada, Sanstad, Wang, and Driks 2003; Beaman, Pankratz, and Gerhardt 1971; Matz, Beaman, and Gerhardt 1970; Todd, Moir, Johnson, and Moir 2003; Panessa-Warren, Tortora, and Warren, 1997; Panessa-Warren, Tortora, and Warren, 1994). Because the exosporium is the outermost layer of the spore, it is the first that makes initial contact with the host cells and tissues (16–18). Therefore, in addition to being the protective layer encasing the spore conferring unique adherence and hydrophobic properties, the exosporium may express molecules that play a significant role in spore attachment and pathogenicity (Driks 2002; Chada, Sanstad, Wang, and Driks 2003; Chada, Sanstad, Wang, and Driks 2003; Beaman, Pankratz, and Gerhardt 1971; Matz, Beaman, and Gerhardt 1970; Todd, Moir, Johnson, and Moir 2003). The present studies were therefore undertaken to investigate whether the exosporium expresses molecule(s) that participate in spore-host cell [surface] attachment/colonization, and if the multifunctional cell surface protein gC1qR, which has affinity for a number of bacterial outer surface antigens serves as one of the spore attachment site (s) (Ghebrehiwet, Peerschke, Willis, Hong, and Reid, 1994; Ghebrehiwet, Lim, Kumar, Feng and Peerschke, 2001).
2 Materials and Methods
2.1 Chemicals and reagents
The following reagents and chemicals were purchased or obtained from the sources indicated. Alkaline phosphatase (AP)-streptavidin, and p-Nitrophenyl Phosphate (pNPP) (Pierce Co,); single walled carbon nanotubes (SWNTs) (Carbolex Inc., Lexington, KY).
2.2 Expression of recombinant gC1qR/p33
The strategy for the construction of a plasmid containing the full-length gC1qR cDNA was described in detail in our earlier publication (Ghebrehiwet, Peerschke, Willis, Hong, and Reid, 1994). Briefly, the cDNAs were subcloned downstream of the glutathione-S-transferase (GST) gene in the expression plasmid, pGex-2T (Pharmacia Biotech Inc.), transformed into E.coli BL-21 (DE3) and protein expression induced by 0.5 mM IPTG (isopropyl β-D-1 thiogalacto pyranoside). The proteins were expressed as GST fusion products with the GST at the N-terminus of the gC1qR. The fusion products were purified on glutathione-Sepharose 4B column. The gC1qR fusion products were then cleaved by thrombin and the GST-free gC1qR purified on fast protein liquid chromatography (FPLC, Pharmacia) using a Mono-Q ion exchange column. After verification by ELISA and Western blotting using monoclonal antibody to gC1q-R (Ghebrehiwet, Zhang, Lim, Eggleton, Leigh, Reid, and Peerschke, 1996), the single peak containing the gC1qR was pooled, concentrated to 1–2 mg/ml, and stored at −80° C. When necessary, 5 mM pro-phe-arg chloromethyl ketone (PPACK), a specific and rapid thrombin inhibitor, was added to eliminate the possibility of the presence of a trace amount of the enzyme in the preparation.
2.3 Monoclonal antibodies
The production, characterization and purification of various monoclonal antibodies to the various domains and epitopes of gC1qR have been described in detail in our earlier publications (Ghebrehiwet, Zhang, Lim, Eggleton, Leigh, Reid, and Peerschke, 1996). The monoclonal antibodies 60.11 and 74.5.2 used in the present studies have been used extensively in various functional studies (Ghebrehiwet, Lim, Kumar, Feng and Peerschke, 2001). They are both IgG1 kappa isotype and recognize the C1q binding site (residues 74–96) and kininogen binding site (residues 204–218) of the molecule.
2.4 Binding studies
To determine the interaction between gC1qR and B.cereus spores, a standard ELISA was used. Briefly, 100 μl (1×107/ml) of B. cereus SB460 [originally isolated from a blood culture of an intravenous drug user (Panessa-Warren, Tortora, Wong, Ghebrehiwet, and Warren, 2003)] spores were first attached to duplicate microtiter plate wells by incubating at either 37° C or 60° C for 2 hr. After blocking with 1% BSA, and washing with Tris-buffered saline, pH 7.5, the spores were incubated with biotinylated gC1qR/p33 in TBS alone or containing either 10 mM Ca2+ or 10 mM EDTA. The bound protein was then detected by sequential reaction with alkaline phosphatase (AP)-streptavidin, and p-Nitrophenyl phosphate (pNPP) and read at 410 nm in a Dynatech, MR 700 ELISA reader.
2.5 Phagocytosis assay
B. cereus spores were preincubated with or without gC1qR (60 min, 37° C) before addition to citrated blood, and incubated for 60 min. Alternatively, mAb 60.11 was added to the blood and prior to addition of spores and incubation (60 min, 37° C). In each of these experiments, the blood was thin smeared onto glass slides and stained with Dif-quick. Visualization of the internalized spores was achieved by spore-specific staining with malachite green (7.6%, in distilled H2O) and counterstained with 0.5% safranin (2.5 g Safranin O, first dissolved in 100 ml of 95% ethanol and brought to 1L with distilled H2O) as described previously (Schaeffer and Fulton, 1933). Phagocytosis was then evaluated by counting under a light microscope, neutrophils or monocytes that ingested ≥3 spores per cell.
2.6 Preparation of carbon nanoloops
Single walled carbon nanotubes (SWNTs) (Carbolex Inc., Lexington, KY) were chemically cleaned in acid, cut and formed into 20–40 nm loops (Panessa-Warren, Wong, Ghebrehiwet, Tortora, and Warren, 2002). The nanoloops were then functionalized with purified recombinant gClqR (gC1qR-nanoloops), a non-immune mouse IgG1k, (IgG1k-nanoloops) or a monoclonal antibody to gC1qR (mAb 60.11, or 74.5.2 mAb-nanoloops). By atomic force microscopy and transmission electron microscopy these functionalized carbon nanoloops measured between 28.2 ± 4.2 and 36.7 ± 10.2 nm mean diameter. The preliminary trials exposing the carbon nanoloops to bacteria and tissue culture cells produced no alterations in normal cell behavior and physiology. The nanoloops provided good visualization for TEM and FESEM without any signs of bioaccumulation within cells, or cytotoxicity over the 4.5 hr incubation period.
The rationale for using antibody functionalized carbon nanoloops is to identify where and when gClqR was expressed on the apical surface of human colon and lung cells. The carbon nanoloops provided a platform for attaching a high density of antibody molecules, and served as a carrier to deliver this excess of antibody to the cell surface using in vitro culture. Unlike more conventional gold labeling, this newer, albeit not much tested, technique does not produce background noise, or cause adverse reactions with the bacteria or human cells in culture. Preliminary exploratory studies showed that there was neither accumulation nor intracellular localization of the nanoloops, nor evidence of necrosis or nuclear damage in control (non-functionalized or IgG and gClqR functionalized nanoloops) or mAb-functionalized carbon nanoloops incubated with cells. Furthermore, cells and bacteria showed no reactivity or binding to the loop itself. However, the ability to carry multiple antibody molecules at one time to the cell surface, insured the attachment and localization of the nanoloop, even at the earliest time periods of gClqR expression.
2.7 Preparation of cells for microscopic analyses
For in vitro experiments, mAb-nanoloops, IgG1k-nanoloops, naked-nanoloops were incubated (36° C) in shell vials with Caco-2 (human colon carcinoma) cells or NCI-292 human epithelial lung cells grown to confluency as monolayers on glass coverslips (Diagnostic Hybrids, Athens, OH). Continual rotary mixing during cell incubation to ensure that the nanoloops did not clump, but were well dispersed with the bacterial spores and evenly distributed over the cell monolayer. Cells were incubated for 20 min (rotary mixing) with control or mAb-nanoloops, followed by spore inoculation with 2 μl of 3 × 108 spore/ml deionized sterile water, and incubated for 30,60,90, and 150–270 min. Cells incubated with naked-nanoloops, and cells with inoculated spores but no nanoloops were also incubated under the same conditions. Following incubation, the media were removed, the surface of the monolayers washed 2X with PBS and the cells fixed in 2.8% glutaraldehyde in 0.1M cacodylate buffer with 0.1% CaCl2 and 10% sucrose overnight. The saved media and PBS washes were spun at 3400 rpm for 30 min and the pellets screened for bacteria, cells, nanoloops, and any contaminants. For microscopy, the cell monolayers were post fixed in osmium tetroxide, dehydrated in acetone, and either critical point dried for FESEM, or embedded in epoxy resin, polymerized, and thin sectioned for TEM. For each time period studied 4 coverslips with culture cells were prepared for electron microscopy. By FESEM, the coverslips were surveyed using five random fields imaged at the same magnifications — 500X, 2000X, 16,000X, 25,000X, 40,000X and 60,000X — at the same tilt angle and accelerating voltage. Microvilli, cell surfaces, and the background were screened for the presence of nanoloops, as well as contaminating cell damage and compared to controls. With TEM samples, control and experimental tissues were thin-sectioned and imaged both stained (uranyl acetate and lead citrate) and unstained to verify the presence of nanoloops.
2.8 Microscopic analyses
2.8.1 Light microscopy
Blood smears on glass slides were reacted with B.cereus spores as described above and stained by a previously detailed method for spore visualization using malachite green (7.6%) and counterstained with safranin (0.25%) (Schaeffer and Fulton, 1933). Slides were examined and photographed (100 ASA Kodak color film) under oil using an Olympus BH2 photomicroscope (Plainview, NY) with a polarizing filter at 1025X.
2.8.2 Electron Microscopy
Glutaraldehyde (2.8% in 0.1M cacodylate buffer, pH 7.2, with added 10% sucrose) fixed Caco-2 and lung monolayers were washed in 0.1 M cacodylate buffer with 10% sucrose, and osmicated, and prepared for microscopy with half of the coverslip processed for TEM, and the other half of the cleaved coverslip prepared for FESEM. Dehydration (dropwise) with acetone and infiltration with epoxy resin was done slowly (2–3 weeks) to prevent the bacterial spores from rupturing. Coverslips were placed on cleaned glass slides and a BEEM capsule filled with epoxy placed over each coverslip and polymerized at 56°C for 2 days. The samples were then frozen in liquid nitrogen and the BEEM capsules broken away from the glass slide, leaving the tissue culture cells in the plastic of the BEEM capsule. This layer of cells was then removed from the BEEM capsule and flat embedded to provide longitudinal cell sections showing the apical and basal cell surfaces. For TEM, thin sections were stained with uranyl acetate and lead citrate and imaged at 80 KV using a Philips 300.
For FESEM, critical point dried monolayers on glass coverslips were attached with silver conducting paint to brass stubs, metal coated with 3–5 nm Pt, and imaged in a JEOL6500F field emission scanning electron microscope at 5–15 KV at 30° tilt. To prevent sample bias, 5 fields were randomly chosen on each sample, and 15 or more images taken at each site at the same magnification. To ensure the correct interpretation of microscopic data, TEM and FESEM images from the same sample were compared to verify nanoloop localizations, cell morphology, and bacterial interactions.
3 Results
3.1 The binding of gC1qR to B. cereus is temperature and calcium dependent
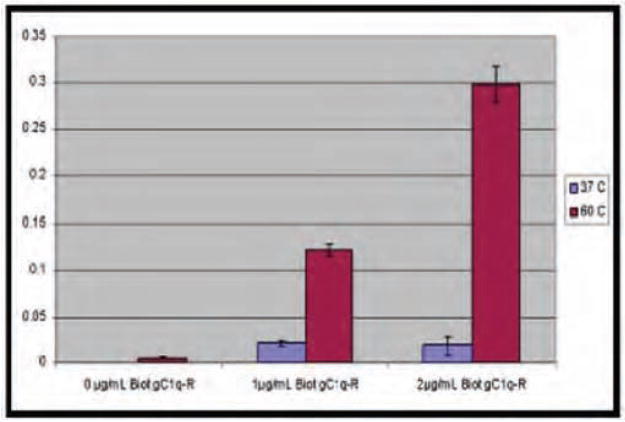
To confirm preliminary observations and to determine the effect of temperature variation on the interaction between gC1qR and spores, a standard solid phase ELISA was used. Spores at a concentration of 1×107 were attached to microtiter plates as described in Methods, incubated with biotinylated gC1qR/p33 and developed. The results (Fig. 1, n=3) not only show that spores attach much more (~10) readily at higher temperatures (60° C ≥ 37° C), but also that gC1qR/p33 binding to B. cereus spores is dose-dependent. Furthermore, although good binding of spores was observed in TBS alone, the addition of exogenous calcium consistently showed a significantly enhanced binding in the presence of additional Ca2, (TBS, pH 7.5, containing 10 mM Ca2+) implying that gC1qR binding to B.cereus is may be Ca2+ dependent (Fig. 2).
Fig. 1.
The binding of gC1qR/p33 to B. cereus is temperature dependent. To determine the effect of temperature variation, a standard ELISA was used. Spores at a concentration of 1×107/ml were attached to microtiter plates by incubation at either, 37°C and 60°C (2 hr). After blocking with 1%BSA, and washing, the spores were incubated with biotinylated gC1qR/p33, detected by sequential reaction with alkaline phosphatase (AP)-streptavidin, and p-Nitrophenyl phosphate (pNPP) and read at 410 nm in an ELISA reader. Each data point is a mean of 3 separate experiments run in duplicates.
Fig. 2.
Exogenous Ca2+ enhances gC1qR/p33 binding to B. cereus. Microtiter plates were first coated with B. cereus spores as in Fig. 1 by incubation at 60°C. Varying concentrations of gC1qR/p33 in Tris buffered saline (TBS), pH 7.5, containing 10 mM Ca2+ were then added to the spores and developed as described. Each data point is a mean of 4 separate experiments run in duplicates.
3.2 EDTA abrogates gC1qR binding to B. cereus
To verify the importance of endogenous or exogenous Ca2+, the experiments in Fig. 2 were repeated except that the added gC1qR was in TBS buffer that contained 0–10 mM EDTA instead of Ca2+. As expected results obtained from this experiment showed a dose-dependent decrease in gC1qR binding (n=3, data not shown). However, complete inhibition was not achieved even with the highest dose of EDTA (10 mM). This is probably due to excessive amount of endogenous calcium, which is released by the spores during activation and attachment (Panessa-Warren, Tortora, and Warren, 1997).
3.3 Neutrophils and monocytes readily take up B. cereus spores
To get insight into the biologic relevance of the interaction between gC1qR and B.cereus spores, we chose a phagocytosis model in which the ability of monocytes or neutrophils to ingest B. cereus spores was examined. To this end spores were incubated with citrated blood for 30 min at 37° C and after incubation, the blood was thin-smeared onto glass slides, stained and evaluated by counting the cells that ingested ≥3 spores under a light microscope. As shown in Fig. 3, B.cereus spores are readily engulfed and taken up by neutrophils and also by monocytes (not shown). To examine whether this ingestion is mediated by gC1qR, experiments were performed in which mAb 60.11 or isotype- and species-matched control antibody was added to the blood together with the spores. After incubation (30 min, 37° C), the degree and quality of ingestion was evaluated by counting the number of cells that ingested spores and the number of ingested spores under a light microscope as described above. The results from these studies (n=3; data not shown) showed a decrease in both in the number of spores ingested and the number of monocytes/neutrophils that were engaged in phagocytosis as compared with controls.
Fig. 3.
Neutrophils readily take up B. cereus spores. After incubation of spores with whole blood, smears of blood were made on glass slides and stained by a previously detailed method for spore visualization using malachite green (7.6%) and counterstained with safranin (0.25%) (23). Slides were examined and photographed (100 ASA Kodak color film) under oil using an Olympus BH2 photomicroscope with a polarizing filter at 1025X. Shown is the image of neutrophils that have ingested an robust number of spores (>10) seen as translucent elliptical spheres.
3.4 Electron microscopic analyses
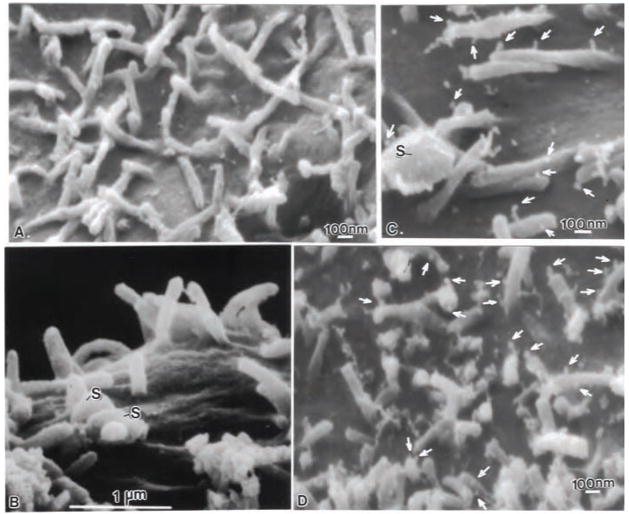
Certain nanotube-fabricated loops (24–40 nm diameter) were used to convey multiple monoclonal antibodies to the specimen surface. Control experiments using plain carbon nanoloops showed no cellular toxicity to tissue culture cells or bacteria. Using these immunocarriers more antibody could be brought to the sample surface and visualized without loosing antibody reactivity. When Caco-2 or lung cells were incubated with naked-nanoloops, no carbon nanoloops were found attached to the apical surface of the cells, the microvilli or cytoskeletal projections in the case of lung cells. Cells appeared robust with turgid microvilli (Fig. 4A), and maintained cell junctions in both TEM (Fig. 4B), and FESEM images (Fig. 4A), indicating that over the time period of 30 min to 4.5 hours, the nanoloops did not cause cell toxicity, bioaccumulation, or produce spurious binding. For all samples analyzed no binding of the nanoloops was observed when IgG1k-nanoloops were incubated with cell monolayers and B.cereus spores. Fig. 4B clearly shows spores (S) on the Caco-2 cell surface adjacent to turgid, clean microvilli, with no binding of nanoloops on the cell or microvilli surface. Nanoloops functionalized with purified recombinant gClqR also failed to bind to the cells or microvilli (not shown). However mAb-functionalized nanoloops readily attached to the surface of microvilli following spore contact with the cell surface (Fig. 4C, D). Increased mAb-binding to gClqR was found overtime as more spores attached to the apical surface (Fig. 4D arrows) with some microvilli showing many bound nanoloops (arrows).
Fig. 4.
FESEM images of Caco-2 cell microvilli incubated for 2.5 hr with mAb-nanoloops in the presence and absence of B. cereus spores. (A) Following incubation with mAb -74.5.2 functionalized nanoloops, the microvillous surface showed no marker uptake in the absence of spores. (B) Caco-2 microvilli incubated with B. cereus spores (s) and control IgG1k-nanoloops showed no marker uptake, even on microvilli adjacent to attached endospores. (C) However, numerous mAb-nanoloops (arrows), attached to microvilli and the exosporia of B. cereus spores. (D) At lower magnification, the extensive localization of gC1qR/p33 with mAb -nanoloops (arrows) is apparent on the microvilli adjacent to B. cereus spores.
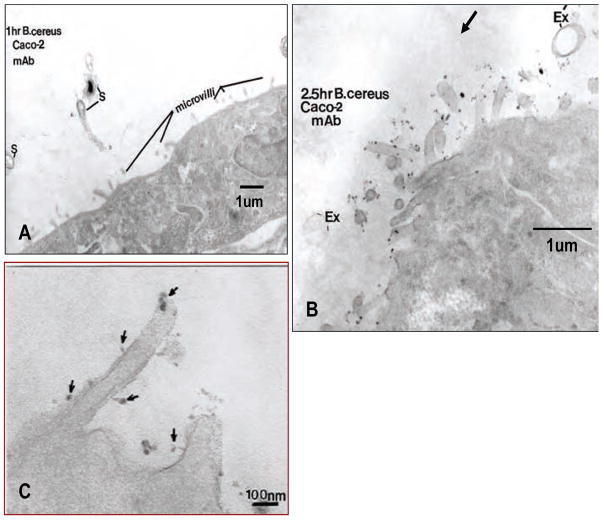
By TEM, cells did not show any mAb-nanoloop binding at 30–60 min incubation (Fig. 5A). Some spores (S) were seen passing towards the Caco-2 apical surface. Some of the spores (S) were still dehydrated (blackened spore), while others were germinating as they approached the host cell apical surface. Surface microvilli showed no mAb-nanoloop binding indicating that no gClqR was being expressed on the cell surface as the spores approached. However at 2.5 hr following the introduction of mAb-nanoloops and B.cereus endospores, numerous sites of mAb binding were seen on the microvilli of the host cells under direct bacterial attack (Fig. 5B). Some empty exosporia (Ex) in contact with microvilli were seen. Numerous clusters of nanoloops decorated the microvilli adjacent to attached spores. At higher magnification individual microvilli revealed clusters of carbon nanotube loops (Fig. 5C).
Fig. 5.
FESEM images showed that the attachment of B. cereus spores to Caco-2 cells induced surface expression of gClqR. The composite images show that: (A) No detectable cell surface gC1qR is expressed after 1 h incubation of Caco-2 cells with spores. (B) After 2.5 h incubation, significant mAb-nanoloop binding indicates sites of gC1qR localization (arrows), which was predominantly localized on the surface of microvilli and occasionally on the surface of the exosporium (Ex). (C). A higher magnification of the microvilli reveals clusters of mAb-nanoloop binding (arrows) along the base and tip of the microvilli outer membranes, revealing sites of gC1qR expression.
A similar gC1qR distribution pattern following B.cereus inoculation of Caco-2 cells, was seen when lung cells revealed attached B.cereus endospores with the adjacent cytoskeletal projections heavily labeled with mAb-functionalized nanoloops (Fig. 6A), localizing sites of gClqR expression. Following analysis of more than 500-field of tissue culture cells, no mAb-nanoloop binding was seen on adjacent cells, or on the same cell in areas lacking bound spores. Intact spores could be seen in surface invaginations (Fig. 6B), with heavy labeling of mAb-nanoloops (arrows) on the cytoskeletal projections and immediate apical surface surrounding the spore. From FESEM and TEM analyses of colon or lung cells incubated with B.cereus, gC1qR expression was only observed at cell surface sites with B.cereus spores, suggesting that spore attachment or the presence on the host cell surface did not cause gC1qR expression on all adjoining cells of the monolayer.
Fig. 6.
Surface expression of gC1qR on NCI-292 human epithelial lung cells. The figure shows TEM images of human lung epithelial cells after incubation with B. cereus spores. Following incubation with B. cereus endospores, lung cells that had attached spores formed large cytoskeletal projections, which revealed profuse surface binding of mAb-functionalized nanoloops [(arrows (A)]. This profuse expression of gClqR was only found focally where a spore had attached. Adjacent areas of the same cell did not show mAb-nanoloop binding, or the cytoskeletal projections. (B) Shows a higher magnification of an apical area of the lung cell with an incorporated intact B. cereus spore with exosporium and spore coats within a vacuole. Just above the vacuole containing the spore on the apical lung surface, extensive mAb binding can be seen in the plasmalemma indicating the presence of gClqR.
4 Discussion
The data presented in this report collectively suggest that B. cereus spores express on their surface a yet unidentified surface molecule(s) through which they bind gC1qR. The binding of microtiter-bound B.cereus spores to purified gC1qR was found to be calcium-dependent, which may be provided by the spore itself during the process of activation (Panessa-Warren, Tortora, and Warren, 1997).
Additional data in support of the involvement of gC1qR in the attachment of B.cereus to cell surfaces is provided by the following electron microscopic observations: (i) Spore attachment induces upregulation of gC1qR on the cell surface of Caco-2 cells, and NCI 292 lung cells. This upregulation appears after 1 hr exposure and peaks at about 2.5 hr. (ii) gC1qR expression is robust at or near sites of spore attachment at least when Caco-2 cells and lung cells are used as target cells for attachment, and this expression is predominantly localized on the surface of microvilli (Caco-2, Fig 4 and 5) or apical cytoskeletal projections (lung cells, Fig. 6). (iii) Concomitant with the appearance of gC1qR on the microvilli, the spore exosporium itself is often decorated with gC1qR. (iv) B. cereus spores normally attach and colonize the surface of Caco-2 cells. However, when mAb-nanoloops block host cell surface gClqR, the B.cereus protoplasts were found to enter the host cell cytoplasm, rather than colonize the host cell surface thus altering the invasion paradigm (Panessa-Warren, Wong, Ghebrehiwet, Tortora, and Warren, 2002). Spore protoplast entry, which occurs only when the target cells are preincubated with blocking antibody, is initiated following attachment of the spore to Caco-2 microvilli and simultaneous shedding of the outer-most coating, or exosporium (not shown). (v) In the absence of antibody blockade, the spores germinate, colonizing the host cell surface, but fail to get inside the cell. A similar finding with E.coli sequestered within bladder cells (Anderson, Palermo, Schilling Roth, Heuser, and Hultgren, 2003) whereby the bacteria could emerge to produce subsequent infections, may be analogous to the storage of B.cereus protoplasts within the colon cells. This process may allow the spore DNA-containing protoplasts to survive for a prolonged period of time undamaged even by the hostile intracellular environment until they are released.
Both Caco-2 and NCI 292 cells allowed B. cereus spore attachment on their cell surfaces regardless of gClqR blockade with mAb-nanoloops. Yet, the presence of activated endospores on the surface of both Caco-2 and human lung cells produced copious overexpression of gClqR only in those areas with spore attachment but not on cells without any bacterial spores. On Caco-2 cells, gClqR surface expression was localized to the microvilli within a 6–10 μm radius of attached spores. However, neighboring cells and the adjacent areas to the responding microvilli showed no gClqR surface expression. Lung cells on the other hand, produced cytoskeletal extensions where B. cereus endospores were present. The cytoskeletal extensions adjacent to the attached spores showed extensive mAb-nanoloop binding indicating the presence of gC1qR, whereas the surrounding cells lacking endospores showed no detectable surface gClqR expression.
The possible involvement of gC1qR in the process of spore attachment is further corroborated by phagocytosis experiments. When spores were exposed to whole blood, there was a rapid and robust uptake by monocytes and neutrophils, but the uptake of spores was significantly reduced (not shown) when mAb-60-11 was added to the blood prior to addition of the spores. The presence of a putative gC1qR binding site on the B.cereus exosporium suggests that spores are able to interact directly with cell surface gC1qR through a molecule on their surface that mimics one of the multiple ligands of gC1qR including C1q and kininogen ( Ghebrehiwet, Lim, Kumar, Feng and Peerschke, 2001). Identification and characterization of this molecule(s) will therefore be of biologic significance with therapeutic potential.
The spore-forming family of Bacillus, which includes B.cereus, B. thuringiensis and B. anthracis, possess a loose balloon-like structure called exosporium (Todd, Moir, Johnson, and Moir, 2003; Panessa-Warren, Tortora, Wong, Ghebrehiwet, Warren, 2003). However, it is not known with certainty whether different strains of the same species including the SB460 isolate used in our studies possess an identical structure or a modified version of the exosporium. The particular adherence (Bowen, Fenton, Lovitt, and Wright, 2002) and hydrophobic properties (Koshikawa, Yamazaki, Yoshimi., Ogawa, Yamada, Watanabe and Torii, 1989) conferred by the exosporium suggest that this structure may be of significance to spore attachment and pathogenicity (Todd, Moir, Johnson, and Moir, 2003). The exosporium of B.cereus contains 43–52% protein, 15–18% lipid, and 23% carbohydrates of dry weight, respectively (Beaman, Pnkratz, and Gerhardt, 1971). It is not surprising therefore that identification and characterization of molecules of the exosporium has become of primordial significance in recent years. For example, a 205 kDa glycoprotein multimer (70 KDa monomer) has been identified and partially characterized from the spore of B.thuringiensis (Garcia-Patrone and Tandecarz) More recently, a major structural protein of Bacillus anthracis exosporium has been identified and molecularly cloned (Sylvestre, Couture-Tosi and Mock, 2002). The gene encodes a protein of 382 amino acid residues, the central part of which contains a region of GXX triplet repeats, similar to those found in the triple-helix region of mammalian collagen. Interestingly, this collagen-like surface glycoprotein, designated BclA (Bacillus collagen-like protein of anthracis) was detected only in spores and sporulating cells but not in vegetative cells suggesting that it is a spore-specific glycoprotein (Sylvestre, Couture-Tosi and Mock, 2002). BclA is a major exosporium protein and is a structural constituent of the filamentous appendages present on its outer layer. Because the exosporium is exposed on the surface and is presumably the first structure of the pathogen to interact with the host cells, it is postulated to play a significant role in the infection process possibly through the interaction of spore proteins such as BclA, with cell surface proteins, of which gC1qR may be a possible candidate. This hypothesis is particularly attractive since gC1qR, is a ubiquitously distributed cellular protein with affinity for a number of viral and bacterial antigens including protein A of Staphylococcus aureus ( Nguyen, Ghebrehiwet, Peerschke, 2000) and internalin B of L. monocytogenes (Braun, Ghebrehiwet, and Cossart), 2000). It has been shown that the genome of Bacillus anthracis is virtually identical to that of B.cereus with the exception of their respective toxin production ((Helgason, Økstad, Caugant, Johansen, Fouet, Mock, Hegna, and Koltsø, 2000; Todd, Moir, Johnson, and Moir, 2003). Therefore, although results obtained from the use of a surrogate bacterium, regardless of how closely related, may not necessarily reflect the function of the desired target bacterium (Finlay and Cossart, 1997), understanding the mechanism of B. cereus spore attachment and entry may nonetheless, serve as a proxy for understanding the mechanism by which spores of B. anthracis exploit the mammalian host cell functions to their advantage Finlay and Cossart, 1997). Identifying a molecule(s) on the B. cereus exosporium, which allows interaction with cell surface proteins or receptors, may therefore predict the existence of a molecularly and functionally related molecule on B. anthracis or vice versa. In support of this hypothesis is the recently published report (Rety, Salamitou, Garcia-Verdugo, Hulmes, Le Hagarat, Chaby and Lewit-Bentley, 2005), which showed that the crystal structure of BclA, the B. anthracis spore surface protein, shares remarkable similarity to the C1q/TNF family of mammalian proteins. It will therefore be interesting to see whether B. cereus spores express a BclA-like molecule that has the ability to bind gC1qR. Experiments are therefore presently underway not only to address the veracity of this postulate but also to determine how the interaction between the spores and the host cell surface contributes to the pathogenicity of B. cereus.
Abbreviations
- gC1qR
33 kDa cellular protein which binds to the globular heads of C1q; MBL
- FESEM
field emission scanning electron microscopy
- TEM
transmission electron microscopy
Footnotes
This work has been authored by Brookhaven Science Associates, LLC under contract No. DE-AC02- 98CH10886 with the US Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes. This work was also supported in part by grants R01-AI 060866 from the National Institutes of Allergy and Infectious Diseases (B.G.) and a generous gift from Larry and Sheila Dalzell (to B.G.).
References
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- Beaman TC, Pankratz HS, Gerhardt P. Paracrystalline sheets reaggregated from solubilized exosporium of Bacillus cereus. J Bacteriol. 1971;107:320–324. doi: 10.1128/jb.107.1.320-324.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher DJ, Pulido JS, Barney NP, Wong ACL. Extracellular virulence factors in Bacillus cereus endophthalmitis: Methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63:632–639. doi: 10.1128/iai.63.2.632-639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WR, Fenton AS, Lovitt RW, Wright CJ. The measurement of Bacillus mycoides spore adhesion using atomic force microscopy, simple counting methods, and spinning disc technique. Biotechnol Bioeng. 2002;79:170–179. doi: 10.1002/bit.10321. [DOI] [PubMed] [Google Scholar]
- Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Engel LS, Hill JM, O’Callaghan RJ. Corneal virulence of Staphylococcus aureus: Roles of alpha-toxin and protein A in corneal pathology. Infect Immun. 1994;62:2478–2482. doi: 10.1128/iai.62.6.2478-2482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada VG, Sanstad EA, Wang R, Driks A. Morphogenesis of bacillus spore surfaces. J Bacteriol. 2003;185:6255–6261. doi: 10.1128/JB.185.21.6255-6261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey RT, Tauber WB. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- Driks A. Maximum shields: the armor plating of the bacterial spore. Trends in Microbiol. 2002;10:251–254. doi: 10.1016/s0966-842x(02)02373-9. [DOI] [PubMed] [Google Scholar]
- Finlay BB, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- Garcia-Patrone M, Tandecarz JS. A glycoprotein multimer from Bacillus thuringiensis sporangia: dissociation into subunits and sugar composition. Mol Cell Biochem. 1995;145:29–37. doi: 10.1007/BF00925710. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim W, Peerschke EIB, Willis AC, Hong Y, Reid KBM. Isolation, cDNA cloning and overexpression of a 33 kDa cell surface glycoprotein which binds to the globular “heads” of C1q (gC1q-R) J Exp Med. 1994;179:1809–1821. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EIB. gC1q-R/p33: a member of a new class of multifunctional and multicompartmental cellular proteins (MMCPs) is involved in inflammation and infection. Immunol Rev. 2001;180:65–77. doi: 10.1034/j.1600-065x.2001.1800106.x. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lu PD, Zhang W, Lim BL, Eggleton P, Leigh LEA, Reid KBM, Peerschke EIB. Identification of functional domains on gC1q-R, a cell surface protein, which binds to the globular “heads” of C1q, using monoclonal antibodies and synthetic peptides. Hybridoma. 1996;15:333–342. doi: 10.1089/hyb.1996.15.333. [DOI] [PubMed] [Google Scholar]
- Gilmore MS, Cruz-Rodz AL, Leimeister-Wachter M, Kreft J, Goebel WA. Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: Nucleotide sequence and genetic linkage. J Bacteriol. 1989;17:744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW, Dring JG. Mechanisms of spore heat resistance. In: Rose AH, Tempest DW, editors. Adv Microbial Physiol. Vol. 11. Academic press; London: 1974. p. 137. [Google Scholar]
- Harrington DJ. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun. 1996;64:1885–1891. doi: 10.1128/iai.64.6.1885-1891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E, Økstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, Hegna I, Koltsø A-B. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl Environm Microbiol. 2000;66:2627–26230. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa T, Yamazaki M, Yoshimi M, Ogawa S, Yamada A, Watabe K, Torii M. Surface hydrophobicity of spores of Bacillus species. J Gen Microbiol. 1989;135:2717–2722. doi: 10.1099/00221287-135-10-2717. [DOI] [PubMed] [Google Scholar]
- Matz LL, Beaman TC, Gerhardt P. Chemical composition of the exosporium from spores of B. cereus. J Bacteriol. 1970;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Ghebrehiwet B, Peerschke EIB. Staphylococcus aureus protein A recognizes platelet gC1q-R/p33: A novel mechanism for staphylococcal interactions with platelets. Infect Immun. 2000;84:2061–2068. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Day DM, Smith RS, Gregg CR, Turnbull PCB, Head WS, Ives JA, Ho PC. The problem of Bacillus species with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- Panessa-Warren B, Tortora G, Warren J. Exosporial membrane plasticity of Clostridium sporogenes and Clostridium difficile. Tissue and Cell. 1997;29:449–461. doi: 10.1016/s0040-8166(97)80031-6. [DOI] [PubMed] [Google Scholar]
- Panessa-Warren B, Tortora G, Warren J. Electron Microscopy of C. sporogenes endospore attachment and germination. Scanning. 1994;16:227–240. [Google Scholar]
- Panessa-Warren B, Tortora G, Wong SS, Ghebrehiwet B, Warren J. Microscopy & Microanalysis. suppl 2. Vol. 9. Cambridge University Press; NY: 2003. Bacillus and Clostridium Spore Attachment/Entry of Human Colon Cells; pp. 1378–1379. [Google Scholar]
- Panessa-Warren B, Wong SS, Ghebrehiwet B, Tortora GT, Warren J. Carbon Nanotube Membrane Probes: Immuno-labelling by LM, TEM & FESEM. In: Voelkl E, Piston D, Gauvin R, Lockley A, Bailey G, McKernan S, editors. Microscopy and Microanalysis. suppl 2. Vol. 8. Cambridge University Press; New York, N.Y: 2002. pp. 726–727CD. [Google Scholar]
- Rety S, Salamitou S, Garcia-Verdugo I, Hulmes DJS, Le Hagarat F, Chaby R, Lewit-Bentley A. The crystal structure of the Bacillus anthracis spore surface protein BclA shows remarkable similarity to mammalian proteins. J Biol Chem. 2005;280:43073–43078. doi: 10.1074/jbc.M510087200. [DOI] [PubMed] [Google Scholar]
- Schaeffer A, Fulton D. A simplified method for staining spores. Science. 1933;77:194–195. doi: 10.1126/science.77.1990.194. [DOI] [PubMed] [Google Scholar]
- Shany S, Bernheimer AW, Grushoff PS, Kim KW. Evidence for membrane cholesterol as the common binding site for cereolysin, strpetolysin O and saponin. Molec Cell Biochem. 1974;3:179–186. doi: 10.1007/BF01686643. [DOI] [PubMed] [Google Scholar]
- Soderling E, Pauino KU. Conditions of production and properties of the collagenolytic enzymes by two Bacillus strains from dental plaque. J Periodontal Res. 1981;16:513–523. [PubMed] [Google Scholar]
- Sylvestre P, Couture-Tosi E, Mock M. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Molec Microbiol. 2002;45:169–178. doi: 10.1046/j.1365-2958.2000.03000.x. [DOI] [PubMed] [Google Scholar]
- Todd SJ, Moir AJG, Johnson MJ, Moir A. Genes of Bacillus cereus and Bacillus anthracis encoding proteins of the exosporium. J Bacteriol. 2003;185(11):3373–3378. doi: 10.1128/JB.185.11.3373-3378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney EAS, Beatty ME, Taylor TH, Jr, Weyant R, Sobel J, Arduino MJ, Ashford DA. Inactivation of Bacillus anthracis spores. Emerging Infect Dis. 2003;9:623–627. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]